Abstract
Erythroid progenitor cells (EPCs) are deficient in mice lacking either the ligand stem cell factor (SCF), its receptor c-Kit, or β1-integrins. In nonhematopoietic cells, integrins and receptor tyrosine kinases can collaborate to modulate cellular functions, providing evidence for cross-talk between signals emerging from these cell surface molecules. Using specific recombinant fibronectin peptides that contain the binding site for the integrin α4β1 (FN-H296) or α5β1 (FN-CH271) or both α4β1 and α5β1(FN-CH296), this study investigated the effect of adhesion alone, or in combination with activation of c-Kit, on functional and biochemical outcomes in an EPC line, G1E-ER2, and primary EPCs. G1E-ER2 cells and primary EPCs cultured on FN-CH271 in the presence of c-Kit activation led to a significant increase in proliferation in comparison with cells grown on FN-H296 or FN-CH296. G1E-ER2 cells cultured on FN-H296 or FN-CH296 resulted in significant cell death in comparison to cells grown on FN-CH271. Activation of c-Kit enhanced the survival of G1E-ER2 cells grown on FN-H296 or FN-CH296; however, the rescue was only partial. The reduced survival of G1E-ER2 cells on FN-H296 correlated with reduced activation of Akt and expression of Bcl-2 and Bcl-xL, whereas increase in proliferation on FN-CH271 correlated with significantly enhanced and sustained activation of focal adhesion kinase (FAK) and extracellular-regulated kinase (ERK) pathways. These data demonstrate that adhesion-induced signals emanating from ligation of α4β1 and α5β1 result in distinct biologic outcomes, including death via α4β1 and survival/proliferation via α5β1.
Introduction
Adhesive interactions between hematopoietic progenitor and stem cells and the hematopoietic microenvironment play a critical role in maintaining hematopoiesis.1-5Hematopoietic growth factors are potent regulators of hematopoiesis. In addition, these proteins have been implicated in modulating adhesion between hematopoietic progenitor cells and extracellular matrix proteins via changes in integrin receptor activation.6-9The role of adhesion molecules alone or with growth factors in maintaining proliferation, differentiation, and survival of hematopoietic cells is less understood,10,11 but collaboration between growth factor receptors and integrins has been hypothesized to be necessary for normal hematopoietic development.12 Specifically, integrin receptors such as alpha 4 beta 1 (α4β1) and/or alpha 5 beta 1 (α5β1), may collaborate in unique ways with receptor tyrosine kinases, to influence cellular events. In this regard, mice deficient in the receptor tyrosine kinase, c-Kit, its ligand stem cell factor (SCF), or β1 integrins demonstrate hematopoietic defects of varying severity, suggesting critical roles for these proteins in normal blood development.1,13 14
Our laboratory and other investigators have shown that receptors of the extracellular matrix protein, fibronectin (FN), are involved in the adhesion of hematopoietic cells, including stem and progenitor cells in the hematopoietic microenvironment.15-20 FN is expressed at high levels throughout the hematopoietic microenvironment.21,22 The FN molecule contains binding sites for heparin, collagen, fibrin, and gelatin, suggesting that it plays an important role in regulating the architecture of the hematopoietic microenvironment. The binding of hematopoietic cells to FN is mediated by at least 2 integrin receptors. The α5β1 receptor recognizes the minimal binding sequence Arg-Gly-Asp (single-letter amino acid code: RGD), as well as 2 other synergistic binding sites, all of which are located within the cell-binding domain of the FN molecule,23,24and α4β1 binds sequences within the alternatively spliced IIICS region of FN defined by the synthetic peptides CS-1 and CS-5.25,26 These receptors play a critical role in normal hematopoietic development.1,27 28
Mutant mice homozygous for null mutations of c-Kit, or its ligand SCF, die in embryonic development or shortly after birth due to severe anemia.14,29-31 Viable homozygous mutants of c-Kit also demonstrate severe anemia and a marked reduction in both immature and mature erythroid progenitors. Data from these mutant mice show a critical role for c-Kit–mediated signaling in normal erythroid development.29,30 Interactions of erythroid cells with FN are also believed to be essential for erythropoiesis, particularly for terminal stages of erythroid differentiation.32-36Erythroid progenitors express both α4β1 and α5β1.37,38 Efficient production of mature cells in vitro requires adhesion to FN in some systems.39-42 In addition, treatment of normal mice with anti-α4β1 antibody completely blocks erythropoiesis.32 Some evidence of collaboration between c-Kit and integrins also exists. Exposure to SCF increases adherence of hematopoietic cells to FN by “inside-out signaling.”6,9 43-47 Together, these data suggest a role for both c-Kit and integrin receptors in normal erythroid development.
In some cell systems, signaling downstream of receptor tyrosine kinases and integrins appear to comodulate cellular events.48 In fibroblasts, autophosphorylation of receptor tyrosine kinases can be enhanced by adhesion to matrix proteins, such as FN.49,50In addition, platelet-derived growth factor receptor and epidermal growth factor receptor phosphorylation following growth factor treatment is greater in adherent cells compared to suspended cells.51-53 Synergy between growth factors and cell adhesion in activation of the mitogen-activated protein kinase (MAPK) and phosphoinositide-3 kinase (PI-3K) cascade has also been observed. Akt has been shown to be synergistically activated by adhesion and epidermal growth factor treatment in fibroblasts. These signaling effects correlated with increased cell survival, consistent with an important role for the PI-3K/Akt pathway in cell survival.54 55
Given the significance of c-Kit and β1-integrin signals in the development of erythroid cells, and the known interaction between receptor tyrosine kinases and integrins in cells of nonhematopoietic origin, we hypothesized that concurrent ligation of integrins and c-Kit has significant effects on the activation of intracellular signaling pathways and subsequent behavior of cells in the erythroid lineage. Because adhesion of hematopoietic cells to FN is a dynamic process, involving coordinated, successive attachment and detachment,12,56 57 we have examined the effect of this dynamic process over time on erythroid progenitor cell (EPC) survival/apoptosis and proliferation using cell cultures grown on FN peptides that specifically bind α4β1(FN-H296) or α5β1 (FN-CH271) or both α4β1 and α5β1(FN-CH296) integrin.
Materials and methods
Cell lines and primary erythroid progenitors
The G1E-ER2 cells have been previously described and were obtained from Dr Mitch Weiss (Ontogeny, Boston, MA). Unless otherwise specified, G1E-ER2 cells were grown in Iscove modified Dulbecco medium (IMDM; GIBCO/BRL, Gaithersburg, MD) with 15% heat-inactivated embryonic stem cell (ES) serum (Hyclone, Logan, UT), recombinant erythropoietin (Epo; 2 U/mL; Amgen, Thousand Oaks, CA), and recombinant rat (rr) SCF (50 ng/mL; Amgen). For starvation experiments, cells were washed 3 times in IMDM, then resuspended in the same medium without serum and growth factors for 6 to 8 hours. Primary EPCs were derived from fetal livers of 12.5-day-old wild-type embryos. Briefly, single-cell suspensions were prepared and fetal liver cells were cultured in IMDM with 10% fetal calf serum (FCS; Hyclone), recombinant Epo (2 U/mL; Amgen), and rrSCF (50 ng/mL; Amgen) for 3 to 4 weeks.
Antibodies and flow cytometric analysis
Phycoerythrin (PE)–conjugated monoclonal antibodies (mAbs) were directed against c-Kit and α5β1. Fluorescence isothyocyanate (FITC)–conjugated antibodies were directed against α4β1. All the PE- and FITC-conjugated mAbs, including the isotype control antibodies, were purchased from Pharmingen (San Diego, CA). G1E-ER2 cells (1 × 106) were incubated at 4°C for 30 minutes with 1 μg of the primary mAb. Cells were washed 3 times with phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA; Sigma, St Louis, MO), and analyzed by fluorescence-activated cell sorter (FACS; Becton Dickinson, San Jose, CA).
Cell adhesion assays
Recombinant human FN peptides H296 and CH271 (Figure1A) were obtained from Takara Shuza (Otsu, Japan). Nontissue culture 6-well plates were coated with FN fragments diluted in PBS at 100 nmol/cm2 overnight as described previously.18 We have also previously demonstrated that adhesion to these various recombinant FNs was mediated specifically on hematopoietic cells, through their integrin receptors α4β1 and α5β1.18 To block nonspecific binding sites, plates were incubated for 30 minutes with 2% BSA in PBS. Wells were then washed 3 times with PBS. Factor-starved G1E-ER2 cells (2 × 106) were allowed to adhere to FN peptides for various time periods at 37°C in the presence of 10 ng/mL rrSCF. After incubation, nonadherent cells were collected by carefully rinsing the plates with medium and cell counts were performed.
Recombinant human FN peptides.
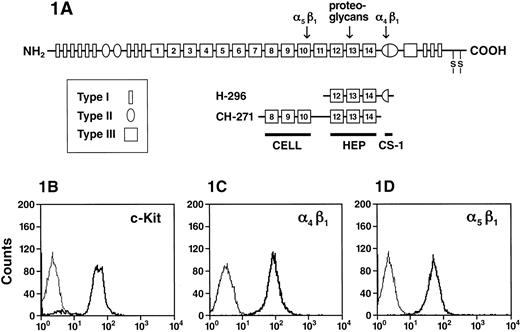
(A) A schematic representation of FN peptides. FN is made up of series of type I, II, and III repeats. Regions of FN with cell-binding activity are shown as the RGD-containing cell-binding domain (CELL), which is recognized by integrin α5β1, the nonintegrin-dependent high-affinity heparin-binding site (HEP), and the alternatively spliced non–type III connecting segment (III CS) that is recognized by integrin α4β1 (CS-1). The recognition sites for integrin α4β1, α5β1, as well as the heparin-binding site on FN are indicated by an arrow. (B) Expression of integrins and c-Kit on G1E-ER2 cells. G1E-ER2 cells were stained with anti–c-Kit-PE and analyzed by flow cytometry. The thin line indicates the level of background staining observed with appropriate isotype control antibody. The thick line indicates the level of c-Kit expression. (C) G1E-ER2 cells were stained with anti–α4β1-FITC and analyzed by flow cytometry. The thick line indicates the level of α4β1 expression. (D) G1E-ER2 cells were stained with anti–α5β1-PE and analyzed by flow cytometry. The thick line indicates the level of α5β1 expression.
Recombinant human FN peptides.
(A) A schematic representation of FN peptides. FN is made up of series of type I, II, and III repeats. Regions of FN with cell-binding activity are shown as the RGD-containing cell-binding domain (CELL), which is recognized by integrin α5β1, the nonintegrin-dependent high-affinity heparin-binding site (HEP), and the alternatively spliced non–type III connecting segment (III CS) that is recognized by integrin α4β1 (CS-1). The recognition sites for integrin α4β1, α5β1, as well as the heparin-binding site on FN are indicated by an arrow. (B) Expression of integrins and c-Kit on G1E-ER2 cells. G1E-ER2 cells were stained with anti–c-Kit-PE and analyzed by flow cytometry. The thin line indicates the level of background staining observed with appropriate isotype control antibody. The thick line indicates the level of c-Kit expression. (C) G1E-ER2 cells were stained with anti–α4β1-FITC and analyzed by flow cytometry. The thick line indicates the level of α4β1 expression. (D) G1E-ER2 cells were stained with anti–α5β1-PE and analyzed by flow cytometry. The thick line indicates the level of α5β1 expression.
Effects of FN on proliferation and survival of G1E-ER2 cells
The effect of FN peptides H296 and CH271 and SCF on proliferation of G1E-ER2 cells and primary EPC was assayed using thymidine incorporation. The 96-well nontissue culture plates were coated with FN peptides as described above. Growth factor-starved G1E-ER2 cells and primary EPC were plated at 5 × 104cells/well for 48 hours, either in the presence or absence of 100 ng/mL rrSCF. Subsequently, 1.0 μCi of [3H]-thymidine (Amersham) was added to each well for 6 to 8 hours at 37°C. Cells were then harvested using an automated cell harvester (96-well harvester, Brandel, Gaithersburg, MD) and thymidine incorporation was determined in a scintillation counter. The effect of FN peptides and SCF on cell death (apoptosis and necrosis) of G1E-ER2 cells was assayed by staining the cells with annexin-FITC and propidium iodide (PI) according to the manufacturer's instructions (Pharmingen, San Diego, CA). The 24-well nontissue culture plates were coated with FN peptides CH296, CH271, and H296 as described above. Growth factor-starved G1E-ER2 cells were plated at 5 × 105 cells/well for 48 hours, either in the presence or absence of 100 ng/mL rrSCF. Subsequently, cells were harvested and stained with annexin-FITC and PI and analyzed by flow cytometry.
Effects of FN on ERK, Akt, and FAK signaling pathways in G1E-ER2 cells
Activation of MAPK (ERK-1 and ERK-2) was determined by using phospho-specific ERK antibody (New England Biolabs, Beverly, MA). This antibody detects ERKs only when they are catalytically activated by phosphorylation. Activation of Akt was determined by using a phospho-specific Akt (S473) antibody (New England Biolabs). Activation and expression of FAK was determined by using an anti-FAK antibody (Upstate Biotechnology, Lake Placid, NY). Expression of Bcl-2 and Bcl-xL was determined by using anti–Bcl-2 and anti–Bcl-xL antibodies (Pharmingen). All antibodies were used at 1:2000 dilution. Briefly, nontissue culture 6-well plates were coated with FN fragments as described above. Factor-starved 5 to 8 × 106 G1E-ER2 cells were loaded onto FN-coated wells and cultured for various time points at 37°C in the presence or absence of SCF. Thereafter, cells were harvested and lysed in lysis buffer at 4°C for 30 minutes. Cell lysates were clarified by centrifugation for 30 minutes at 10 000g at 4°C. Equal amount of protein was fractionated on 12% polyacrylamide/sodium dodecyl sulfate (SDS) gel and electrophoretically transferred to nitrocellulose membrane. Western blot analysis was performed according to the manufacturer's instructions (New England Biolabs).
Results
Expression of c-Kit, α4β1, and α5β1 on EPCs
To study potential interactions between c-Kit and integrins α4β1 and α5β1, we confirmed c-Kit, α4β1, and α5β1 expression on the erythroid cell surface by flow cytometric analysis using anti–c-Kit, anti-α4β1, and anti-α5β1 mAbs coupled to either FITC or PE. G1E-ER2 cells uniformly express c-Kit (Figure 1B), α4β1 (Figure 1C), and α5β1 (Figure 1D). One hundred percent of G1E-ER2 cells express integrins α4β1 and α5β1 (Figure 1C,D). To confirm that α4β1 or α5β1 or both mediate the adhesion of G1E-ER2 cells to FN, we used 2 recombinant peptides containing the single binding domain for α4β1 (H296) or α5β1 (CH271) (Figure 1A).11,18G1E-ER2 cells were plated on FN-H296 or FN-CH271 and adhesion measured over 2 hours. Significant adhesion to FN fragments was observed by either α4β1 or α5β1. 71% ± 3.1% G1E-ER2 cells were adherent to FN-CH271 via α5β1 and 77% ± 5.8% via α4β1 to FN-H296 (data not shown). Previous studies using primary EPCs have shown similar levels of adhesion to FN as well as to FN peptides.58 In contrast, the majority of the cells (90%) were in suspension in BSA-coated dishes used as control cultures. In previous studies using these same fragments and other hematopoietic cell lines or primary cells, we have shown specificity of adhesion on these fragments with blocking antibodies.18 Because the majority of EPCs were adherent to FN peptides and because previous studies have shown that adhesion of hematopoietic cells to FN is a dynamic process, in subsequent studies we have examined the impact of this process on cell cultures incubated in dishes coated with FN-H296, FN-CH271, or BSA.
Cooperation between c-Kit and α5β1enhances proliferation of EPCs
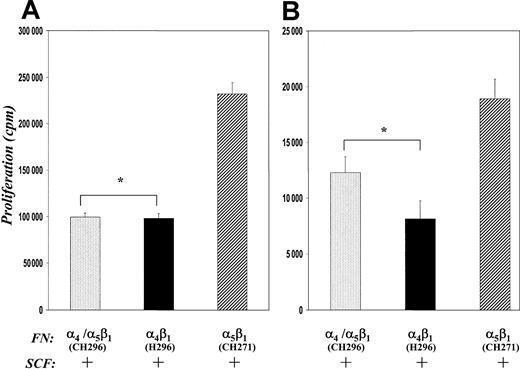
The role of integrins in erythroid cell proliferation has not been determined, although studies have suggested that FN is necessary both in vitro and in vivo to provide the appropriate niche for erythroid development.34-40 To determine if integrins are important mediators of mitogenesis in erythroid cells, we measured DNA synthesis in G1E-ER2 cells and primary fetal liver-derived EPCs cultured on FN peptides in the presence or absence of SCF. After 48 hours in culture on FN peptides, [3H]-thymidine was added for 6 hours and [3H]-thymidine incorporation was determined. In experiments in which no SCF was added to cultures, factor-starved G1E-ER2 cells cultured on BSA or FN-CH271 showed similar proliferative response (11 071 ± 1257 counts per minute [cpm] for BSA versus 10 085 ± 1108 cpm for CH271, mean ± SEM, respectively, from 6 different experiments, P > .05). In contrast, cells cultured on FN-H296 or FN-CH296 resulted in significantly less DNA synthesis (6958 ± 756 cpm for H296 and 5689 ± 614 cpm for CH296 versus 10 085 ± 1108 cpm for CH271, mean ± SEM, respectively, from 6 different experiments, P < .05).
In experiments in which SCF was added to cultures, G1E-ER2 cells cultured on FN-CH271 (mediating adhesion via α5β1) demonstrated significantly higher proliferation compared with cells stimulated with SCF in suspension (BSA) (231 301 ± 12 114 cpm CH271 versus 145 173 ± 10 832 cpm BSA, mean ± SEM, respectively, from 6 different experiments,P < .05). In contrast, even in the presence of SCF, culturing these cells on FN-H296 (mediating adhesion via α4β1) or FN-CH296 (mediating adhesion via both α5β1 and α4β1) was associated with reduced proliferation compared to cells cultured in suspension or FN-CH271 (Figure 2A). A similar decrease in proliferation was also noted in primary fetal liver-derived erythroid progenitors cultured on FN-H296 or FN-CH296 compared to FN-CH271 (Figure 2B). Thus, these studies demonstrate that different biologic outcomes are stimulated by adhesion of erythroid cells to FN via α4β1 compared with α5β1.
Effects of adhesion.
Comparison of the effects of adhesion to FN peptides on proliferation of G1E-ER2 cells (A) and primary EPCs (B). Cells were cultured on FN-peptide–coated dishes mediating adhesion via both α4β1 and α5β1(CH296) or α4β1 (H296) or α5β1 (CH271) in the presence of SCF for 48 hours. Proliferation was measured by thymidine incorporation assay. Bars denote the mean thymidine incorporation (cpm ± SEM) of 6 different experiments performed in replicates of 6 (A), and one of the 2 representative experiments performed in replicates of 4 (B). Asterisk indicates P < .05 α4β1 (H296) and α4β1 and α5β1 (CH296) versus α5β1 (CH271).
Effects of adhesion.
Comparison of the effects of adhesion to FN peptides on proliferation of G1E-ER2 cells (A) and primary EPCs (B). Cells were cultured on FN-peptide–coated dishes mediating adhesion via both α4β1 and α5β1(CH296) or α4β1 (H296) or α5β1 (CH271) in the presence of SCF for 48 hours. Proliferation was measured by thymidine incorporation assay. Bars denote the mean thymidine incorporation (cpm ± SEM) of 6 different experiments performed in replicates of 6 (A), and one of the 2 representative experiments performed in replicates of 4 (B). Asterisk indicates P < .05 α4β1 (H296) and α4β1 and α5β1 (CH296) versus α5β1 (CH271).
Cooperation between α5β1 and c-Kit results in enhanced and sustained FAK and MAPK (ERK-1 and ERK-2) activation
To test whether the increase in proliferation of erythroid progenitors that occurs after engagement of α5β1 was associated with activation of downstream kinase signaling pathways, we measured FAK and MAPK (ERK-1 and ERK-2) activation. These pathways have previously been implicated in integrin-mediated signal transduction in hematopoietic cells.59 G1E-ER2 cells were starved for 7 hours, allowed to adhere to FN via α4β1 or α5β1 for various time points, lysed, and subjected to Western blot analysis using an anti-FAK or antiphospho-MAPK antibody. In the presence of SCF, engagement of α5β1 strongly induced FAK activation as early as 12 hours after stimulation, reaching maximum levels at 48 hours (Figure 3A). Ligation induced by α4β1 also resulted in FAK activation, however, at significantly reduced levels in comparison to activation via α5β1 (Figure 3A). We next examined the activation of MAPK (ERKs), and first measured activation of ERKs in G1E-ER2 cells stimulated with SCF in suspension. Stimulation of these cells by SCF resulted in significant but only transient activation of ERK-1 and ERK-2 (Figure 3B). Activation peaked 10 minutes after SCF stimulation and dropped to baseline thereafter. In contrast, in the presence of engagement of α5β1, SCF strongly induced ERK-1 and ERK-2 activation in erythroid cells as early as 10 minutes after stimulation that persisted and reached maximum levels at 120 minutes (Figure 3C). Once again, engagement via α4β1 also resulted in ERK-1 and ERK-2 activation, however, at significantly reduced levels in comparison to α5β1 (Figure 3C). These data suggest that increased proliferation of erythroid cells associated with ligation via α5β1 may in part be due to sustained and enhanced activation of FAK/MAPK (ERK-1 and ERK-2) cascade in these cells.
FAK and MAPK (ERKs) activation after integrin-mediated adhesion.
Factor-starved G1E-ER2 cells were cultured on FN peptides in the presence of SCF and analyzed at the indicated time points. (A) Cell lysates were collected and subjected to Western blot analysis with an anti-FAK antibody. Bottom panels indicate the position of phosphorylated (pFAK) and unphosphorylated (FAK) FAK. Upper panels (bars) demonstrate the relative phosphorylation of FAK; 48 hours taken as 100. Bars denote the mean relative phosphorylation (± SEM) of 3 different experiments. Asterisk indicates P < .05 α5β1 (CH271) versus α4β1 (H296). (B,C) Factor-starved G1E-ER2 cells were left unstimulated or cultured on BSA (B) or on indicated FN peptides mediating adhesion via α4β1 (H296) and α5β1 (CH271) (C) in the presence of SCF. Cell lysates were collected and subjected to Western blot analysis with a rabbit antiphospho-ERK antibody that specifically detects phosphorylated T202 and Y204. Bottom panels show total Erk in each lane. The positions of the phosphorylated ERK-1 (pErk-1) and ERK-2 (pErk2) are indicated. Upper panels (bars) demonstrate the relative phosphorylation of ERK-1 and ERK-2 at residues T202 and Y204. Data are presented relative to the phosphorylation of ERK-1 and ERK-2 after 10 minutes (B) (with the level at 10 minutes taken as 100) and 120 minutes (C) (with the level at 120 minutes taken as 100). Bars denote the mean relative phosphorylation (± SEM) of 3 different experiments. Asterisk indicates P < .05 α5β1(CH271) versus α4β1 (H296). (D) G1E-ER2 erythroid progenitors were cultured on BSA or on FN peptides H296 or CH271 in the presence of SCF and the MEK inhibitor (PD98059). Proliferation was measured by thymidine incorporation assay. Bars denote the inhibition in proliferation (± SEM) of 3 independent experiments performed in replicates of 6. Asterisk indicatesP < .05 α4β1 (H296) versus α5β1 (CH271) and BSA; and double asterisks indicate P < .05 α4β1 (H296) and α5β1 (CH271) versus BSA.
FAK and MAPK (ERKs) activation after integrin-mediated adhesion.
Factor-starved G1E-ER2 cells were cultured on FN peptides in the presence of SCF and analyzed at the indicated time points. (A) Cell lysates were collected and subjected to Western blot analysis with an anti-FAK antibody. Bottom panels indicate the position of phosphorylated (pFAK) and unphosphorylated (FAK) FAK. Upper panels (bars) demonstrate the relative phosphorylation of FAK; 48 hours taken as 100. Bars denote the mean relative phosphorylation (± SEM) of 3 different experiments. Asterisk indicates P < .05 α5β1 (CH271) versus α4β1 (H296). (B,C) Factor-starved G1E-ER2 cells were left unstimulated or cultured on BSA (B) or on indicated FN peptides mediating adhesion via α4β1 (H296) and α5β1 (CH271) (C) in the presence of SCF. Cell lysates were collected and subjected to Western blot analysis with a rabbit antiphospho-ERK antibody that specifically detects phosphorylated T202 and Y204. Bottom panels show total Erk in each lane. The positions of the phosphorylated ERK-1 (pErk-1) and ERK-2 (pErk2) are indicated. Upper panels (bars) demonstrate the relative phosphorylation of ERK-1 and ERK-2 at residues T202 and Y204. Data are presented relative to the phosphorylation of ERK-1 and ERK-2 after 10 minutes (B) (with the level at 10 minutes taken as 100) and 120 minutes (C) (with the level at 120 minutes taken as 100). Bars denote the mean relative phosphorylation (± SEM) of 3 different experiments. Asterisk indicates P < .05 α5β1(CH271) versus α4β1 (H296). (D) G1E-ER2 erythroid progenitors were cultured on BSA or on FN peptides H296 or CH271 in the presence of SCF and the MEK inhibitor (PD98059). Proliferation was measured by thymidine incorporation assay. Bars denote the inhibition in proliferation (± SEM) of 3 independent experiments performed in replicates of 6. Asterisk indicatesP < .05 α4β1 (H296) versus α5β1 (CH271) and BSA; and double asterisks indicate P < .05 α4β1 (H296) and α5β1 (CH271) versus BSA.
To further examine the extent of involvement of the ERK pathway in c-Kit and/or integrin-mediated proliferation of erythroid cells, we used a specific pharmacologic inhibitor (PD98059) of the MAPK (ERK) cascade. Factor-starved G1E-ER2 cells were cultured on FN-CH271 or H296 or in suspension in the presence or absence of SCF and PD98059. After 48 hours of coculture, [3H]-thymidine was added for 6 hours and [3H]-thymidine incorporation was determined. Despite evidence of transient ERK activation (Figure 3B), PD98059 had minimal effect on the proliferation of G1E-ER2 cells grown in suspension in presence of SCF (Figure 3D). In contrast, proliferation of G1E-ER2 cells cultured on FN-H296 and FN-CH271 was inhibited by 65% and 30%, respectively, in the presence of PD98059. These data suggest that integrin- and c-Kit-stimulated growth of G1E-ER2 cells is dependent on activation of the MAPK (ERK) pathway, although the extent of use of this pathway differs significantly after ligation of α4β1 and α5β1.
Adhesion via α4β1 induces cell death in EPCs
Stem cell factor is an important survival factor for c-Kit+ cells and protects some cells from growth factor withdrawal-induced apoptosis.29 Integrin-mediated interactions with FN also play an important role in regulating cell survival and apoptosis in some cells. To determine if the reduction in proliferation of G1E-ER2 cells noted in cells cultured on FN–H296 or FN-CH296 was in part due to increased cell death, we compared apoptosis in G1E-ER2 cells cultured on different FN peptides in the presence or absence of SCF using a combination of PI and annexin V staining. G1E-ER2 cells were factor starved for 6 to 8 hours and then cultured for 48 hours in suspension or on FN-H296 or FN-CH296 or FN-CH271 in medium with or without SCF. Cells were harvested, stained with annexin V and PI, and scored for the percentage of apoptotic (annexin V+/PI−) and necrotic (annexin V+/PI+) cells using FACS analysis. In experiments performed in the absence of c-Kit stimulation, G1E-ER2 cells cultured on FN-H296 demonstrated significantly more cell death compared to cells cultured on FN-CH271 (52% ± 1 [H296] versus 33.4% ± 1 [CH271], mean ± SD, respectively,P < .05; Figure 4A). A similar increase in cell death was noted in cells cultured on FN-CH296 (mediating adhesion via both α4β1 and α5β1) in comparison to FN-CH271 (55% ± 3 [CH296] versus 33.4% ± 1 [CH271], mean ± SD, respectively, P < .05). The survival of cells grown in suspension was similar to that observed in cultures grown on FN-CH271 (29.3% ± 2 [BSA] versus 33.4% ± 1 [CH271], mean ± SD, respectively P > .05). These data suggest that adhesion to FN via α4β1 stimulates apoptosis of G1E-ER2 cells in the absence of growth factor. Further, these data demonstrate that adhesion to FN via α4β1has a dominant effect on the survival of G1E-ER2 cells.
Ligation via α4β1 induces cell death in G1E-ER2 cells.
G1E-ER2 erythroid progenitors were cultured on FN peptides mediating adhesion to α4β1 (H296) or α5β1 (CH271) or both α4β1 and α5β1(CH296) in the absence (A) or presence (B) of SCF for 48 hours. Cell death was quantitated by performing annexin V and PI staining as described in “Materials and methods.” Bars denote the percentage of total cell death (± SD) of 2 independent experiments performed in replicates of 3. Asterisk indicates P < .05 α4β1 (H296) and α4β1 and α5β1(CH296) versus α5β1 (CH271).
Ligation via α4β1 induces cell death in G1E-ER2 cells.
G1E-ER2 erythroid progenitors were cultured on FN peptides mediating adhesion to α4β1 (H296) or α5β1 (CH271) or both α4β1 and α5β1(CH296) in the absence (A) or presence (B) of SCF for 48 hours. Cell death was quantitated by performing annexin V and PI staining as described in “Materials and methods.” Bars denote the percentage of total cell death (± SD) of 2 independent experiments performed in replicates of 3. Asterisk indicates P < .05 α4β1 (H296) and α4β1 and α5β1(CH296) versus α5β1 (CH271).
To investigate the effect of c-Kit activation on reversal of apoptosis, G1E-ER2 cells were cultured on FN peptides in the presence of SCF and apoptosis measured. G1E-ER2 cells cultured on FN-H296 in the presence of SCF demonstrated significant improvement in survival in comparison with cells grown in absence of SCF. However, the rescue was only partial and significantly less in comparison to cells cultured on FN-CH271 and SCF (24.8% ± 5.3% α4β1versus 8.6% ± 0.2% α5β1, mean ± SD, P < .05; Figure 4B). Similar results were seen when these cells were cultured on FN-CH296 and SCF (27.2% ± 2.2% α4β1 and α5β1versus 8.6% ± 0.2% α5β1, mean ± SD, P < .05). The percentage of surviving cells in suspension culture in the presence of SCF was similar to that seen in cells cultured on FN-CH271 (7.45% ± 1.45% BSA versus 8.6% ± 0.2% CH271, mean ± SD, respectively). These results suggest that the reduced proliferation seen in erythroid cells following adhesion to FN via α4β1 is due in part due to an increase in the number of erythroid cells undergoing apoptosis.
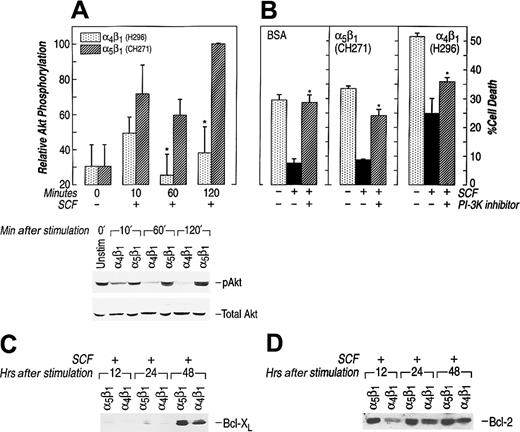
The downstream effector of PI-3K, Akt, has been implicated as an important antagonist of apoptosis.55 Previous studies have linked reduced activation of Akt to enhanced apoptosis.55Further, in some cells expression of constitutively activated forms of Akt has been shown to block apoptosis, whereas use of an Akt inhibitor, wortmannin, augments apoptosis.60 61 Therefore, we measured Akt activation in the cell culture conditions described above. Ligation of either α4β1 or α5β1 induced Akt activation in erythroid cells, but to different degrees (Figure5A). Akt activation induced by α4β1 was significantly less throughout the incubation period compared with Akt activation mediated by α5β1 in G1E-ER2 cells. This 3- to 4-fold reduction in Akt activation via α4β1attachment was seen at all time points examined (Figure 5A). To further investigate the importance of Akt activation in preventing apoptotsis of these cells, we cultured G1E-ER2 cells on FN-CH271 or in suspension (BSA control) in the presence of SCF and wortmannin. The presence of wortmannin under these culture conditions resulted in a significant increase in apoptosis of G1E-ER2 cells grown on FN-CH271 (Figure 5B). A similar increase in apoptosis was also observed in G1E-ER2 cells grown on FN-H296 (Figure 5B). In addition to reduced Akt activation, adhesion of G1E-ER2 cells to FN-H296 also resulted in reduced Bcl-2 and Bcl-xL expression (Figure 5C,D). Together, these data suggest that the PI-3K/Akt signaling cascade plays an important role in mediating erythroid cell apoptosis downstream from c-Kit and integrins. Adhesion of G1E-ER2 cells to FN via α4β1significantly impairs Akt activation and the expression of downstream antiapoptotic proteins; this impairment may be one of the mechanism(s) of enhanced apoptosis of these cells in comparison to cells grown in suspension or on FN via α5β1.
Inhibition of Akt activation via α4β1 enhances cell death in G1E-ER2.
(A) Reduced activation of Akt at Ser473 via α4β1 (H296) adhesion. Factor-starved G1E-ER2 cells were left unstimulated or cultured on FN peptides mediating adhesion via α4β1 (H296) or α5β1 (CH271) in the presence of SCF for various time points. Subsequently, at various times, cell lysates were collected and subjected to Western blot analysis with a rabbit antiphospho-Akt antibody that specifically detects phosphorylated S473. Bottom panel shows total Akt in each lane. The position of the activated Akt (pAkt) is indicated. Upper panel (bars) quantitatively demonstrates the relative phosphorylation of Akt. Data are presented relative to the phosphorylation of Akt after 120 minutes (taken as 100). Bars denote the mean relative phosphorylation (± SEM) of at least 3 independent experiments. Asterisk indicatesP < .05 α4β1 (H296) versus α5β1 (CH271). (B) G1E-ER2 cells were cultured on BSA or FN peptide CH271 or H296 in the presence of SCF and the PI-3K/Akt inhibitor (wortmannin) for 48 hours. Cell death was quantitated by performing annexin and PI staining as described in “Materials and methods.” Bars denote the percentage of total cell death (± SD) of 2 independent experiments performed in replicates of 3. Asterisk indicates P < .05 BSA, α5β1 (CH271), α4β1 (H296) (wortmannin) versus BSA, α5β1 (CH271), α4β1 (H296) (no inhibitor). (C,D) G1E-ER2 cells were cultured on FN peptides in the presence of SCF and analyzed at the indicated time points. Cell lysates were collected and subjected to Western blot analysis with an anti–Bcl-2 antibody. The position of Bcl-2 and Bcl-xL is indicated.
Inhibition of Akt activation via α4β1 enhances cell death in G1E-ER2.
(A) Reduced activation of Akt at Ser473 via α4β1 (H296) adhesion. Factor-starved G1E-ER2 cells were left unstimulated or cultured on FN peptides mediating adhesion via α4β1 (H296) or α5β1 (CH271) in the presence of SCF for various time points. Subsequently, at various times, cell lysates were collected and subjected to Western blot analysis with a rabbit antiphospho-Akt antibody that specifically detects phosphorylated S473. Bottom panel shows total Akt in each lane. The position of the activated Akt (pAkt) is indicated. Upper panel (bars) quantitatively demonstrates the relative phosphorylation of Akt. Data are presented relative to the phosphorylation of Akt after 120 minutes (taken as 100). Bars denote the mean relative phosphorylation (± SEM) of at least 3 independent experiments. Asterisk indicatesP < .05 α4β1 (H296) versus α5β1 (CH271). (B) G1E-ER2 cells were cultured on BSA or FN peptide CH271 or H296 in the presence of SCF and the PI-3K/Akt inhibitor (wortmannin) for 48 hours. Cell death was quantitated by performing annexin and PI staining as described in “Materials and methods.” Bars denote the percentage of total cell death (± SD) of 2 independent experiments performed in replicates of 3. Asterisk indicates P < .05 BSA, α5β1 (CH271), α4β1 (H296) (wortmannin) versus BSA, α5β1 (CH271), α4β1 (H296) (no inhibitor). (C,D) G1E-ER2 cells were cultured on FN peptides in the presence of SCF and analyzed at the indicated time points. Cell lysates were collected and subjected to Western blot analysis with an anti–Bcl-2 antibody. The position of Bcl-2 and Bcl-xL is indicated.
Discussion
In the developing embryo and in adult animals c-Kit and integrin-mediated signaling are necessary for erythroid cell survival, proliferation and differentiation.1,14,29,30 Therefore, erythroid progenitors provide a unique model to study the mechanism of c-Kit- and integrin-mediated signaling in a physiologically relevant context. To facilitate studies on the mechanisms governing the pleiotropic responses of c-Kit and integrins we have used an EPC line (G1E-ER2) that resembles primary cells at the progenitor stage of development. These cells are similar to primary erythroid progenitors with respect to globin- and erythroid-specific transcription factor expression and differentiation.62 63 These cells were derived from ES cells of mice deficient in GATA-1 expression. Instead of undergoing apoptosis, these cells grow continuously in culture as developmentally arrested precursors. We demonstrate that G1E-ER2 cells express c-Kit, α4β1, and α5β1 and adhere to FN peptides at levels similar to primary EPCs.
The role of cell adhesion to FN during erythroid cell development per se has not been completely determined, although several studies have suggested that FN is necessary both in vitro and in vivo to provide an appropriate niche for erythroid development and also to provide proliferative stimulus for erythroid cells.32-34 36 The studies presented here demonstrate significantly greater proliferation and enhanced activation of ERKs in cells grown in cultures containing α5β1 adhesion sites compared with cells grown in suspension or in cultures containing the α4β1 binding site. In addition, our results show significant quantitative differences in the activation of both FAK and MAPK (ERK-1 and ERK-2) in cells grown on FN mediating adhesion via these 2 different β1-integrin receptors. Specifically, engagement of α4β1 on G1E-ER2 cells results in significant reduction of FAK, ERK-1, and ERK-2 activation in comparison with cells grown in cultures containing α5β1 binding sites. The decrease in FAK, ERK-1, and ERK-2 activation correlated with reduced proliferation of these cells. In addition, the differences in activation appear to be important in cell proliferation because cells cultured on FN containing α4β1 binding sites were more resistant to the growth-inhibitory effects of the specific MEK inhibitor PD98059.
In contrast, adhesion of cells via α4β1 resulted in significant cell death. In addition to reduced activation of FAK and MAPK, inhibition of growth in response to ligation via α4β1 correlated with significant reduction in Akt activation in these cells. Although, SCF-induced activation of c-Kit partially prevented apoptosis in these cells, in comparison to cells grown on FN mediating adhesion via α5β1 or in suspension this reversal of apoptosis was incomplete. Studies in fibroblasts have shown that integrins and growth factors can jointly regulate Akt activation. In fibroblasts, activation of Akt leads to suppression of apoptosis, whereas in other cells activated Akt can protect cells from apoptosis in response to growth factor withdrawal, and apoptosis can be accelerated in these cells by dominant-negative Akt.61Recently, we have demonstrated that prolonged activation of Akt by stimulation of c-Kit via membrane-associated SCF is associated with erythroid cell survival/proliferation.64 Our finding that α4β1-mediated ligation results in reduced Akt activation and consequently greater apoptosis is consistent with these previous studies implicating Akt in hematopoietic cell survival. A key role for PI-3K-dependent Akt activation in erythroid cell survival via integrins and/or growth factor receptor stimulation was further demonstrated in the studies presented here by the use of the PI-3K inhibitor wortmannin. The results suggest that the PI-3K/Akt pathway is a key antagonist of cell death downstream of c-Kit and integrins in erythroid cells.
The mechanism(s) of the opposing effects of ligation of α4β1 and α5β1on growth and survival of erythroid cells is not clear. Although, G1E-ER2 cells express both α4β1 and α5β1 at comparable levels, we cannot rule out the possibility that the overall avidity of α4β1 and α5β1for FN might be different. These differences in overall avidity may result in different biologic and biochemical outcomes. Alternatively, the observed differences may be due to the generation of unique signals via the engagement of specific α5 and α4chains of α5β1 and α4β1 with FN, respectively. For instance, the studies presented here using EPCs demonstrate significantly greater activation of FAK in cells grown in the presence of FN containing α5β1 binding sites compared to α4β1. In this regard, it is possible that enhanced FAK activation via α5β1 and c-Kit could lead to more efficient recruitment of Ras, and hence to downstream kinase cascade of Raf-1, MEK, and MAPK. In fibroblasts evidence supports this model. Adhesion-mediated autophosphorylation of FAK leads to Src recruitment, increasing tyrosine phosphorylation of FAK, and the subsequent binding of SH2-domain proteins including Shc and the Grb2/Sos complex.65-67 The formation of a FAK/Src/Grb2 complex suggests the possibility of further signaling to MAPK. In addition, studies have shown that overexpression of FAK results in Ras-dependent activation of MAPK.68 Thus, enhanced phosphorylation of FAK via α5β1and c-Kit activation may explain the enhanced activation of ERKs in cells grown on FN mediating adhesion via α5β1. In contrast, recent studies using primary human periodontal ligament fibroblasts have shown that the adhesion to FN via α4β1 results in reduced FAK expression. Changes in FAK expression after adhesion via α4β1 in these cells were shown to be due to the activation of the caspase cascade.69 Our preliminary data, using the caspase-3 inhibitor, also demonstrate inhibition of apoptosis in cells cultured on FN-H296 (R.K. and D.A.W., unpublished observations, July 1999). Thus, our data in addition to these published results provide a mechanism by which opposing effects of integrin ligation could be mediated in erythroid cells.
Previous studies, using long-term human bone marrow cultures have demonstrated inhibition of hematopoietic cell proliferation in response to direct adhesion to bone marrow stroma via integrin receptors.17 The data presented here suggest that α4β1 compared to α5β1-mediated ligation to FN triggers unique signals in erythroid progenitors. The results of these signals are divergent, death versus proliferation. Taken together, these data support the notion that specific integrin-FN interactions in hematopoietic cells can regulate cell survival and proliferation and these effects can be modulated via ligand-induced stimulation of growth factor receptors.
We thank Eva Meunier and Sharon Smoot for assistance in preparation of this manuscript and expert administrative assistance. We thank Takara Shuzo, Biomedical Group (Otsu, Japan) for providing fibronectin peptides. We thank Drs Mervin Yoder and Don Durden for review of the manuscript and members of our laboratories for useful discussions.
Supported by National Institutes of Health grant 2R01 DK48605-06. R.K. is a recipient of an American Society of Hematology Junior Faculty Scholar Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Reuben Kapur, Herman B Wells Center for Pediatric Research, Cancer Research Building, 1044 W Walnut St, Rm 425, Indianapolis, IN 46202.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal