Dendritic cells (DCs) play a central role in the initiation and regulation of the immune response. The modalities by which DCs are committed to undergo apoptosis are poorly defined. Here it is shown that, unlike death receptor ligands, UVB radiation triggers apoptosis of human DCs very efficiently. UVB exposure is followed by the activation of caspases 8, 9, and 3, by the loss of mitochondrial transmembrane potential (ΔΨm), and by cellular and nuclear fragmentation. Caspase inhibitors substantially prevented the occurrence of cellular and nuclear fragmentation but had no effect on UVB-induced ΔΨm dissipation. Importantly, mature DCs (MDCs) displayed relative resistance to UVB; UVB-induced caspase activation and apoptosis were substantially delayed compared to immature DCs (IDCs). Resistance correlated with the strong up-regulation of cellular FLIP and bcl2 observed in MDCs compared to IDCs.
Introduction
Most tissues are equipped with interstitial dendritic cells (DCs) as an efficient alert system against foreign antigens for the exquisite ability of DCs in capturing macromolecules.1,2 Tissue-associated DCs are commonly referred to as immature DCs (IDCs) because they lack some key surface accessory molecules and are, therefore, unable to trigger T-cell activation effectively. After antigen capture and processing, IDCs undergo extensive morphologic and biochemical changes and migrate to specialized lymphoid areas. Such mature DCs (MDCs) dismiss antigen capture functions, become competent antigen-presenting cells (APCs), and productively interact with T cells to initiate an antigen-specific immune response.3
Two types of bone marrow–derived DCs colonize human skin from peripheral blood to serve as professional APCs of environmental antigens. These are Langerhans cells (LCs), which reside mostly in the basal and suprabasal layers of the epidermis, and dermal IDCs, which are confined within the dermis.4-6 On antigen capture and maturation, mature LCs and MDCs relocate from the skin to draining lymph nodes in search of antigen-specific T cells.
Ultraviolet B (280-320 nm) radiation is a major stress-inducing agent for most body surfaces. Exposure of the human skin to chronic and acute UVB irradiation is known to cause immunosuppression.7 This results largely from local effects, including both massive depletion of LCs,8,9 likely due to apoptosis,10,11 and functional impairment in the costimulatory abilities of the residual LCs.12 UVB exposure may also suppress the T-cell stimulatory capacity of human DCs.13 UV-induced intracellular mediators such as ceramide, moreover, may profoundly affect DC functions.14 Little is known, however, about whether dermal human DCs trigger the apoptotic program under UVB exposure or how they activate a protective response to UVB-induced cellular stress.
Here we show that high-dose UVB radiation may induce very efficient apoptosis of human DCs. This is associated with early mitochondrial changes and is mediated by multiple caspase activation, resulting in cytosolic and nuclear fragmentation. The up-regulation of FLIP and bcl2, which occurs during DC maturation, may provide protection from UVB-induced effects, conferring a survival advantage to MDCs.
Materials and methods
In vitro culture of human dendritic cells
IDCs and MDCs were prepared according to the method of Sallusto and Lanzavecchia.15 Briefly, peripheral blood mononuclear cells obtained by standard Ficoll-Paque (Nycomed Pharma AS, Oslo, Norway) were separated on multistep Percoll gradients (Pharmacia Fine Chemicals, Uppsala, Sweden), and the light-density fraction from the 42.5% to 50% interface was recovered and depleted of CD19+ and CD2+ cells using magnetic beads coated with specific antibodies (Dynal, Oslo, Norway). Remaining cells were resuspended in RPMI 1640 (supplemented with 2 mM L-glutamine, 1% nonessential amino acids, 1% pyruvate, penicillin-streptomycin, 5 × 10−5β-2-mercaptoethanol, 10% fetal bovine serum) in the presence of 50 ng/mL granulocyte-macrophage colony-stimulating factor (Mielogen 300; Schering-Plough, Milan, Italy) and 5 ng/mL human recombinant IL-4 (R&D Systems, Minneapolis, MN) and were cultured in 6-well plates (Costar; Corning, Corning, NY) at the concentration of 5 × 105cells/mL. Five-day culture cells (defined as IDCs) were then cultured for an additional 48 hours in the presence of 400 ng/mL lipopolysaccharide (LPS; Sigma Chemical, St Louis, MO) to induce differentiation into MDCs. Purity and maturation of DCs was assessed by immunostaining and FACS analysis with a FACScan (Becton Dickinson, San Jose, CA), using fluorescein isothiocyanate–conjugated monoclonal antibodies (mAbs) to CD14, CD83, and CD80 and phycoerythrin-conjugated mAbs to CD1a, HLA-DR, and CD86 (Pharmingen, San Diego, CA).
UVB irradiation
Dendritic cells at a concentration of 5 × 105/mL were irradiated for 2 minutes with a UV transilluminator (Spectronics; New York, NY), with a peak intensity of 9000 μW/cm2 at the filter surface and a peak emission of 313 nm. In some cases cells were pretreated with the broad-spectrum caspase inhibitor zVAD-fmk (40 μM; Bachem, Bubendorf, Switzerland) for 20 minutes before UVB irradiation.
Evaluation of nuclear hypodiploidy and condensation
Irradiated cells were washed in cold phosphate-buffered saline (PBS) and resuspended in hypotonic fluorochrome solution (50 μg/mL propidium iodide in 0.1% sodium citrate plus 0.1% Triton X-100), kept for 4 to 8 hours at 4°C in the dark, and analyzed by FACScan cytofluorometer (Becton Dickinson) for the evaluation of the percentage of the hypodiploid nuclei. Irradiated cells were incubated for 10 minutes at 37°C with 500 ng/mL Hoechst (Molecular Probes, Eugene, OR), and nuclear condensation was analyzed by fluorescence microscopy.
Quantification of mitochondrial membrane permeability transition
Cells (5 × 105/mL) were incubated for 15 minutes with 10 μg/mL JC1 (Molecular Probes). Cells were washed twice with cold PBS and immediately analyzed by flow cytometry. JC1 forms red fluorescent J-aggregates (590 nm) at higher ΔΨm and green fluorescent monomers (527 nm) at low-membrane potential. Changes in ΔΨm were, therefore, evaluated by the shift in fluorescence emission.
Western blotting
Cells (2 × 106) were washed in PBS, and the pellet was resuspended in lysis buffer (150 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA, 1 mM EGTA, 2% Triton X-100, 0.5% NP40) and protease inhibitors. Samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane (Protran; Schleicher & Schuell, Keene, NH). Membranes were incubated with mouse mAb antihuman caspase 8 (clone 5F7; Upstate Biotechnology, Lake Placid, NY), rabbit polyclonal antihuman caspase 9 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse mAb antihuman caspase 3 (clone19; Transduction Laboratories, Lexington, KY), rabbit polyclonal antihuman-FLIP (kindly provided by Dr J. Tschopp, Institute of Biochemistry, University of Lausanne, Switzerland), mouse mAb antihuman FADD (clone 1; Transduction Laboratories), rabbit polyclonal antihuman bcl2 (Santa Cruz Biotechnology), rabbit polyclonal antihuman bclx-L (Santa Cruz Biotechnology), and rabbit polyclonal antihuman bax (Santa Cruz Biotechnology). For detection, secondary antibodies conjugated to horseradish peroxidase were used, followed by enhanced chemiluminescence (ECL; Amersham, Buckinghamshire, United Kingdom) and autoradiography.
Poly(ADP-ribose) polymerase in vitro translation and cleavage assay
Full-length human poly(ADP-ribose) polymerase (PARP) cDNA, cloned in Pgem vector, was used to synthesize [S35]methionine-labeled PARP by coupled T7 RNA polymerase-mediated transcription and translation in a reticulocyte lysate system (Promega, Madison, WI). Cell pellets were resuspended in 100 μL lysis buffer (50 mM NaCl, 2 mM MgCl2, 40 mM glycerophosphate, 5 mM EGTA, and 10 mM HEPES, pH 7.0). Cleavage reactions were performed in a volume of 36 μL containing 25 mM HEPES, pH 7.5, 100 mM NaCl, 2 mM MgCl2, 5 mM dithiothreitol, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and 2 μg/mL aprotinin, leupeptin, and pepstatin with 3 μL [35S]methionine-labeled PARP and 15 μL cell lysates. The reaction was incubated for 1 hour at 37°C. Samples were analyzed by SDS-PAGE. Gels were fixed (acetic acid 60%, methanol 40%, and glycerol 5%), treated with the Amplify solution (Amersham), and dried. Cleavage products were visualized by autoradiography.
Results
UVB radiation induces apoptosis in human dendritic cells
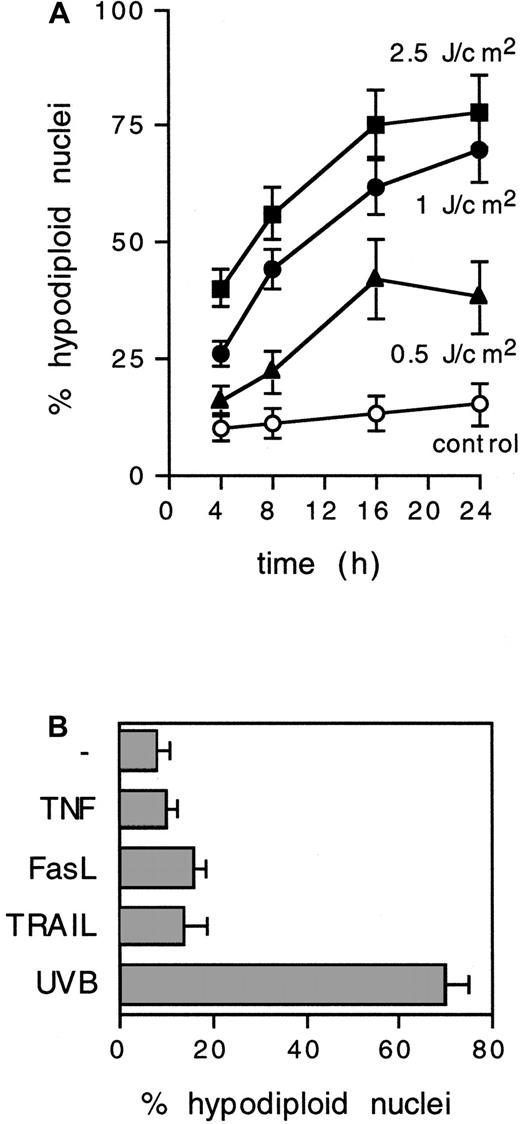
To establish whether DCs undergo apoptosis in response to UVB radiation, in vitro–cultured peripheral blood–derived DCs15 were exposed to doses of UVB ranging from 0.5 to 2.5 J/cm2. Approximately 75% of IDCs showed hypodiploid nuclei within 24 hours when exposed to 1 J/cm2 (2-minute exposure to a 9000 μW/cm2 source; peak emission, 313 nm) (Figure1A). UVB doses less than 0.1 J/cm2 did not affect cell survival (not shown), but 1 J/cm2 UVB induced classical morphologic features of cellular apoptosis in IDCs, including nuclear fragmentation and formation of apoptotic bodies (see below). Only a minor proportion (less than 15%) of cells displayed a necrotic phenotype.
UVB irradiation induces nuclear hypodiploidy in IDCs.
(A) IDCs were exposed for 2 minutes to different doses of UVB, and the percentage of hypodiploid nuclei, as detected by propidium iodide staining and FACS analysis, was determined at the indicated time points. Means ± SD from 3 experiments are shown. (B) Nuclear hypodiploidy induced at 24 hours after exposure to tumor necrosis factor (200 ng/mL), FasL (1 μg/mL), TRAIL (1 μg/mL), or UVB (1 J/cm2, 2 minutes). Means ± SD from 3 experiments are shown.
UVB irradiation induces nuclear hypodiploidy in IDCs.
(A) IDCs were exposed for 2 minutes to different doses of UVB, and the percentage of hypodiploid nuclei, as detected by propidium iodide staining and FACS analysis, was determined at the indicated time points. Means ± SD from 3 experiments are shown. (B) Nuclear hypodiploidy induced at 24 hours after exposure to tumor necrosis factor (200 ng/mL), FasL (1 μg/mL), TRAIL (1 μg/mL), or UVB (1 J/cm2, 2 minutes). Means ± SD from 3 experiments are shown.
Variable levels of apoptosis can be induced in human DCs after cross-linking surface death receptors by ligands or agonistic antibodies.16-18 We observed that UVB-exposed IDCs trigger apoptosis efficiently in comparison with the approximately 15% to 20% nuclear hypodiploidy induced in 24 hours by optimal doses of soluble cross-linked FasL or TRAIL (Figure 1B).
UVB-induced apoptosis of dendritic cells is largely mediated by caspase activation
UVB-induced apoptosis may be triggered by both ligand-independent19,20 and ligand-dependent21 22 Fas clustering, with consequent caspase activation. The caspase cascade then mediates signal progression and proteolytic disassembling of apoptotic cells.
We investigated the activation of caspase 8, caspase 9, and caspase 3 as key effectors responsible for the initiation, integration, and effector phases of the caspase cascade in cells undergoing apoptosis. Activation and processing of caspases 8, 9, and 3 could be detected in IDCs as early as 2 hours after UVB exposure and was completed by 8 hours (Figure 2A-C). Caspase activity in cell lysates derived from IDCs 4 hours after UVB treatment is fully competent in cleaving in vitro–translated PARP (Figure 2D).
UVB induces caspase activation and processing in IDC.
IDCs were exposed to 1 J/cm2 UVB, and the activation and processing of caspase 8 (A), caspase 9 (B), and caspase 3 (C) was analyzed at the indicated time points by Western blot analysis. P43 and p45, derived from processing of both caspase 8 isoforms, as well as the p18 subunit, are indicated by arrows. Similarly, p18 and p17 subunits derived from caspase 9 and caspase 3 proteolytic processing, respectively, are indicated by arrows. (D) Caspase activation in cell lysates was functionally analyzed by the cleavage of in vitro–translated PARP. [S35]-PARP was incubated with cell lysates from untreated IDCs or IDCs exposed to 1 J/cm2UVB and incubated at 5% CO2, 37°C for 4 hours. Arrows indicate the 85-kd and 25-kd PARP cleavage products.
UVB induces caspase activation and processing in IDC.
IDCs were exposed to 1 J/cm2 UVB, and the activation and processing of caspase 8 (A), caspase 9 (B), and caspase 3 (C) was analyzed at the indicated time points by Western blot analysis. P43 and p45, derived from processing of both caspase 8 isoforms, as well as the p18 subunit, are indicated by arrows. Similarly, p18 and p17 subunits derived from caspase 9 and caspase 3 proteolytic processing, respectively, are indicated by arrows. (D) Caspase activation in cell lysates was functionally analyzed by the cleavage of in vitro–translated PARP. [S35]-PARP was incubated with cell lysates from untreated IDCs or IDCs exposed to 1 J/cm2UVB and incubated at 5% CO2, 37°C for 4 hours. Arrows indicate the 85-kd and 25-kd PARP cleavage products.
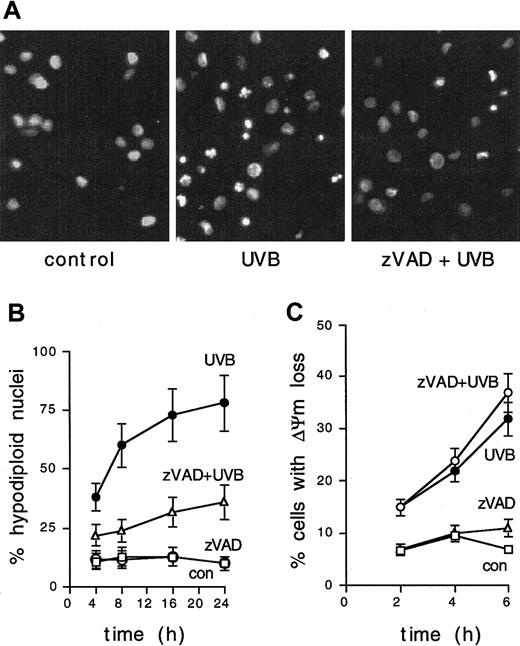
To investigate the requirement for caspase activation in UVB-induced apoptosis, IDCs were exposed to 1 J/cm2 UVB in the presence of the general caspase inhibitor zVAD. Blocking zVAD-sensitive caspases resulted in reduced UVB-induced nuclear fragmentation (Figure3A) and in a substantial inhibition of UVB-induced nuclear hypodiploidy in IDCs (Figure 3B), suggesting a central role for caspases in the process.
UVB-induced apoptosis of IDCs is inhibited by zVAD.
(A) IDCs were left untreated, exposed to 1 J/cm2 UVB, or pretreated with the broad-spectrum caspase inhibitor zVAD-fmk (40 μM) 20 minutes before UVB irradiation. Nuclear fragmentation was revealed after 8 hours by Hoechst staining and fluorescence microscopy. Results are shown from 1 of 5 representative experiments. (B) zVAD inhibition of UVB-induced DNA fragmentation in IDCs (as detected by propidium iodide staining and FACS analysis) at the indicated time points. Means ± SD from 3 experiments are shown. (C) IDCs were UVB irradiated (1 J/cm2), and ΔΨm was evaluated at the indicated time points by FACS analysis. UVB-induced ΔΨm loss is not inhibited by zVAD pretreatment (40 μM, 20 minutes). Means ± SD from 3 experiments are shown.
UVB-induced apoptosis of IDCs is inhibited by zVAD.
(A) IDCs were left untreated, exposed to 1 J/cm2 UVB, or pretreated with the broad-spectrum caspase inhibitor zVAD-fmk (40 μM) 20 minutes before UVB irradiation. Nuclear fragmentation was revealed after 8 hours by Hoechst staining and fluorescence microscopy. Results are shown from 1 of 5 representative experiments. (B) zVAD inhibition of UVB-induced DNA fragmentation in IDCs (as detected by propidium iodide staining and FACS analysis) at the indicated time points. Means ± SD from 3 experiments are shown. (C) IDCs were UVB irradiated (1 J/cm2), and ΔΨm was evaluated at the indicated time points by FACS analysis. UVB-induced ΔΨm loss is not inhibited by zVAD pretreatment (40 μM, 20 minutes). Means ± SD from 3 experiments are shown.
UVB radiation induces ΔΨm loss in dendritic cells by caspase-independent mechanisms
Loss of mitochondrial transmembrane potential (ΔΨm) from inner membrane permeability transition is a key feature of the cellular apoptotic program and is often responsible for the amplification and postmitochondrial progression of apoptotic signals.23 We therefore examined the induction of permeability transition in IDCs exposed to 1 J/cm2 UVB. Disruption of ΔΨm occurred in a substantial proportion of IDCs after 4 to 6 hours (Figure 3C). Interestingly, zVAD pretreatment could not inhibit UVB-induced ΔΨm loss, indicating that UVB exposure causes the induction of mitochondrial inner membrane permeability transition in IDCs without the involvement of caspases.
Mature dendritic cells are relatively resistant to UVB
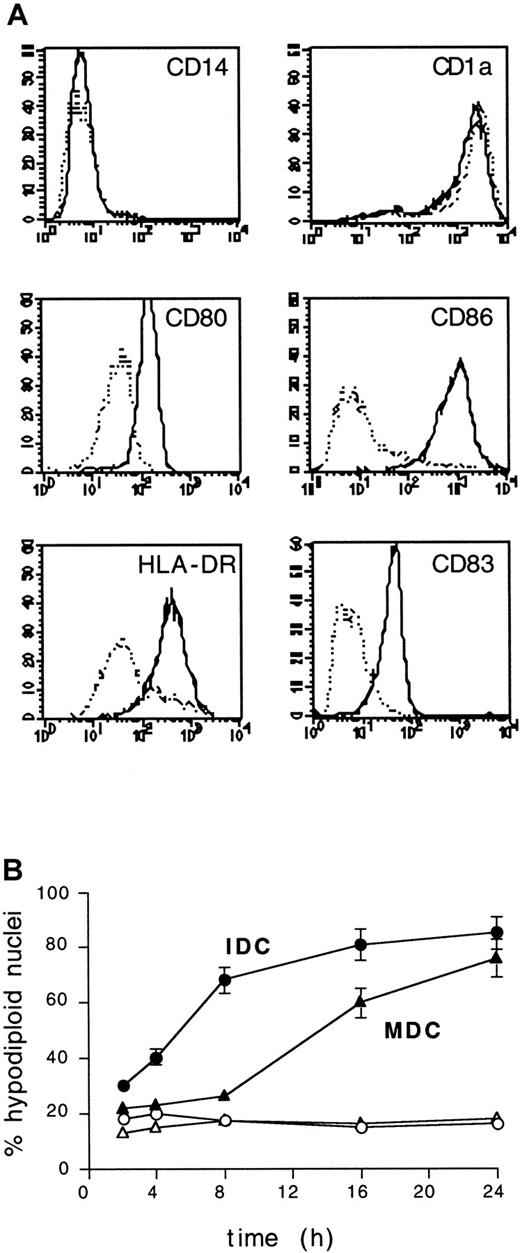
In vitro maturation of IDCs is promoted by cytokines and bacterial products and is associated with a variety of morphologic, structural, and functional changes that enable MDCs to act as fully competent APCs. We therefore asked whether the maturation of DCs could alter their UVB sensitivity. CD14−CD1a+ IDCs were cultured for 48 hours in the presence of lipopolysaccharide to induce maturation, as assessed by the up-regulation of surface HLA-DR, CD80, CD86, and CD83 (Figure 4A). Then IDCs and MDCs from the same donors were exposed to 1 J/cm2 UVB. MDCs displayed significant resistance to UVB because nuclear hypodiploidy was not detected until after 8 hours from UVB exposure, and it occurred with an average delay of approximately 8 hours compared to IDCs (Figure 4B).
MDCs acquire resistance to UVB irradiation.
(A) Surface phenotype of IDCs and MDCs used in this study. Cells were studied for the expression of the indicated surface markers and analyzed by FACS. Relative fluorescence intensity, x-axis; relative cell number, y-axis. Dotted lines, IDCs; solid lines, MDCs. Control mAb staining histograms were confined within the first decade on the x-axis (not shown). (B) IDCs and MDCs from the same donor were exposed to UVB (1 J/cm2), and the percentage of hypodiploid nuclei was evaluated by propidium iodide staining and FACS analysis at the indicated time points. ○ and ▵, untreated cells; ▪ and ▴, UVB-treated cells; ▴ and ▵, MDCs; ● and ○, IDCs. Means ± SD from 5 experiments are shown.
MDCs acquire resistance to UVB irradiation.
(A) Surface phenotype of IDCs and MDCs used in this study. Cells were studied for the expression of the indicated surface markers and analyzed by FACS. Relative fluorescence intensity, x-axis; relative cell number, y-axis. Dotted lines, IDCs; solid lines, MDCs. Control mAb staining histograms were confined within the first decade on the x-axis (not shown). (B) IDCs and MDCs from the same donor were exposed to UVB (1 J/cm2), and the percentage of hypodiploid nuclei was evaluated by propidium iodide staining and FACS analysis at the indicated time points. ○ and ▵, untreated cells; ▪ and ▴, UVB-treated cells; ▴ and ▵, MDCs; ● and ○, IDCs. Means ± SD from 5 experiments are shown.
Role for FLIP and bcl2 up-regulation in mature dendritic cells
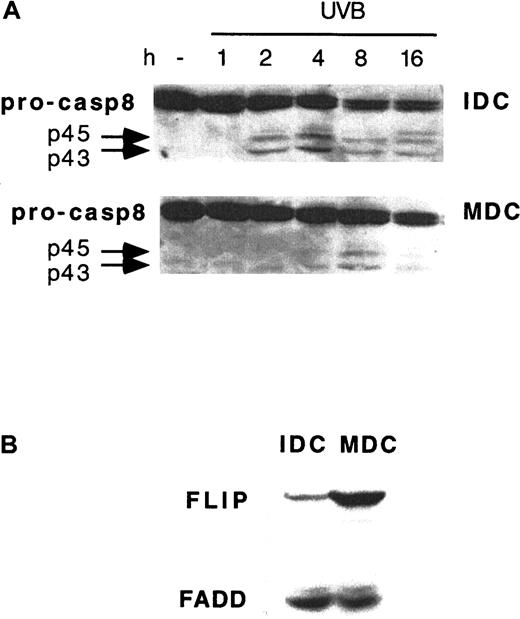
To gain insights into the molecular mechanisms mediating UVB resistance in MDCs, the kinetics of early signaling events that follow UVB exposure was investigated in further detail. Caspase 8 activation could not be detected in MDCs until 8 hours from UVB exposure, with a triggering delay of approximately 6 hours compared to IDCs from the same donors (Figure 5A). The activation of caspase 8 is controlled by endogenous FLIP at the death receptor signaling complex level.24 We, therefore, investigated the expression of FLIP during DC maturation. IDCs cultured for 24 hours in the presence of LPS showed approximately 10-fold up-regulation in FLIP expression levels compared to untreated IDCs from the same donor. The increase in FLIP expression occurred in MDCs without concomitant changes in FADD (Figure 5B) or caspase 8 expression (Figure5A).
Delayed UVB-induced caspase 8 activation and FLIP up-regulation in MDCs.
(A) IDCs and MDCs from the same donor were exposed to 1 J/cm2 UVB and analyzed by Western blotting for caspase 8 processing (pro-casp8) at the time points indicated. (B) FLIP and FADD expression in IDCs and MDCs from the same donor. Representative blots from 1 of 4 donors giving similar results are shown.
Delayed UVB-induced caspase 8 activation and FLIP up-regulation in MDCs.
(A) IDCs and MDCs from the same donor were exposed to 1 J/cm2 UVB and analyzed by Western blotting for caspase 8 processing (pro-casp8) at the time points indicated. (B) FLIP and FADD expression in IDCs and MDCs from the same donor. Representative blots from 1 of 4 donors giving similar results are shown.
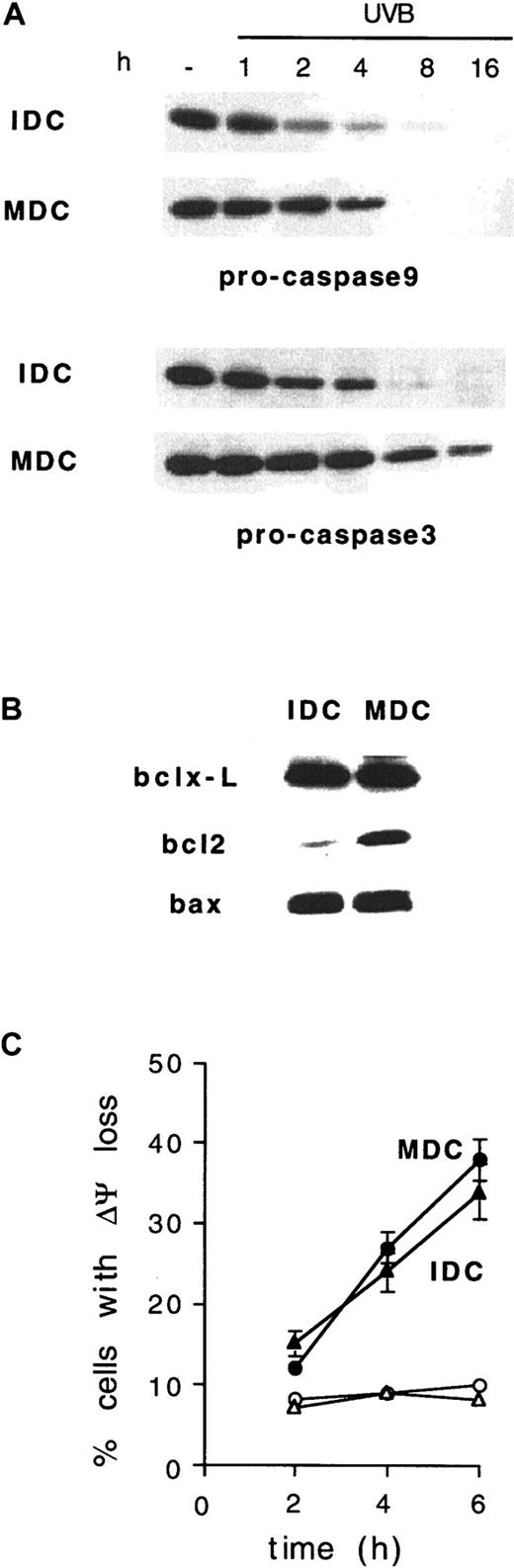
As with caspase 8, UVB-induced activation of caspases 9 and 3 was significantly delayed in MDCs compared to IDCs (Figure6A). Antiapoptotic members of the bcl2 family, such as bcl2 and bclxL, may negatively regulate the activation of postmitochondrial caspases 9 and 3.25-27 We observed that MDCs massively up-regulated bcl2, but not bclxL or bax, compared to IDCs from the same donors (Figure 6B).
Delayed UVB-induced caspases 9 and 3 activation and bcl2 up-regulation in MDCs.
(A) IDCs and MDCs from the same donor were exposed to 1 J/cm2 UVB and analyzed by Western blotting at the time points indicated for caspase 9 (pro-caspase9) and caspase 3 processing. (B) Expression of bcl2, bclxL, and bax was investigated in IDCs and MDCs from the same donor. Representative blots from 1 of 3 donors giving similar results are shown. (C) Time course of UVB-induced ΔΨm dissipation in IDCs and MDCs derived from the same donor. ○ and ▵, untreated cells; ● and ▴, UVB-treated cells; ▴ and ▵, IDCs; ● and ○, MDCs. Means ± SD from 4 experiments are shown.
Delayed UVB-induced caspases 9 and 3 activation and bcl2 up-regulation in MDCs.
(A) IDCs and MDCs from the same donor were exposed to 1 J/cm2 UVB and analyzed by Western blotting at the time points indicated for caspase 9 (pro-caspase9) and caspase 3 processing. (B) Expression of bcl2, bclxL, and bax was investigated in IDCs and MDCs from the same donor. Representative blots from 1 of 3 donors giving similar results are shown. (C) Time course of UVB-induced ΔΨm dissipation in IDCs and MDCs derived from the same donor. ○ and ▵, untreated cells; ● and ▴, UVB-treated cells; ▴ and ▵, IDCs; ● and ○, MDCs. Means ± SD from 4 experiments are shown.
Delay in the execution of the apoptotic program finally appeared to spare caspase-independent events, such as UV-induced mitochondrial changes, because the UVB-induced loss of ΔΨm occurred simultaneously in IDCs and MDCs (Figure 6C). This further indicates that the caspase activation and dissipation of ΔΨm that follow UVB irradiation in DCs may proceed independently. Together these data strongly suggest that the increased expression of endogenous FLIP and bcl2 allows delayed caspase activation and prolonged survival of MDCs, compared to IDCs, in response to UVB irradiation.
Discussion
Here we show that human DCs execute a caspase-dependent apoptotic program in response to UVB irradiation. Importantly, the up-regulation of endogenous FLIP and bcl2 during maturation may delay UVB-induced caspase activation and confer a survival advantage to MDCs.
Acute irradiation of body surfaces with UVB, which may occur after severe sunburn (0.5-5 J/cm2), results in transient but profound depletion of APCs that contributes to immunosuppression. Loss of LCs after human skin UVB irradiation might be attributed to apoptosis; apoptosis induction of LCs after in vitro or ex vivo UVB exposure has been reported.10,11 Murine LCs are sensitized by in vitro UVB exposure to undergo apoptosis after interaction with antigen and T cells.28 No information, however, is available about the effects of acute UVB irradiation on human dermal DCs. We report here that high-dose UVB irradiation triggers efficient apoptosis in human DCs. The apoptotic program includes multiple caspase activation, disruption of mitochondrial transmembrane potential, fragmentation of nuclear DNA, and apoptotic body formation.
It has been proposed that UV light triggers protein synthesis–independent apoptosis by energy transfer and cell membrane perturbation, with consequent clustering of surface death receptors such as Fas.19,20,29 It has also been shown that homocellular Fas–FasL interactions contribute to UV-induced apoptosis in specific cell types.21 22 The latter mechanism seems not to play a major role in UVB-induced DC apoptosis; we observed that DCs display substantial resistance to FasL.
On UV-induced receptor oligomerization, therefore, the cytosolic adaptor FADD binds to clustered death domains and allows the recruitment of caspase 8.19 This results in the proteolytic autoactivation of caspase 8 and initiation of the caspase cascade. We observed that UVB irradiation of DCs triggered the activation of caspases 8, 9, and 3 within 2 hours. Accordingly, the general caspase inhibitor zVAD could prevent most of the nuclear features of the apoptotic response to UVB. Blocking caspase activation could not, however, prevent the induction of mitochondrial permeability transition observed in DCs after UVB exposure. Moreover, the delay in caspase activation observed in MDCs compared to IDCs after UVB exposure had no effect on the kinetics of mitochondrial changes induced by UVB exposure. Together these results indicate that acute UVB irradiation triggers 2 pathways in human DCs—a caspase-dependent pathway responsible for cytosolic and nuclear fragmentation and a caspase-independent pathway directly targeting mitochondria.
Cytokines or bacterial product–induced maturation of IDCs is accompanied by profound functional and structural changes. By up-regulating selected surface receptors, MDCs relocate from antigen-capturing areas to specialized lymphoid tissue and make productive contact with T lymphocytes. Here we show that DC maturation is associated with the acquisition of resistance to UVB irradiation and with the up-regulation of key antiapoptotic products, such as FLIP and bcl2. FLIP can compete with caspase 8 for binding to FADD, thus preventing caspase 8 clustering and autoactivation.30Because the expression levels of caspase 8 and FADD do not change with maturation, the approximately 10-fold increase in FLIP is likely to be responsible for the delay (compared to IDCs) in caspase 8 activation observed in MDCs after UVB irradiation.
The expression of FLIP is acquired during the differentiation of peripheral blood monocytes, conferring resistance to death ligands to both macrophages and IDCs.31-33 Most IDCs express relatively low levels of FLIP, which appear nevertheless sufficient to prevent caspase 8 activation after death receptor oligomerization by ligands. In this report we show that more dramatic environmental stressors, such as acute UVB irradiation, overcome the protective effects of FLIP in IDCs and result in efficient caspase 8 activation. However, further up-regulation of FLIP during DC maturation may confer additional resistance, perhaps critical for the survival of MDCs in vivo.
Our data indicate, moreover, that UVB irradiation in DCs may damage mitochondrial membranes by a caspase-independent pathway. This is associated with a rapid dissipation of ΔΨm, an event that apparently cannot be prevented or delayed by the up-regulation of bcl2 during DC maturation. However, high levels of bcl2 expression may raise the threshold for irreversible mitochondrial damage by limiting the release of cytochrome c,25 26 which follows ΔΨm loss, mitochondrial swelling, and rupture of the external mitochondrial membranes. Accordingly, high bcl2 expression in MDCs affects the downstream activation of caspase 9 and, consequently, of caspase 3, and it contributes to preventing the triggering of apoptosis after UVB irradiation.
Coordinated FLIP and bcl2 induction during DC maturation, therefore, is likely to represent a key event in granting protection to UVB-induced cellular stress and underscores the need for prolonged survival of MDCs at sites of lymphocyte challenge and activation.
We thank Dr J. Tschopp (Institute of Biochemistry, University of Lausanne) for the anti-FLIP antibody and Dr Cristina Gagliardi (Department of Public Health, University of Tor Vergata) for help with flow cytometry. M.R.R. is a Fondazione Adriano Buzzati-Traverso fellowship holder.
Supported by Associazione Italiana Ricerca sul Cancro, Agenzia Spaziale Italiana, Consiglio Nazionale delle Ricerche Progetto Biotecnologie, Ministero dell' Universita' e della Ricerca Scientifica e Tecnologica, and European Commission Training and Mobility of Researchers Program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Roberto Testi, Laboratory of Signal Transduction, Department of Experimental Medicine and Biochemical Sciences, University of Rome “Tor Vergata,” via di Tor Vergata 135, 00133 Rome, Italy; e-mail: tesrob@flashnet.it.

![Fig. 2. UVB induces caspase activation and processing in IDC. / IDCs were exposed to 1 J/cm2 UVB, and the activation and processing of caspase 8 (A), caspase 9 (B), and caspase 3 (C) was analyzed at the indicated time points by Western blot analysis. P43 and p45, derived from processing of both caspase 8 isoforms, as well as the p18 subunit, are indicated by arrows. Similarly, p18 and p17 subunits derived from caspase 9 and caspase 3 proteolytic processing, respectively, are indicated by arrows. (D) Caspase activation in cell lysates was functionally analyzed by the cleavage of in vitro–translated PARP. [S35]-PARP was incubated with cell lysates from untreated IDCs or IDCs exposed to 1 J/cm2UVB and incubated at 5% CO2, 37°C for 4 hours. Arrows indicate the 85-kd and 25-kd PARP cleavage products.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/6/10.1182_blood.v97.6.1803/6/m_h80610787002.jpeg?Expires=1767736365&Signature=EeNqgB~qOFXQOsSD994BxDY0Ck8287382oQt4VxV~wOCbVNBjEV6y61oLzn~e4Lsbedc0gcOUpBSwuUxNo~vE8lF3sWL1NlZBHyf2TqaFry3O6J~iY9XnvHTm6ivPZIPpZZpiNdgz6JcllXPQTKP3QUJx7Vb8BlGYYBdlUWRHc~wbklpnPRDceNEtY3j5HRbnHOdH21A2z8XrkWkQzxJIcVHT-i6OxVmYoKRpArJhzt7RpyUCB4ASmY1tBVZ0cMhIbljAvw6ssXnpy5BncSrxL1jjGBfZpn2T~wdvS4kdwQBvkMr3DuV0MWBkdWZXXgCWhIiOuP5gQwlBFQDp4GntQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal