B7-H1 is a recently described B7-like molecule that costimulates T-cell growth and cytokine secretion without binding to CD28, cytotoxic T-lymphocyte antigen-4 (CTLA-4), and inducible costimulator (ICOS). In this report, a mouse homologue of human B7-H1 is identified, and its immunologic functions are studied in vitro and in vivo. Mouse B7-H1 shares 69% amino acid homology to the human counterpart. Similar to human B7-H1, mouse B7-H1 can be induced to express on macrophages, T cells, and B cells and to enhance T-cell proliferation and secretion of interleukin-10 (IL-10), interferon-γ, and granulocyte-macrophage colony-stimulating factor but not IL-2 and IL-4. Furthermore, B7-H1 preferentially costimulates CD4+ T cells independently of CD28 and enhances mixed lymphocyte responses to allogeneic antigens. In contrast to B7-1, expression of B7-H1 on murine P815 tumor cells by transfection fails to increase allogeneic and syngeneic cytolytic T-cell responses in vitro and in vivo. Administration of B7-H1Ig fusion protein, however, enhances keyhole limpet hemocyanin– specific T-cell proliferation and 2,4,6-trinitrophenyl–specific immunoglobulin G2a antibody production. The study thus identifies a unique costimulatory pathway that preferentially affects T-helper cell functions.
Introduction
The antigen-specific induction of proliferation and differentiation of lymphocytes requires a first signal delivered through T-cell or B-cell receptor together with a second, costimulatory signal. Stimulation of T cells by antigen in the absence of costimulation can result in unproductive T-cell responses or T-cell anergy. Engagement of CD28 by its natural ligand B7-1 or B7-2 promotes T-cell activation, proliferation, and the differentiation of effector functions for both CD4+ and CD8+ T cells.1-3 CD28 triggering augments both Th1 and Th2 cytokine secretion including interleukin-2 (IL-2), interferon-γ (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor-α (TNF-α), IL-4, IL-5, and IL-62,4 and up-regulates bcl-xLexpression, which promotes T-cell survival.5 Expression of B7-1 or B7-2 by tumor cells can enhance CD8+ cytotoxic T lymphocyte (CTL) generation and differentiation in vitro and in vivo.6-10 CTLA-4, the second receptor for B7-1 and B7-2, is believed to inhibit T-cell activation and IL-2 production by delivering an inhibitory signal.11 Recently, several new B7-like molecules have been described to costimulate T-cell growth and cytokine production by binding to receptors other than CD28 and CTLA-4. Human and mouse B7h/B7RP-1 (GL50/LICOS/B7-H2) interact with an inducible receptor, ICOS, on T cells to costimulate T-cell growth in vitro.12-18 More importantly, mice treated with B7RP-1 fusion protein had enhanced experimental contact hypersensitivity, and overexpression of soluble B7RP-1 in transgenic mice showed hyperplasia on several secondary lymphoid organs.13 Taken together, these data suggest that the interaction between B7h/B7RP-1 and ICOS regulate cell-mediated responses in vivo.
We have recently identified a new member of human B7 family designated B7-H1.19 B7-H1 gene encodes a putative type I transmembrane protein of 290 amino acids and consists of an immunoglobulin (Ig)V–like domain and an IgC-like domain in its extracellular portion. Although homology between B7-H1, B7h/B7RP-1, B7-1, and B7-2 is limited, the putative secondary and tertiary structures of these molecules are very similar (Chen et al, unpublished data, 2001). B7-H1 messenger RNA (mRNA) is found in various tissues, including lung, heart, skeletal muscle, and placenta, as well as several lymphoid organs, including spleen, thymus, and liver. B7-H1 can be detected on macrophages but not on T and B cells. Activation by various stimuli, however, can up-regulate the expression of B7-H1 on these cells. Stimulation of purified T cells by immobilized B7-H1Ig fusion protein in the presence of anti-CD3 antibodies induces a profound proliferative response and secretion of cytokines. Examination of cytokines on costimulation by B7-H1 ligation reveals a unique pattern: B7-H1 selectively costimulates the production of IL-10 and IFN-γ but not IL-2 and IL-4. Furthermore, soluble B7-H1Ig enhances mixed lymphocyte responses to allogeneic antigens.19 These results suggest that B7-H1 may participate in the regulation of cell-mediated and humoral immune responses. However, the potential role of B7-H1 in immune regulation in vivo is not known because of limitations of in vitro human T-cell culture system.
By cloning mouse B7-H1, immunologic functions of B7-H1 are evaluated both in vitro and in vivo. We demonstrate that, in contrast to a more broad immunomodulatory functions of B7-1 and B7-2, B7-H1 appears to selectively enhance antigen-specific helper T-cell responses but has minimal effect on the generation and differentiation of cytolytic T cells.
Materials and methods
Mice and cell lines
Female C57BL/6 (B6), DBA/2, and BALB/c mice were purchased from the National Cancer Institute (Frederick, MD). CD28−/−mice with a B6 background were kindly provided by Dr Moses Rodrigues (Mayo Clinic, Rochester, MN). P815 mastocytoma, L1210 lymphoma, EL4 mouse T-cell lymphoma, and 293 human kidney epithelial cells were purchased from the American Type Culture Collection (Rockville, MD). Cell lines were maintained in a complete medium of RPMI 1640 (Life Technologies, Rockville, MD) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT), 25 mM HEPES, 100 U/mL penicillin G, and 100 μg/mL streptomycin sulfate.
Cloning and identification of mouse B7-H1 complementary DNA
A search of the NCBI database revealed that 2 mouse expressed sequence tags (ESTs) had high homology (GenBank accession no. AA823166 and AA896104) to the published human B7-H1 sequence.19 The missing 5′ end and 3′ end of mouse B7-H1 (mB7-H1) sequence was obtained by a rapid amplification of complementary DNA (cDNA) ends, using the SMART PCR cDNA synthesis kit (Clontech, Palo Alto, CA) from a mouse T-cell library. The full-length cDNA of mB7-H1 was cloned into pcDNA3 vector (mB7-H1.pcDNA3) (Invitrogen, Carlsbad, CA). The homology to human B7-H1, mouse B7-1, B7-2, and B7h/B7RP-1 was analyzed, using the ClustalW program of MacVector 6.5.
RNA analysis
Tissue distribution of mB7-H1 was carried out, using mouse RNA dot blot (Clontech) according to the manufacture's instructions. The random-primed cDNA probe containing the full-length of mB7-H1 cDNA (895 base pairs) was labeled, using 32P-dCTP. The hybridization was performed for 16 hours at 65°C. After a 4-time wash with 2× SSC-sodium dodecyl sulfate, the membrane was exposed at −80°C to x-ray films.
Antibodies and fusion proteins
Rabbit antibodies against mB7-H1 protein were prepared by the Cocalico Biologicals (Reamstown, PA) by immunization with keyhole limpet hemocyanin (KLH)–conjugated hydrophilic peptides, spanning mB7-H1 amino acid sequence 95-119 (GNAALQITDVKLQDAGVYCCIISYG). The antibodies were purified from rabbit serum by using peptide-conjugated affinity columns. Both enzyme-linked immunosorbent assay (ELISA) and flow-activated cell sorter (FACS) staining of mB7-H1 transfected COS cells demonstrated that the antibodies bound specifically to mB7-H1. Purified monoclonal antibody (mAb) against CD3, CD4 (GK1.5), and CD28; fluorescein isothiocyanate (FITC)–conjugated mAb against CD3, CD4, CD8, and CD40L; phycoerythrin (PE)–conjugated CD3; B220; and Mac-1 were purchased from Pharmingen (San Diego, CA). FITC- and PE-conjugated goat antibody against rabbit IgG was purchased from Southern Biotechnology Associates (Birmingham, AL). Purified rabbit IgG and hamster IgG (Rockland, Gilbertsville, PA) and rat IgG (Sigma, St Louis, MO) were used as controls. To prepare the mB7-H1Ig fusion protein, cDNA encoding mouse B7-H1 extracellular domain was generated by reverse transcriptase–polymerase chain reaction (RT-PCR), using the sense primer 5′-CAGGAATTCACCATGAGGATATTTGCTG-3′ and the antisense primer 5′-CATCAGATCTATGTGAGTCCTGTTCTGTG-3′ from mouse T-cell mRNA. After digestion with EcoRI and BglII, the PCR products were fused to CH2-CH3 domain of mouse IgG2a in the expression plasmid pmIgV.19 The resulting plasmid, pmB7-H1Ig, was transfected into Chinese hamster ovary (CHO) cells and cultured by using serum-free CHO media (Life Technologies). The mB7-H1Ig in the supernatants was purified by a protein G–Sepharose column (Pierce, Rockford, IL) and dialyzed in lipopolysaccharide (LPS)–free phosphate-buffered saline (PBS). The endotoxin concentration was less than 1 pg/mg of purified protein according to limulus amebocyte lysate assays (Cape Cod, Woods Hole, MA). The mB7-1 immunoglobulin fusion protein (mB7-1Ig) was prepared by the same methods.
Flow cytometric analysis
The method for FACS analysis of surface molecules by antibodies has been previously described.6 20 For indirect immunofluorescence, the cells were incubated with the antibodies at 4°C for 30 minutes in the presence of blocking mAb to CD16/32 (Pharmingen). The cells were washed and further incubated with FITC- or PE-conjugated antirabbit IgG. For activation of T cells, nylon-wool (NW)–purified T cells (> 75% CD3+ cells) at 2 × 106/mL were cultured with Con A (3 μg/mL), anti-CD3 (5 μg/mL), or a combination of anti-CD3 and anti-CD28 (5 μg/mL). For preparation of activated B cells, splenocytes were cultured with 10 μg/mL LPS for 24 to 48 hours (Sigma). Monocytes were obtained from the peritoneal cavity of mice, which had been injected with thioglycollate 7 days before, and these cells were cultured with IFN-γ at 10 U/mL and LPS at 100 ng/mL. All cultures were collected and analyzed at 48 hours. To detect CD40L expression, CD4+T cells were purified by magnetic sorting (see the followings) and further incubated with FITC-conjugated mAb to CD40L. Fluorescence was analyzed by a FACS Calibur flow cytometry and analyzed with Cell Quest software (Becton Dickinson, Mountain View, CA).
T-cell costimulation and cytokine assay
NW column-purified T cells were further positively selected by magnetic sorting, using FITC-conjugated mAb against CD4 or CD8 and anti-FITC microbeads according to the manufacture's instructions (Miltenyi Biotec, Auburn, CA). The purity of isolated CD4+and CD8+ T cells was more than 95% by FACS analysis, using mAb to CD4 and CD8, respectively. Purified T cells at 2 × 106/mL from mouse spleens were cultured in 96-well plates that were precoated with anti-CD3 in the presence of 10 μg/mL mB7-H1Ig or control mouse IgG2a. mAb against CD28 (2.5 μg/mL) was used in soluble form as a positive control. Proliferation of T cells was determined by incorporation of 1 μCi/well of 3H-TdR during the last 15 hours of the 3-day culture. 3H-TdR incorporation was counted by a MicroBeta TriLux liquid scintillation counter (Wallac, Turku, Finland). To detect cytokines, supernatants were collected at 18 to 72 hours of cultures, and the concentrations of IFN-γ, IL-2, IL-10, IL-4, and GM-CSF were measured by sandwich ELISA following the manufacture's instructions (Pharmingen). Assay for proliferative responses of CD4+ T cells in mixed lymphocyte responses to allogeneic antigens has been described.19
Generation of mB7-H1–expressing P815 cells and induction of cytotoxic lymphocyte
P815 cells were transfected with mB7-H1.pcDNA3 plasmid by Fugene (Roche, Mannheim, Germany) according to manufacture's instructions. The transfected cells were selected in complete medium containing 1 mg/mL G418 (Life Technologies) and were subsequently cloned by limiting dilution. mB7-H1+ P815 cells were identified by FACS analysis, using anti–B7-H1 antibody. A representative clone, mB7-H1+ P815, was selected for further study. P815 clones transfected with pcDNA vector (mock.P815) and mB7-1.pcDNA (mB7-1+ P815) were also generated as described previously.6
To induce alloantigen-specific CTL activity in vitro, NW-purified T cells (2.5 × 106/mL) from B6 splenocytes were stimulated with irradiated (10 000 rad) mock.P815, mB7-1+ P815, or mB7-H1+ P815 cells (2.5 × 105/mL). After the 5-day stimulation, CTL activities against P815 (H-2d) and EL4 (H-2b) were measured by a standard 51Cr release assay.6 20 Alternatively, NW-purified T cells from BALB/c splenocytes at 3 × 106/mL were stimulated with irradiated B6 splenocytes at 1 × 106/mL in the presence of immobilized B7-H1Ig for 5 days, and CTL activity against allogeneic (EL4) or syngeneic target cells (P815) was measured by 51Cr release assay.
To induce tumor-specific CTL activity in vivo, DBA/2 mice were inoculated subcutaneously with 1 × 106 lived cells from mock.P815, mB7-1+ P815, or mB7-H1+ P815. The draining lymph nodes were removed 7 to 10 days after tumor injection, and the suspended lymph node cells (3 × 106/mL) were restimulated with P815 cells (3 × 105/mL) for 5 days. The CTL activity was measured in a standard 51Cr release assay.6 20
In vivo induction and assay of 2,4,6-trinitrophenyl– specific antibody
2,4,6-trinitrophenyl (TNP)–KLH (Biosearch Technologies, Novato, CA) at 100 μg/mouse in PBS was injected intraperitoneally into B6 mice on day 0. On days 1 and 4, the mice were injected intraperitoneally with 100 μg of control mIg, mB7-1Ig, or mB7-H1Ig. Sera were collected on days 7 and 14. To examine TNP-specific antibodies in sera, 0.3 mg/mL TNP-BSA (Biosearch Technologies) was bound to 96-well ELISA plates overnight at 4°C. Nonspecific binding was blocked with 10% FBS in PBS for 90 minutes at room temperature. After extensive washing, samples, diluted by 1/200 to 1/2000 with PBS, were added and incubated for 2 hours. The plates were then washed, and biotinylated rat antimouse IgM, IgG1, IgG2a, or IgG3 (Pharmingen) was added to the wells. The plates were further incubated for 1 hour at room temperature. Bound biotinylated rat antibodies were detected by incubation with horseradish peroxidase-conjugated streptavidin (Caltag Laboratories, Burlingame, CA) for 1 hour at room temperature. Color reactions were developed by 3,3′,5,5′-tetramethyl-benzidine substrate (Sigma) and the optical density (OD) reading at 450 nm was evaluated.
T-cell proliferation to KLH
B6 mice were immunized with 100 μg TNP-KLH in incomplete Freund's adjuvant (IFA) subcutaneously or in PBS intraperitoneally on day 0 and were subsequently treated with fusion protein or control immunoglobulin intraperitoneally on days 1 and 4. To detect T-cell responses to KLH, draining lymph nodes and spleens were removed from immunized mice on days 7 and 14, respectively. Suspended cells were cultured with KLH at 1.56 to 100 μg/mL as indicated. Proliferation of T cells to KLH was determined by addition of 1 μCi/well 3H-TdR during the last 15 hours of the 3-day culture. 3H-TdR incorporation was counted by a Microbeta Trilux liquid scintillation counter (Wallac).
Results
Characterization of mouse B7-H1 gene
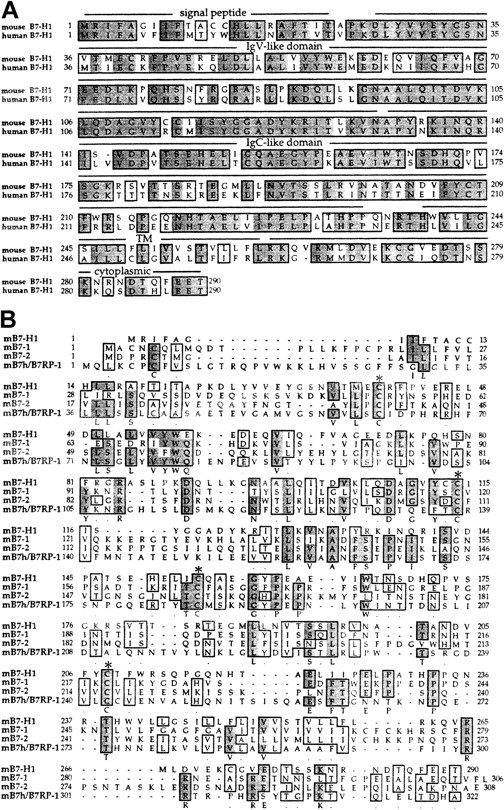
The mB7-H1 gene encodes a putative type I transmembrane protein of 290 amino acids and has 69% overall amino acid homology to human B7-H1 (Figure 1A). Similar to other members of B7 family, mB7-H1 consists of an IgV-like domain, an IgC-like domain, a hydrophobic transmembrane domain, and a cytoplasmic tail. B7-H1 shares 20% homology to mB7-1, 14% to mB7-2, and 19% to mB7h/B7RP-1, based on analysis using McVector 6.5 software (Figure 1B). Four structural cysteines are well conserved, the same as other B7 members.
Putative amino acid sequence of mouse B7-H1 and alignment with human B7-H1.
(A) Predicted amino acid sequence of mB7-H1 and alignment with human B7-H1. Signal peptide, IgV-like domain, IgC-like domain, transmembrane (TM), and cytoplasmic tail are indicated. (B) Alignment of mB7-H1 with other members of the B7 family. Conserved cysteine residues, which may be important to form the disulfide bounds of IgV and IgC domains, are marked by star signs. A few gaps (−) were introduced for the optimal alignment. Identical amino acid residues are shaded and conserved residues are boxed. The nucleic acid and amino acid sequences of mouse B7-H1 are available from GenBank under accession no. AAG31810.
Putative amino acid sequence of mouse B7-H1 and alignment with human B7-H1.
(A) Predicted amino acid sequence of mB7-H1 and alignment with human B7-H1. Signal peptide, IgV-like domain, IgC-like domain, transmembrane (TM), and cytoplasmic tail are indicated. (B) Alignment of mB7-H1 with other members of the B7 family. Conserved cysteine residues, which may be important to form the disulfide bounds of IgV and IgC domains, are marked by star signs. A few gaps (−) were introduced for the optimal alignment. Identical amino acid residues are shaded and conserved residues are boxed. The nucleic acid and amino acid sequences of mouse B7-H1 are available from GenBank under accession no. AAG31810.
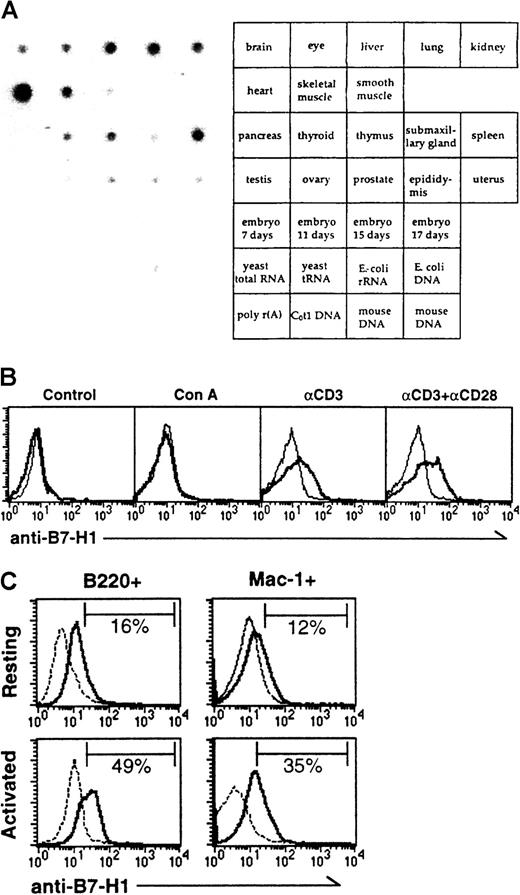
RNA analysis revealed that mB7-H1 mRNA was abundant in heart, spleen, lung, skeletal muscle, and liver, less abundant but positive in kidney, liver, thymus, and thyroid (Figure 2A). Therefore, the expression pattern is similar to human B7-H1 mRNA.19 Negligible expression of the B7-H1 mRNA was observed in pancreas and testis.
Expression pattern of mB7-H1.
(A) Tissue distribution of mB7-H1 mRNA in mouse tissue dot blot (Clontech). The random-primed cDNA probe containing the full length of mB7-H1 cDNA was prepared and labeled using 32P-dCTP. The tissues corresponding to the dot are indicated in the right panel. (B) Expression of mB7-H1 on resting and activated T cells. NW-purified T cells were analyzed without (control) or with stimulation of Con A, anti-CD3, or anti-CD3 plus anti-CD28 for 48 hours. The cells were stained by FITC-conjugated mAb against CD3 and rabbit anti–B7-H1 antibodies (solid line) or control immunoglobulin (dotted line) followed by PE-conjugated goat antirabbit IgG. Positive cells for CD3 were gated and analyzed for the expression of mB7-H1 by FACS. (C) Expression of mB7-H1 on B cells and macrophages. Freshly isolated mouse splenocytes were analyzed without (upper panel) or with stimulation (Activated; lower left panel) by LPS for 48 hours. Macrophages obtained from the peritoneal cavity of B6 mice were stimulated with LPS and IFN-γ (lower right panel). The cells were doubly stained by FITC-conjugated mAb against B220 or Mac-1 and rabbit anti–B7-H1 antibodies (solid line) or control immunoglobulin (dotted line) followed by PE-conjugated goat antirabbit IgG. Positive cells for B220 or Mac-1 were gated and analyzed for the expression of mB7-H1 by FACS. The percentages of B7-H1+ cells in the gated fraction are indicated in each histogram.
Expression pattern of mB7-H1.
(A) Tissue distribution of mB7-H1 mRNA in mouse tissue dot blot (Clontech). The random-primed cDNA probe containing the full length of mB7-H1 cDNA was prepared and labeled using 32P-dCTP. The tissues corresponding to the dot are indicated in the right panel. (B) Expression of mB7-H1 on resting and activated T cells. NW-purified T cells were analyzed without (control) or with stimulation of Con A, anti-CD3, or anti-CD3 plus anti-CD28 for 48 hours. The cells were stained by FITC-conjugated mAb against CD3 and rabbit anti–B7-H1 antibodies (solid line) or control immunoglobulin (dotted line) followed by PE-conjugated goat antirabbit IgG. Positive cells for CD3 were gated and analyzed for the expression of mB7-H1 by FACS. (C) Expression of mB7-H1 on B cells and macrophages. Freshly isolated mouse splenocytes were analyzed without (upper panel) or with stimulation (Activated; lower left panel) by LPS for 48 hours. Macrophages obtained from the peritoneal cavity of B6 mice were stimulated with LPS and IFN-γ (lower right panel). The cells were doubly stained by FITC-conjugated mAb against B220 or Mac-1 and rabbit anti–B7-H1 antibodies (solid line) or control immunoglobulin (dotted line) followed by PE-conjugated goat antirabbit IgG. Positive cells for B220 or Mac-1 were gated and analyzed for the expression of mB7-H1 by FACS. The percentages of B7-H1+ cells in the gated fraction are indicated in each histogram.
FACS analysis using the antibody against mB7-H1 showed that freshly isolated as well as Con A-stimulated CD3+ T cells express negligible amounts of B7-H1. However, stimulation by anti-CD3 or a combination of anti-CD3/CD28 mAb moderately increased the expression of B7-H1 (Figure 2B). A small fraction of B220+ B cells and Mac-1+ macrophages expressed a low level of B7-H1 (Figure2C, upper panel). Activation of B cells with LPS and macrophages with LPS plus IFN-γ significantly increased the expression of B7-H1 on the cell surface (Figure 2C, lower panel). We conclude that B7-H1 is an inducible cell surface molecule.
B7-H1 costimulation is CD28 independent and preferential for CD4+ T cells
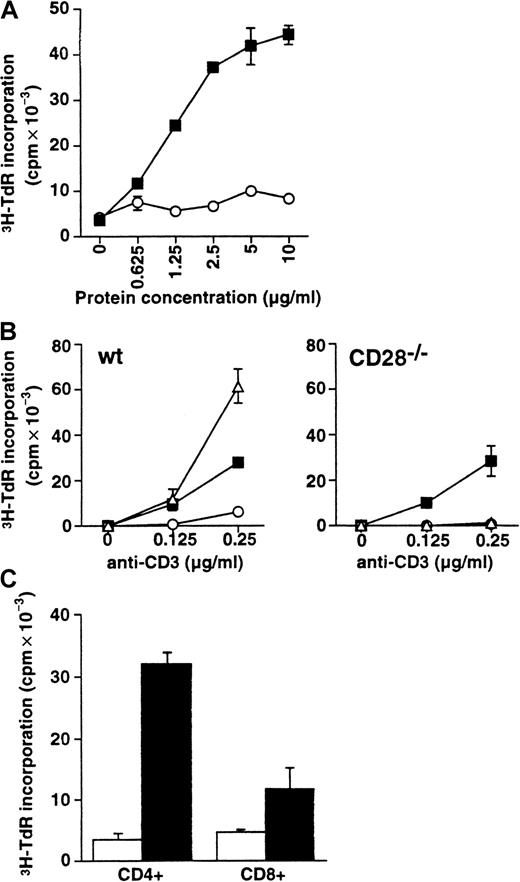
To investigate the costimulatory effect of B7-H1, we stimulated NW-purified T cells with B7-H1Ig and suboptimal doses of anti-CD3 mAb. B7-H1 can enhance T-cell growth up to 5-fold compared to that of the control immunoglobulin (Figure 3A). The costimulatory effect of the B7-H1 is dose dependent and is dependent on anti-CD3 mAb because, in the absence of anti-CD3 mAb, B7-H1Ig up to 10 μg/mL did not stimulate the proliferation of T cells (Figure 3B, left panel). When purified T cells were cultured with 293 cells transfected with mB7-H1.pcDNA3 or control vector in the presence of suboptimal doses of anti-CD3 mAb, B7-H1-transfected 293 cells also enhanced T-cell proliferation substantially compared with that of the control vector-transfected 293 cells (data not shown). We conclude that, similar to human B7-H1, mB7-H1 costimulates T-cell proliferation.
Costimulatory effect of B7-H1 on T-cell growth.
(A) The costimulatory effect of the B7-H1 is dose dependent. NW-purified T cells were cultured with the indicated doses of immobilized mB7-H1Ig (▪) or control immunoglobulin (○) in the presence of a suboptimal dose (200 ng/mL) of precoated anti-CD3 mAb. The proliferation of T cells was determined by 3H-thymidine incorporation assay. (B) B7-H1 costimulation is CD28 independent. NW-purified T cells obtained from spleens of wild-type (wt, left panel) and CD28−/− (right panel) mice were cultured with control immunoglobulin (○), anti-CD28 mAb (▵), or mB7-H1Ig (▪) at 10 μg/mL in the presence of suboptimal doses (0.125 and 0.25 μg/mL) of immobilized anti-CD3. The proliferation of T cells was determined by3H-TdR incorporation assay. (C) B7-H1 preferentially costimulates CD4+ T-cell growth. Affinity-purified CD4+ or CD8+ T cells were cultured in the presence of a suboptimal dose of anti-CD3 and immobilized B7-H1Ig. Control Ig, ■; B7-H1Ig, ▪. The proliferation of T cells was determined by 3H-TdR incorporation assay. Data represent one experiment of 3 and 3H-TdR incorporation in counts per minute (cpm) ± SD.
Costimulatory effect of B7-H1 on T-cell growth.
(A) The costimulatory effect of the B7-H1 is dose dependent. NW-purified T cells were cultured with the indicated doses of immobilized mB7-H1Ig (▪) or control immunoglobulin (○) in the presence of a suboptimal dose (200 ng/mL) of precoated anti-CD3 mAb. The proliferation of T cells was determined by 3H-thymidine incorporation assay. (B) B7-H1 costimulation is CD28 independent. NW-purified T cells obtained from spleens of wild-type (wt, left panel) and CD28−/− (right panel) mice were cultured with control immunoglobulin (○), anti-CD28 mAb (▵), or mB7-H1Ig (▪) at 10 μg/mL in the presence of suboptimal doses (0.125 and 0.25 μg/mL) of immobilized anti-CD3. The proliferation of T cells was determined by3H-TdR incorporation assay. (C) B7-H1 preferentially costimulates CD4+ T-cell growth. Affinity-purified CD4+ or CD8+ T cells were cultured in the presence of a suboptimal dose of anti-CD3 and immobilized B7-H1Ig. Control Ig, ■; B7-H1Ig, ▪. The proliferation of T cells was determined by 3H-TdR incorporation assay. Data represent one experiment of 3 and 3H-TdR incorporation in counts per minute (cpm) ± SD.
The role of CD28 in B7-H1 costimulation was evaluated by comparing the effects of mB7-H1Ig costimulation on T cells isolated from CD28−/− mice and from normal mice. As shown in Figure 3B, although there was no costimulatory effect of anti-CD28 mAb on CD28−/− T cells, mB7-H1Ig induced the proliferation of both CD28−/− and CD28+/+ T cells to a similar degree. Therefore, B7-H1 can costimulate T-cell growth in a CD28-independent fashion.
We next examined the preference of B7-H1 costimulation on CD4+ and CD8+ T cells. Purified CD4+ and CD8+ T cells were stimulated with the same concentration of mB7-H1Ig and anti-CD3. Proliferation of CD4+ T cells was up to 10-fold, and the proliferation of CD8+ T cells was only enhanced 2- to 3-fold (Figure 3C). Thus, costimulatory effect of B7-H1 is more favorable for CD4+ T cells.
Effects of B7-H1 costimulation on cytokine secretion
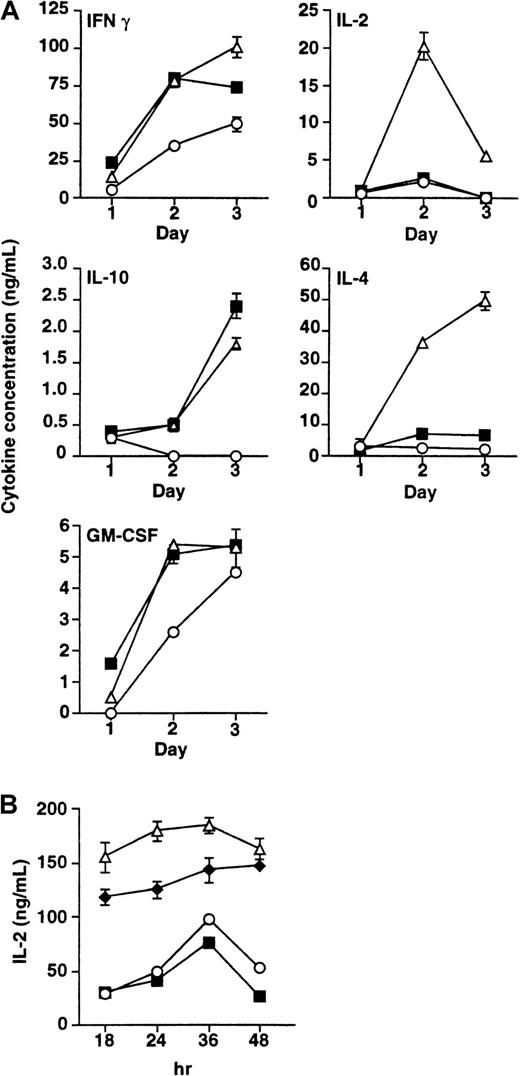
We have previously shown that human B7-H1 preferentially stimulated secretion of IL-10 and IFN-γ but not IL-2 and IL-4.19 To examine whether mB7-H1 functions similarly, we measured the levels of these cytokines as well as GM-CSF from T cells after stimulation with mB7-H1Ig or anti-CD28 mAb in the presence of anti-CD3 mAb. Figure 4A shows that mB7-H1, similar to anti-CD28 mAb, can induce high levels of IL-10 production in the 72-hour culture supernatants. IL-10 was not detectable at the same time point when T cells were treated with control immunoglobulin and anti-CD3. mB7-H1 also promotes the secretion of IFN-γ and GM-CSF, which are also induced by anti-CD28 mAb. In sharp contrast to anti-CD28 mAb, which induces high levels of IL-2 and IL-4, mB7-H1Ig induces low or negligible levels of IL-2 and IL-4 up to 3 days (Figure 4A). We conclude that mouse B7-H1 and human B7-H1 have similar selectivity for cytokine production.
Cytokine secretion of mB7-H1-stimulated T cells.
(A) NW-purified T cells were cultured with immobilized control immunoglobulin (○) or mB7-H1Ig (▪, 10μg/mL) in the presence of precoated anti-CD3 (2μg/mL). The mAb to CD28 (▵, 2.5 μg/mL) was used in soluble form. Supernatants were collected at days 1, 2, and 3 on the culture, and indicated cytokine concentrations in the supernatants were determined by sandwich ELISA (Pharmingen). Data represent one experiment of 3. Error bars represent the SD of the mean of triplicate T-cell culture wells. (B) Effect of B7-H1 costimulation on IL-2 production. The levels of IL-2 were determined in the supernatants, in which purified T cells were cultured in the presence of 2.5 μg/mL anti-CD28, 10 μg/mL mB7-H1Ig, or anti-CD28 plus mB7-H1Ig (♦) in the precoated plate with immobilized anti-CD3 (1 μg/mL). Supernatants were collected at 18, 24, 36, and 48 hours, and IL-2 concentrations in the supernatants were determined by sandwich ELISA. Data present one experiment of 3. Error bars represent the SD of the mean of triplicate T-cell culture wells.
Cytokine secretion of mB7-H1-stimulated T cells.
(A) NW-purified T cells were cultured with immobilized control immunoglobulin (○) or mB7-H1Ig (▪, 10μg/mL) in the presence of precoated anti-CD3 (2μg/mL). The mAb to CD28 (▵, 2.5 μg/mL) was used in soluble form. Supernatants were collected at days 1, 2, and 3 on the culture, and indicated cytokine concentrations in the supernatants were determined by sandwich ELISA (Pharmingen). Data represent one experiment of 3. Error bars represent the SD of the mean of triplicate T-cell culture wells. (B) Effect of B7-H1 costimulation on IL-2 production. The levels of IL-2 were determined in the supernatants, in which purified T cells were cultured in the presence of 2.5 μg/mL anti-CD28, 10 μg/mL mB7-H1Ig, or anti-CD28 plus mB7-H1Ig (♦) in the precoated plate with immobilized anti-CD3 (1 μg/mL). Supernatants were collected at 18, 24, 36, and 48 hours, and IL-2 concentrations in the supernatants were determined by sandwich ELISA. Data present one experiment of 3. Error bars represent the SD of the mean of triplicate T-cell culture wells.
Because IL-2 is undetectable in the presence of B7-H1 costimulation, it is thus possible that B7-H1 ligation inhibits IL-2 secretion. To test this possibility, we examined the effect of B7-H1 costimulation in IL-2 secretion from T cells stimulated by anti-CD3/28 mAb. Figure 4B shows that inclusion of immobilized mB7-H1Ig up to 10 μg/mL in the culture results in a small but not significant decrease in IL-2 production during 18 to 48 hours. Similarly, mB7-H1Ig did not inhibit IL-2 production from the culture, in which T cells were stimulated with anti-CD3 alone (Figure 4B). Our results thus indicate that mB7-H1 ligation does not inhibit the production of IL-2.
Effect of B7-H1 costimulation on antigen-specific T-cell responses
To examine the effect of B7-H1 costimulation on the generation of CTL, we transfected mouse P815 tumor cells with mB7-H1.pcDNA3 plasmid. Clones that stably express mB7-H1 on their surface were selected for further experiments. A representative clone, B7-H1+ P815, expresses high levels of mB7-H1 as indicated by FACS staining, using the anti–mB7-H1 antibodies (Figure 5A). The binding of the antibody is specific because mock.P815 cells (Figure5A, left panel) and mB7-1+ P815 cells (data not shown) are negative for the staining. Furthermore, inclusion of the peptide of B7-H1, which was used for immunization, completely blocks the binding of anti–B7-H1 antibodies to mB7-H1+ P815 (Figure 5A, right panel).
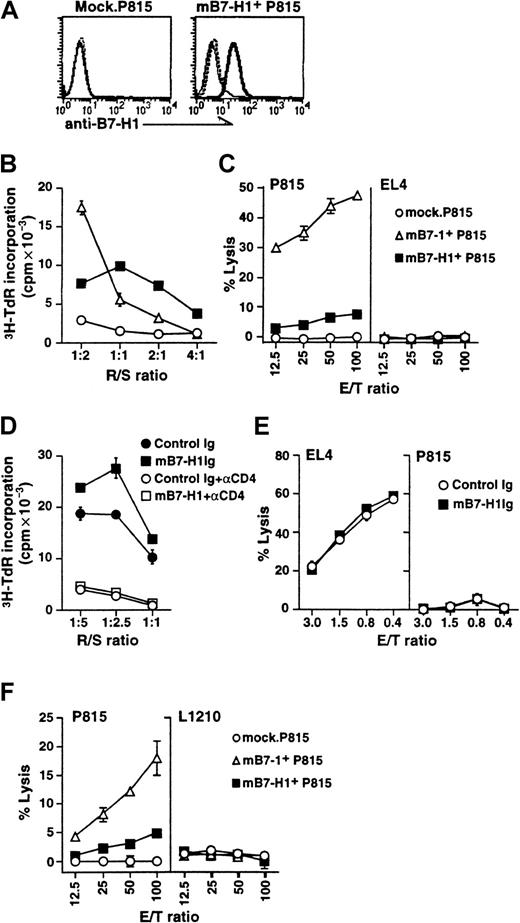
Effect of B7-H1 costimulation in T-cell responses to allogeneic and tumor antigens.
(A) The expression of mB7-H1 by transfected P815 tumor cells. P815 tumor cells were transfected with pcDNA3 vector (Mock; left panel) or B7-H1.pcDNA3 (B7-H1+ P815; right panel). Cells were stained by rabbit anti–B7-H1 antibodies (bold line) or control rabbit immunoglobulin (dashed line) followed by FITC-conjugated goat antirabbit IgG. In addition, B7-H1+ P815 cells were stained by anti–B7-H1 antibodies in the presence of the peptide of B7-H1, which had been used for immunization to make the antibodies (solid line). (B) The effect of mB7-H1 expression by P815 on T-cell proliferative responses to allogeneic antigen. NW-purified T cells obtained from B6 mice (H-2b) were cultured with irradiated mock, mB7-1+, or mB7-H1+ P815 cells (H-2d) at the indicated responder/stimulator (R/S) ratio. After 3-day culture, the proliferative responses of T cells were determined by 3H-TdR incorporation assay. (C) The effect of mB7-H1 expression by P815 on the generation of alloreactive CTL in vitro. NW-purified T cells (2.5 × 106/mL) obtained from B6 spleen cells were cultured with irradiated mock, mB7-1+, or mB7-H1+ P815 cells at 2.5 × 105/mL for 5 days. The cells were harvested, and their cytolytic activity against allogeneic P815 (left panel) or syngeneic EL4 cells (right panel) was determined in a standard 51Cr-release assay. (D) The effect of mB7-H1Ig on the proliferative responses to allogeneic antigen. NW-purified T cells of BALB/c mice (H-2d) (1 × 106/mL) were cocultured with irradiated B6 spleen cells (H-2b) in the presence of immobilized control immunoglobulin or mB7-H1Ig at the indicated responder/stimulator (R/S) ratio. Anti-CD4 (GK1.5) or control rat immunoglobulin was added at 10 μg/mL. After 3-day culture, 3H-TdR incorporation was measured. (E) The effect of mB7-H1Ig on the generation of alloreactive CTL in vitro. NW-T cells (H-2d) (3 × 106) were cultured with 6 × 106 irradiated B6 spleen cells (H-2b) in the presence of immobilized control immunoglobulin or mB7-H1Ig in 2mL of 24-well plates for 5 days. The CTL activity against allogeneic EL4 (left panel) or syngeneic P815 (right panel) was determined in a standard 51Cr-release assay. (F) The effect of B7-H1 expression by P815 cells on the induction of P815-specific CTL in vivo. DBA/2 mice were injected subcutaneously with live cells of mock.P815, mB7-1+ P815, or mB7-H1+ P815 at 1 × 106/mouse. The draining lymph nodes were removed 7 to 10 days after tumor injection, and prepared suspension cells were restimulated with irradiated wild-type P815 cells for 5 days. The cells were harvested, and their cytolytic activity against P815 (left panel) and L1210 cells (right side) was determined in a standard 51Cr release assay. Data represent one experiment of 3 or more. Results are expressed as the mean ± SD of triplicate wells.
Effect of B7-H1 costimulation in T-cell responses to allogeneic and tumor antigens.
(A) The expression of mB7-H1 by transfected P815 tumor cells. P815 tumor cells were transfected with pcDNA3 vector (Mock; left panel) or B7-H1.pcDNA3 (B7-H1+ P815; right panel). Cells were stained by rabbit anti–B7-H1 antibodies (bold line) or control rabbit immunoglobulin (dashed line) followed by FITC-conjugated goat antirabbit IgG. In addition, B7-H1+ P815 cells were stained by anti–B7-H1 antibodies in the presence of the peptide of B7-H1, which had been used for immunization to make the antibodies (solid line). (B) The effect of mB7-H1 expression by P815 on T-cell proliferative responses to allogeneic antigen. NW-purified T cells obtained from B6 mice (H-2b) were cultured with irradiated mock, mB7-1+, or mB7-H1+ P815 cells (H-2d) at the indicated responder/stimulator (R/S) ratio. After 3-day culture, the proliferative responses of T cells were determined by 3H-TdR incorporation assay. (C) The effect of mB7-H1 expression by P815 on the generation of alloreactive CTL in vitro. NW-purified T cells (2.5 × 106/mL) obtained from B6 spleen cells were cultured with irradiated mock, mB7-1+, or mB7-H1+ P815 cells at 2.5 × 105/mL for 5 days. The cells were harvested, and their cytolytic activity against allogeneic P815 (left panel) or syngeneic EL4 cells (right panel) was determined in a standard 51Cr-release assay. (D) The effect of mB7-H1Ig on the proliferative responses to allogeneic antigen. NW-purified T cells of BALB/c mice (H-2d) (1 × 106/mL) were cocultured with irradiated B6 spleen cells (H-2b) in the presence of immobilized control immunoglobulin or mB7-H1Ig at the indicated responder/stimulator (R/S) ratio. Anti-CD4 (GK1.5) or control rat immunoglobulin was added at 10 μg/mL. After 3-day culture, 3H-TdR incorporation was measured. (E) The effect of mB7-H1Ig on the generation of alloreactive CTL in vitro. NW-T cells (H-2d) (3 × 106) were cultured with 6 × 106 irradiated B6 spleen cells (H-2b) in the presence of immobilized control immunoglobulin or mB7-H1Ig in 2mL of 24-well plates for 5 days. The CTL activity against allogeneic EL4 (left panel) or syngeneic P815 (right panel) was determined in a standard 51Cr-release assay. (F) The effect of B7-H1 expression by P815 cells on the induction of P815-specific CTL in vivo. DBA/2 mice were injected subcutaneously with live cells of mock.P815, mB7-1+ P815, or mB7-H1+ P815 at 1 × 106/mouse. The draining lymph nodes were removed 7 to 10 days after tumor injection, and prepared suspension cells were restimulated with irradiated wild-type P815 cells for 5 days. The cells were harvested, and their cytolytic activity against P815 (left panel) and L1210 cells (right side) was determined in a standard 51Cr release assay. Data represent one experiment of 3 or more. Results are expressed as the mean ± SD of triplicate wells.
We showed previously that inclusion of B7-H1Ig fusion protein enhances mixed lymphocyte responses to allogeneic antigen in human T-cell culture.19 Similar to this observation, stimulation of purified T cells from B6 mice with allogeneic mB7-H1+ P815 cells induces proliferative responses superior to that stimulated with mock.P815 cells. As a control, stimulation with mB7-1+ P815 cells also induces high-level proliferative responses (Figure 5B). The proliferative responses enhanced by both mB7-H1+ P815 as well as mB7-1+ P815 cells can be completely inhibited by anti-CD4 mAb (data not shown). However, mB7-H1+ P815 cells as well as mock.P815 are poor stimulators of P815-specific CTL activity, whereas mB7-1+ P815 elicited a strong P815-specific CTL activity as assayed in standard 51Cr release assay (Figure 5C). The CTL induced by mB7-1 was allogeneic antigen specific because it did not lyse H-2b EL4 cells. Similar results were also obtained in a different system in which purified T cells from BALB/c mice were stimulated with irradiated B6 spleen cells as allogeneic antigen-presenting cells. As shown in Figure5D, proliferative responses are enhanced by inclusion of B7-H1Ig fusion protein, and the response can be completely blocked by an anti-CD4 antibody. CTL activity against allogeneic antigens in this system is not affected (Figure 5E). Therefore, B7-H1 costimulation does not facilitate the generation of allogeneic CTL in vitro.
We also examined the ability of mB7-H1+ P815 cells to stimulate P815-specific CTL in vivo. DBA/2 mice were injected subcutaneously with lived cells from mock.P815, mB7-1+P815, or mB7-H1+ P815 lines. T cells from tumor-draining lymph nodes were removed 7 to 10 days later and cocultivated with wild-type (wt).P815 cells for 5 days. In mice injected with mB7-H1+ P815, a small, but not significant increase in CTL activity against P815 cells was detected compared to CTL from the mock.P815-injected mice. In contrast, mB7-1+ P815 elicited a strong CTL activity in vivo. CTL activity is P815 specific since syngeneic but antigenically irrelevant L1210 cells were not lysed (Figure 5F). We conclude that expression of B7-H1 in P815 cells does not enhance the induction of CTL activity against P815 tumor antigens.
B7-H1 costimulation amplifies antigen-specific T-helper cell responses and T-cell–dependent humoral responses in vivo
To investigate the effect of B7-H1 costimulation on T-helper cell function, we immunized B6 mice with TNP-conjugated KLH and subsequently injected the mice with mB7-H1Ig at day 1 and day 4. The proliferation of T cells obtained from both lymph nodes and spleens of the immunized mice (control mice) was examined by exposure to KLH in vitro. As shown in Figure 6, T cells from both spleens and lymph nodes of TNP-KLH–immunized mice proliferated to KLH in a dose-dependent fashion. Administration of mB7-H1Ig to TNP-KLH–immunized mice amplified the proliferative responses up to 2- to 3-fold. Our result indicates that B7-H1 costimulation can enhance T-helper cell responses in vivo.
T-helper cell response to KLH.
B6 mice were injected with 100 μg TNP-KLH in IFA subcutaneously (left panel) or in PBS intraperitoneally (right panel) on day 0, and subsequently treated with 100 μg control immunoglobulin (○) or B7-H1Ig (▪) on days 1 and 4. The draining lymph nodes were prepared at day 7 (left panel), and the splenocytes were prepared on day 14 (right panel). The suspension cells were stimulated with KLH at the indicated concentrations. The proliferative responses of T cells to KLH were determined by the addition of 1 μCi/well3H-thymidine during the last 15 hours of the 3-day culture.
T-helper cell response to KLH.
B6 mice were injected with 100 μg TNP-KLH in IFA subcutaneously (left panel) or in PBS intraperitoneally (right panel) on day 0, and subsequently treated with 100 μg control immunoglobulin (○) or B7-H1Ig (▪) on days 1 and 4. The draining lymph nodes were prepared at day 7 (left panel), and the splenocytes were prepared on day 14 (right panel). The suspension cells were stimulated with KLH at the indicated concentrations. The proliferative responses of T cells to KLH were determined by the addition of 1 μCi/well3H-thymidine during the last 15 hours of the 3-day culture.
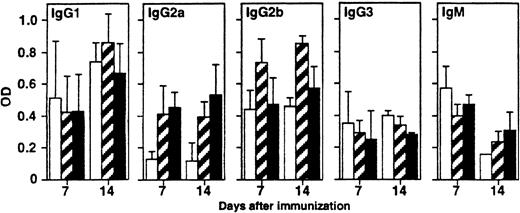
We next examined the effect of B7-H1 costimulation in the generation of antigen-specific antibodies to TNP. This is a well-established system in which antibody production against TNP is dependent on helper T-cell response to KLH.21 22 The production of antibodies against TNP in the sera from TNP-KLH–immunized mice was measured after treatment with control immunoglobulin, mB7-1Ig, or mB7-H1Ig. In preliminary experiments, we have found a significant increase of total IgG levels in mice treated with mB7-H1Ig compared to those treated with control mIg (data not shown). The subclasses of IgG, including IgG1, IgG2a, IgG2b, and IgG3 were further examined. As shown in Figure7, TNP-specific IgG2a antibody was increased significantly in mice treated with mB7-H1Ig, and there were no significant changes on other immunoglobulin subtypes. This effect is different from mice treated with mB7-1Ig since other IgG components, including IgG1 and IgG2b, were also increased significantly (Figure 7). Therefore, B7-H1 costimulation enhances T-helper cell proliferation and T-helper–dependent humoral immune responses.
The effect of B7-H1 on humoral response.
B6 mice at 8 per group were injected intraperitoneally with 100 μg TNP-KLH in PBS on day 0 and subsequently treated with 100 μg of control immunoglobulin (■) or B7-H1Ig (▪) on days 1 and 4. ▨, mB7-1Ig. Serum from individual mice was collected on days 7 and 14. The concentrations of anti-TNP–specific IgG of all subclasses and IgM were determined by specific sandwich ELISA as described in “Materials and methods.” The results of the OD450 reading are expressed as the mean ± SD.
The effect of B7-H1 on humoral response.
B6 mice at 8 per group were injected intraperitoneally with 100 μg TNP-KLH in PBS on day 0 and subsequently treated with 100 μg of control immunoglobulin (■) or B7-H1Ig (▪) on days 1 and 4. ▨, mB7-1Ig. Serum from individual mice was collected on days 7 and 14. The concentrations of anti-TNP–specific IgG of all subclasses and IgM were determined by specific sandwich ELISA as described in “Materials and methods.” The results of the OD450 reading are expressed as the mean ± SD.
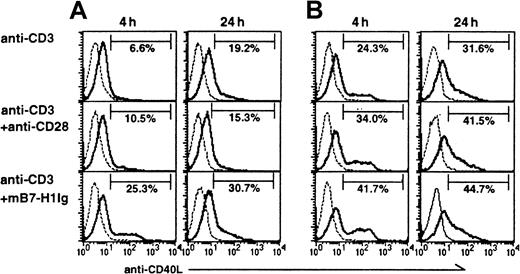
CD40–CD40L interaction is critical for T-helper cell–B-cell interaction for the generation of antibody responses and for immunoglobulin class switching.23 We examined whether B7-H1 costimulation up-regulates CD40L on T cells. Purified CD4+ T cells from B6 mice were stimulated with suboptimal anti-CD3 mAb in the presence of mB7-H1Ig. Anti-CD28 mAb was also included as a control. Expression of CD40L on T cells was detected by a specific mAb to CD40L by flow cytometry analysis. B7-H1Ig up-regulated CD40L rapidly (25.3% in 4-hour incubation) compared with that of the control IgG (6.6%) or anti-CD28 (10.5%). The expression of CD40L was further increased on 24-hour stimulation (Figure8A). Similar results were also obtained with CD4+ T cells, using optimal dose of anti-CD3 stimulation (Figure 8B). Our results suggest that triggering of B7-H1 counter-receptor on T cells rapidly up-regulates the expression of CD40L that may contribute to enhanced antibody production.
Up-regulation of CD40L on B7-H1–costimulated CD4+ T cells.
Affinity-purified CD4+ T cells from B6 mice were stimulated with either immobilized control immunoglobulin, B7-H1Ig, or soluble anti-CD28 mAb in the presence of anti-CD3 mAb at either 0.2 μg/mL (A) or 1 μg/mL (B) for 4 or 24 hours. CD40L on the T cells was stained with FITC-conjugated anti-CD40L mAb and analyzed by FACS.
Up-regulation of CD40L on B7-H1–costimulated CD4+ T cells.
Affinity-purified CD4+ T cells from B6 mice were stimulated with either immobilized control immunoglobulin, B7-H1Ig, or soluble anti-CD28 mAb in the presence of anti-CD3 mAb at either 0.2 μg/mL (A) or 1 μg/mL (B) for 4 or 24 hours. CD40L on the T cells was stained with FITC-conjugated anti-CD40L mAb and analyzed by FACS.
Discussion
In this study, we have identified the mouse homologue of human B7-H1 and shown its characteristic functions in vitro and in vivo. Similar to its human counterpart, mouse B7-H1 can markedly costimulate T-cell proliferation in the presence of suboptimal doses of anti-CD3 and stimulate secretion of a unique pattern of cytokines. Both CD28−/− and CD28+/+ T cells respond to B7-H1 costimulation to a similar degree, suggesting that B7-H1 costimulates T-cell growth through a CD28-independent event. In sharp contrast to B7-1, B7-H1 does not enhance the induction of CTL to major histocompatibility complex (MHC) class I–restricted allogeneic and syngeneic antigens. Rather, administration of B7-H1Ig enhances proliferation of T-cell response to KLH and the antibody response to hapten antigen TNP. Our results, thus, indicate that B7-H1 costimulation preferentially induces T-helper cell responses with minimal effect on CTL generation.
Costimulation mediated by B7-1 and B7-2 plays a crucial role in the induction of CTLs. The expression of B7-1 and B7-2 on tumor cells, including P815 cells, has been shown to enhance MHC class I–restricted CTL responses specific for allogeneic or syngeneic tumor antigens in vitro and in vivo.7,8 Augmentation of MHC class II–restricted responses by B7-1 costimulation has also been demonstrated.24 Several studies indicate that priming of T cells by B7-transfected tumor cells requires cross presentation by host professional antigen-presenting cells, direct presentation by tumor cells, or both.7,25-27 One of the hypotheses for enhanced cross presentation of tumor antigens after transfection of B7-1 is increased sensitivity of tumor cells to natural killer (NK) cells.28 We have found that both B7-1+ and B7-H1+ P815 cells express high levels of MHC class I antigens and are resistant to NK-cell lysis in vitro (Tamura et al, unpublished data, 2001). In addition, the expression of B7-H1 by P815 cells appears to have a marginal effect on the stimulation of CTL both in vitro and in vivo, whereas the growth of CD4+ T cells in the presence of B7-H1 costimulation are augmented in the same cultures (Figure 5). The failure of B7-H1–transfected P815 cells to costimulate CTL response may be partially due to less responsiveness of CD8+ T cells to B7-H1 costimulation than that of CD4+ T cells (Figure 3C). It is not known whether this difference is caused by differential expression of the counter-receptor of B7-H1 on CD4+ versus CD8+ T cells. Human CD28 receptor has been found to express on the majority of CD4+ T cells, but only 50% of CD8+ T cells are CD28 positive.2
CD28 engagement has been shown to enhance the production of various cytokines, including IL-2, IL-4, IFN-γ, and TNF-α2,4and play a role in the early development and differentiation of both Th1/Th2 T-cell subsets. For example, purified naive T cells stimulated with suboptimal anti-CD3 in the absence of anti-CD28 enhanced the secretion of Th1 cytokine such as IFN-γ and GM-CSF, whereas the addition of anti-CD28 mAb promotes IL-4 and IL-10 production in addition to IFN-α and GM-CSF (Figure 4A). In some studies, the differentiation of Th1/Th2 subsets was shown to be dependent on CD28 ligation. In CD28−/− mice, Th2-dependent antibody response is reduced, whereas the Th1-dependent delayed type hypersensitivity (DTH) response remains intact.24Similar to human B7-H1, mouse B7-H1 preferentially costimulates secretion of IL-10, GM-CSF, and IFN-γ but has little effects on IL-2 and IL-4 (Figure 4A). This observation implies that B7-H1 triggering is not involved in the differentiation of Th1 and Th2 cytokine-producing cells. However, it appears that B7-H1 costimulation does provide an advantage for the secretion of IL-10 and IFN-γ. An alternative interpretation is that B7-H1 activates a unique subset of Th cells that is distinct from Th1 and Th2. Further analysis, including cloning and identification of the counter-receptor for B7-H1 as well as distribution of the counter-receptor on different T cell subsets, will help address these questions.
During the review process of this paper, Freeman et al29reported the sequence of mouse PD-1L that is identical to mB7-H1. They have found that PD-1LIg inhibits the proliferation of mouse T cells in vitro in the presence of a high dose (10 μg/mL) of anti-CD3 mAb. In our system, anti-CD3 mAb in suboptimal doses (0.125 to 0.25 μg/mL) costimulates the growth of mouse T cells (Figure 3). Therefore, the effect of B7-H1 on T-cell growth or inhibition may depend on antigen strength. It is also possible there is additional counter-receptor of B7-H1 than PD-1 on T cells.
It has been reported that the production of IgG1 and IgG2b was decreased in CD28−/− mice.24 In addition, mice lacking both B7-1 and B7-2 had a decreased anti-vesicular stomatitis virus (VSV) IgG2a response.30Consistent with these observations, we have found that treatment of mice with mB7-1Ig enhanced the production of anti-TNP IgG1, IgG2a, and IgG2b antibodies (Figure 7). However, in contrast to B7-1Ig, mB7-H1Ig promoted only TNP-specific IgG2a antibody response. Several potential mechanisms may be responsible for this phenomenon. B7-H1 costimulation of T cells leads to secretion of IL-10 that has been shown to promote antibody production.31-33 In addition, IFN-γ facilitates immunoglobulin class switching to IgG2a.34,35 IL-10, together with anti-CD40 mAb, has been found to enhance IgG1 and IgG3 isotypes.36 37 In B7-H1–costimulated T cells, although there is both increased IL-10 release and CD40L up-regulation observed (Figure 3 and 8), there is no significant elevation of IgG1 and IgG3 in mB7-H1Ig–treated mice (Figure 7). Therefore, there is no simple explanation for selective increase of IgG2a antibody by B7-H1 costimulation. IgG class switching is regulated by multiple cytokines and accessory molecules, and thus the end result is likely to be a balance of these interactions. Cytokines other than IL-10 and IFN-γ released from B7-H1–costimulated T cells may also play a role in the regulation of antibody production. It is tempting to speculate that B7-H1 costimulation might participate in the generation and progression of some autoimmune diseases. In summary, our results show a preferential effect of B7-H1 costimulation in enhancement of T-helper cell functions, including growth, cytokine production, and T-dependent humoral immunity.
We thank Dr Moses Rodrigues for providing CD28-deficient mice and Kathy Jensen for editing the manuscript.
Supported in part by the Mayo Foundation. G.Z. is supported by NIH postdoctoral fellow training grant CA09127, and K.T. is a US Army Breast Cancer Research Program postdoctoral fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lieping Chen, Department of Immunology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail:chen.lieping@mayo.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal