Abstract
We have previously shown that CD4+ T cell–mediated allergic inflammation is diminished in signal transducer and activator of transcription (Stat)5a-deficient (Stat5a−/−) mice. To determine whether Stat5a regulates T helper cell differentiation, we studied T helper (Th)1 and Th2 cell differentiation of Stat5a−/−CD4+ T cells at single-cell levels. First, Th2 cell differentiation from antigen-stimulated splenocytes was significantly decreased in Stat5a−/− mice as compared with that in wild-type mice. Further, Th2 cell differentiation was also impaired in Stat5a−/− mice even when purified CD4+ T cells were stimulated with anti-CD3 plus anti-CD28 antibodies in the presence of interleukin-4. Moreover, the retrovirus-mediated gene expression of Stat5a in Stat5a−/−CD4+ T cells restored the Th2 cell differentiation at the similar levels to that in wild-type CD4+ T cells. In addition, interleukin-4 normally phosphorylated Stat6 in CD4+ T cells from Stat5a−/− mice. Second, the development of CD4+CD25+ immunoregulatory T cells was impaired in Stat5a−/− mice, as indicated by a significant decrease in the number of CD4+CD25+ T cells in Stat5a−/− mice. Furthermore, the depletion of CD4+CD25+ T cells from wild-type splenocytes significantly decreased Th2 cell differentiation but increased Th1 cell differentiation, whereas the depletion of CD4+CD25+ T cells from Stat5a−/−splenocytes had no significant effect on the Th1 and Th2 cell differentiation. Together, these results indicate that the intrinsic expression of Stat5a in CD4+ T cells is required for Th2 cell differentiation and that Stat5a is involved in the development of CD4+CD25+ immunoregulatory T cells that modulate T helper cell differentiation toward Th2 cells.
Introduction
Newly activated CD4+ T helper cells differentiate into at least 2 functionally distinct subsets as defined by their pattern of cytokine production.1,2 T helper (Th)1 cells produce interferon γ (IFN-γ) and lymphotoxin, while Th2 cells produce interleukin 4 (IL-4), IL-5, IL-10, and IL-13.1,2Over the last few years, significant progress has been made in identification of the transcription factors that control the transition of T helper precursor cells into mature Th1 or Th2 cells.3-5 It has been clearly demonstrated that in mice lacking signal transducer and activator of transcription (Stat)4 and Stat6, these transcription factors play important roles in Th1 and Th2 cell differentiation, respectively.6,7 In addition to Stat6, it has recently been shown that several developmentally regulated transcription factors, such as GATA-3 and c-Maf, are critical for the generation of IL-4–producing Th2 cells.8,9 In contrast, ERM and T-bet have been shown to be involved in Th1 cell differentiation.10 11 However, the transcriptional regulation of T helper cell differentiation is still largely unknown.
Stat5a and Stat5b are cytosolic latent transcription factors that are activated by a very wide range of cytokines, including immunologically important cytokines, IL-2 and IL-7.12,13Although Stat5a and Stat5b are highly homologous, the different phenotypes of Stat5a−/− mice and Stat5b−/−mice underscore the distinctive roles of Stat5a and Stat5b.13 It has been demonstrated that, while Stat5a−/− T cells exhibit only a partial defect in anti-CD3–induced proliferation that can be overcome by high doses of IL-2,14 defective proliferation in Stat5b−/−T cells cannot be corrected by this treatment.15 On the other hand, Stat5a and Stat5b may also have overlapping functions because Stat5a/Stat5b double-deficient mice exhibit a severe defect in T-cell proliferation.16
Recently, we have shown that antigen-induced IL-5 production and subsequent eosinophil recruitment in the airways are severely decreased in Stat5a−/− mice.17 We have also shown that antigen-specific immunoglobulin E (IgE) and IgG1 production is diminished but antigen-specific IgG2a production is increased in Stat5a−/− mice.17 These findings suggest that T helper cell differentiation is biased toward Th1 type in Stat5a−/− mice. However, the precise role of Stat5a in T helper cell differentiation remains to be determined.
Therefore, to determine whether Stat5a regulates Th1 and Th2 cell differentiation, we studied T helper cell differentiation of Stat5a−/−CD4+ T cells at single-cell levels. We found that the development of Th2 cells was impaired in Stat5a−/−CD4+ T cells even in the presence of IL-4 and that the retrovirus-mediated expression of Stat5a restored Th2 cell differentiation in Stat5a−/−CD4+ T cells. We also found that the development of CD4+CD25+ immunoregulatory T cells, which promoted Th2 cell differentiation from CD4+ T cells, was impaired in Stat5a−/− mice. Together, these results indicate that Stat5a regulates T helper cell differentiation toward Th2 type by several distinct mechanisms.
Materials and methods
Mice
Stat5a-deficient (Stat5a−/−) mice18and Stat5b-deficient (Stat5b−/−) mice19 were backcrossed to BALB/c mice (Charles River Laboratories, Atsugi, Japan) for 7 generations. Ovalbumin (OVA)-specific DO11.10 (DO10+) T-cell receptor transgenic mice20 were backcrossed over 10 times onto BALB/c mice and crossed with Stat5a−/− mice. DO10+Stat5a−/− mice (H-2d/d) and the littermate DO10+ mice (H-2d/d) were analyzed in the following experiments. Stat6−/−mice21 in C57BL/6 background were kind gifts from Drs S. Akira and K. Takeda (Osaka University, Osaka, Japan). Mice were housed in microisolator cages under pathogen-free conditions. All experiments were performed according to the guidelines of Chiba University.
T-cell purification
Splenic CD4+ T cells were purified (> 90% pure by flow cytometry) using T-cell enrichment columns (R&D Systems, Minneapolis, MN) as described previously.22
Cell culture
Splenocytes (2 × 106 cells/mL) from wild-type mice, Stat5a−/− mice, or Stat5b−/− mice were stimulated with platebound anti-CD3ε monoclonal antibody (mAb) (5 μg/mL, clone 145-2C11, Pharmingen, San Diego, CA) in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 50 μM β-mercaptoethanol, 2 mM L-glutamine, and antibiotics in a 24-well microtiter plate at 37°C for 48 hours. Splenocytes (2 × 106 cells/mL) from DO10+ mice or DO10+Stat5a−/− mice were stimulated with OVA323-339 peptide (0.1 μg/mL) at 37°C for 48 hours. Purified splenic CD4+ T cells (1 × 106 cells/mL) from DO10+ mice or DO10+Stat5a−/− mice were also stimulated with platebound anti-CD3ε mAb plus anti-CD28 mAb (5 μg/mL, clone 37.51, Pharmingen) at 37°C for 48 hours. Where indicated, IL-12 (7.5 ng/mL, R&D Systems) was added to polarize toward Th1 cells (Th1 condition), and IL-4 (15 ng/mL, R&D Systems), anti–IL-12 mAb (15 μg/mL, clone C17.8, Pharmingen), and anti–IFN-γ mAb (15 μg/mL, clone XMG1.2, Pharmingen) were added to polarize toward Th2 cells (Th2 condition) as described previously.23 Cells were then washed with phosphate-buffered saline (PBS) and cultured for another 3 days in the presence of indicated cytokines without antigen stimulation. IL-2 (20 U/mL, R&D Systems) was added in the second culture to support the survival.
CFSE labeling of splenocytes
Splenocytes from DO10+ mice or DO10+Stat5a−/− mice were labeled with 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE, Molecular Probes, Eugene, OR) according to the manufacturer's instruction.
Retrovirus-mediated gene expression
Retrovirus-mediated gene expression for splenic T cells was performed as described elsewhere.24 Bicistronic retrovirus vectors, pMX-Stat5a-IRES-GFP, pMX-Stat5b-IRES-GFP, and pMX-IRES-GFP (as a negative control), were constructed as previously described.25 High-titer retroviruses (average titer 1 × 107/mL) were produced with a transient retrovirus packaging cell line, Plat-E,26 and stored at −80°C until use. A fraction of the retroviruses was tested for the ability of infection on Ba/F3 cells, and the retroviruses that reached over 70% of infection (as determined by green fluorescent protein [GFP]+ population using fluorescence-activated cell sorting [FACS]) were used in the following experiments. For infection on T cells, after purified CD4+ T cells from DO10+ mice or DO10+Stat5a−/− mice were stimulated with anti-CD3 mAb plus anti-CD28 mAb for 48 hours as described above, cells were washed with PBS and incubated with 500 μL of the retroviruses in the presence of IL-2 (20 U/mL) in a 24-well microtiter plate that was coated with recombinant fibronectin fragment CH-296, Retronectin (27 μg/mL, Takara, Ohtsu, Japan). After 4 hours of infection, 500 μL fresh medium was added to the culture and cells were allowed to grow for another 60 hours before being subjected to intracellular cytokine analysis. Under these conditions, the efficiency of infection to CD4+ T cells was 15% to 30% as assessed by GFP+ cells by FACS.
Flow cytometric analysis
Cells were stained and analyzed on a FACScaliber (Becton Dickinson, San Jose, CA) using CellQuest software. The following antibodies were purchased: anti-CD4 fluorescein isothiocynate (FITC), Allophycocyanin (APC), peridinin chlorophyll protein (PerCP) (RM4-5, Pharmingen), anti-CD25 FITC (7D4, Pharmingen), anti-CD25 phycoerythrin (PE) (PC61, Pharmingen), anti-γc PE (4G2, Pharmingen), anti-CDw124 (IL-4Rα) (1688-01, Genzyme, Cambridge, MA), KJI-26 FITC,22 and antirat IgG2a FITC (RG7/1.30, Pharmingen). Prior to staining, Fc receptors were blocked with anti-CD16/32 antibody (2.4G2, Pharmingen). Negative controls consisted of isotype-matched, directly conjugated, nonspecific antibodies (Pharmingen).
Intracellular cytokine analysis
Cultured cells were washed with PBS and restimulated with platebound anti-CD3 mAb at 37°C for 6 hours in the presence of IL-2 (10 U/mL), with monensin (2 μM) (Sigma, St Louis, MO) added for the final 4 hours to prevent cytokine release. Cells were harvested, washed with PBS, and stained with anti-CD4 PerCP for 30 minutes at 4°C. Cells were washed with PBS, fixed with IC Fix (Biosource International, Camarillo, CA), permeabilized with IC Perm (Biosource International), and stained with anti–IL-4 PE (BVD4-1D11, Pharmingen) and anti–IFN-γ APC (XMG1.2, Pharmingen) for 30 minutes at 4°C. After washing, cytokine profile (IL-4 vs IFN-γ) on CD4+ cells or CD4+GFP+ cells (in the case of retrovirus experiments) was analyzed on a FACScaliber using CellQuest software. In preliminary experiments, we found that these procedures did not significantly decrease the frequency of GFP+ cells within CD4+ T cells.
Western blotting
Cells were washed with PBS and lysed in lysis buffer (1% Nonidet P-40, 20 mM Tris-HCl [pH 8.0], 50 mM NaCl, 2 mM dithiothreitol, 4 mM EGTA, 10 mM NaF, 1 mM Na3VO4, 5 μg/mL aprotinin, 5 μg/mL leupeptin, 2 μg/mL pepstatin, 0.5 mM phenylmethylsulfonyl fluoride, and 10% glycerol) on ice for 30 minutes, and cell lysates were prepared by centrifugation. A total of 15 μg of cell lysates was separated on 6% or 10% sodium dodecyl sulfate polyacrylamide gels and transferred to Immobilon-P membranes (Millipore, Bedford, MA). After blocking with PBS containing 0.15% Tween 20 and 3% bovine serum albumin for 1 hour at room temperature, the membranes were incubated with antisera to mouse Stat5 (Transduction Laboratories, Lexington, KY), mouse Stat6 (M-200) (Santa Cruz Biotechnology, Santa Cruz, CA), phospho-Stat5 (New England Biolabs, Beverly, MA), or phospho-Stat6 (New England Biolabs) for 1 hour at room temperature. After washing 3 times with PBS containing 0.15% Tween 20, the membranes were incubated with antirabbit IgG or antimouse IgG antibodies conjugated with horseradish peroxidase (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) in PBS containing 0.15% Tween 20 and 3% bovine serum albumin for 1 hour at room temperature. The membranes were then washed with PBS containing 0.15% Tween 20 and developed with an enhanced chemiluminescent substrate (Roche Diagnostics, Mannheim, Germany).
Depletion of CD4+CD25+ T cells from splenocytes
Splenocytes from DO10+ mice or DO10+Stat5a−/− mice were incubated with biotinylated anti-CD25 antibody (clone PC61, Immunotech, Marseilles, France) at 4°C for 20 minutes. After washing with PBS, cells were incubated with streptavidin-magnetic microbeads (Miltenyi Biotec, Sunnyvale, CA) at 4°C for 20 minutes, washed 3 times with PBS, and passed through the magnetic-activated cell separation BS column (Miltenyi Biotec) according to the manufacturer's instructions. As a control, cells were incubated with isotype-matched biotinylated antibody (Pharmingen), followed by the incubation with streptavidin-magnetic microbeads, and passed through the BS column. The efficiency of depletion was evaluated by FACS with anti-CD4 APC and anti-CD25 FITC (clone 7D4, Pharmingen), which recognizes the different epitope of murine CD25 from that of PC61.27 More than 99% of CD4+CD25+ T cells were removed from splenocytes by this procedure, whereas other cell types, including CD4+CD25− T cells, CD8+ cells, B220+ cells, Mac-1+ cells, and NK cells, were not eliminated (data not shown).
Data analysis
Data are summarized as mean ± SD. The statistical analysis of the results was performed by the unpaired t test.P values below .05 were considered significant.
Results
Development of Th2 cells from splenocytes is decreased in Stat5a−/− mice
We have previously shown that antigen-induced IL-5 production and subsequent eosinophil recruitment in the airways are decreased in Stat5a−/− mice,17 suggesting that Th2 cell–mediated immune responses are diminished in Stat5a−/− mice. Therefore, to determine whether Stat5a plays a regulatory role in T helper cell differentiation, we first examined T helper cell differentiation in Stat5a−/− mice at single-cell levels by intracellular cytokine analysis. When stimulated with anti-CD3 mAb, CD4+ T cells that produced IL-4 but not IFN-γ (Th2 cells) were significantly decreased in Stat5a−/− mice as compared with those in wild-type mice (wild-type mice 6.0% ± 1.1% vs Stat5a−/− mice 0.2% ± 0.1%, mean ± SD, n = 5 mice in each group,P < .001) (Figure 1). In contrast, CD4+ T cells that produced IFN-γ but not IL-4 (Th1 cells) were significantly increased in Stat5a−/−mice (wild-type mice 25.8% ± 3.7% vs Stat5a−/− mice 42.0% ± 4.9%, P < .001) (Figure 1), suggesting that T helper cell differentiation is biased toward Th1 cells in Stat5a−/− mice. In contrast, both Th1 cells and Th2 cells were decreased in Stat5b−/− mice as compared with those in wild-type mice (Figure 1).
Th2 cell differentiation is decreased in Stat5a−/− T cells.
Splenocytes from wild-type (WT) mice, Stat5a−/− mice, or Stat5b−/− mice were stimulated with platebound anti-CD3 mAb for 48 hours in nonpolarizing condition. Cells were cultured in the presence of IL-2 for another 3 days and then restimulated with platebound anti-CD3 mAb for 6 hours. Intracellular cytokine profiles for IL-4 versus IFN-γ were determined on CD4+ T cells as described in “Materials and methods.” Shown are representative FACS profiles from 5 mice in each group.
Th2 cell differentiation is decreased in Stat5a−/− T cells.
Splenocytes from wild-type (WT) mice, Stat5a−/− mice, or Stat5b−/− mice were stimulated with platebound anti-CD3 mAb for 48 hours in nonpolarizing condition. Cells were cultured in the presence of IL-2 for another 3 days and then restimulated with platebound anti-CD3 mAb for 6 hours. Intracellular cytokine profiles for IL-4 versus IFN-γ were determined on CD4+ T cells as described in “Materials and methods.” Shown are representative FACS profiles from 5 mice in each group.
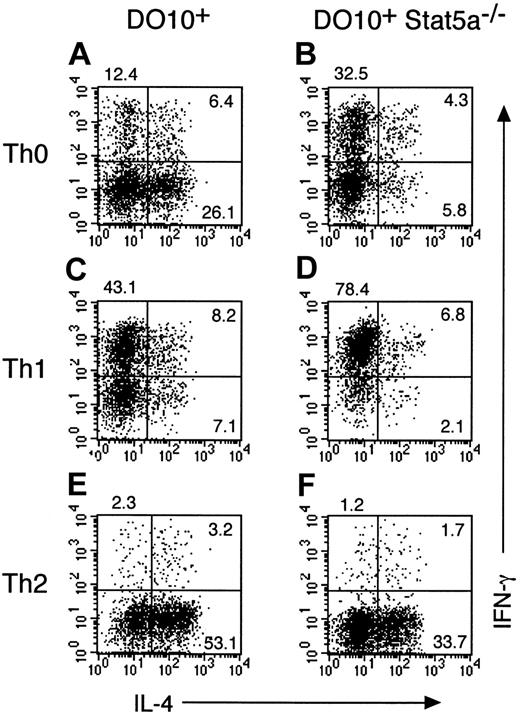
To address the role of Stat5a in T helper cell differentiation in more detail, Stat5a−/− mice were crossed with OVA-specific DO10+ T-cell receptor transgenic mice, and antigen-specific T helper cell differentiation in DO10+Stat5a−/− mice was compared with that in the littermate DO10+ mice. Consistent with previous reports on Stat5a−/− mice,14 15 the number of splenocytes in DO10+Stat5a−/− mice was approximately 70% of DO10+ mice. However, no significant differences were observed in the frequency of CD4+ T cells, CD8+ T cells, B220+ cells, and Mac-1+ cells between DO10+ mice and DO10+Stat5a−/− mice (data not shown). When splenocytes from DO10+ mice or DO10+Stat5a−/− mice were stimulated with antigenic peptide (OVA323-339) in nonpolarizing Th0 condition, Th2 cells were significantly decreased in DO10+Stat5a−/− mice as compared with those in DO10+ mice (DO10+ mice 25.4% ± 5.2% vs DO10+Stat5a−/− mice 5.5% ± 1.4%, n = 5 mice in each group, P < .001) (Figure2A,B). In contrast, Th1 cells were significantly increased in DO10+Stat5a−/−mice (DO10+ mice 12.0% ± 2.2% vs DO10+Stat5a−/− mice 31.6% ± 5.2%,P < .001) (Figure 2A,B). Moreover, IL-4 and IL-5 levels were decreased, but IFN-γ levels were increased in the culture supernatant of splenocytes in DO10+Stat5a−/−mice (data not shown). These results indicate that the expression of Stat5a modulates antigen-induced T helper cell differentiation toward Th2 cells.
Development of Th2 cells from splenocytes is decreased in DO10+ T cell receptor transgenic Stat5a−/−mice.
Splenocytes from DO10+ mice (A,C,E) or DO10+Stat5a−/− mice (B,D,F) were stimulated with OVA323-339 peptide (0.1 μg/mL) for 48 hours in Th0- (A,B), Th1- (C,D), or Th2- (E,F) polarizing condition. Cells were cultured in the presence of IL-2 for another 3 days and then restimulated with platebound anti-CD3 mAb for 6 hours. Intracellular cytokine profiles for IL-4 versus IFN-γ were determined on CD4+ T cells as described in “Materials and methods.” Shown are representative FACS profiles from 5 mice in each group.
Development of Th2 cells from splenocytes is decreased in DO10+ T cell receptor transgenic Stat5a−/−mice.
Splenocytes from DO10+ mice (A,C,E) or DO10+Stat5a−/− mice (B,D,F) were stimulated with OVA323-339 peptide (0.1 μg/mL) for 48 hours in Th0- (A,B), Th1- (C,D), or Th2- (E,F) polarizing condition. Cells were cultured in the presence of IL-2 for another 3 days and then restimulated with platebound anti-CD3 mAb for 6 hours. Intracellular cytokine profiles for IL-4 versus IFN-γ were determined on CD4+ T cells as described in “Materials and methods.” Shown are representative FACS profiles from 5 mice in each group.
We further examined the effect of Th1 or Th2 polarization on T helper cell differentiation in DO10+Stat5a−/− mice. In Th1-polarizing condition (the presence of IL-12), the development of Th1 cells was significantly enhanced in DO10+Stat5a−/− mice as compared with that in DO10+ mice (DO10+ mice 42.0% ± 4.9% vs DO10+Stat5a−/− mice 75.6% ± 8.8%, n = 5, P < .001) (Figure 2C,D). In contrast, in Th2-polarizing condition (the presence of IL-4, anti–IL-12 mAb, and anti–IFN-γ mAb), the development of Th2 cells was significantly decreased in DO10+Stat5a−/− mice (DO10+ mice 54.5% ± 6.8% vs DO10+Stat5a−/− mice 32.5% ± 5.9%, n = 5, P < .002) (Figure 2E,F), suggesting that the possible defect in IL-4 production at an early phase of immune responses was not solely responsible for the decreased Th2 cell differentiation in DO10+Stat5a−/− mice.
Development of Th2 cells from purified CD4+ T cells is impaired in Stat5a−/− mice
To determine whether the intrinsic expression of Stat5a in CD4+ T cells is required for Th2 cell differentiation, we examined the T helper cell differentiation using purified CD4+ T cells from DO10+ mice or DO10+Stat5a−/− mice. While Th2 cells were readily observed when CD4+ T cells were stimulated with antigenic peptide in the presence of antigen-presenting cells (Figure2A), almost no Th2 cells were observed when purified CD4+ T cells were stimulated with anti-CD3 mAb plus anti-CD28 mAb (Figure3A). As a result, no significant difference was observed in the frequency of Th1 cells and Th2 cells in DO10+ mice and DO10+Stat5a−/−mice in nonpolarizing Th0 condition (n = 4 mice, each) (Figure 3A,B). In addition, similar frequency of Th1 cells was observed in DO10+ mice and DO10+Stat5a−/−mice in Th1-polarizing condition (Figure 3C and 3D). Interestingly, in contrast to Th1 cell differentiation, Th2 cell differentiation was significantly decreased in DO10+Stat5a−/−mice even when purified CD4+ T cells were stimulated with anti-CD3 mAb plus anti-CD28 mAb in Th2-polarizing condition (DO10+ mice 30.0% ± 3.8% vs DO10+Stat5a−/− mice 13.7% ± 2.3%, n = 4, P < .001) (Figure 3E,F). These results indicate that the intrinsic expression of Stat5a in CD4+ T cells is required for the IL-4–dependent Th2 cell differentiation.
Development of Th2 cells from purified CD4+T cells is decreased in Stat5a−/− mice.
Purified splenic CD4+ T cells from DO10+ mice (A,C,E) or DO10+Stat5a−/− mice (B,D,F) were stimulated with platebound anti-CD3 mAb and anti-CD28 mAb for 48 hours in Th0-, Th1-, or Th2-polarizing condition. Activated T cells were cultured in the presence of IL-2 for another 3 days and then restimulated with platebound anti-CD3 mAb for 6 hours. Intracellular cytokine profiles for IL-4 versus IFN-γ were determined on CD4+ T cells as described in “Materials and methods.” Shown are representative FACS profiles from 4 mice in each group.
Development of Th2 cells from purified CD4+T cells is decreased in Stat5a−/− mice.
Purified splenic CD4+ T cells from DO10+ mice (A,C,E) or DO10+Stat5a−/− mice (B,D,F) were stimulated with platebound anti-CD3 mAb and anti-CD28 mAb for 48 hours in Th0-, Th1-, or Th2-polarizing condition. Activated T cells were cultured in the presence of IL-2 for another 3 days and then restimulated with platebound anti-CD3 mAb for 6 hours. Intracellular cytokine profiles for IL-4 versus IFN-γ were determined on CD4+ T cells as described in “Materials and methods.” Shown are representative FACS profiles from 4 mice in each group.
Impaired T helper cell differentiation in Stat5a−/−CD4+ T cells is independent of cell-cycle progression
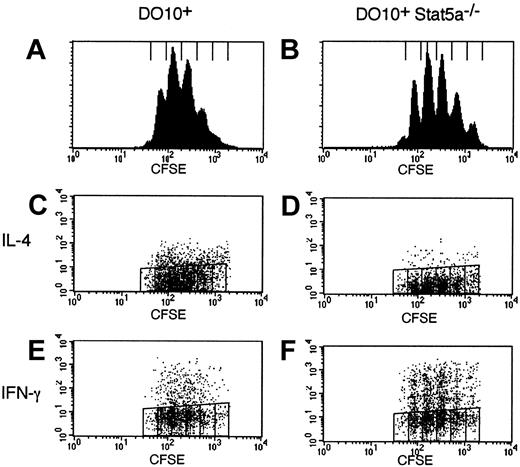
Recently, it has been suggested that the frequency of Th2 cells is correlatively increased in CD4+ T cells with cell divisions.28,29 Thus, the possible defect of cell-cycle progression in Stat5a−/−CD4+ T cells might be involved in the increased Th1 cell differentiation in Stat5a−/− mice. Therefore, we examined cell-cycle progression of activated CD4+ T cells in DO10+Stat5a−/− mice using CFSE labeling methods.30 As shown in Figure4, the frequency of cell division was indistinguishable between DO10+CD4+ T cells and DO10+Stat5a−/−CD4+ T cells (Figure 4A vs 4B). Moreover, the frequency of IL-4–producing cells was decreased but that of IFN-γ-producing cells was increased in DO10+Stat5a−/− mice as compared with those in DO10+ mice regardless of the rounds of cell-cycle progression (Figure 4C vs 4D for IL-4, Figure 4E vs 4F for IFN-γ). Thus, these results indicate that the impaired T helper cell differentiation in Stat5a−/−CD4+ T cells is independent of cell-cycle progression.
Impaired Th2 cell differentiation in Stat5a−/− T cells is cell-cycle independent.
Splenocytes from DO10+ mice (A,C,E) or DO10+Stat5a−/− mice (B,D,F) were labeled with CFSE and stimulated with platebound anti-CD3 mAb for 60 hours in nonpolarizing condition. Intracellular staining for cytokines was performed as described in “Materials and methods.” Shown are representative FACS profiles (panels A,B for the intensity of CFSE; C,D for IL-4 versus CFSE staining; and E,F for IFN-γ versus CFSE staining of CD4+ T cells) from 4 mice in each group.
Impaired Th2 cell differentiation in Stat5a−/− T cells is cell-cycle independent.
Splenocytes from DO10+ mice (A,C,E) or DO10+Stat5a−/− mice (B,D,F) were labeled with CFSE and stimulated with platebound anti-CD3 mAb for 60 hours in nonpolarizing condition. Intracellular staining for cytokines was performed as described in “Materials and methods.” Shown are representative FACS profiles (panels A,B for the intensity of CFSE; C,D for IL-4 versus CFSE staining; and E,F for IFN-γ versus CFSE staining of CD4+ T cells) from 4 mice in each group.
IL-4 normally phosphorylates Stat6 in the absence of Stat5a
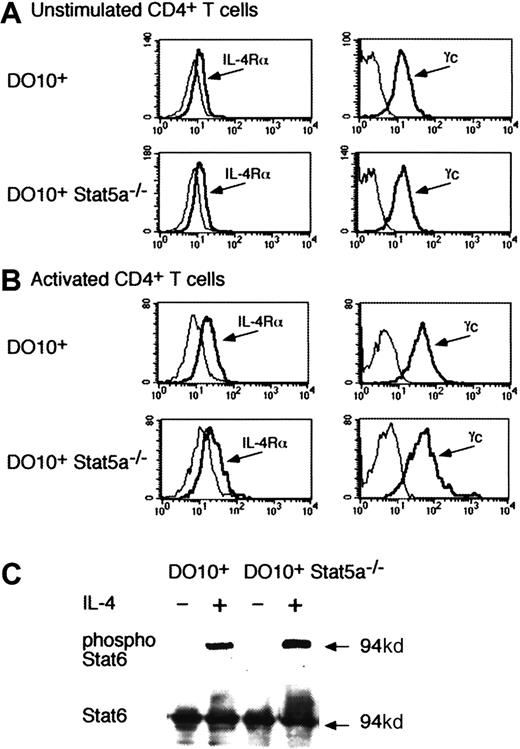
Because the development of Th2 cells was decreased in DO10+Stat5a−/−CD4+ T cells even in the presence of IL-4 (Figures 2 and 3), it was possible that IL-4 signaling was impaired in Stat5a−/−CD4+ T cells. Therefore, we analyzed the expression of IL-4 receptor components (IL-4Rα and γc) and IL-4–induced Stat6 phosphorylation in DO10+Stat5a−/− mice. As shown in Figure5, no significant difference was observed in the expression of IL-4Rα on CD4+ T cells between DO10+ mice and DO10+Stat5a−/−mice (Figure 5A for unstimulated CD4+ T cells and Figure 5B for activated CD4+ T cells). The expression of γc was also normal in CD4+ T cells from DO10+Stat5a−/− mice (Figure 5A,B). Moreover, Stat6 was equally expressed and phosphorylated upon IL-4 stimulation in CD4+ T cells from DO10+ mice and DO10+Stat5a−/− mice (Figure 5C).
IL-4 phosphorylates Stat6 in the absence of Stat5a.
(A,B) Expression of IL-4 receptor components on Stat5a−/−CD4+ T cells. Freshly isolated splenic CD4+ T cells (A) or activated CD4+ T cells that were stimulated by anti-CD3 mAb and anti-CD28 mAb for 48 hours (B) from DO10+ mice or DO10+Stat5a−/− mice were analyzed for the expression of IL-4Rα and γc by FACS. Shown are representative FACS profiles of IL-4Rα (left panels) and γc (right panels) staining from 4 independent experiments. Dashed lines are FACS profiles for the isotype-matched controls. (C) IL-4 induces Stat6 phosphorylation in the absence of Stat5a. Purified splenic CD4+ T cells from DO10+ mice or DO10+Stat5a−/− mice were stimulated with platebound anti-CD3 mAb and anti-CD28 mAb for 48 hours. Cells were starved for 2 hours from cytokines and stimulated with IL-4 (20 ng/mL) for 15 minutes. Shown is representative antiphospho-Stat6 (upper panel) and anti-Stat6 (lower panel) Western blotting from 4 independent experiments. Stat6 was also equally phosphorylated in DO10+CD4+ T cells and DO10+Stat5a−/−CD4+ T cells 30 minutes after IL-4 stimulation (data not shown).
IL-4 phosphorylates Stat6 in the absence of Stat5a.
(A,B) Expression of IL-4 receptor components on Stat5a−/−CD4+ T cells. Freshly isolated splenic CD4+ T cells (A) or activated CD4+ T cells that were stimulated by anti-CD3 mAb and anti-CD28 mAb for 48 hours (B) from DO10+ mice or DO10+Stat5a−/− mice were analyzed for the expression of IL-4Rα and γc by FACS. Shown are representative FACS profiles of IL-4Rα (left panels) and γc (right panels) staining from 4 independent experiments. Dashed lines are FACS profiles for the isotype-matched controls. (C) IL-4 induces Stat6 phosphorylation in the absence of Stat5a. Purified splenic CD4+ T cells from DO10+ mice or DO10+Stat5a−/− mice were stimulated with platebound anti-CD3 mAb and anti-CD28 mAb for 48 hours. Cells were starved for 2 hours from cytokines and stimulated with IL-4 (20 ng/mL) for 15 minutes. Shown is representative antiphospho-Stat6 (upper panel) and anti-Stat6 (lower panel) Western blotting from 4 independent experiments. Stat6 was also equally phosphorylated in DO10+CD4+ T cells and DO10+Stat5a−/−CD4+ T cells 30 minutes after IL-4 stimulation (data not shown).
IL-4 does not phosphorylate Stat5 even in the absence of Stat6
Recently, it was reported that IL-4 activated Stat5 in a pro-B cell line and that the activation of Stat5 was involved in IL-4–induced cell proliferation.31 Thus, it was possible that Stat5a was activated directly by IL-4 and enhanced the IL-4–induced Th2 cell expansion. To determine whether IL-4 activates Stat5 in CD4+ T cells, we analyzed the phosphorylation of Stat5 in IL-4–stimulated CD4+ T cells. Inconsistent with the previous report,31 no Stat5 phosphorylation was detected by IL-4 stimulation in wild-type CD4+ T cells (Figure 6). To exclude the possibility that Stat5 activation via IL-4R (IL-4 receptor) was blocked by the presence of Stat6, we analyzed IL-4–induced phosphorylation of Stat5 in Stat6−/−CD4+ T cells. As shown in Figure6, IL-4 did not phosphorylate Stat5 even in the absence of Stat6. In contrast, IL-2 efficiently phosphorylated Stat5 in both wild-type CD4+ T cells and Stat6−/−CD4+ T cells (Figure 6). As expected, both Stat5a and Stat5b were equally expressed in wild-type CD4+ T cells and Stat6−/−CD4+ T cells (Figure 6). Therefore, it is unlikely that the impaired Th2 cell differentiation results from the direct involvement of Stat5a in IL-4 signaling.
IL-4 does not phosphorylate Stat5 even in the absence of Stat6.
Splenic CD4+ T cells were purified from wild-type (WT) mice or Stat6−/− mice and stimulated with platebound anti-CD3 mAb and anti-CD28 mAb for 48 hours. Cells were starved for 2 hours and stimulated with either IL-2 (5 ng/mL) or IL-4 (20 ng/mL) for 15 minutes. Shown is representative antiphospho-Stat5 (upper panel) and antipan Stat5 (lower panel) Western blotting from 4 independent experiments. Note that only a single band was detected with antiphospho-Stat5 antiserum, which was produced by immunization with a synthetic phosphopeptide corresponding to residues around Tyr694 of mouse Stat5a. The mobility of the band is identical to that of Stat5a but not of Stat5b.
IL-4 does not phosphorylate Stat5 even in the absence of Stat6.
Splenic CD4+ T cells were purified from wild-type (WT) mice or Stat6−/− mice and stimulated with platebound anti-CD3 mAb and anti-CD28 mAb for 48 hours. Cells were starved for 2 hours and stimulated with either IL-2 (5 ng/mL) or IL-4 (20 ng/mL) for 15 minutes. Shown is representative antiphospho-Stat5 (upper panel) and antipan Stat5 (lower panel) Western blotting from 4 independent experiments. Note that only a single band was detected with antiphospho-Stat5 antiserum, which was produced by immunization with a synthetic phosphopeptide corresponding to residues around Tyr694 of mouse Stat5a. The mobility of the band is identical to that of Stat5a but not of Stat5b.
Retrovirus-mediated expression of Stat5a restores Th2 cell differentiation in Stat5a−/− mice
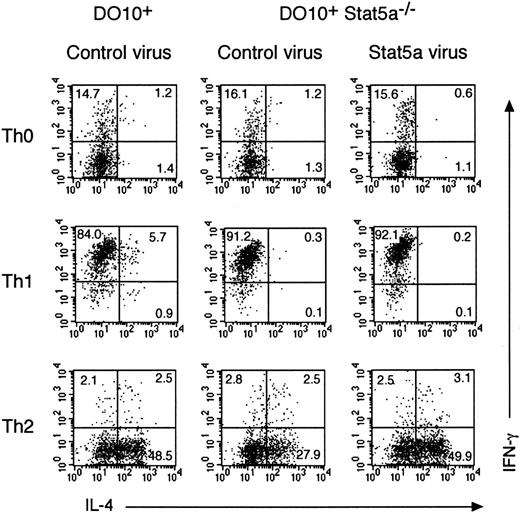
To determine whether enforced expression of Stat5a rescues Th2 cell differentiation in DO10+Stat5a−/− mice, we employed a bicistronic retrovirus-mediated gene expression system in which infected cells were identified by coexpressed GFP.32Purified CD4+ T cells from DO10+Stat5a−/− mice were stimulated with anti-CD3 mAb plus anti-CD28 mAb and infected with either Stat5a or control retrovirus in either Th0-, Th1-, or Th2-polarizing condition. As a control, CD4+ T cells from DO10+ mice were stimulated with anti-CD3 mAb plus anti-CD28 mAb and infected with control retrovirus. Three days after infection, cells were analyzed for intracellular cytokines (IL-4 vs IFN-γ) on GFP-expressing CD4+ T cells (GFP+CD4+ T cells) (Figure 7). As shown in the upper panels of Figure 7, the expression of Stat5a itself did not induce Th2 cell differentiation in DO10+Stat5a−/−CD4+ T cells in Th0 condition (Figure 7). However, the retrovirus-mediated expression of Stat5a rescued Th2 cell differentiation in DO10+Stat5a−/−CD4+ T cells at the similar levels to that in DO10+CD4+ T cells in Th2-polarizing condition (n = 4 experiments) (Figure 7, lower panels). In contrast to infected GFP+CD4+ T cells, uninfected GFP−CD4+ T cells were not affected for their T helper cell differentiation by the presence of GFP+CD4+ T cells (data not shown). These results suggest that the intrinsic expression of Stat5a is required for the appropriate Th2 cell differentiation of CD4+ T cells and that soluble factor(s) from Stat5a-expressing GFP+CD4+ T cells is not sufficient for the Th2 cell differentiation of uninfected GFP−CD4+ T cells. On the other hand, the retrovirus-mediated expression of Stat5a did not affect the Th2 cell differentiation of CD4+ T cells in DO10+ mice (data not shown), suggesting that the endogenous levels of Stat5 protein are sufficient for Th2 cell differentiation. Moreover, the enforced expression of Stat5a did not affect the Th1 cell differentiation in DO10+Stat5a−/−CD4+ T cells in Th1-polarizing condition (Figure 7).
Retrovirus-mediated gene transduction of Stat5a rescues Th2 cell differentiation in Stat5a−/−CD4+ T cells.
Purified splenic CD4+ T cells from DO10+ mice or DO10+Stat5a−/− mice were stimulated with platebound anti-CD3 mAb and anti-CD28 mAb for 48 hours in Th0-, Th1-, or Th2-polarizing condition. Activated T cells were then infected with retroviruses of pMX-Stat5a-IRES-GFP or pMX-IRES-GFP (as a negative control) as described in “Materials and methods.” Cells were cultured with IL-2 for another 60 hours and then restimulated with platebound anti-CD3 antibody for 6 hours. Intracellular cytokine profiles for IL-4 versus IFN-γ were evaluated on infected CD4+ T cells (GFP+CD4+ cells). Shown is representative intracellular cytokine staining from 4 independent experiments.
Retrovirus-mediated gene transduction of Stat5a rescues Th2 cell differentiation in Stat5a−/−CD4+ T cells.
Purified splenic CD4+ T cells from DO10+ mice or DO10+Stat5a−/− mice were stimulated with platebound anti-CD3 mAb and anti-CD28 mAb for 48 hours in Th0-, Th1-, or Th2-polarizing condition. Activated T cells were then infected with retroviruses of pMX-Stat5a-IRES-GFP or pMX-IRES-GFP (as a negative control) as described in “Materials and methods.” Cells were cultured with IL-2 for another 60 hours and then restimulated with platebound anti-CD3 antibody for 6 hours. Intracellular cytokine profiles for IL-4 versus IFN-γ were evaluated on infected CD4+ T cells (GFP+CD4+ cells). Shown is representative intracellular cytokine staining from 4 independent experiments.
Retrovirus-mediated expression of Stat5b also restores Th2 cell differentiation in Stat5a−/− mice
To determine whether the role of Stat5a in Th2 cell differentiation is specific to Stat5a, we examined the effect of retrovirus-mediated expression of Stat5b on the Th2 cell differentiation of CD4+ T cells in DO10+Stat5a−/− mice. Interestingly, the retrovirus-mediated expression of Stat5b rescued Th2 cell differentiation in DO10+Stat5a−/−CD4+ T cells as efficiently as Stat5a-expressing retroviruses (Figure8). These results suggest that the role of Stat5a in Th2 cell differentiation is shared with that of Stat5b and that the expression levels of Stat5 proteins may be important for the regulation of Th2 cell differentiation.
Retrovirus-mediated expression of Stat5b restores Th2 cell differentiation in Stat5a−/−CD4+ T cells.
Purified splenic CD4+ T cells from DO10+Stat5a−/− mice were stimulated with platebound anti-CD3 mAb and anti-CD28 mAb for 48 hours in Th2-polarizing condition. Activated T cells were then infected with retroviruses of pMX-Stat5a-IRES-GFP, pMX-Stat5b-IRES-GFP, or pMX-IRES-GFP (as a negative control) as described in “Materials and methods.” Cells were cultured with IL-2 for another 60 hours and then restimulated with platebound anti-CD3 antibody for 6 hours. Intracellular cytokine profiles for IL-4 versus IFN-γ were evaluated on infected CD4+ T cells (GFP+CD4+cells). Shown is representative intracellular cytokine staining from 4 independent experiments.
Retrovirus-mediated expression of Stat5b restores Th2 cell differentiation in Stat5a−/−CD4+ T cells.
Purified splenic CD4+ T cells from DO10+Stat5a−/− mice were stimulated with platebound anti-CD3 mAb and anti-CD28 mAb for 48 hours in Th2-polarizing condition. Activated T cells were then infected with retroviruses of pMX-Stat5a-IRES-GFP, pMX-Stat5b-IRES-GFP, or pMX-IRES-GFP (as a negative control) as described in “Materials and methods.” Cells were cultured with IL-2 for another 60 hours and then restimulated with platebound anti-CD3 antibody for 6 hours. Intracellular cytokine profiles for IL-4 versus IFN-γ were evaluated on infected CD4+ T cells (GFP+CD4+cells). Shown is representative intracellular cytokine staining from 4 independent experiments.
CD4+CD25+ immunoregulatory T cells are decreased in Stat5a−/− mice
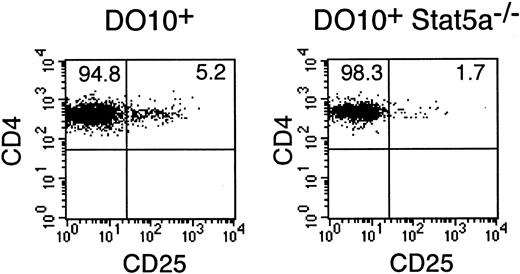
Because Stat5a is known to potently induce the expression of CD25 by directly binding to the 5′ regulatory region of the CD25 gene,33,34 we then examined the development of CD4+CD25+ T cells, which represent a unique population of immunoregulatory T cells and constitutively express CD25,35 36 in DO10+Stat5a−/−mice. Interestingly, the number of CD4+CD25+ T cells was significantly decreased in DO10+Stat5a−/− mice as compared with that in DO10+ mice (DO10+ mice 5.4% ± 0.8% vs DO10+Stat5a−/− mice 1.7% ± 0.3% in CD4+ T cells, n = 5 mice in each group,P < .001) (Figure 9).
CD4+CD25+ T cells are decreased in DO10+Stat5a−/− mice.
Splenocytes from DO10+ mice and DO10+Stat5a−/− mice were stained with anti-CD25 PE, KJI-26 FITC, and anti-CD4 APC. Shown is representative CD4 versus CD25 staining on CD4+ KJI-26+splenocytes from 5 independent experiments.
CD4+CD25+ T cells are decreased in DO10+Stat5a−/− mice.
Splenocytes from DO10+ mice and DO10+Stat5a−/− mice were stained with anti-CD25 PE, KJI-26 FITC, and anti-CD4 APC. Shown is representative CD4 versus CD25 staining on CD4+ KJI-26+splenocytes from 5 independent experiments.
Depletion of CD4+CD25+ T cells prevents Th2 cell differentiation
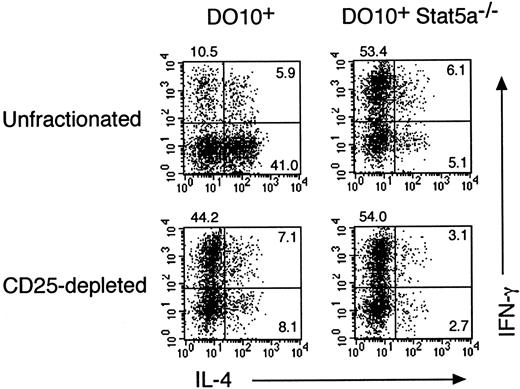
We have recently shown that Th2 cell–mediated eosinophil recruitment into the airways is decreased by the depletion of CD4+CD25+ immunoregulatory T cells (A. Suto, unpublished data, 2000), suggesting that CD4+CD25+ immunoregulatory T cells modulate T helper cell differentiation toward Th2-type. To determine whether CD4+CD25+ T cells regulate the differentiation of Th1 and Th2 cells in the presence or absence of Stat5a, we further examined the effect of CD4+CD25+ T-cell depletion on antigen-induced Th1 and Th2 cell differentiation from splenocytes in DO10+ mice and DO10+Stat5a−/− mice. As shown in the left panels of Figure 10, the depletion of CD4+CD25+ T cells from DO10+splenocytes significantly decreased the development of Th2 cells (41.0% vs 8.1%) but increased the development of Th1 cells (10.5% vs 44.2%). However, the depletion of CD4+CD25+ T cells from DO10+Stat5a−/− splenocytes had no effect on the differentiation of Th1 cells and Th2 cells (Figure 10, right panels). Taken together, these results suggest that the decreased CD4+CD25+ T cells may account in part for the decreased Th2 cell differentiation of CD4+ T cells in DO10+Stat5a−/− mice.
Depletion of CD4+CD25+T cells increases Th1 cell differentiation but decreases Th2 cell differentiation.
CD4+CD25+ T cells were depleted from splenocytes of DO10+ mice and DO10+Stat5a−/− mice by magnetic cell sorting as described in “Materials and methods.” CD4+CD25+ T cell–depleted or unfractionated splenocytes were stimulated with OVA323-339 peptide (0.1 μg/mL) for 48 hours. Cells were cultured with IL-2 for another 3 days and then restimulated with platebound anti-CD3 antibody for 6 hours. Intracellular staining for IL-4 and IFN-γ on CD4+ T cells was then analyzed by FACS. Shown are representative FACS profiles from 5 mice in each group.
Depletion of CD4+CD25+T cells increases Th1 cell differentiation but decreases Th2 cell differentiation.
CD4+CD25+ T cells were depleted from splenocytes of DO10+ mice and DO10+Stat5a−/− mice by magnetic cell sorting as described in “Materials and methods.” CD4+CD25+ T cell–depleted or unfractionated splenocytes were stimulated with OVA323-339 peptide (0.1 μg/mL) for 48 hours. Cells were cultured with IL-2 for another 3 days and then restimulated with platebound anti-CD3 antibody for 6 hours. Intracellular staining for IL-4 and IFN-γ on CD4+ T cells was then analyzed by FACS. Shown are representative FACS profiles from 5 mice in each group.
Discussion
In this study, we first show that Stat5a modulates T helper cell differentiation of CD4+ T cells toward Th2 cells. We found that Th2 cell differentiation of CD4+ T cells was significantly decreased in DO10+Stat5a−/−mice as compared with that in DO10+ mice even in the presence of IL-4 (Figures 2 and 3). Moreover, we found that the retrovirus-mediated gene expression of Stat5a in Stat5a−/−CD4+ T cells rescued the Th2 cell differentiation at the similar levels to that in wild-type CD4+ T cells (Figure 7). Taken together, these results indicate that the intrinsic expression of Stat5a in CD4+ T cells is required for the appropriate Th2 cell differentiation.
Second, we also show that Stat5a is involved in the development of CD4+CD25+ immunoregulatory T cells, as indicated by a significant decrease in the number of CD4+CD25+ T cells in DO10+Stat5a−/− mice (Figure 9). Furthermore, we show that CD4+CD25+ immunoregulatory T cells modulate T helper cell differentiation toward Th2 type. We found that the depletion of CD4+CD25+ T cells from DO10+ splenocytes significantly decreased Th2 cell differentiation but increased Th1 cell differentiation (Figure 10). Consequently, the cytokine profile of CD4+CD25+T cell–depleted DO10+ splenocytes resembled that of unfractionated DO10+Stat5a−/− splenocytes (Figure 10). In contrast, the depletion of CD4+CD25+ T cells from DO10+Stat5a−/− splenocytes did not significantly affect the Th1 and Th2 cell differentiation (Figure 10). Therefore, the decreased CD4+CD25+ T cells may account in part for the decreased Th2 cell differentiation of CD4+ T cells in DO10+Stat5a−/−mice (Figure 2).
Because Th2 cell differentiation was impaired in CD4+ T cells from Stat5a−/− mice even in the presence of high concentrations of IL-4 (Figures 2 and 3), we examined IL-4–induced signaling in Stat5a−/−CD4+ T cells. We found, however, that the expression of IL-4 receptors (IL-4Rα and γc) as well as IL-4–induced phosphorylation of Stat6 was normal in Stat5a−/−CD4+ T cells (Figure 5), suggesting that the IL-4/Stat6 pathway for Th2 cell differentiation is normal in Stat5a−/− mice. In addition, it has recently been reported that Stat5 is activated directly by IL-4 in a pro-B cell line and that IL-4–induced Stat5 activation might be involved in cell expansion.31 However, we found that IL-4 did not phosphorylate Stat5a in CD4+ T cells even in the absence of Stat6 (Figure 6). Therefore, it is unlikely that the direct activation of Stat5a by IL-4 stimulation is responsible for the decreased Th2 cell differentiation in Stat5a−/− mice.
The precise mechanisms by which Stat5a promotes Th2 cell differentiation are unclear. Stat5 has been shown to up-regulate a number of molecules, including cytokine-inducible SH2 proteins (CIS family, also referred to as the SOCS or SSI family).37 Accumulating evidence suggests that some CIS family proteins might be involved in the cross-regulation of cytokine network and may regulate Th1 cell and Th2 cell differentiation.38,39 CIS1, a prototype of CIS family proteins, is induced by Stat5 and inhibits Stat5 activation by blocking the interaction between Stat5 and cytokine receptors.37Thus, CIS1 seems to function in a classical negative feedback of Stat5 signaling. Recently, the CIS1 transgenic mice were reported and the phenotype of these mice remarkably resembled that found in Stat5a−/− mice in many aspects, including the decreased number of γδ T cells and the impaired IL-2Rα expression on activated T cells.40 However, in contrast to Stat5a−/− mice, CIS1 transgenic mice exhibited the enhanced Th2 cell differentiation,40 suggesting that CIS1 may not be responsible for the decreased Th2 cell differentiation of Stat5a−/−CD4+ T cells. It has recently been shown that Stat5 also up-regulates the expression of SOCS1/JAB/SSI-1,25 which is another CIS family protein and an important negative regulator of IFN-γ signaling.41 42Therefore, SOCS1/JAB/SSI-1 might be responsible for the increased Th1 cell differentiation in Stat5a−/− mice. This possibility is now under investigation.
Stat5a is known to potently regulate the expression of CD25 by directly binding to the 5′ regulatory region of the CD25 gene.33,34Our findings that the development of CD4+CD25+T cells was impaired in DO10+Stat5a−/− mice (Figure 9) and that the depletion of CD4+CD25+T cells from DO10+ splenocytes resulted in the similar cytokine profile as that in DO10+Stat5a−/−splenocytes (Figure 10) suggest that Stat5a is essential not only for the expression of CD25 molecules but also for the functional maturation of CD4+CD25+ T cells. Because CD4+CD25+ T cells lack the expression of IL-2Rβ 43 (A. Suto unpublished data, 2000), an essential receptor component for IL-2 signaling, the CD25 on CD4+CD25+ T cells may not compose high-affinity IL-2 receptors. Alternatively, it is conceivable that CD25 may be expressed on CD4+CD25+ T cells just as a result of Stat5a activation, and an undefined Stat5a-inducible gene(s) may be essential for the development or the function of CD4+CD25+ T cells.
We demonstrate that CD4+CD25+ T cells play an important regulatory role in Th2 cell differentiation (Figure 10). We have also recently found that CD4+CD25+ T cells enhance Th2 cell–mediated allergic inflammation in vivo (A. Suto, unpublished data 2000). Regarding Th2 cell differentiation induced by the presence of CD4+CD25+ T cells, cytokines produced by CD4+CD25+ T cells may not significantly affect the differentiation of CD4+CD25− T cells to Th2 cells, because previous studies showed that IL-2, IL-4, IL-10, or transforming growth factor–β (TGF-β) was not detectable in the culture supernatants of anti-CD3–stimulated CD4+CD25+T cells.44,45 Alternatively, CD4+CD25+ T cells may preferentially inhibit Th1-type CD4+CD25− T cells through the cell surface adhesion molecules, because it has been shown that CD4+CD25+ T cells inhibit the proliferation of CD4+CD25− T cells in a cell contact–dependent manner.44 45
We show that both Th1 cells and Th2 cells are decreased in Stat5b−/− mice (Figure 1). However, these data on T helper cell differentiation in Stat5b−/− mice might be inconclusive, because the number of CD4+ T cells recovered from the culture was significantly lower in Stat5b−/−mice than that from Stat5a−/− mice or wild-type mice, and the cytokine profile of CD4+ T cells has been suggested to be affected by cell-cycle progression.28,29 The decreased T-cell recovery in Stat5b−/− mice is consistent with the previous findings that the impaired proliferation of T cells can be overcome by high doses of IL-2 in Stat5a−/− mice but not in Stat5b−/− mice.14 15 Therefore, Stat5b may play a role in the proliferation or survival of activated T cells, which may not be shared with Stat5a. On the other hand, our finding that the enforced expression of Stat5b restored the impaired Th2 cell differentiation in Stat5a−/− T cells (Figure 8) indicates that the role of Stat5a in Th2 cell differentiation is shared with Stat5b.
In conclusion, our results indicate that the expression of Stat5a is required both for the appropriate differentiation of Th2 cells upon antigen stimulation and for the development of CD4+CD25+ immunoregulatory T cells that modulate T helper cell differentiation toward Th2 cells. Thus, specific inactivation of Stat5a could potentially represent a basis for the treatment of Th2-biased, allergic diseases such as asthma.
We thank Drs L. Hennighausen and X. Liu for Stat5a−/− mice, Dr H. W. Davey for Stat5b−/− mice, Drs S. Akira and K. Takeda for Stat6−/− mice, Drs D. Y. Loh and K. M. Murphy for DO10+ mice, Ms S. Yoshimura for Retronectin, Drs W. J. Leonard and A. Yoshimura for valuable discussion, and Dr K. Fujio for technical help.
Supported in part by grants from the Ministry of Education, Science and Culture of Japan, the Japan Allergy Foundation, and a Chiba University research grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hiroshi Nakajima, Dept of Internal Medicine II, Chiba University School of Medicine, 1-8-1 Inohana, Chiba City, Chiba 260-8670, Japan; e-mail: nakajimh@intmed02.m.chiba-u.ac.jp.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal