Splenomegaly is one of the characteristics of agnogenic myeloid metaplasia and other syndromes associated with myelofibrosis. If spleen enlargement is massive, patients may be severely symptomatic with abdominal pain, early satiety, and other manifestations. Splenectomy is effective in relieving symptoms, but surgical mortality is in the range of 5%-10%. Furthermore, there is concern about postsplenectomy thrombocytosis and associated complications, and some reports have suggested an increased probability of leukemic transformation.1 Tefferi et al recently reviewed results in 223 patients with agnogenic myeloid metaplasia who underwent splenectomy at the Mayo Clinic.2 The most frequent indications were transfusion-dependent anemia and symptomatic spleen enlargement. Patients with thrombocytopenia had an increased probability of postsplenectomy blast transformation, although this did not result in shortened survival. Symptoms related to splenomegaly resolved permanently in two thirds of the patients.
Hemopoietic cell transplantation offers the potential of cure for patients with agnogenic myeloid metaplasia and other hematopoietic disorders associated with marrow fibrosis.3 Patients with myelofibrosis who are being considered for transplantation generally have marked splenomegaly. In addition to problems as discussed above, splenomegaly in these patients also raises concerns about excessive sequestration of transplanted stem cells and difficulties with transfusion support after transplantation,1 4 which in turn may affect transplant outcome. We reviewed our experience in 26 patients with myelofibrosis and splenomegaly who underwent hemopoietic cell transplantation and who have been followed for a minimum of 2 years. Eleven patients had undergone splenectomy before receipt of transplant, and 15 received their transplant with an enlarged spleen in place. Patient and transplant characteristics are summarized in Table1.
Patient and transplantation characteristics
| Characteristic . | Splenectomy . | |
|---|---|---|
| Yes (n = 11) . | No (n = 15) . | |
| Diagnosis (no. patients) | ||
| AMM | 7 | 7 |
| MF after ET or P vera | 2 | 7 |
| AMM with excess blasts | 2 | 1 |
| Patient age (y), range (median) | 26-59 (44) | 18-54 (36) |
| Patient sex (male/female; no. patients) | 5/6 | 9/6 |
| Duration of disease (mo), range (median) | 4-252 (37.5) | 1-144 (14) |
| Therapy before transplantation (no. patients) | ||
| None | 0 | 2 |
| RBC transfusion | 7 | 8 |
| Platelet transfusions | 0 | 3 |
| Hydroxyurea | 0 | 2 |
| Splenectomy | 11 | 0 |
| Donor (no. patients) | ||
| HLA-identical sibling | 4 | 8 |
| HLA-nonidentical, related | 3 | 3 |
| HLA-matched, unrelated | 4 | 4 |
| Conditioning regimen (no. patients) | ||
| BUCY (total/targeted)* | 8/5 | 11/8 |
| CYTBI | 1 | 2 |
| BUTBI | 2 | 2 |
| Characteristic . | Splenectomy . | |
|---|---|---|
| Yes (n = 11) . | No (n = 15) . | |
| Diagnosis (no. patients) | ||
| AMM | 7 | 7 |
| MF after ET or P vera | 2 | 7 |
| AMM with excess blasts | 2 | 1 |
| Patient age (y), range (median) | 26-59 (44) | 18-54 (36) |
| Patient sex (male/female; no. patients) | 5/6 | 9/6 |
| Duration of disease (mo), range (median) | 4-252 (37.5) | 1-144 (14) |
| Therapy before transplantation (no. patients) | ||
| None | 0 | 2 |
| RBC transfusion | 7 | 8 |
| Platelet transfusions | 0 | 3 |
| Hydroxyurea | 0 | 2 |
| Splenectomy | 11 | 0 |
| Donor (no. patients) | ||
| HLA-identical sibling | 4 | 8 |
| HLA-nonidentical, related | 3 | 3 |
| HLA-matched, unrelated | 4 | 4 |
| Conditioning regimen (no. patients) | ||
| BUCY (total/targeted)* | 8/5 | 11/8 |
| CYTBI | 1 | 2 |
| BUTBI | 2 | 2 |
AMM, agnogenic myeloid metaplasia; MF, myelofibrosis; BUCY, busulfan (16 mg/kg) + cyclophosphamide (2 × 60 mg/kg); CYTBI, cyclophosphamide (2 × 60 mg/kg) + total body irradiation (6 × 200 cGy); BUTBI, busulfan (7 mg/kg) + total body irradiation (6 × 200 cGy); ET, essential thrombocythemia; P vera, polycythemia vera; RBC, red blood cell; HLA, human leukocyte antigen.
In patients given “targeted” busulfan, the dose was adjusted to a steady-state plasma level of 900 ng/mL.
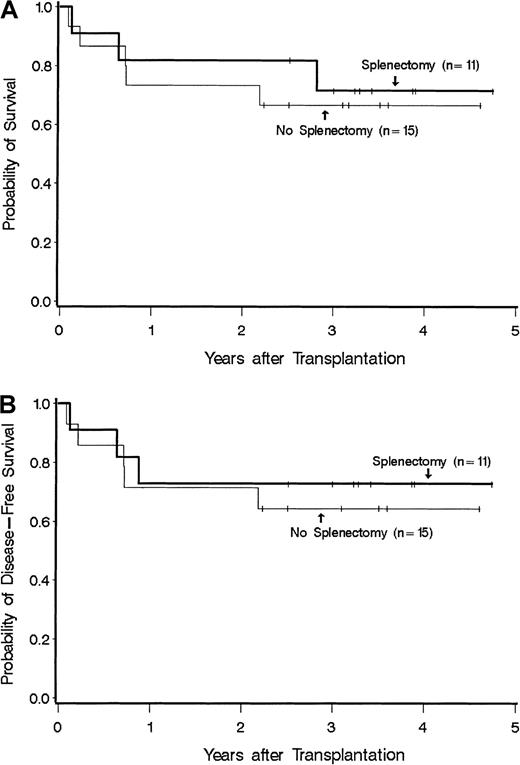
Disease duration was 4-252 months (median, 37.5 months) for patients with splenectomy and 1-144 months (median, 14 months) for patients without splenectomy (P = NS). Among patients without splenectomy, all had splenomegaly with spleen sizes ranging from 4 cm to 23 cm below the costal margin. In splenectomized patients a specific description of spleen size was not uniformly available, but in all cases the spleen was described as markedly or massively enlarged. Patients in the 2 groups received a median of 3.5 (range 0.6-14.9) and 3.5 (1.8-31) × 108 nucleated cells/kg, respectively (P = NS). Two patients with splenectomy and one without splenectomy received peripheral blood stem cells; the remaining patients received transplants of marrow cells. All were given methotrexate plus cyclosporine for graft-versus-host disease (GVHD) prophylaxis. All patients achieved sustained engraftment. The median time (range) to reach granulocyte counts of 0.5 and 1.0 × 109/L among splenectomized patients was 18 (14-51) and 22 (15-54) days, respectively. The corresponding time points for patients without splenectomy were 23 (19-85) and 25 (20-28) days, respectively. These differences were significant, both for 0.5 and 1.0 × 109 ANC/L (P = .04 andP = .03, respectively). (Information for 1 × 109 granulocytes was missing in one splenectomized patient.) Platelet counts of at least 20 × 109/L were reached at 15.5 (8-93) days in 9 of 11 splenectomized patients and at 19 (11-48) days in 10 of 15 nonsplenectomized patients (P = NS); 7 patients died without reaching platelet counts of at least 20 × 109/L. The incidence rates of acute GVHD of at least grade II were comparable (63% with splenectomy and 66% without splenectomy); no patient had grade IV, and only 1 had grade III acute GVHD. Chronic GVHD developed in 6 of 10 patients at risk with splenectomy and in 5 of 13 patients without splenectomy. Disease-free survival at 3 years was 73% for splenectomized and 64% for nonsplenectomized patients (P = NS; Figure 1). Two patients in the splenectomy group experienced a disease relapse after transplantation. Overall, 3 splenectomized patients and 5 nonsplenectomized patients have died. Among splenectomized patients, causes of death included organ failure and infection in 2 patients and disease recurrence in 1 patient. Among patients with the spleen intact, 5 patients died either from organ failure and infection (n = 2), GVHD and infection (n = 1), infection (n = 1), or lymphoma (n = 1).
Survival among splenectomized and nonsplenectomized patients who received a hemopoietic cell transplant for myelofibrosis. (A) Overall survival. (B) Disease-free survival.
Survival among splenectomized and nonsplenectomized patients who received a hemopoietic cell transplant for myelofibrosis. (A) Overall survival. (B) Disease-free survival.
Thus patients with splenectomy had faster posttransplantation granulocyte recovery than nonsplenectomized patients. Otherwise, the present results provide no evidence for either a significant advantage or disadvantage of splenectomy on posttransplantation outcome. This analysis has all the shortcomings of a small, retrospective, nonrandomized study. There may well be other important differences between the splenectomized and nonspelenctomized patients. For example, pretransplantation disease duration was somewhat longer (albeit not significantly so) in splenectomized patients, and although not the case in the present study, disease duration has been found to be inversely correlated with posttransplantation outcome for other indications. In addition, splenectomized patients tended to be older and more often received a transplant from an alternative donor. On the other hand, nonsplenectomized patients more often were transfusion dependent prior to transplantation.5
Without data from a large, prospective randomized trial, it is impossible to address the issue of the effect of pretransplantation splenectomy on transplantation for myelofibrosis with certainty. Such a trial is not currently available. From the present data it appears that, similar to the situation in patients not receiving transplants with agnogenic myeloid metaplasia and splenomegaly as reported by Tefferi et al,2 broad, general recommendations are difficult to formulate for patients who plan to receive a hemopoietic cell transplant. For now, the decision about splenectomy should be determined by patient symptomatology and not based on a presumed effect of splenectomy on posttransplantation outcome.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal