Tissue inhibitors of metalloproteinases (TIMPs), first described as specific inhibitors of matrix metalloproteinases, have recently been shown to exert growth factor activities. It was previously demonstrated that TIMP-1 inhibits apoptosis in germinal center B cells and induces further differentiation. Interleukin-10 (IL-10) is reported as a vital factor for the differentiation and survival of germinal center B cells and is also a negative prognostic factor in non-Hodgkin lymphoma (NHL). However, the mechanism of IL-10 activity in B cells and the regulation of its expression are not well understood. IL-10 has been shown to up-regulate TIMP-1 in tissue macrophages, monocytes, and prostate cancer cell lines, but IL-10 modulation of TIMP-1 in B cells and the effect of TIMP-1 on IL-10 expression has not been previously studied. It was found that TIMP-1 expression regulates IL-10 levels in B cells and that TIMP-1 mediates specific B-cell differentiation steps. TIMP-1 inhibition of apoptosis is not IL-10 dependent. TIMP-1 expression in B-cell NHL correlates closely with IL-10 expression and with high histologic grade. Thus, TIMP-1 regulates IL-10 expression in B-cell NHL and, through the inhibition of apoptosis, appears responsible for the negative prognosis associated with IL-10 expression in these tumors.
Introduction
Tissue inhibitors of metalloproteinases (TIMPs) are a family of closely related proteins that were first described1-3 as the specific inhibitors of matrix metalloproteinases (MMP). However, in addition to blocking MMP activity, TIMPs have also been shown to exert growth factor activities that are independent of their enzymatic inhibitory function.4-10 We have previously reported on the high-level expression of TIMP-1 by normal activated tonsillar B cells and centroblast-like Burkitt lymphoma cell lines.11 TIMP-1 expression is operative at a specific stage in B-cell development and is associated with the germinal center phenotype that occurs with the generation of lymphoblasts.12 TIMP-1 expression by tonsillar germinal center B cells is dependent on the germinal center milieu, with expression decreasing rapidly on in vitro isolation.11 Induction of endogenous TIMP-1, or treatment with recombinant TIMP-1 protein, effects further B-cell differentiation in centroblast-like Burkitt lymphoma cell lines. This is evidenced by the TIMP-1 modulation of expression of the differentiation-specific antigens CD10, CD38, surface immunoglobulin (sIg), CD77, CD40, and CD23.12 We have also previously shown that TIMP-1 inhibits apoptosis in normal germinal center B cells and Burkitt lymphoma cell lines.13 In B cells, this is at least partially attributed to TIMP-1 up-regulation of the antiapoptotic factor Bcl-XL. However, TIMP-1 may also inhibit apoptosis by regulating the expression of CD40, CD77, and CD23. Li et al14 have recently demonstrated that TIMP-1 inhibits apoptosis in human breast epithelial cells and is induced by the antiapoptotic factor Bcl-2, indicating this effect is not tissue specific.
The phenotypic changes induced by TIMP-1 normally occur in the germinal center during the interaction of B cells and T cells and in proximity to the TIMP-1–containing germinal center stroma.15-18CD77 expression is highly restricted to germinal-center B lymphocytes and is a neutral glycolipid expressed by a subset of B lymphocytes that readily enter programmed cell death.19-21 Apoptosis in these cells is prevented by CD40 engagement and by soluble CD23.22 A model of B-cell maturation has been proposed in which the ligation of CD40 drives differentiating B cells to lose CD77 surface expression and to express membrane and soluble CD23, all of which are associated with an antiapoptotic state. Thus, TIMP-1—by up-regulating the expression of CD40 and CD23 and by decreasing the expression of CD77—is implicated in the normal development of B cells and the survival of normal and neoplastic B cells.
Human interleukin-10 (IL-10) is a cytokine normally produced by activated T cells, monocytes, B cells, and thymocytes.15IL-10 contributes to antigen- or mitogen-driven B-cell differentiation and, in non-Hodgkin lymphomas, acts as a growth factor.23,24 Activation of CD40 ligand induces IL-10 secretion and modulates immunoglobulin production. Furthermore, IL-10 is an important factor for the differentiation and survival of germinal center B cells.15 Because TIMP-1 expression in Burkitt lymphoma cell lines induces a more mature phenotype and affects CD40 and immunoglobulin expression,12 a possible effect of TIMP-1 on IL-10 expression was contemplated. IL-10 has been shown to up-regulate TIMP-1 in tissue macrophages, peripheral blood monocytes, and prostate cancer cell lines,25 26 but the IL-10 modulation of TIMP-1 in B cells and the effect of TIMP-1 on IL-10 expression have not been studied. Therefore, we examined the relation between TIMP-1 and IL-10 expression in a series of cell lines and non-Hodgkin lymphoma specimens.
Materials and methods
Cells
The following Burkitt lymphoma cell lines were kindly provided by Dr Ian Magrath, (National Cancer Institute) or were purchased from ATCC (Rockville, MD): AG876, PA682, Jijyoe, DW6, Daudi, ST846, and JD38. LXSN retroviral transduction was used to induce TIMP-1 expression in the TIMP-1− cell line JD38, as described.13 Briefly, TIMP-1 cDNA was obtained by polymerase chain reaction and subcloned into LXSN by DNA recombinant techniques. Empty LXSN or LXSN–TIMP-1 constructs were transfected into packaging cell lines. Nonadherent JD38 cells were co-cultured with adherent LXSN or LXSN–TIMP-1 packaging cells (GP + envAM12) for 48 hours. LXSN and LXSN–TIMP-1–infected cells were selected by using 2500 mg/mL G418 (Gibco-BRL, Rockville, MD) for 10 days. Individual clones were selected by repeated limiting dilution. Clonality was confirmed by restriction enzyme digestion with SmaI and Southern blot analysis, with unique sites of integration detected in the JD38 clones used in this study. JD38 clones have been stabile for TIMP-1 expression and phenotype for more than 3 years. Cell lines were cultured in RPMI 1640 obtained from Gibco BRL, supplemented with 10% fetal bovine serum (without serum, as indicated) and antibiotics in 5% CO2, at 37°C. Cells were also incubated in AIM-V obtained from Gibco in 5% CO2 at 37°C when indicated.
Consecutive high-grade (n = 10) and low-grade (n = 21) B-cell non-Hodgkin lymphomas were retrieved from the Hematopathology Section frozen-tissue bank at the National Cancer Institute (National Institutes of Health, Bethesda, MD). Hematoxylin–eosin-stained sections from the original case were reviewed, and complete immunophenotyping was performed to confirm the diagnosis. Immunophenotyping was performed on frozen sections and paraffin sections by the immunoperoxidase technique and on cell suspensions by flow cytometry. High-grade cases included 8 diffuse large B-cell lymphomas and 2 Burkitt lymphomas. Low-grade cases included 8 small lymphocytic lymphomas and 13 follicle-center lymphoma (all follicular, nondiffuse, and grade I or II).
cDNA production
Viable frozen-cell suspensions were thawed on ice and RNA extracted using the RNAzol kit (Cinna/Biotecx Laboratories, Houston, TX) and following the manufacturer's instructions. RNA was treated with DNAase (Gibco BRL) and subjected to polymerase chain reaction (PCR) for β actin to confirm the absence of genomic DNA before cDNA preparation. cDNA was prepared using 2 μg RNA and the superscriptase II preamplification system for first-strand cDNA synthesis (Gibco BRL, Gaithersburg, MD) with the random primers supplied by the manufacturer.
Reverse transcription–polymerase chain reaction
TIMP-1 and IL-10 expression was determined by semiquantitative reverse transcription (RT)–PCR. Multiple quantities of cDNA and cycle numbers were used to optimize the range for PCR determinations. Equal quantities of cDNA were then used in PCR amplification. The following primers were used for TIMP-1 RT-PCR: 5′-ATAGTCGACATGGCCCCCTTTGAG CCCCTG-3′ and 5′GGAATTCCTC AGGCTATCTGGGACCGCAGGGA-3′. The following primers (specific for human IL-10; do not amplify viral IL-10) were used for IL-10: 5′-ACCAAGACCCAGACATCAAG-3′ and 5′-GAGGTACAATAAGGTTTCTCAAG-3′. β-Actin primers were as follows: 5′-AAGAGAGGCATCCCTCACCT-3′ and 5′-TACATGGCTGGGGTGTTGAA-3′. Thirty-one B-cell non-Hodgkin lymphomas (10 high grade, 21 low grade) were examined for TIMP-1 expression. Twenty-seven B-cell non-Hodgkin lymphomas (10 high grade, 17 low grade) were examined for IL-10 expression because of the availability of adequate cDNA.
Amplified products were electrophoresed on a 4% low melting point agarose gel, and bands were visualized with 2% ethidium bromide and transferred onto a nylon membrane (Dupont-NEN Research, Boston, MA) by Southern blot analysis. Blots were hybridized using standard conditions with TIMP-1, IL-10, and β-actin probes labeled with [32P] dCTP by using a random primer labeling kit from Gibco BRL (Gaithersburg, MD). Radiographs were scanned by an AGFA Arcus flat-bed scanner, and densities were determined using NIH Image 1.41.
Enzyme-linked immunosorbent assay determinations
Secreted TIMP-1 was quantitated by using a TIMP-1 enzyme-linked immunosorbent assay (ELISA; Oncogene Research, Cambridge, MA) kit that detects free TIMP-1 and MMP-bound TIMP-1. Secreted IL-10 was quantitated using an Immunotech (Westbrook, ME) ELISA kit that detects human IL-10 and the Epstein-Barr virus (EBV) IL-10 viral homolog. Equal numbers of cells (5 × 105/mL) were cultured in fresh media. After 48 hours, cells were centrifuged and supernatants were tested for TIMP-1 or IL-10 following the manufacturer's instructions. All ELISA determinations were performed 3 times, with triplicate samples within each determination.
Reverse zymography
Metalloproteinase inhibitory activity was assayed by electrophoresis in polyacrylamide gels containing gelatin as matrix metalloproteinase substrate and HT1080 fibroblast culture conditioned media as a source of matrix metalloproteinase, as previously described.27 Briefly, 12% or 15% polyacrylamide gels were prepared with Tris-HCl containing 0.1% sodium dodecyl sulfate (SDS), 2.5 mg/mL gelatin, and 10% (vol) HT1080 fibroblast culture-conditioned media. Aliquots of conditioned media (15 μL) were suspended in sample buffer (50 mol/L Tris-HCl, 2% SDS, 0.1% bromophenol blue, 10% glycerol, pH 6.8) and applied to gels followed by electrophoresis at 20 mA/gel. Gels were incubated in 2.5% (vol/vol) Triton X-100 for 60 minutes to remove SDS and then were incubated overnight in developing buffer (50 M Tris-HCl, 0.2 M NaCl, 5 mmol/L CaCl2, 0.02% (wt/vol) Brij-35, adjusted to pH 7.6). This restores the matrix metalloproteinase activity, resulting in gelatin degradation. Gels were stained for 3 hours in 30% methanol, 10% glacial acetic acid, 0.5% Coomassie blue G-250 (Bio-Rad, Richmond, CA), destained for 1.5 to 2 hours in 30% methanol and 10% glacial acetic acid, and dried overnight. TIMPs were visualized as nondegraded gelatin staining positive with Coomassie blue.
TIMP-1–IL-10 incubations
JD38 cells (3 × 105 cells/mL) were incubated in serum-free media with both native recombinant TIMP-1 and reduced and alkylated TIMP-1 (0-500 ng/mL) for 24 and 48 hours. JD38 cells (3 × 105 cells/mL) were incubated in serum-free media with 0 to 25 ng/mL recombinant IL-10 (Genzyme, Cambridge, MA). TIMP-1–expressing clones were incubated with 1 μg/mL neutralizing anti–TIMP-1 monoclonal antibody (Oncogene Research Products). IL-10 was inactivated by incubation with neutralizing antibodies to IL-10 (0.5 μg/mL) and with 50 pg/mL soluble IL-10 receptor (R & D Systems, Minneapolis, MN). Incubation with an equivalent quantity of isotype control antibody protein was performed in each experiment. Conditioned media were collected after 24- and 48-hour incubation in serum-free RPMI or AIM V medium (Gibco BRL). Supernatants were cleared of cells by centrifugation and, in some experiments, whole cell lysates prepared using RIPA buffer (150 nM NaCl, 1% NP-40, 0.1% SDS, 50 mM Tris-HCL, pH 8.0) containing protease inhibitors. Protein concentration of supernatants and whole cell lysates was determined by BCA protein assay from Pierce (Rockville, IL).
Flow cytometric immunophenotyping
Cells (1 × 106) were washed twice in phosphate-buffered saline containing 1% bovine serum albumin before staining, as described,12 with fluorochrome-conjugated monoclonal antibodies against the following antigens: fluorescein isothiocyanate (FITC)–CD38, FITC-CD20, phycoerythrin (PE)–CD5, FITC-CD45, PE-CD14, FITC–mouse IgG1, and PE IgG2 (DAKO, Carpinteria, CA); PE- CD38 (Becton Dickinson, San Jose, CA); PE-CD40 and unconjugated CD77 (Immunotech, Miami, FL); and FITC-CD10 (Coulter, Miami, FL). Labeled cells were analyzed in a Facscan (Becton Dickinson, San Jose, CA) with Cellquest software to determine the percentage of positive cells and fluorescence intensity (ie, quantitation of antibody-staining intensity). After data acquisition, fluorescent calibrated beads (Flow Cytometry Standards, San Juan, Puerto Rico) were used to standardize fluorescence intensity of different antigens and data were expressed as molecule equivalent of surface fluorochrome units.
Cell viability–proliferation assays
All cells were reviewed microscopically for morphologic evidence of apoptosis such as blebbing and nuclear condensation. Viability was determined by cellular exclusion of trypan blue. Apoptosis was measured by light scatter as previously described28 and by staining with Annexin V–FITC from Caltag (Burlington, CA) according to the manufacturer's protocol. To measure proliferation, cells were incubated with bromodeoxyuridine (BrdU) for 30 minutes at 37°C. S-phase and BrdU incorporation were simultaneously detected by flow cytometric analysis of staining with the DNA-binding fluorescent dye propidium iodide– and FITC-labeled anti-BrdU (Becton Dickinson, San Jose, CA), according to manufacturer's guidelines.
Results
TIMP-1 up-regulation of IL-10 expression
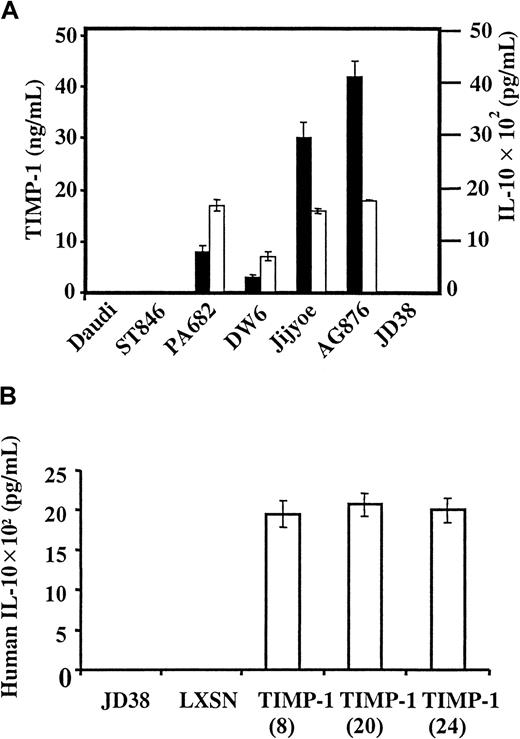
IL-10 expression was studied in a series of Burkitt lymphoma cell lines with known TIMP-1 expression.12 13 Figure1A shows that IL-10 is expressed and secreted by all TIMP-1+ Burkitt lymphoma lines studied (AG876, PA682, Jijyoe, DW6). In contrast, TIMP-1− Burkitt cell lines do not secrete IL-10 (Daudi, ST846, JD38). Furthermore, IL-10 expression is proportional to levels of TIMP-1 secretion. To determine whether IL-10 expression was dependent on TIMP-1 expression, we analyzed the TIMP-1− Burkitt lymphoma cell line JD38 transfected by retroviral infection with the human TIMP-1 gene. ELISA used for the IL-10 determination also detected viral IL-10. The EBV− Burkitt cell line JD38 offered another advantage: we could examine the effects of TIMP-1 and IL-10 in the absence of viral IL-10. Figure 1B shows the effects of TIMP-1 expression, mediated by retroviral vector, on human IL-10 expression by JD38 cells. Up-regulation of TIMP-1 induces the secretion of IL-10, as shown by 3 independent TIMP-1 clones (clones 8, 20, 24). TIMP-1–induced IL-10 levels are similar to those seen in TIMP-1–expressing, IL-10+ Burkitt cell lines. In contrast, TIMP-1− JD38 cells carrying empty LXSN vector and parental cells do not secrete IL-10.
TIMP-1 induces IL-10 expression.
(A) TIMP-1 and IL-10 expression in Burkitt lymphoma lines. A series of Burkitt lymphoma cell lines was examined for secreted TIMP-1 (▪) and IL-10 (■). All ELISA determinations were performed 3 times, with triplicate samples within each determination. (B) TIMP-1 induction of IL-10. LXSN retroviral transduction was used to induce TIMP-1 expression in the TIMP-1− cell line JD38 and IL-10 secretion measured by ELISA (y-axis).
TIMP-1 induces IL-10 expression.
(A) TIMP-1 and IL-10 expression in Burkitt lymphoma lines. A series of Burkitt lymphoma cell lines was examined for secreted TIMP-1 (▪) and IL-10 (■). All ELISA determinations were performed 3 times, with triplicate samples within each determination. (B) TIMP-1 induction of IL-10. LXSN retroviral transduction was used to induce TIMP-1 expression in the TIMP-1− cell line JD38 and IL-10 secretion measured by ELISA (y-axis).
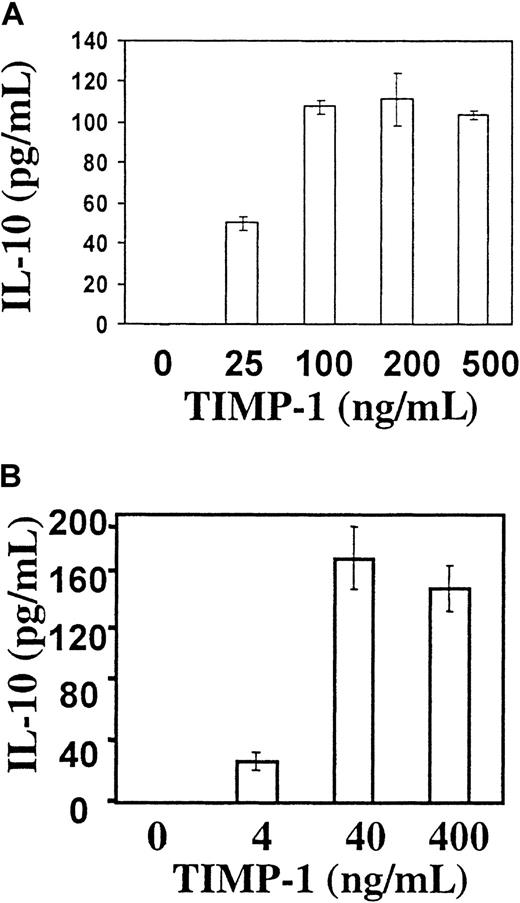
To further determine the role of TIMP-1 in controlling IL-10 expression, JD38 cells were cultured in the presence of increasing concentrations of human recombinant TIMP-1 and analyzed for IL-10 secretion. Figure 2A shows that when cells are cultured in serum-free conditions, exogenous TIMP-1 not only maintains high viability (as shown previously) but also induces JD38 cells to secrete IL-10. Furthermore, inhibition of secreted TIMP-1 in the TIMP-1–expressing JD38 clone 24 by a neutralizing anti–TIMP-1 antibody results in the decreased expression of IL-10 (data not shown). This demonstrates that TIMP-1 can regulate the production of IL-10 by Burkitt lymphomas. This activity of TIMP-1 on IL-10 induction is independent of its enzymatic inhibitory function. Figure 2B demonstrates that reduced and alkylated TIMP-1 (a form devoid of all MMP inhibitory function but retaining growth factor activity13,29) can still induce IL-10 secretion in a concentration-dependent manner. Induction of IL-10 secretion by TIMP-1 is evidence supportive of an autocrine loop. We have previously demonstrated saturable cell surface binding of TIMP-1 to JD38 cells that is consistent with a TIMP-1 receptor.13 This suggests that TIMP-1 binding to a cell surface receptor mediates IL-10 expression.
Recombinant TIMP-1 induces IL-10 expression by a mechanism independent of inhibition of matrix metalloproteinases.
TIMP-1− JD38 cells were incubated in serum-free media with native recombinant TIMP-1 (A) and reduced and alkylated TIMP-1 (B) (destroys enzyme inhibitory but not growth factor activity) and secreted IL-10 measured by ELISA.
Recombinant TIMP-1 induces IL-10 expression by a mechanism independent of inhibition of matrix metalloproteinases.
TIMP-1− JD38 cells were incubated in serum-free media with native recombinant TIMP-1 (A) and reduced and alkylated TIMP-1 (B) (destroys enzyme inhibitory but not growth factor activity) and secreted IL-10 measured by ELISA.
Biologic activity of IL-10 versus TIMP-1 in B cells
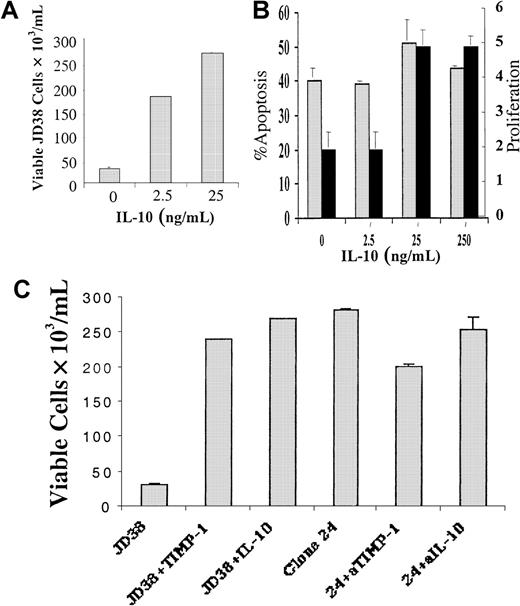
In previous studies we have shown that TIMP-1 is a survival and differentiation factor for B cells.12,13 These effects could be directly mediated by TIMP-1 activity or TIMP-1 induction of IL-10. We therefore studied the effect of recombinant IL-10 on B-cell differentiation and inhibition of apoptosis. JD38 cells are highly serum dependent and rapidly undergo apoptosis in serum-free media. Although rTIMP-1 prevents the induction of programmed cell death in JD38 cells under serum-free conditions,13 it also induces IL-10 expression. The number of viable cells under serum deprivation was dose dependent in the JD38 cells incubated with 0 to 25 ng/mL rIL-10 (Figure 3A). However, treatment with rIL-10 at concentrations observed in TIMP-1+ cell lines did not prevent apoptosis of the TIMP-1− JD38 cells in serum-free media (Figure 3B). Il-10 increased proliferation in the cells, as measured by BrdU incorporation (Figure 3B-C). Although the differences in BrdU incorporation are modest, they are significant and only represent a 30-minute incubation. Differences in the number of viable cells were observed after 48 hours, a much more sensitive indicator of the action of IL-10. Thus, the increased number of viable cells was attributed to increased cell growth in the presence of identical levels of apoptosis. Although IL-10 has been reported to prevent germinal center B-cell apoptosis,30 this effect is differentiation dependent—IL-10 also induces B-cell apoptosis in specific B-cell populations.31 It is apparent that it is TIMP-1, not IL-10, that prevents apoptosis in the current model; rTIMP-1 prevents apoptosis and IL-10 has been shown to induce TIMP-1 expression in monocytes and prostate cancer cells.25 26 To determine whether IL-10 induces TIMP-1 in B cells, we analyzed the supernatant of IL-10–treated cells for TIMP-1 protein by both ELISA and reverse zymography (data not shown). In addition, RT-PCR for TIMP-1 cDNA was performed on the IL-10–treated cells. IL-10 induction of TIMP-1 was not observed by any of the methods used. To further prove that TIMP-1–mediated inhibition of apoptosis occurs independently of IL-10, JD38 clones 20 and 24 (positive for both TIMP-1 and IL-10 expression) were incubated in serum-free conditions in the presence of both IL-10 neutralizing antibody and IL-10 receptor blocking (nonactivating) antibody (ie, IL-10 activity simultaneously blocked by 2 methods). After the addition of both neutralizing antibodies, the JD38 clones 20 and 24 did not undergo apoptosis (TIMP-1 is still active). However, the addition of TIMP-1–neutralizing antibody did induce apoptosis (Figure 3C). Isotype controls for anti–IL-10, anti–IL-10 receptor, and anti–TIMP-1 antibodies did not induce apoptosis. In summary, apoptosis of these Burkitt lymphoma cells is prevented by the presence of TIMP-1 alone, whereas IL-10 stimulates cell proliferation. Neutralization of IL-10 in the presence of TIMP-1 did not result in programmed cell death.
TIMP-1 and IL-10 inhibit apoptosis in JD38 cells.
(A) JD38 cells (3 × 105 cells/mL) were incubated for 48 hours in serum-free media with 0 to 25 ng/mL recombinant IL-10, and viability was determined. (B) JD38 cells (3 × 105cells/mL) were incubated for 48 hours in serum-free media with 0 to 250 ng/mL recombinant IL-10. Apoptosis (░) and proliferation (▪) were determined. (C) JD38 cells and TIMP-1 clone 24 (3 × 105cells/mL) were incubated for 48 hours in serum-free media and viability was determined. Viability is shown on the y-axis. JD38: JD38 cells incubated in serum-free media. JD38 + TIMP-1: JD38 cells incubated with 143 nM rTIMP-1. JD38 + IL-10: JD38 cells incubated with 24 ng/mL rIL-10. Clone 24: TIMP-1–expressing JD38 clone. 24 + aTIMP-1: TIMP-1–expressing clone 24 was incubated with 1 μg/mL neutralizing anti–TIMP-1 monoclonal antibody. 24 + aIL-10: IL-10 was inactivated in TIMP-1–expressing clone 24 by incubation with neutralizing antibodies to IL-10 (0.5 μg/mL) and with 50 pg/mL soluble IL-10 receptor. Incubation with equivalent quantity of isotype control antibody protein was performed in each experiment.
TIMP-1 and IL-10 inhibit apoptosis in JD38 cells.
(A) JD38 cells (3 × 105 cells/mL) were incubated for 48 hours in serum-free media with 0 to 25 ng/mL recombinant IL-10, and viability was determined. (B) JD38 cells (3 × 105cells/mL) were incubated for 48 hours in serum-free media with 0 to 250 ng/mL recombinant IL-10. Apoptosis (░) and proliferation (▪) were determined. (C) JD38 cells and TIMP-1 clone 24 (3 × 105cells/mL) were incubated for 48 hours in serum-free media and viability was determined. Viability is shown on the y-axis. JD38: JD38 cells incubated in serum-free media. JD38 + TIMP-1: JD38 cells incubated with 143 nM rTIMP-1. JD38 + IL-10: JD38 cells incubated with 24 ng/mL rIL-10. Clone 24: TIMP-1–expressing JD38 clone. 24 + aTIMP-1: TIMP-1–expressing clone 24 was incubated with 1 μg/mL neutralizing anti–TIMP-1 monoclonal antibody. 24 + aIL-10: IL-10 was inactivated in TIMP-1–expressing clone 24 by incubation with neutralizing antibodies to IL-10 (0.5 μg/mL) and with 50 pg/mL soluble IL-10 receptor. Incubation with equivalent quantity of isotype control antibody protein was performed in each experiment.
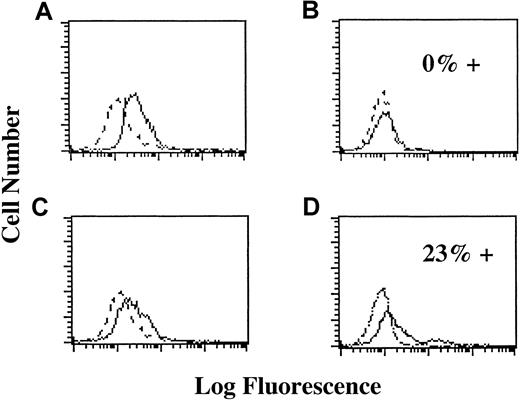
TIMP-1 effects further B-cell differentiation, and TIMP-1–expressing lines are mature, activated B cells in a pre–plasma cell stage.12 Induction of TIMP-1 (Figure4A-B) or addition of recombinant TIMP-1 protein down-regulates CD10, CD38, CD77, and surface immunoglobulins while up-regulating CD23 and CD40 in JD38 cells. Neutralization of TIMP-1 by anti–TIMP-1 antibody reverses this effect (Figure 4C). The induction of a more mature phenotype may be a direct action of TIMP-1 or secondary to IL-10 expression. Therefore, we incubated JD38 cells with rIL-10 and analyzed for the changes in immunophenotype. We did not observe any changes in levels of expression of CD40, CD10, or CD77 after the addition of IL-10 to TIMP-1− parental JD38 cells (Figure 4D). This demonstrates that TIMP-1 regulation of these antigens is not caused by IL-10 but is, in fact, dependent on TIMP-1 acting directly or through an IL-10 independent pathway.
TIMP-1 induces further B-cell differentiation in IL-10–independent manner:
Y axis: Cell number. X axis: Log fluorescence staining with FITC-labeled antibody. Dotted lines indicate staining with isotype control. Solid lines indicate staining with anti-CD77. (A) Expression of CD77 in JD38 cells (do not express TIMP-1 or IL-10 protein). Cells express CD77. (B) Expression of CD77 in TIMP-1 JD38 clone 24 (expresses and secretes TIMP-1 and IL-10). Cells are negative for CD77 (0% positive for CD77). (C) Expression of CD77 in TIMP-1 JD38 clone 24 after 48-hour incubation with neutralizing anti–TIMP-1 antibody (no active TIMP-1). CD77 expression up-regulated. (D) Expression of CD77 in JD38 cells incubated with rIL-10 (no TIMP-1, active IL-10). Cells express CD77 (23% of the cells stain higher with anti-CD77 than with isotype control).
TIMP-1 induces further B-cell differentiation in IL-10–independent manner:
Y axis: Cell number. X axis: Log fluorescence staining with FITC-labeled antibody. Dotted lines indicate staining with isotype control. Solid lines indicate staining with anti-CD77. (A) Expression of CD77 in JD38 cells (do not express TIMP-1 or IL-10 protein). Cells express CD77. (B) Expression of CD77 in TIMP-1 JD38 clone 24 (expresses and secretes TIMP-1 and IL-10). Cells are negative for CD77 (0% positive for CD77). (C) Expression of CD77 in TIMP-1 JD38 clone 24 after 48-hour incubation with neutralizing anti–TIMP-1 antibody (no active TIMP-1). CD77 expression up-regulated. (D) Expression of CD77 in JD38 cells incubated with rIL-10 (no TIMP-1, active IL-10). Cells express CD77 (23% of the cells stain higher with anti-CD77 than with isotype control).
Expression of TIMP-1 and IL-10 in B-cell non-Hodgkin lymphoma
The in vitro effects of TIMP-1 on the survival and induction of IL-10 by B cells could have important implications in the clinical behavior and response to therapy of B-cell malignancies. IL-10 expression has been associated with a poor prognosis in non-Hodgkin lymphoma and may be important in the development of B-cell neoplasia. These findings prompted us to determine the relation between TIMP-1 and IL-10 expression in a series of B-cell non-Hodgkin lymphoma of either high grade (n = 10) or low grade (n = 21) using semiquantitative RT-PCR normalized to β actin expression. A statistically significant difference (P < .00001) in the expression of TIMP-1 is observed between high- and low-grade tumors (Figure5A), as determined by the Wilcoxon rank sum test. In high-grade B-cell lymphomas, 100% had high level TIMP-1 (greater than 0.7 relative density units). All low-grade tumors were either negative for TIMP-1 (n = 2 of 21) or had low level expression (19 of 21; 90%), as determined by less than 0.65 relative density. These data clearly establish a direct correlation between TIMP-1 expression and the histologic grade of B-cell lymphomas and further support our hypothesis that TIMP-1 may play a role in the pathogenesis of B-cell lymphomas.
TIMP-1 and IL-10 expression in B-cell non-Hodgkin lymphoma.
(A) Correlation of TIMP-1 expression, by RT-PCR, with histologic grade in B-cell non-Hodgkin lymphoma. Y-axis: relative density of TIMP-1 versus beta actin bands. A significant difference is noted between high- and low-grade tumors (P < .00001). (B) Correlation of IL-10 expression, by RT-PCR, with histologic grade in B-cell non-Hodgkin lymphoma. Y-axis: relative density of IL-10 versus beta actin bands. A significant difference is noted between high- and low-grade tumors (P < .00001).
TIMP-1 and IL-10 expression in B-cell non-Hodgkin lymphoma.
(A) Correlation of TIMP-1 expression, by RT-PCR, with histologic grade in B-cell non-Hodgkin lymphoma. Y-axis: relative density of TIMP-1 versus beta actin bands. A significant difference is noted between high- and low-grade tumors (P < .00001). (B) Correlation of IL-10 expression, by RT-PCR, with histologic grade in B-cell non-Hodgkin lymphoma. Y-axis: relative density of IL-10 versus beta actin bands. A significant difference is noted between high- and low-grade tumors (P < .00001).
The same lymphoma cases were examined for human IL-10 mRNA by semiquantitative RT-PCR using primers that specifically detect human IL-10 mRNA, and not viral IL-10. IL-10 levels are strongly associated with tumor grade (P < .00001) by the Wilcoxon rank sum test. As demonstrated in Figure 5B, all high-grade lymphomas expressed IL-10, whereas only 4 of 17 (23.5%) low-grade lymphomas were positive. There was a high correlation between TIMP-1 and IL-10 expression in these tumors (P = .00001; Fisher exact test). Data from our in vitro studies demonstrate that IL-10 expression correlates with and is dependent on TIMP-1 expression. Clinical data on B cell lymphomas further demonstrate a strong correlation between TIMP-1 expression, IL-10 expression, and histologic grade. Together these data suggest that TIMP-1 controls the expression of IL-10 in B-cell tumors. In view of TIMP-1 antiapoptosis activity in B cells,13TIMP-1 may affect prognosis by determining the expression of factors involved in the survival and growth of B-cell tumors.
Discussion
B-cell differentiation follows a complex series of steps and is tightly regulated. Naive B cells are produced in the bone marrow and travel to T-cell–rich extrafollicular areas, where they may be stimulated to proliferate by T cells and antigen-presenting interfollicular dendritic cells.32,33 Stimulated B cells invade germinal centers to either undergo apoptosis or to differentiate into highly proliferative CD77-expressing centroblasts. The factors controlling the process of B-cell differentiation versus apoptosis in the germinal center are not completely understood. Stimulated T cells play an important role in B-cell stimulation. Germinal center stromal cells, such as follicular dendritic cells, are also essential for germinal center B-cell survival and differentiation. Proliferation within the germinal center is dependent on CD40 activation and on IL-2 and IL-4. CD40 activation is important in the early survival of these cells because CD40 engagement drives B cells to lose CD77 (a neutral glycolipid associated with susceptibility to apoptosis).19-21 Activation of CD40 by its ligand is also reported to induce IL-10 secretion and to modulate immunoglobulin production. On stimulation with IL-10 in conjunction with CD40 activation, centroblasts differentiate into nonproliferating centrocytes, with down-regulation of CD77 and up-regulation of CD40. Because TIMP-1 alone affects all these changes, CD40-induced B-cell differentiation may be dependent on TIMP-1. Centrocytes can differentiate into either memory cells or plasma cells, and IL-10 and CD40 are both important in this process.34-37
TIMP-1 expression by B cells is dependent on their environment and on their state of differentiation. We have demonstrated11that TIMP-1 is expressed by isolated germinal center B cells and that expression decreases rapidly, within 24 hours, under conventional culture conditions. Cell death on the culturing of isolated germinal center B cells is coincident with decreased TIMP-1 expression by these B cells.15 Addition of exogenous TIMP-1 prevents the germinal center B-cell apoptosis that normally occurs in vitro. Therefore, germinal center B cells express TIMP-1 in the appropriate environment, and TIMP-1 inhibits programmed cell death in these cells, indicating that TIMP-1 plays an important role in germinal center B-cell survival.13 Culturing of the isolated B cells with germinal center stromal cells also prolongs their survival. This observation is interesting because TIMP-1 is expressed by germinal center stromal cells and macrophages.41,25 Thus, germinal center stromal cells may promote B-cell survival directly by secreting TIMP-1 or possibly by stimulating germinal center B cells to produce TIMP-1. In addition to promoting survival, TIMP-1 affects germinal center B-cell differentiation by up-regulating CD40 and CD23 and down-regulating CD77.12 Our current study demonstrates that TIMP-1 induces IL-10 expression, an important step in B-cell differentiation. Furthermore, it is TIMP-1, not IL-10, that regulates the expression of CD40, CD77, and CD10. IL-10 cannot substitute for TIMP-1 in its actions. This is consistent with previous studies indicating that CD77+ germinal center B cells do not express IL-10.38 Our data indicate that exogenous IL-10 promotes proliferation of the centrocytes in the absence of TIMP-1 but that TIMP-1 alone is able to inhibit apoptosis and to induce further differentiation in these cells. Based on these data, it appears that TIMP-1 plays an important role in B-cell differentiation. Previous reports of the effects of IL-10 and CD40 activation in normal germinal center B cells were likely biased by the presence of contaminating TIMP-1 from fetal calf serum, which contains high levels of TIMP-1, or germinal center stromal cells, which expresses TIMP-1. Monocytes or tissue macrophages, which express TIMP-1 on IL-10 stimulation,25 might also have been present. These studies should be interpreted with caution because observed effects may, in part, result from TIMP-1 in the growth media from exogenous (serum) or endogenous (IL-10–stimulated macrophage) sources.
We hypothesize that TIMP-1 expression is induced in centroblasts by the appropriate germinal center environment and that TIMP-1 not only inhibits apoptosis but also induces differentiation to the centrocyte stage. TIMP-1 induces IL-10 expression (Figure6) and up-regulates CD40, which makes the cells responsive to CD40 activation. In CD40-activated B cells, IL-10 is known to induce cell proliferation and to stimulate the further differentiation of B cells into plasma cells. The phenotypic changes induced by TIMP-1 normally occur in the germinal center during the interaction of B cells and T cells and in proximity to the TIMP-1–containing germinal center stroma.15-18Therefore, a view of B-cell differentiation evolves in which germinal center environment induces TIMP-1 expression in differentiating B cells. This effect could be initiated or augmented by germinal center stromal cell TIMP-1. TIMP-1 then inhibits apoptosis and directly induces further B-cell differentiation. TIMP-1 also induces IL-10 expression, which, in the presence of CD40 ligand activation, results in further differentiation to the plasma cell stage. T cells and monocytes can affect B-cell differentiation by the secretion of IL-10, which not only stimulates B-cell differentiation but also induces TIMP-1 expression by macrophages and monocytes. Thus, TIMP-1 may play a pivotal role in humoral-mediated immunity by controlling B-cell differentiation from the centroblast to the plasma cell stage. TIMP-1–deficient mice display altered immunity.42However, B-cell function has not been studied in these mice.
Model for TIMP-1 control of B cell differentiation from the centroblast to plasma cell.
Model for TIMP-1 control of B cell differentiation from the centroblast to plasma cell.
IL-10 is a strong B-cell activator and is proposed to act as an autocrine growth factor in certain non-Hodgkin lymphomas.23,24,39 It has been proposed that IL-10 can contribute to B-cell malignant disorders, in particular those associated with EBV infection, through B-cell activation or immunosuppression.39 TIMP-1 is important in the growth and survival of B cells, and, in the current study, we have shown that TIMP-1 can regulate IL-10 expression in B cells. To examine the clinical relevance of this correlation, we studied the relation between TIMP-1, IL-10, and tumor grade in a series of high-grade and low-grade B-cell lymphomas. We found a highly significant correlation between TIMP-1 expression and histologic grade. This is consistent with previous findings40 and indicates that TIMP-1 may be a negative prognostic indicator in non-Hodgkin lymphoma. Furthermore, TIMP-1 expression in this series of B-cell non-Hodgkin lymphomas correlates with human (ie, not EBV) IL-10 expression. TIMP-1 appears to play a role in B-cell neoplasia and may up-regulate IL-10 in these tumors. Because TIMP-1 is known to inhibit apoptosis, the reported association of IL-10 with a negative prognosis may be a function of the association of IL-10 with TIMP-1 expression in addition to IL-10 activity as a proliferation factor.
The mechanism(s) of IL-10 action in B-cell tumors and in normal B-cell maturation is poorly understood. Because these mechanisms are crucial to understanding B-cell differentiation and because they are potential therapeutic targets for B-cell lymphomas, they are actively studied by a number of investigators. In the current study, we have shown that TIMP-1 regulates IL-10 production in B cells and that the induction of TIMP-1 expression may be a key effector in the regulation of IL-10 in B-cell malignancies. In addition, there is a strong correlation of high histologic grade with TIMP-1 and IL-10 expression. Furthermore, IL-10 expression directly correlates with TIMP-1 expression in these tumor samples. These observations confirm previous reports of IL-10 as a negative prognostic indicator in lymphoma. However, our data indicate that TIMP-1 expression controls IL-10 expression and is responsible for the poor prognosis in these tumors. Additional studies are needed to determine the specific role TIMP-1 plays in the development of B-cell malignancies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maryalice Stetler-Stevenson, Flow Cytometry Unit, Laboratory of Pathology, Division of Clinical Sciences, National Cancer Institute, National Institutes of Health, Bldg 10, Rm 2N-108, Bethesda, MD 20892; e-mail: stetler@box-s.nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal