Lehmann et al1 recently reported results suggesting that the impaired binding of perforin (PFN) to the surface of tumor cells is associated with resistance to cytotoxic effector cell killing. Using natural killer (NK)–sensitive (K562) and –resistant (ML-2) cell lines, they found that supernatants from freeze-thawed human CD56+ NK cells (NK lysates) did not damage ML-2 but that K562 were permeabilized, an effect inhibited by the anti-PFN antibody (clone δG9). Using dual fluorescence analysis, the authors then directly demonstrated that K562 cells that were permeabilized (propidium iodide, PI+) also stained positive for PFN. The inference was made that most permeabilized (dead) cells had PFN on their surface. ML-2 cells, on the other hand, showed no binding of PFN and did not undergo lysis; therefore the authors attributed this resistance of the ML-2 line to the inability of PFN to bind to plasma membrane of these resistant tumor cells.
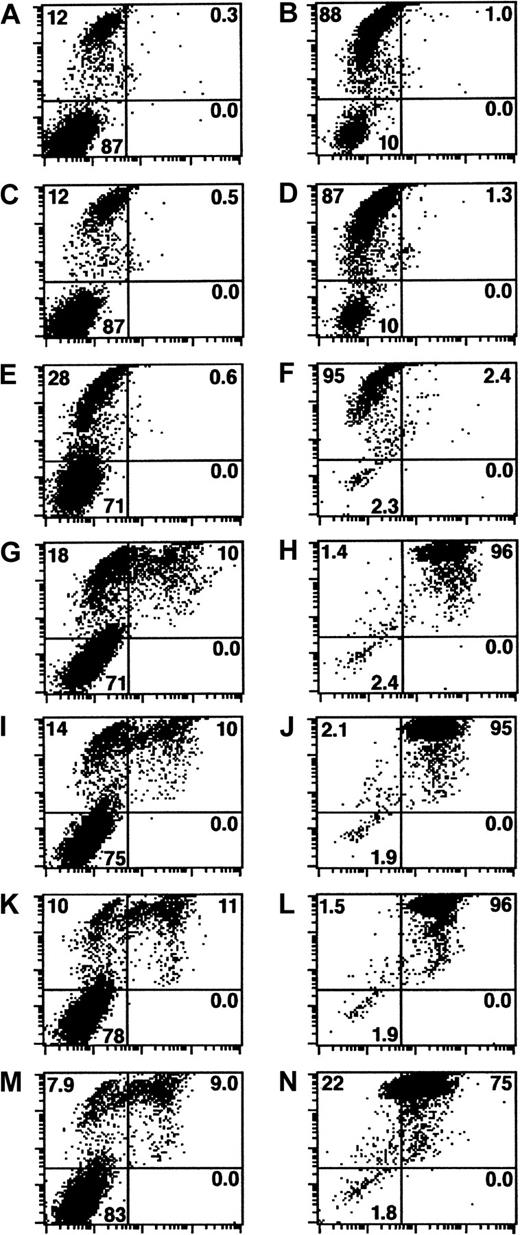
In our opinion, the authors overlooked a crucial control, which evaluates whether the PFN detected by flow cytometry (FCM) represents protein that interacted with targets after membrane damage has occurred. We hypothesize that PFN, in amounts below the level of detection by FCM, actually permeabilizes the target cells. But due to its charged state,2 PFN monomers in solution could then bind nonspecifically to sites on the membrane of necrotic cells, as well as intracellularly. To test this hypothesis, we examined the capacity of PFN contained in granule extracts and also isolated PFN3 to bind to detergent permeabilized (0.01% NP-40, 37°C, 30 minutes) Jurkat cells. The cells were then incubated with the preparations (37°C, 60 minutes), washed with phosphate buffered saline (PBS)–2% bovine serum albumin (BSA), and then reacted with either FITC-anti-PFN antibody (clone δG9) or an FITC-IgG2b isotype control antibody (BD Pharmingen, San Diego, CA). After a wash step (PBS-2% BSA), the cells were resuspended in the same buffer containing PI (5 μg/mL) and analyzed on a FACSCALIBUR (Becton Dickinson Immunocytometry Systems, San Jose, CA) instrument. Figure1 shows PI reactivity versus PFN reactivity of nonpermeabilized and permeabilized Jurkat cells. After treatment with PFN containing YT granule extract in a concentration range that was previously determined to minimally permeabilize the target (300-37.5 ng/mL), only 10% of the detergent untreated cells were present in the double positive quadrant (Figure1G, I, K, and M, respectively). In comparison, the NP40 permeabilized cells exposed to PFN at similar concentrations resulted in dual positive events ranging from 96% to 75% (Figure 1H, J, L, and N). The percentage of double positive events was comparable for PFN concentrations ranging from 300 ng/mL to 75 ng/mL. This absence of concentration-dependent increase in PFN reactivity further suggests that PFN interacted nonspecifically with the detergent permeabilized target cells. Finally, under a fluorescent microscope, target cells possess an intracellular rather than a cell surface staining pattern (data not shown).
Permeabilized cells nonspecifically bind PFN.
PI reactivity (y-axis) versus PFN reactivity (x-axis) of nonpermeabilized (left column of panels) and NP-40 permeabilized (right) Jurkat cells. PFN untreated cells that were exposed to isotype control mAb or anti-PFN mAb are shown respectively as panels A-B and C-D. Similarly manipulated subsets were treated with PFN containing YT granule extract for 1 hour (37°C) and stained with either control antibody (E-F) or anti-PFN antibody (G-N). The concentrations of PFN were 300 ng/mL (E-H), 150 ng/mL (I-J), 75 ng/mL (K-L), and 37.5 ng/mL (M-N). These concentrations were estimated based on densitometric calculations where granules from 106 YT cells yield approximately 3 ng of PFN. The resultant dot plots have intensity of PI reactivity on the y-axis (FL3) and PFN reactivity on the x-axis (FL1). Gates were established based on the antibody control groups (A-D) allowing the quadrants to be defined as follows: upper left, PI+ PFN−; upper right, PI+PFN+; lower left, PI−PFN−; and lower right, PI− PFN+. The percentage of events in each quadrant is provided.
Permeabilized cells nonspecifically bind PFN.
PI reactivity (y-axis) versus PFN reactivity (x-axis) of nonpermeabilized (left column of panels) and NP-40 permeabilized (right) Jurkat cells. PFN untreated cells that were exposed to isotype control mAb or anti-PFN mAb are shown respectively as panels A-B and C-D. Similarly manipulated subsets were treated with PFN containing YT granule extract for 1 hour (37°C) and stained with either control antibody (E-F) or anti-PFN antibody (G-N). The concentrations of PFN were 300 ng/mL (E-H), 150 ng/mL (I-J), 75 ng/mL (K-L), and 37.5 ng/mL (M-N). These concentrations were estimated based on densitometric calculations where granules from 106 YT cells yield approximately 3 ng of PFN. The resultant dot plots have intensity of PI reactivity on the y-axis (FL3) and PFN reactivity on the x-axis (FL1). Gates were established based on the antibody control groups (A-D) allowing the quadrants to be defined as follows: upper left, PI+ PFN−; upper right, PI+PFN+; lower left, PI−PFN−; and lower right, PI− PFN+. The percentage of events in each quadrant is provided.
If our hypothesis is invalid, then PFN should be detectable by FCM on nonpermeabilized cells. When isolated PFN was added to targets, only PI+, PFN+ cells were identified (data not shown). We then repeated the experiment without Ca++ (4mM EDTA) to minimize membrane permeabilization. Under this condition, approximately 10%-20% of cells were found to be PFN+ and PI− (data not shown). We were unable, therefore, to identify cells that were PFN+ and PI− unless permeabilization was blocked.
In conclusion, our observations suggest that FCM only detects PFN in treated cells after membrane permeabilization. Therefore this technique is not suitable to accurately detect the amount of PFN that binds and mediates membrane damage and cannot be correlated with resistance to PFN mediated damage. An important corollary of these studies is that the cell membrane associated PFN described by Lehman et al actually represents primarily intracellular PFN. Our findings do not minimize the fundamental observation reported by Lehman et al, where the results show that tumor cell lines display varying degrees of susceptibility to lysis when exposed to equivalent amounts of PFN. This conclusion, however, must be based solely on the differences in PI reactivity.
Mechanisms of perforin resistance: the differentiation between perforin binding and perforin-mediated lysis remains difficult
Metkar et al report that previously permeabilized Jurkat cells show an intense staining of perforin with an FITC-labeled antibody after incubation with YT granule-extract/purified perforin compared to nonpermeabilized Jurkat cells. They hypothesize that the reason for their observation is an unspecific intracellular binding of perforin (due to its cationic nature) to the cell membrane of the permeabilized target cells. Although we are convinced that the data provided by Metkar et al represent an interesting contribution that may help to further explain our findings published recently,1-1 we are not able to follow all of their conclusions. Several points of their interpretation of their own and our data need to be critically discussed:
First, Metkar et al conclude from the observation that Jurkat cells permeabilized by NP-40 are strongly positive for perforin after incubation with granule extract or purified perforin that perforin might get into the cells, once the membrane is permeabilized, and bind unspecifically to the inner side of the cell membrane. But to our knowledge, it is not yet clear whether perforin pores are big enough to allow the FITC-labeled antibody to enter the cell. In the case of the NP-40 permeabilized cells, it is obvious that the antibody might get into the cell since the detergent will strongly disintegrate the cell membrane (which is often used in experiments to permeabilize cells for an intracellular antibody staining). But this has not been shown for perforin pores: In our opinion it would be necessary to prove that an FITC-labeled antibody is able to get into a cell by a perforin pore (eg, by permeabilizing cells with perforin and then adding an antibody specific for intracellular proteins). Only then would it be justified to talk about “intracellular perforin” detected by the perforin antibody (as Metkar et al claim is the case in our experiments), although there is still the possibility that the antibody binds mainly to surface bound perforin.
Second, we are concerned about the fact that the experiments Metkar et al performed were with Ca-free buffer. They justify the use of Ca-free media by the need to minimize membrane permeabilization. But using a buffer without Ca must result in a strongly impaired perforin binding, as indicated by several groups that have uniformly reported that Ca is mandatory for perforin binding (and of course subsquent lysis); see, for example, Uellner et al.1-2 In our opinion, it is not possible to obtain conclusive data on the mechanisms of perforin-binding and perforin-mediated lysis in the absence of Ca. The observation of Metkar et al that they were unable to find PFN+PI− cells unless permeabilization is blocked corresponds with our hypothesis: only cells that bind perforin and are thus lysed can become PI+. These experiments demonstrate that it is very difficult (if not impossible) to differentiate between perforin binding and perforin-mediated lysis, and it is at least questionable whether these 2 events can be investigated separately at all. Despite our concerns regarding the interpretation of their results, we agree with Metkar et al that the proposed mechanism could indeed be responsible for the perforin-positive staining of PI+ target cells and should be further investigated.
In our study we used the working hypothesis that perforin-resistant tumor cells bind less perforin on their surface than perforin-sensitive tumor cells and found evidence that this might indeed be the case. Although Metkar et al have presented interesting results that could lead to another explanation for the observations we made, we think that definitive conclusions are not possible at the present time. As we have pointed out, further experiments are clearly necessary to finally elucidate the precise molecular mechanisms responsible for the heretofore undetected phenomen of tumor-cell resistance against perforin-mediated lysis. It is important to note that the description of this phenomenon, which was the central issue of our work, was confirmed by Metkar et al and is not affected by the present discussion.
References
Supported by NIH RO-1 grant # AI 44941-01A1 to C.J.F.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal