Analysis of T-cell receptor (TCR) repertoire usage made by peripheral T lymphocytes during the chronic phase of HIV-1 infection has revealed the presence of clonal expansions of CD8 T cells that are also shown to be largely HIV-specific. Yet, it remains unclear whether the global repertoire perturbation observed during the chronic phase of the infection is also HIV-related and reversible in the long term with the application of highly active antiretroviral therapy. Furthermore, the diversity and the stability of repertoire usage after a relapse of viral replication were never examined. Eight patients were observed longitudinally up to 31 months under triple-association therapy. When viral replication was steadily suppressed, CD8 repertoires were significantly stabilized. Conversely, in situations of incomplete or only transient viral suppression, persistence or rebound in repertoire perturbation was observed. Finally, a T-cell response remarkably different from baseline, as reflected by a repertoire switch, was generated after the discontinuation of highly active therapy. In conclusion, a sustained control of HIV replication correlated with profound modifications of the CD8 repertoire usage. These data also suggested that autovaccination by the withdrawal of antiviral drugs would result in the selection and expansion of T-cell clones that were not necessarily dominant before the onset of treatment.
Introduction
In adults, the size of the peripheral lymphocyte population is under homeostatic control, and the total number of T cells remains constant.1 In mice expressing a transgenic T-cell receptor (TCR), it has been elegantly shown that memory cells are maintained for extended periods of time in peripheral pools (though the size of individual clones decays progressively), whereas new thymic emigrants expressing a diverse repertoire constantly replace autoreactive and anergic T cells.2 It was proposed that in HIV-infected patients, CD4+ (and ultimately CD8+) T cell loss translates into massive T-cell repertoire deletions.3 In a previous work,4 we showed that CD4 T-cell repertoires are indeed perturbed in patients with advanced HIV disease. However, the CD4 repertoire perturbation was not, as previously concluded,3 the result of massive clonal deletions but could also be solely explained by the existence of multiple clonal expansions in that subset.4 5
Nevertheless, it could be feared that the effects of repetitive antigenic stimulations that bias the peripheral repertoire in HIV patients could never be compensated, even after the retrieval of the antigenic stimuli, because of irreversible damage to the thymic micro-environment.6 However, after 3 to 6 months of efficient treatment, immunoscope profiles derived from peripheral blood CD4 T cells of HIV patients were found to be comparable to the corresponding control data obtained from uninfected controls.4 Long-lasting recovery of CD4 T-cell function was also evidenced in the successfully treated patients from that group.7 In comparison, a bias in the usage of the CD8 repertoire appeared to persist in 10 patients observed through 6 months of treatment.4 In another study,8 persistent alterations in CD8 T-cell repertoire were also reported after 11 months of highly active antiretroviral therapy (HAART) in 3 patients, though HIV replication had been suppressed below the detection level.
The latter results could appear to contradict the conclusions of other studies that relied on the use of peptide–major histocompatibility tetrameric complexes because a relatively rapid decrease in the frequency of tetramer-stained cells was noted in most patients receiving HAART.9,10 HLA-tetrameric complexes have allowed, for the first time, a direct ex vivo quantitative analysis of HIV-specific T-cell immunity, but their use is limited to the analysis of few predefined epitopes that are not necessarily immunodominant in the patients analyzed. The tetramer-positive populations observed during HAART represented less 0.4%9 or less than 2%10 of all CD8+ T cells at baseline and probably corresponded to only a fraction of the HIV-specific cellular response. In a subsequent study it was indeed shown, using the same technique, that chronically expanded CD8 T cells are also HIV specific.11 HIV-specific cytotoxic T lymphocytes are found in lymphoid organs,12 genital mucosae,13 and skin of HIV-1–infected patients.14 Under HAART, the redistribution15 and subsequent down-regulation of such amplified subsets could take a substantial period of time; this explains the lack of global repertoire stabilization previously reported during the initial phase of HAART.4 Indeed, it was recently shown that it takes 7 months to stabilize CD8 repertoires when therapy is initiated during the primary phase of the infection.16
Here, the follow-up of 8 chronically infected patients, taken from the group previously defined in Gorochov et al,4 was extended to the second semester of treatment (and in 4 cases at least 28 months after the initiation of therapy) to analyze the long-term evolution of the CD8 repertoire. For all patients, Immunoscope analysis was performed at each time point. More detailed information on the perturbations reported was obtained by combining the Immunoscope CDR3 length analysis with cytofluorometric analysis of BV subset frequencies and DNA sequencing of selected BV subsets. The strategy used in the current work for the evaluation of CD8 repertoire perturbation allows a quantitative and sensitive survey of a large fraction of the expressed repertoire. We show that the strong CD8 TCR repertoire bias observed in the peripheral blood of HIV patients results from the accumulation in this compartment of relatively few CD8 T-cell clones, whereas the polyclonal repertoire appears, as a result, vastly underrepresented. That apparent reduction of diversity is not irreversible. Indeed, a lasting suppression of HIV replication is followed by a dramatic size reduction of the CD8 clonal expansions. One year after the onset of a highly active antiretroviral treatment, and in the absence of any other ongoing viral infection, the TCRBV repertoire of peripheral CD8 T cells has returned to a predominantly polyclonal configuration. In 2 such patients, a lack of adherence to HAART resulted in a resumption of active viral replication and in the recall of several baseline CD8 clones, but also in the expansion of others not dominant at the onset of therapy.
Patients, materials, and methods
Patients
The study was performed after obtaining informed consent from 8 HIV-1–infected male patients. Immunologic status virologic parameters of these patients at the time of repertoire analysis are listed in Table 1. Patients T17, T18, T19, and T20 did not receive any previous antiviral therapy. Patient T13 was under a bi-therapeutic regimen (AZT + 3TC) before the onset of the current study. Patients T8, T13, and T16 were classified in group C according to the Centers for Disease Control and Prevention criteria,17 whereas patients T11, T17, T18, T19, and T20 were classified in CDC group B. All patients received triple-combination therapy, as indicated in Table 1. Drugs and daily doses were: Ritonavir (Abbott), 1200 mg; Zidovudine (Glaxo Wellcome), 500 mg; Didanosine (Bristol Myers Squibb), 400 mg; Stavudine (Bristol Myers Squibb), 80 mg; lamivudine (Glaxo Wellcome), 300 mg; Indinavir (Merck Sharp and Dohme), 2400 mg; and Nevirapine (Boehringer Ingelheim), 400 mg. Blood samples were collected for repertoire analysis on the day before the introduction of Ritonavir or Indinavir. Consecutive samples were taken over a period of 24 months, except in HIV-seronegative controls C4 and C5, who are 30- and 35-year-old healthy men, respectively. Three more control subjects were included to establish the profiles corresponding to the theoretical, nonperturbed repertoires used for quantitative analysis (see below). The groups of patients and controls have all been previously described.4
Clinical data
| Patient/ sample* . | Sex . | Age (y) . | Current infectious manifestations† . | Treatment‡ (starting) . | CD4 (cells/μL) . | CD8 (cells/μL) . | HIV RNA (copies/mL) . |
|---|---|---|---|---|---|---|---|
| T8/M0 | M | 38 | d4T/ddI | 65 | 1 230 | 29 260 | |
| T8/M1 | Ind/d4T/ddI | 82 | 1 059 | 7 989 | |||
| T8/M4 | Ind/d4T/ddI | 131 | 1 369 | 1 064 | |||
| T8/M19 | B3 | Ind/d4T/3TC | 318 | n.d. | < 200 | ||
| T8/M30 | Ind/d4T/3TC | 485 | n.d. | < 200 | |||
| T11/M0 | M | 38 | AZT/3TC | 27 | 499 | 322 000 | |
| T11/M1 | Ind/AZT/3TC | 100 | 1 028 | 12 000 | |||
| T11/M6 | Ind/AZT/3TC | 122 | 1 466 | 331 | |||
| T11/M17 | Ind/AZT/3TC | 207 | 1 817 | < 200 | |||
| T11/M31 | Ind/AZT/3TC | 261 | 1 573 | 175 | |||
| T13/M0 | M | 61 | Ind/AZT/3TC | 30 | 378 | 420 000 | |
| T13/M1 | C9 | Ind/AZT/3TC | 18 | 598 | 11 506 | ||
| T13/M6 | Ind/d4T/3TC | 15 | 480 | 9 620 | |||
| T13/M10 | Ind/d4T/3TC | 7 | 544 | 18 200 | |||
| T16/M0 | M | 41 | Ind/AZT/ddC | 40 | 520 | 515 000 | |
| T16/M1 | Ind/AZT/ddC | 169 | 1 410 | 93 900 | |||
| T16/M6 | Ind/AZT/ddC | 152 | 660 | 400 | |||
| T16/M14 | B3 | Ind/AZT/3TC | 163 | 788 | 104 600 | ||
| T16/M26 | Ind/NVP/d4T/ddI | 144 | 886 | 345 200 | |||
| T16/M30 | Ind/NVP/d4T/ddI | 150 | 663 | 22 300 | |||
| 1-153T17/M0 | M | 40 | Ind/AZT/ddI | 36 | 560 | 1 500 000 | |
| T17/M1 | Ind/AZT/ddI | 93 | 599 | 5 363 | |||
| T17/M6 | Ind/AZT/ddI | 197 | 1 000 | 323 | |||
| T17/M9 | Ind/AZT/ddI | 155 | 566 | < 200 | |||
| T17/M12 | Ind/AZT/ddI | 230 | 693 | < 200 | |||
| 1-153T18/M0 | M | 39 | Ind/AZT/3TC | 136 | 1 091 | 99 365 | |
| T18/M1 | Ind/AZT/3TC | 181 | 1 156 | 1 124 | |||
| T18/M6 | Ind/AZT/3TC | 195 | 1 335 | < 200 | |||
| T18/M9 | Ind/AZT/3TC | 280 | 841 | < 200 | |||
| T18/M12 | Ind/AZT/3TC | 225 | 788 | < 200 | |||
| T18/M28 | Ind/AZT/3TC | 162 | 858 | 2 674 | |||
| 1-153T19/M0 | M | 31 | Rit/d4T/ddI | 299 | 479 | 129 800 | |
| T19/M1 | Rit/d4T/ddI | 465 | 579 | 4 400 | |||
| T19/M3 | Rit/d4T/ddI | 579 | 540 | 680 | |||
| T19/M6 | Rit/d4T/ddI | 565 | 474 | < 200 | |||
| T19/M12 | 3TC/d4T | 679 | 503 | 1 000 | |||
| T19/M20 | 3TC/d4T | 977 | 734 | 700 | |||
| 1-153T20/M0 | M | 36 | Ind/AZT/ddC | 92 | 1 635 | 101 393 | |
| T20/M1 | Ind/AZT/ddC | 186 | 953 | 1 213 | |||
| T20/M3 | Ind/AZT/ddC | 217 | 1 346 | < 200 | |||
| T20/M6 | Ind/AZT/ddC | 276 | 1 017 | < 200 | |||
| T20/M12 | Ind/AZT/ddC | 203 | 888 | < 200 |
| Patient/ sample* . | Sex . | Age (y) . | Current infectious manifestations† . | Treatment‡ (starting) . | CD4 (cells/μL) . | CD8 (cells/μL) . | HIV RNA (copies/mL) . |
|---|---|---|---|---|---|---|---|
| T8/M0 | M | 38 | d4T/ddI | 65 | 1 230 | 29 260 | |
| T8/M1 | Ind/d4T/ddI | 82 | 1 059 | 7 989 | |||
| T8/M4 | Ind/d4T/ddI | 131 | 1 369 | 1 064 | |||
| T8/M19 | B3 | Ind/d4T/3TC | 318 | n.d. | < 200 | ||
| T8/M30 | Ind/d4T/3TC | 485 | n.d. | < 200 | |||
| T11/M0 | M | 38 | AZT/3TC | 27 | 499 | 322 000 | |
| T11/M1 | Ind/AZT/3TC | 100 | 1 028 | 12 000 | |||
| T11/M6 | Ind/AZT/3TC | 122 | 1 466 | 331 | |||
| T11/M17 | Ind/AZT/3TC | 207 | 1 817 | < 200 | |||
| T11/M31 | Ind/AZT/3TC | 261 | 1 573 | 175 | |||
| T13/M0 | M | 61 | Ind/AZT/3TC | 30 | 378 | 420 000 | |
| T13/M1 | C9 | Ind/AZT/3TC | 18 | 598 | 11 506 | ||
| T13/M6 | Ind/d4T/3TC | 15 | 480 | 9 620 | |||
| T13/M10 | Ind/d4T/3TC | 7 | 544 | 18 200 | |||
| T16/M0 | M | 41 | Ind/AZT/ddC | 40 | 520 | 515 000 | |
| T16/M1 | Ind/AZT/ddC | 169 | 1 410 | 93 900 | |||
| T16/M6 | Ind/AZT/ddC | 152 | 660 | 400 | |||
| T16/M14 | B3 | Ind/AZT/3TC | 163 | 788 | 104 600 | ||
| T16/M26 | Ind/NVP/d4T/ddI | 144 | 886 | 345 200 | |||
| T16/M30 | Ind/NVP/d4T/ddI | 150 | 663 | 22 300 | |||
| 1-153T17/M0 | M | 40 | Ind/AZT/ddI | 36 | 560 | 1 500 000 | |
| T17/M1 | Ind/AZT/ddI | 93 | 599 | 5 363 | |||
| T17/M6 | Ind/AZT/ddI | 197 | 1 000 | 323 | |||
| T17/M9 | Ind/AZT/ddI | 155 | 566 | < 200 | |||
| T17/M12 | Ind/AZT/ddI | 230 | 693 | < 200 | |||
| 1-153T18/M0 | M | 39 | Ind/AZT/3TC | 136 | 1 091 | 99 365 | |
| T18/M1 | Ind/AZT/3TC | 181 | 1 156 | 1 124 | |||
| T18/M6 | Ind/AZT/3TC | 195 | 1 335 | < 200 | |||
| T18/M9 | Ind/AZT/3TC | 280 | 841 | < 200 | |||
| T18/M12 | Ind/AZT/3TC | 225 | 788 | < 200 | |||
| T18/M28 | Ind/AZT/3TC | 162 | 858 | 2 674 | |||
| 1-153T19/M0 | M | 31 | Rit/d4T/ddI | 299 | 479 | 129 800 | |
| T19/M1 | Rit/d4T/ddI | 465 | 579 | 4 400 | |||
| T19/M3 | Rit/d4T/ddI | 579 | 540 | 680 | |||
| T19/M6 | Rit/d4T/ddI | 565 | 474 | < 200 | |||
| T19/M12 | 3TC/d4T | 679 | 503 | 1 000 | |||
| T19/M20 | 3TC/d4T | 977 | 734 | 700 | |||
| 1-153T20/M0 | M | 36 | Ind/AZT/ddC | 92 | 1 635 | 101 393 | |
| T20/M1 | Ind/AZT/ddC | 186 | 953 | 1 213 | |||
| T20/M3 | Ind/AZT/ddC | 217 | 1 346 | < 200 | |||
| T20/M6 | Ind/AZT/ddC | 276 | 1 017 | < 200 | |||
| T20/M12 | Ind/AZT/ddC | 203 | 888 | < 200 |
Blood was sampled just before treatment (M0) and at 1 to 32 months (M1 to M32) after the onset of treatment.
CDC 1993 definition of AIDS stage and AIDS-related infections (B3, herpes zoster; C9, esophageal candidiasis).
d4T, stavudine; ddI, didanosine; Ind, indinavir; AZT, zidovudine; 3TC, lamivudine; NVP, nevirapine; Rit, ritonavir.
Naive patients. All other patients receiving triple therapy were pretreated.
Viral load measurement
Plasma samples were stored at −70°C. Plasma HIV-1 RNA levels were measured by quantitative reverse transcription–polymerase chain reaction (RT-PCR) (Amplicor HIV Monitor Test; Roche Molecular Systems, Branchburg, NJ). The detection threshold was 200 copies/mL.
Purification of CD8 T cells
Peripheral blood mononuclear cells (PBMCs) were prepared by centrifugation on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). CD4 and CD8 cells were purified immediately after blood sampling. Cells were washed twice in phosphate-buffered saline (PBS)–0.5% bovine serum albumin (BSA), and CD4+ lymphocytes were first separated using anti-CD4 monoclonal antibodies coated on magnetic beads according to the manufacturer's instructions (Dynabeads; Dynal, Oslo, Norway). The CD8+ cells were purified after removal of the CD4+ cells using anti-CD8 magnetic beads. Bead-coated CD8 lymphocytes were washed 3 times in PBS–0.5% BSA. Purity was assessed by FACS analysis and also by PCR analysis using CD4- and CD8-specific primers on both fractions (not shown). At least 106CD8+ cells (98% purity) were required for further analysis.
Flow cytometry
For double- or triple-fluorescence analysis of PBMCs, 5 × 105 cells were incubated for 30 minutes at 4°C with 5μg each monoclonal antibody (mAb) washed with PBS, then fixed with 2% paraformaldehyde in PBS and analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Ten thousand events per sample were collected and analyzed using the CellQuest software (Becton Dickinson). All antibodies were obtained from Immunotech (Marseilles, France). All BV-specific mAbs listed in Table2 were used as phycoerythrin conjugates, except for the anti-BV3 mAb, which was fluorescein isothiocyanate-labeled. The BV-specific monoclonal antibodies were a kind gift of Drs F. Romagne and A. Necker (Immunotech). Denominations correspond to the gene products recognized; the corresponding original clones are listed as follows: BV1S1 (clone BL37.2), BV2S1 (clone MPB2D5), BV3S1 (clone CH92), BV5S1 (clone IMMU157), BV5S2 (clone 36213), BV5S3 (clone 3D11), BV7S1 (clone ZOE), BV8S1/2 (clone 56C5), BV9S1 (clone FIN9), BV11S1 (clone C21), BV12S1 (clone VE R2.32), BV13S1 (clone IMMU222), BV13S6 (clone JU74), BV14S1 (clone CAS1.1.3), BV16S1 (clone TAMAYA), BV17S1 (clone E17SF3), BV18S1 (clone BA62.6), BV20S1 (clone ELL1.4), BV21S3 (clone IG125), BV22S1 (clone IMMU546), and BV23S1 (clone AF23). TCRBV gene segment denomination is in accordance with that of Wei et al.18 Specificities of the panel of antibodies used are based on the findings of the first workshop on human TCR monoclonal antibody19 and of Diu et al20 and Romagne et al.21,22 Those reagents have been tested on a group of 20 healthy seronegative controls to establish reference values and standard deviations.23
Quantitative cytofluorometric analysis of BV subsets among CD8 T cells of patients
| . | T13/M6 . | T13/M10 . | T17/M9 . | T18/M9 . | T18/M12 . | T19/M9 . | T19/M14 . | T19/M20 . | T20/M6 . | T20/M12* . |
|---|---|---|---|---|---|---|---|---|---|---|
| BV1S1 | ND | ND | 8.55 | 4.03 | 5.11 | 1.29 | 1.76 | 1.41 | 4.30 | 5.62 |
| BV2S1 | 19.00 | 34.00 | 8.47 | 4.41 | 5.24 | 2.16 | 2.30 | 2.48 | 4.11 | 5.32 |
| BV3S1 | ND | ND | 1.65 | 0.38 | 2.24 | 2.25 | 0.00 | 1.02 | 2.28 | 1.54 |
| BV5S1 | ND | ND | 4.43 | 1.79 | 3.42 | 3.67 | 3.80 | 4.07 | 9.18 | 10.40 |
| BV5S2 | ND | ND | 0.60 | 0.49 | 0.48 | 0.20 | 0.42 | ND | 0.81 | 0.98 |
| BV5S3 | ND | ND | 0.85 | 0.49 | 0.70 | 0.25 | 0.57 | ND | 0.25 | 0.26 |
| BV7S1 | ND | ND | 7.16 | 2.04 | 3.62 | 9.22 | 13.28 | 22.51† | 1.10 | 0.96 |
| BV8S1 | ND | ND | 4.59 | 2.04 | 3.29 | 2.60 | 3.14 | 3.84 | 1.49 | 1.73 |
| BV9S1 | ND | ND | 1.23 | 0.80 | 0.90 | 0.77 | 1.14 | ND | 1.06 | 0.90 |
| BV11S1 | ND | ND | 0.81 | 3.37 | 0.53 | 0.50 | 0.45 | ND | 1.00 | 0.57 |
| BV12S1 | ND | ND | 1.92 | 0.66 | 1.05 | 0.25 | 0.60 | ND | 0.57 | 0.59 |
| BV13S1 | ND | ND | 3.03 | 10.98 | 1.83 | 0.80 | 1.21 | ND | 2.01 | 1.82 |
| BV13S6 | ND | ND | 1.86 | 0.77 | 1.16 | 0.45 | 0.61 | ND | 0.71 | 0.50 |
| BV14S1 | ND | ND | 6.4 | 13.05 | 4.9 | 6.77 | 7.26 | 6.53 | 7.50 | 4.63 |
| BV16S1 | ND | ND | 1.35 | 0.21 | 1.15 | 0.29 | 0.52 | ND | 0.60 | 3.95 |
| BV17S1 | ND | ND | 10.89 | 7.84 | 14.94 | 2.29 | 2.19 | 2.31 | 4.91 | 5.09 |
| BV18S1 | ND | ND | 0.30 | 0.39 | 0.6 | 0.10 | 0.26 | ND | 0.13 | 0.24 |
| BV20S1 | ND | ND | 0.76 | 0.77 | 1.5 | ND | 0.35 | ND | 2.23 | 1.40 |
| BV21S3 | ND | ND | 1.52 | 1.22 | 2.5 | 0.51 | 1.45 | ND | 1.33 | 1.24 |
| BV22S1 | ND | ND | 2.72 | 2.55 | 5.37 | 4.00 | 10.47 | 8.96 | 1.72 | 2.53 |
| BV23S1 | ND | ND | 2.15 | 2.01 | 2.38 | 0.32 | 2.07 | ND | 0.91 | 1.33 |
| Total | ND | ND | 71.33 | 60.36 | 62.90 | 38.87 | 53.92 | ND | 48.30 | 51.72 |
| . | T13/M6 . | T13/M10 . | T17/M9 . | T18/M9 . | T18/M12 . | T19/M9 . | T19/M14 . | T19/M20 . | T20/M6 . | T20/M12* . |
|---|---|---|---|---|---|---|---|---|---|---|
| BV1S1 | ND | ND | 8.55 | 4.03 | 5.11 | 1.29 | 1.76 | 1.41 | 4.30 | 5.62 |
| BV2S1 | 19.00 | 34.00 | 8.47 | 4.41 | 5.24 | 2.16 | 2.30 | 2.48 | 4.11 | 5.32 |
| BV3S1 | ND | ND | 1.65 | 0.38 | 2.24 | 2.25 | 0.00 | 1.02 | 2.28 | 1.54 |
| BV5S1 | ND | ND | 4.43 | 1.79 | 3.42 | 3.67 | 3.80 | 4.07 | 9.18 | 10.40 |
| BV5S2 | ND | ND | 0.60 | 0.49 | 0.48 | 0.20 | 0.42 | ND | 0.81 | 0.98 |
| BV5S3 | ND | ND | 0.85 | 0.49 | 0.70 | 0.25 | 0.57 | ND | 0.25 | 0.26 |
| BV7S1 | ND | ND | 7.16 | 2.04 | 3.62 | 9.22 | 13.28 | 22.51† | 1.10 | 0.96 |
| BV8S1 | ND | ND | 4.59 | 2.04 | 3.29 | 2.60 | 3.14 | 3.84 | 1.49 | 1.73 |
| BV9S1 | ND | ND | 1.23 | 0.80 | 0.90 | 0.77 | 1.14 | ND | 1.06 | 0.90 |
| BV11S1 | ND | ND | 0.81 | 3.37 | 0.53 | 0.50 | 0.45 | ND | 1.00 | 0.57 |
| BV12S1 | ND | ND | 1.92 | 0.66 | 1.05 | 0.25 | 0.60 | ND | 0.57 | 0.59 |
| BV13S1 | ND | ND | 3.03 | 10.98 | 1.83 | 0.80 | 1.21 | ND | 2.01 | 1.82 |
| BV13S6 | ND | ND | 1.86 | 0.77 | 1.16 | 0.45 | 0.61 | ND | 0.71 | 0.50 |
| BV14S1 | ND | ND | 6.4 | 13.05 | 4.9 | 6.77 | 7.26 | 6.53 | 7.50 | 4.63 |
| BV16S1 | ND | ND | 1.35 | 0.21 | 1.15 | 0.29 | 0.52 | ND | 0.60 | 3.95 |
| BV17S1 | ND | ND | 10.89 | 7.84 | 14.94 | 2.29 | 2.19 | 2.31 | 4.91 | 5.09 |
| BV18S1 | ND | ND | 0.30 | 0.39 | 0.6 | 0.10 | 0.26 | ND | 0.13 | 0.24 |
| BV20S1 | ND | ND | 0.76 | 0.77 | 1.5 | ND | 0.35 | ND | 2.23 | 1.40 |
| BV21S3 | ND | ND | 1.52 | 1.22 | 2.5 | 0.51 | 1.45 | ND | 1.33 | 1.24 |
| BV22S1 | ND | ND | 2.72 | 2.55 | 5.37 | 4.00 | 10.47 | 8.96 | 1.72 | 2.53 |
| BV23S1 | ND | ND | 2.15 | 2.01 | 2.38 | 0.32 | 2.07 | ND | 0.91 | 1.33 |
| Total | ND | ND | 71.33 | 60.36 | 62.90 | 38.87 | 53.92 | ND | 48.30 | 51.72 |
BV indicates variable region of beta chain; ND, not determined.
Percentage CD3+ CD8+ cells stained by the BV-specific mAbs listed in the first column. For each patient (Tn), after the indicated duration of treatment in months (Mn). Values exceeding the corresponding average control value +3 SD, are shown in boldface.
The BV7 subset was further typed CD45RO−, CD45RA+, CD62L−, CD57+, CD28−.
TCRBV analysis
Total RNA was extracted directly from 106 to 107 bead-coated cells and reverse-transcribed using the single-strand synthesis kit (Stratagene, La Jolla, CA). Amplification reactions were performed using a BC1/BC2-specific primer (CGGGCTGCTCCTTGAGGGGCTGCG) and a BV-specific primer, along with a Cα primer set as an internal control of amplification.24Briefly, 2 μL RT product (corresponding to 2 × 104 to 2 × 105 CD4+ or CD8+ T cells) were brought to a final reaction volume of 50 μL containing 10 mM Tris-HCl, 1.5 mM MgCl2, 50 mM KCl, pH 8.3, 20 pmol each oligonucleotide, 0.2 mM each dNTP, and 2.5 U Taq DNA polymerase blocked by the addition of an anti-Taq mAb (Taq Start; Clontech, San Diego, CA). After an initial denaturation step of 3 minutes at 95 °C, the reactions were subjected to 30 cycles of PCR (94°C for 30 seconds, 60°C for 1 minute, 74°C for 1 minute), followed by a final extension step of 5 minutes at 74°C. One nested BC oligonucleotide (GTGCACCTCCTTCCCATTCA) was used dye-labeled (Joe Fluorophore; Applied Biosystems, Foster City, CA) in runoff reactions. Two microliters PCR product was added to 8 μL of a mixture containing 10 mM Tris-HCl, 1.5 mM MgCl2, 50 mM KCl, pH 8.3, 0.2 mM each dNTP, 0.2 U Taq DNA polymerase, and 0.1 μM Joe Fluorophore-labeled oligonucleotide. The extension reaction consisted of a 3-minutes denaturation step at 95°C followed by 12 cycles of 30 seconds at 94°C, 30 seconds at 60°C, and 2 minutes at 72°C. A final 10-minute incubation at 72°C was performed. Runoff products were then loaded on a 4% acrylamide–4 M urea sequencing gel and run on an ABI 377 DNA sequencer (Applied Biosystems). A mixture of dye-labeled size standards was also loaded on the sequencing gel to allow the precise determination of the sizes of the BC-BV runoff reaction products. The sizes and areas of the peaks corresponding to the DNA products were determined using the Immunoscope software.25,26 The percentage of representation of each peak size among all BC-BV segments was subsequently calculated. Observed peaks were usually separated by 3 bases and corresponded to in-frame transcripts of TCRs. Windows of analysis were centered on expected sizes corresponding to TCR transcripts encoding a 10 residue-long CDR3 region, defined according to Kabat.27
BV family-specific primers used were as follows: BV1, CCGCACAACAGTTCCCTGACTTGC; BV2, GGCCACATACGAGCAAGGCGTCGA; BV3, CGCTTCTTCCGGATTCTGGAGTCC; BV4, TTCCCATCAGCCGCCCAAACCTAA; BV5, AGCTCTGAGCTGAATGTGAACGCC; BV7, CCTGAATGCCCCAACAGCTCTCTC; BV8, CCATGATGCGGGGACTGGAGTTGC; BV15, CAGGCACAGGCTAAATTCTCCCTG; BV16, GCCTGCAGAACTGGAGGATTCTG.
BV1, BV2, BV3, BV4, BV5, BV7, and BV8 families were among the most frequently represented in the periphery, whereas BV15 and BV16 were infrequent. BV amplicons of interest were gel-purified and directly ligated into the PCR II vector (TA cloning kit; Invitrogen, San Diego, CA). After amplification into Escherichia coli, plasmids were purified and their inserts were sequenced using an automated sequencer with M13R and T7 dye-labeled primers and AmpliTaq DNA polymerase FS (Perkin Elmer, Foster City, CA). Analysis of controlled serial dilutions of T cells showed that the proportions in the CDR3 length profiles are well conserved down to 4 × 105 cells used for RNA extraction 28
Quantitative analysis of repertoire perturbation
The strategy adopted for quantitative analysis of repertoire perturbation was reported previously.4 Briefly, each CDR3 profile obtained from the Immunoscope analysis was translated into a probability distribution, P(i) = Ai/(ΣiAi), using the fraction of the area (Ai) under the profile for each CDR3 length i, from minimal to maximal length in 3 nucleotide steps. The TCR-BV repertoire for each sample j is represented by the set of corresponding probability distributions for the various BV families analyzed in the CD8 subset. Thus, the probability distribution P(i) corresponds to the CD8 repertoires of BVk in samplej. Five control subjects (mean age, 35 years; range, 25-39 years) were randomly selected from among healthy, HIV-seronegative persons from the laboratory. A control profile, representing the theoretical nonperturbed repertoire, was established for each BVk by calculating the average probability distribution of the corresponding BVk CD4 profiles of the control samples, P(i) = Σj[P(i)]/N. The resultant control profiles, P(i), have a very good Gaussian distribution (P < .001, using the Kolmogorov-Smirnov test). We chose to use as control seronegative CD4 profiles for analysis of the CD8 repertoires because it was shown29 that, even in healthy persons, CD8 CDR3 profiles are sometimes skewed. Nevertheless, we also tested our analysis with controls derived from seronegative CD8 profiles and obtained similar results.
The extent of perturbation in a TCR profile was calculated for each BVk by the distance between the probability distributions of sample j and the control, D(i) = P(i) − P(i). The sum of the absolute value of the distances (generalized Hamming distance), D = 100 Σi D(i)/2, in each BV family over all TCR lengths i yields the perturbation of that TCR profile in percentages. Lack of perturbation is represented by no difference in the probability distributions of the sample and the control (D = 0%), whereas a sample that has a repertoire completely nonoverlapping with the control should give an absolute distance value of D = 100%. Two randomly drawn probability distributions would yield D = 50%. The average perturbation, Dj = Σk D /n, of alln BV families in a CD8 sample represents the repertoire perturbation in that sample. The perturbations in TCR profiles of seronegative CD8 samples range between 9% and 25%, with an average of D = 15% ± 5% per BV family.
The statistical significance (P) of differences among different subgroups of samples or patients was calculated using the nonparametric Wilcoxon rank sum test. Perturbations per BV family were used when assessing the difference in perturbation between samples (Figure 4). To evaluate the extent of normalization or further perturbation in a patient, we calculated the changes in perturbation per BV family (ΔD) between an initial time point (t0) and the subsequent time point available (t), ΔD = D(t) − D(t0). A negative change denotes a decrease in perturbations and possible restoration of the repertoire if the perturbations return to the normal range of seronegative persons (Figure 4). Intrinsic levels of variability of D (6%) and (0.7%) ΔD were previously assessed on control blood samples taken over a period of 3 days.4 28
In addition, to study the evolution of particular clonal expansions and not only the general properties of the total repertoire, overrepresented peak sizes among the CDR3 profiles were tracked longitudinally. It has been consistently shown that such peaks usually correspond to single clonal expansions.4,14,24 28 A threshold (θ = 10%) of 2 times the standard deviation above the normal distribution was defined, and Immunoscope peaks (i) in every BV family profile (k) in each sample (j) that were above that threshold (D(i) > θ) were identified as corresponding to at least one clonal T-cell expansion. It was then verified whether a clonal expansion detected in a given sample already existed in previous samples or whether it recently appeared. Note that the definition of the threshold level (θ) for expanded peaks is arbitrary. Therefore, all the data were analyzed using other threshold levels (5%-25%) as well. Varying threshold levels, the evolution reported in Figure 2, followed the same pattern, though the number of peaks in each sample varied as a function of the threshold.
Results
Long-term persistence of CD8+ clonal expansions in patients with active HIV replication
Cytofluorometric analysis of BV subsets, even when combined with semiquantitative RT-PCR analysis, underscores the extent of CD8 repertoire perturbation found in patients with HIV. As shown in Table2, few BV subsets were clearly overrepresented in the total CD8 population. In contrast, with the use of Immunoscope, we found that repertoire perturbation was a consistent feature of the CD8 subset before active antiviral treatment because most of the CDR3 length-distribution profiles were notably disrupted. Such disruptions usually correspond to the presence of dominant clonal expansions within the corresponding BV subset.4,14,24,28 Several clonal expansions were stable over a follow-up period of 1 year to 3 years in patients with no highly active antiviral therapy.4
To confirm that the repertoire perturbations we measured reflected expansions of cell subsets, we measured the relative size of various BV families using a large panel of antibodies covering at least 50% of the BV repertoire. Expansions were defined based on published data performed on healthy controls (see “Patients, materials, and methods”). When FACS and CDR3 length analysis could be performed simultaneously, the BV subsets with the most perturbed CDR3 profiles were usually found to be expanded—for example, BV7 for patient T19/M20, BV2 for patient T13/M6/M10, and BV5 for patient T20/M6 (Table2). A dedicated algorithm was used to define and track longitudinally overrepresented peak sizes among the CDR3 length profiles of the patients. On average, in the 3 patients who remained constantly viremic, 64% of the peaks detected at baseline persisted during the 10 to 31 months of follow-up. Finally, DNA sequencing confirmed in selected patients (T13/BV2, T19/BV2/BV7/BV8, T20/BV2/BV5) the monoclonal origin of the dominant peaks detected using Immunoscope (data not shown and Tables 3,4).
Predicted amino acid sequences of the CDR3 loops of the BV5+ CD8+ PBMCs of patient T20
| Sample . | BV . | BJ . | CDR3 . | Frequency . | Length (aa) . |
|---|---|---|---|---|---|
| T20/M3 | 2 | 2S1 | PQTGDHEQ | 3/11 | 8 |
| T20/M3 | 2 | 2S3 | RGQTAQETQ | 5/11 | 9 |
| T20/M3 | 2 | 2S1 | RVGRANYNEQ | 1/11 | 10 |
| T20/M3 | 2 | 2S1 | RDHTGTGDNE | 1/11 | 10 |
| T20/M3 | 2 | 2S5 | RERDRETSGSR | 1/11 | 11 |
| T20/M12 | 2 | 1S1 | SGGTEA | 1/18 | 6 |
| T20/M12 | 2 | 2S3 | PTRSDTQ | 1/18 | 7 |
| T20/M12 | 2 | 1S1 | GETGGLGA | 1/18 | 8 |
| T20/M12 | 2 | 2S3 | RGQTAQETQ | 2/18 | 9 |
| T20/M12 | 2 | 2S3 | WIAERADTQ | 1/18 | 9 |
| T20/M12 | 2 | 2S1 | RGLASYNEQ | 1/18 | 9 |
| T20/M12 | 2 | 2S3 | GRGDSQETQ | 1/18 | 9 |
| T20/M12 | 2 | 1S3 | RVGTGDTEA | 1/18 | 9 |
| T20/M12 | 2 | 2S3 | SFTSGQETQ | 1/18 | 9 |
| T20/M12 | 2 | 2S3 | LPGLATTDTQ | 2/18 | 10 |
| T20/M12 | 2 | 2S6 | SGGMPGANVL | 1/18 | 10 |
| T20/M12 | 2 | 2S2 | RKRERGTGEL | 1/18 | 10 |
| T20/M12 | 2 | 2S3 | RGLAGGPGETQ | 1/18 | 11 |
| T20/M12 | 2 | 2S2 | REEGRRSTGEL | 1/18 | 11 |
| T20/M12 | 2 | 2S3 | TGLADLLRGGYEQ | 2/18 | 12 |
| T20/M0 | 5 | 1S6 | LGGRNSPLH | 11/13 | 9 |
| T20/M0 | 5 | 1S1 | LAGEGKGTEAF | 1/13 | 11 |
| T20/M0 | 5 | 1S6 | QQPWWQNSPLH | 1/13 | 11 |
| T20/M6 | 5 | 1S6 | LGGRNSPLH | 9/9 | 9 |
| T20/M12 | 5 | 1S1 | SRATEA | 1/18 | 6 |
| T20/M12 | 5 | 2S3 | PPPLYEQ | 1/18 | 7 |
| T20/M12 | 5 | 1S5 | FGWNQPQ | 1/18 | 7 |
| T20/M12 | 5 | 1S1 | SNWDTEA | 1/18 | 7 |
| T20/M12 | 5 | 2S3 | FGTSQETQ | 1/18 | 8 |
| T20/M12 | 5 | 1S1 | FLGFVTEA | 1/18 | 8 |
| T20/M12 | 5 | 2S3 | GNRAQETQ | 1/18 | 8 |
| T20/M12 | 5 | 2S3 | LWRAPSTQ | 1/18 | 8 |
| T20/M12 | 5 | 2S3 | FRGGADTQ | 1/18 | 8 |
| T20/M12 | 5 | 2S2 | SGGNTGEL | 1/18 | 8 |
| T20/M12 | 5 | 2S3 | HFPTAAYEQ | 1/18 | 9 |
| T20/M12 | 5 | 2S3 | ALASGPETL | 1/18 | 9 |
| T20/M12 | 5 | 2S3 | LYVDGSYEQ | 1/18 | 9 |
| T20/M12 | 5 | 2S3 | FWRGSTDTQ | 1/18 | 9 |
| T20/M12 | 5 | 2S3 | GRTAYTDTQ | 1/18 | 9 |
| T20/M12 | 5 | 1S1 | AARTSGDEQ | 1/18 | 9 |
| T20/M12 | 5 | 2S1 | RRTGTGYSEQ | 1/18 | 10 |
| T20/M12 | 5 | 1S2 | LGSTADSYGY | 1/18 | 10 |
| Sample . | BV . | BJ . | CDR3 . | Frequency . | Length (aa) . |
|---|---|---|---|---|---|
| T20/M3 | 2 | 2S1 | PQTGDHEQ | 3/11 | 8 |
| T20/M3 | 2 | 2S3 | RGQTAQETQ | 5/11 | 9 |
| T20/M3 | 2 | 2S1 | RVGRANYNEQ | 1/11 | 10 |
| T20/M3 | 2 | 2S1 | RDHTGTGDNE | 1/11 | 10 |
| T20/M3 | 2 | 2S5 | RERDRETSGSR | 1/11 | 11 |
| T20/M12 | 2 | 1S1 | SGGTEA | 1/18 | 6 |
| T20/M12 | 2 | 2S3 | PTRSDTQ | 1/18 | 7 |
| T20/M12 | 2 | 1S1 | GETGGLGA | 1/18 | 8 |
| T20/M12 | 2 | 2S3 | RGQTAQETQ | 2/18 | 9 |
| T20/M12 | 2 | 2S3 | WIAERADTQ | 1/18 | 9 |
| T20/M12 | 2 | 2S1 | RGLASYNEQ | 1/18 | 9 |
| T20/M12 | 2 | 2S3 | GRGDSQETQ | 1/18 | 9 |
| T20/M12 | 2 | 1S3 | RVGTGDTEA | 1/18 | 9 |
| T20/M12 | 2 | 2S3 | SFTSGQETQ | 1/18 | 9 |
| T20/M12 | 2 | 2S3 | LPGLATTDTQ | 2/18 | 10 |
| T20/M12 | 2 | 2S6 | SGGMPGANVL | 1/18 | 10 |
| T20/M12 | 2 | 2S2 | RKRERGTGEL | 1/18 | 10 |
| T20/M12 | 2 | 2S3 | RGLAGGPGETQ | 1/18 | 11 |
| T20/M12 | 2 | 2S2 | REEGRRSTGEL | 1/18 | 11 |
| T20/M12 | 2 | 2S3 | TGLADLLRGGYEQ | 2/18 | 12 |
| T20/M0 | 5 | 1S6 | LGGRNSPLH | 11/13 | 9 |
| T20/M0 | 5 | 1S1 | LAGEGKGTEAF | 1/13 | 11 |
| T20/M0 | 5 | 1S6 | QQPWWQNSPLH | 1/13 | 11 |
| T20/M6 | 5 | 1S6 | LGGRNSPLH | 9/9 | 9 |
| T20/M12 | 5 | 1S1 | SRATEA | 1/18 | 6 |
| T20/M12 | 5 | 2S3 | PPPLYEQ | 1/18 | 7 |
| T20/M12 | 5 | 1S5 | FGWNQPQ | 1/18 | 7 |
| T20/M12 | 5 | 1S1 | SNWDTEA | 1/18 | 7 |
| T20/M12 | 5 | 2S3 | FGTSQETQ | 1/18 | 8 |
| T20/M12 | 5 | 1S1 | FLGFVTEA | 1/18 | 8 |
| T20/M12 | 5 | 2S3 | GNRAQETQ | 1/18 | 8 |
| T20/M12 | 5 | 2S3 | LWRAPSTQ | 1/18 | 8 |
| T20/M12 | 5 | 2S3 | FRGGADTQ | 1/18 | 8 |
| T20/M12 | 5 | 2S2 | SGGNTGEL | 1/18 | 8 |
| T20/M12 | 5 | 2S3 | HFPTAAYEQ | 1/18 | 9 |
| T20/M12 | 5 | 2S3 | ALASGPETL | 1/18 | 9 |
| T20/M12 | 5 | 2S3 | LYVDGSYEQ | 1/18 | 9 |
| T20/M12 | 5 | 2S3 | FWRGSTDTQ | 1/18 | 9 |
| T20/M12 | 5 | 2S3 | GRTAYTDTQ | 1/18 | 9 |
| T20/M12 | 5 | 1S1 | AARTSGDEQ | 1/18 | 9 |
| T20/M12 | 5 | 2S1 | RRTGTGYSEQ | 1/18 | 10 |
| T20/M12 | 5 | 1S2 | LGSTADSYGY | 1/18 | 10 |
For the corresponding Immunoscope analysis, see Figure 3. Redundant sequences corresponding to identical DNA sequences and, thus, to clonal expansions, are in boldface.
aa indicates amino acid.
Predicted amino acid sequences of the CDR3 loops of the BV2+ CD8+ peripheral blood mononuclear cells s of patient T19
| Sample . | BV . | BJ . | CDR3 . | Frequency . | Length (aa) . |
|---|---|---|---|---|---|
| T19/M0 | 2 | 2S1 | VLRGGGEQ | 1/18 | 8 |
| T19/M0 | 2 | 2S2 | EGGSGNTI | 1/18 | 8 |
| T19/M0 | 2 | 1S1 | VRDRGNTEA | 1/18 | 9 |
| T19/M0 | 2 | 2S1 | RRASSYNEQ | 1/18 | 9 |
| T19/M0 | 2 | 2S1 | IPRFADYEQ | 1/18 | 9 |
| T19/M0 | 2 | 2S2 | LEGTNTGEL | 1/18 | 9 |
| T19/M0 | 2 | 1S2 | PRQVLNYGY | 1/18 | 9 |
| T19/M0 | 2 | 1S1 | RDLKGLNTEA | 4/18 | 10 |
| T19/M0 | 2 | 2S1 | PSFTGRRDGY | 1/18 | 10 |
| T19/M0 | 2 | 2S5 | GAGGPHGETQ | 1/18 | 10 |
| T19/M0 | 2 | 2S5 | GRGGRNGRAQ | 1/18 | 10 |
| T19/M0 | 2 | 1S1 | RDSDRDNTEA | 2/18 | 10 |
| T19/M0 | 2 | 1S1 | NPGDSGYSPLH | 2/18 | 11 |
| T19/M12 | 2 | 1S2 | RTGGYGY | 1/9 | 7 |
| T19/M12 | 2 | 1S2 | SVPGNGY | 1/9 | 7 |
| T19/M12 | 2 | 1S1 | RDWDSEEA | 6/9 | 8 |
| T19/M12 | 2 | 1S1 | RDLKGLNTEA | 1/9 | 10 |
| T19/M20 | 2 | 1S1 | RGGIRA | 1/21 | 6 |
| T19/M20 | 2 | 1S1 | KQGNTEA | 1/21 | 7 |
| T19/M20 | 2 | 1S1 | RDWDSEEA | 11/21 | 8 |
| T19/M20 | 2 | 2S3 | RDLGQASW | 1/21 | 8 |
| T19/M20 | 2 | 1S2 | RKTGGAQN | 1/21 | 8 |
| T19/M20 | 2 | 2S6 | RDTDRANVL | 1/21 | 9 |
| T19/M20 | 2 | 1S5 | RDRQSNQPQ | 1/21 | 9 |
| T19/M20 | 2 | 1S1 | RDPPTASWYN | 1/21 | 10 |
| T19/M20 | 2 | 1S1 | TYRAISNQPQ | 1/21 | 10 |
| T19/M20 | 2 | 2S7 | RDGGFSTDTQ | 1/21 | 10 |
| T19/M20 | 2 | 1S1 | RETGGWGYTEA | 1/21 | 11 |
| T19/M0 | 7 | 2S3 | QDSGSEQ | 2/5 | 7 |
| T19/M0 | 7 | 1S1 | QAGGMRTEA | 3/5 | 9 |
| T19/M1 | 7 | 1S5 | LAAVSYEQ | 1/6 | 8 |
| T19/M1 | 7 | 2S5 | QGRSSYNEQ | 4/6 | 9 |
| T19/M1 | 7 | 1S1 | QAGGMRTEA | 1/6 | 9 |
| T19/M3 | 7 | 2S3 | QVQETQ | 1/8 | 6 |
| T19/M3 | 7 | 1S2 | HLTGAGGY | 1/8 | 8 |
| T19/M3 | 7 | 2S5 | QGRSSYNEQ | 6/8 | 9 |
| T19/M6 | 7 | 2S3 | QGTAINEQ | 1/9 | 8 |
| T19/M6 | 7 | 2S5 | QGRSSYNEQ | 6/9 | 9 |
| T19/M6 | 7 | 2S3 | QLYQFTYEQ | 1/9 | 9 |
| T19/M6 | 7 | 1S5 | QDGTGRRTPQ | 1/9 | 10 |
| T19/M12 | 7 | 2S5 | QGRSSYNEQ | 10/10 | 9 |
| T19/M20 | 7 | 2S5 | QGRSSYNEQ | 5/5 | 9 |
| T19/M0 | 8 | 1S1 | LWNTEA | 1/20 | 6 |
| T19/M0 | 8 | 1S1 | AGPGTEA | 1/20 | 7 |
| T19/M0 | 8 | 2S1 | LPGGSEQ | 1/20 | 7 |
| T19/M0 | 8 | 2S3 | LFWEYEQ | 1/20 | 7 |
| T19/M0 | 8 | 2S3 | LEEVYEQ | 1/20 | 7 |
| T19/M0 | 8 | 2S3 | SGPIYEQ | 1/20 | 7 |
| T19/M0 | 8 | 2S3 | LASGQETQ | 1/20 | 8 |
| T19/M0 | 8 | 2S1 | LTSAYNEQ | 1/20 | 8 |
| T19/M0 | 8 | 2S3 | FGGGADTQ | 1/20 | 8 |
| T19/M0 | 8 | 2S1 | GLLVLGDEQ | 5/20 | 9 |
| T19/M0 | 8 | 2S3 | LGQGTTYEQ | 1/20 | 9 |
| T19/M0 | 8 | 1S3 | RPRGSGNTI | 1/20 | 9 |
| T19/M0 | 8 | 2S3 | PSRDDSNTGEL | 1/20 | 11 |
| T19/M0 | 8 | 1S1 | LEDRDPRNTEA | 2/20 | 11 |
| T19/M0 | 8 | 1S2 | LLIGGQPFNYGY | 1/20 | 12 |
| T19/M20 | 8 | 2S3 | LFWEYEQ | 2/3 | 7 |
| T19/M20 | 8 | 2S1 | TQAGGDTQ | 1/13 | 8 |
| T19/M20 | 8 | 2S1 | LTTSAYEQ | 1/13 | 8 |
| T19/M20 | 8 | 2S1 | LGRGGNEQ | 1/13 | 8 |
| T19/M20 | 8 | 2S1 | GLLVLGDEQ | 4/13 | 9 |
| T19/M20 | 8 | 2S5 | LGQGTTYEQ | 1/13 | 9 |
| T19/M20 | 8 | 1S1 | LEDRDPRNTEA | 2/13 | 11 |
| T19/M20 | 8 | 1S2 | LLIGGQPFNYGY | 1/13 | 12 |
| Sample . | BV . | BJ . | CDR3 . | Frequency . | Length (aa) . |
|---|---|---|---|---|---|
| T19/M0 | 2 | 2S1 | VLRGGGEQ | 1/18 | 8 |
| T19/M0 | 2 | 2S2 | EGGSGNTI | 1/18 | 8 |
| T19/M0 | 2 | 1S1 | VRDRGNTEA | 1/18 | 9 |
| T19/M0 | 2 | 2S1 | RRASSYNEQ | 1/18 | 9 |
| T19/M0 | 2 | 2S1 | IPRFADYEQ | 1/18 | 9 |
| T19/M0 | 2 | 2S2 | LEGTNTGEL | 1/18 | 9 |
| T19/M0 | 2 | 1S2 | PRQVLNYGY | 1/18 | 9 |
| T19/M0 | 2 | 1S1 | RDLKGLNTEA | 4/18 | 10 |
| T19/M0 | 2 | 2S1 | PSFTGRRDGY | 1/18 | 10 |
| T19/M0 | 2 | 2S5 | GAGGPHGETQ | 1/18 | 10 |
| T19/M0 | 2 | 2S5 | GRGGRNGRAQ | 1/18 | 10 |
| T19/M0 | 2 | 1S1 | RDSDRDNTEA | 2/18 | 10 |
| T19/M0 | 2 | 1S1 | NPGDSGYSPLH | 2/18 | 11 |
| T19/M12 | 2 | 1S2 | RTGGYGY | 1/9 | 7 |
| T19/M12 | 2 | 1S2 | SVPGNGY | 1/9 | 7 |
| T19/M12 | 2 | 1S1 | RDWDSEEA | 6/9 | 8 |
| T19/M12 | 2 | 1S1 | RDLKGLNTEA | 1/9 | 10 |
| T19/M20 | 2 | 1S1 | RGGIRA | 1/21 | 6 |
| T19/M20 | 2 | 1S1 | KQGNTEA | 1/21 | 7 |
| T19/M20 | 2 | 1S1 | RDWDSEEA | 11/21 | 8 |
| T19/M20 | 2 | 2S3 | RDLGQASW | 1/21 | 8 |
| T19/M20 | 2 | 1S2 | RKTGGAQN | 1/21 | 8 |
| T19/M20 | 2 | 2S6 | RDTDRANVL | 1/21 | 9 |
| T19/M20 | 2 | 1S5 | RDRQSNQPQ | 1/21 | 9 |
| T19/M20 | 2 | 1S1 | RDPPTASWYN | 1/21 | 10 |
| T19/M20 | 2 | 1S1 | TYRAISNQPQ | 1/21 | 10 |
| T19/M20 | 2 | 2S7 | RDGGFSTDTQ | 1/21 | 10 |
| T19/M20 | 2 | 1S1 | RETGGWGYTEA | 1/21 | 11 |
| T19/M0 | 7 | 2S3 | QDSGSEQ | 2/5 | 7 |
| T19/M0 | 7 | 1S1 | QAGGMRTEA | 3/5 | 9 |
| T19/M1 | 7 | 1S5 | LAAVSYEQ | 1/6 | 8 |
| T19/M1 | 7 | 2S5 | QGRSSYNEQ | 4/6 | 9 |
| T19/M1 | 7 | 1S1 | QAGGMRTEA | 1/6 | 9 |
| T19/M3 | 7 | 2S3 | QVQETQ | 1/8 | 6 |
| T19/M3 | 7 | 1S2 | HLTGAGGY | 1/8 | 8 |
| T19/M3 | 7 | 2S5 | QGRSSYNEQ | 6/8 | 9 |
| T19/M6 | 7 | 2S3 | QGTAINEQ | 1/9 | 8 |
| T19/M6 | 7 | 2S5 | QGRSSYNEQ | 6/9 | 9 |
| T19/M6 | 7 | 2S3 | QLYQFTYEQ | 1/9 | 9 |
| T19/M6 | 7 | 1S5 | QDGTGRRTPQ | 1/9 | 10 |
| T19/M12 | 7 | 2S5 | QGRSSYNEQ | 10/10 | 9 |
| T19/M20 | 7 | 2S5 | QGRSSYNEQ | 5/5 | 9 |
| T19/M0 | 8 | 1S1 | LWNTEA | 1/20 | 6 |
| T19/M0 | 8 | 1S1 | AGPGTEA | 1/20 | 7 |
| T19/M0 | 8 | 2S1 | LPGGSEQ | 1/20 | 7 |
| T19/M0 | 8 | 2S3 | LFWEYEQ | 1/20 | 7 |
| T19/M0 | 8 | 2S3 | LEEVYEQ | 1/20 | 7 |
| T19/M0 | 8 | 2S3 | SGPIYEQ | 1/20 | 7 |
| T19/M0 | 8 | 2S3 | LASGQETQ | 1/20 | 8 |
| T19/M0 | 8 | 2S1 | LTSAYNEQ | 1/20 | 8 |
| T19/M0 | 8 | 2S3 | FGGGADTQ | 1/20 | 8 |
| T19/M0 | 8 | 2S1 | GLLVLGDEQ | 5/20 | 9 |
| T19/M0 | 8 | 2S3 | LGQGTTYEQ | 1/20 | 9 |
| T19/M0 | 8 | 1S3 | RPRGSGNTI | 1/20 | 9 |
| T19/M0 | 8 | 2S3 | PSRDDSNTGEL | 1/20 | 11 |
| T19/M0 | 8 | 1S1 | LEDRDPRNTEA | 2/20 | 11 |
| T19/M0 | 8 | 1S2 | LLIGGQPFNYGY | 1/20 | 12 |
| T19/M20 | 8 | 2S3 | LFWEYEQ | 2/3 | 7 |
| T19/M20 | 8 | 2S1 | TQAGGDTQ | 1/13 | 8 |
| T19/M20 | 8 | 2S1 | LTTSAYEQ | 1/13 | 8 |
| T19/M20 | 8 | 2S1 | LGRGGNEQ | 1/13 | 8 |
| T19/M20 | 8 | 2S1 | GLLVLGDEQ | 4/13 | 9 |
| T19/M20 | 8 | 2S5 | LGQGTTYEQ | 1/13 | 9 |
| T19/M20 | 8 | 1S1 | LEDRDPRNTEA | 2/13 | 11 |
| T19/M20 | 8 | 1S2 | LLIGGQPFNYGY | 1/13 | 12 |
Sequences were deduced after DNA sequencing of the polymerase chain reaction products previously used for Immunoscope analysis (Figure 5). Redundant amino acid sequences, corresponding to identical DNA sequences and, thus, to clonal expansions, are in boldface.
aa indicates amino acid.
By multiplying the fraction of cells in a certain BV family (as measured by FACS) by the fraction of cells of a certain CDR3 length in that BV family (as measured by Immunoscope), we obtained a maximal estimate of the frequency of a certain clone from among all the CD8 cells in the blood. The largest clonal expansion documented in our study (21% of all CD8 cells at M10) was found in patient T13. In that case, a BV2 clone (with a CDR3 loop 10 AA long) persisted for at least 10 months. In 3 other patients (T8, T11, T16), several clones appeared to persist for at least 30 months in the peripheral blood (Figures1, 2).
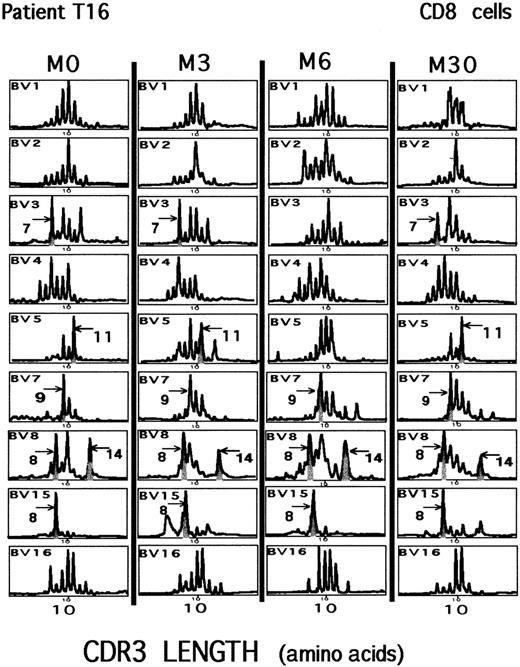
Repertoire analysis in a patient with a partial response to HAART.
CD8+ PBMCs were studied for a 30-month period (M0 to M30) for patient T16. Viral replication was never completely suppressed during that period of analysis. Gray areas represent significant CD8 clonal expansions (see “Patients, materials, and methods”) found at M0 that remained present 30 months later. The CDR3 length of the major size peaks detected is indicated (arrow and number of amino acid residues). Graphs represent the intensity of fluorescence in arbitrary units, as a function of the CDR3 length, in amino acids of BV-BC single-strand DNA runoff products. Windows of analysis are centered on expected sizes corresponding to TCR transcripts encoding a 10-residue-long CDR3 region.
Repertoire analysis in a patient with a partial response to HAART.
CD8+ PBMCs were studied for a 30-month period (M0 to M30) for patient T16. Viral replication was never completely suppressed during that period of analysis. Gray areas represent significant CD8 clonal expansions (see “Patients, materials, and methods”) found at M0 that remained present 30 months later. The CDR3 length of the major size peaks detected is indicated (arrow and number of amino acid residues). Graphs represent the intensity of fluorescence in arbitrary units, as a function of the CDR3 length, in amino acids of BV-BC single-strand DNA runoff products. Windows of analysis are centered on expected sizes corresponding to TCR transcripts encoding a 10-residue-long CDR3 region.
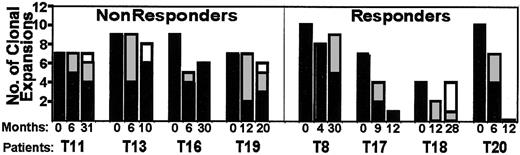
Longitudinal tracking of clonal expansions.
For patients with successful therapy (responders), there was a significant decrease in the number of overrepresented CDR3 peak sizes. Clonal expansions that were stable over the indicated time of follow-up are black. New clones (those not found dominant in previous sample) are gray or white. Note the repertoire shift for patients T18 and T19. A dedicated algorithm was used to track overrepresented areas (compared to control profiles) among all Immunoscope profiles from the study.
Longitudinal tracking of clonal expansions.
For patients with successful therapy (responders), there was a significant decrease in the number of overrepresented CDR3 peak sizes. Clonal expansions that were stable over the indicated time of follow-up are black. New clones (those not found dominant in previous sample) are gray or white. Note the repertoire shift for patients T18 and T19. A dedicated algorithm was used to track overrepresented areas (compared to control profiles) among all Immunoscope profiles from the study.
Down-regulation of clonal expansion after successful and sustained antiviral treatment
If the drastic CD8 repertoire perturbations were directly related to the presence of persistent HIV antigens, one would expect that on removal of the latter, CD8 repertoires would, like CD4 repertoires, return to normal. We were, therefore surprised to observe4 that CD8 repertoires remained biased after 6 months of antiviral treatment in most patients, even in those responding very well to therapy. The same patients were followed up for a longer time period. In 4 patients (T8, T17, T18, and T20), viral replication was efficiently suppressed during at least 3 months to levels below the detection threshold. In all 4 patients, the pretreatment CD8+ repertoires was significantly more perturbed (average, D = 30% ± 5%) than in noninfected controls (average, D = 15% ± 5%; P < .001). However, after at least 12 months of efficient therapy, the calculated average CD8 repertoire perturbation was significantly lower (D = 18% ± 8%;P < .001) than before treatment and comparable to that in HIV− controls. The reduction of repertoire perturbation corresponded to a down-regulation of baseline clonal expansions because only 19% (6 of 31) of the baseline overrepresented peaks were still dominant after 12 to 30 months of evolution in these 4 successfully treated patients (Figure 2). For patient T20 the largest BV subset among CD8 PBMCs was BV5. Immunoscope analysis revealed that BV5 CD8 cells indeed appeared to be dominated by a single clone during the first 6 months of therapy, but the CDR3 length distribution profile obtained at 12 months after therapy was characteristic of a polyclonal subset (Figure 3). This interpretation was confirmed by DNA sequencing of BV5-specific PCR products obtained just before the onset of treatment (M0 = day 1 of first month of treatment) and after 3 or 12 months of treatment (M12 = day 360). Table 3 shows that a single dominant CDR3 sequence (LGGRNSPLH) of CDR3 length 9 was indeed found at day 0 and day 180. In contrast, all CDR3 sequences from M12 were unrelated, and the above sequence was not found anymore. Therefore, during the last 6 months of follow-up, the frequency of the single CD8 T-cell clone, accounting previously for most of the BV5 subset, was dramatically reduced (from 85% of the BV5 subset on day 0 to less than 5.5% a year later).
Repertoire analysis in a good responder to HAART.
CD8+ PBMCs were sampled over a 12-month period of antiviral treatment for patient T20 that resulted in efficient suppression of HIV replication. Sample M0 was taken just before the onset of treatment. Note the normalization of the CD8 repertoire at M12.
Repertoire analysis in a good responder to HAART.
CD8+ PBMCs were sampled over a 12-month period of antiviral treatment for patient T20 that resulted in efficient suppression of HIV replication. Sample M0 was taken just before the onset of treatment. Note the normalization of the CD8 repertoire at M12.
In the remaining 4 nonresponding patients (T11, T13, T16, and T19), viral replication was never efficiently suppressed, and the CD8 repertoire remained significantly more perturbed (D = 29% ± 18%) than in controls (D = 15% ± 5%; P < .001), even during treatment (Figure 2). A large fraction (59%; 19 of 32) of the overexpanded peaks found at baseline in the nonresponding patients persisted after 12 to 30 months, significantly higher than in the responding patients (19%; P < .001). In one of them, patient T19, a transient control of HIV replication after 6 months of therapy was associated with a corresponding transient reduction in repertoire perturbation (Figure 4).
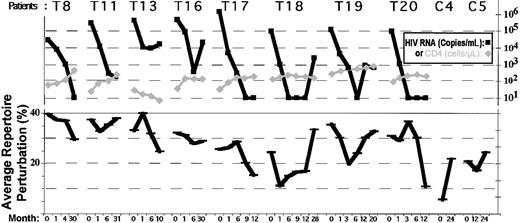
Summary of longitudinal repertoire studies in 8 patients and 2 controls.
Each black bar represents the average CD8 repertoire perturbation calculated from Immunoscope data on 9 BV families, at a given time point. CD4 absolute counts (gray diamonds) and HIV viremia (black squares) are shown on top of repertoire data. Patients T8, T17, T18, and T20 had successful therapy, as defined by an increase in CD4 absolute counts and a decrease of HIV viral load (remaining below detection level at M12). For patients with successful therapy, there was a decrease in average CD8 repertoire perturbation (black bars). Note for patient T19 the transient decrease in perturbation that parallels an initial efficient control of HIV replication. Data for seronegative controls C4 and C5 are also presented.
Summary of longitudinal repertoire studies in 8 patients and 2 controls.
Each black bar represents the average CD8 repertoire perturbation calculated from Immunoscope data on 9 BV families, at a given time point. CD4 absolute counts (gray diamonds) and HIV viremia (black squares) are shown on top of repertoire data. Patients T8, T17, T18, and T20 had successful therapy, as defined by an increase in CD4 absolute counts and a decrease of HIV viral load (remaining below detection level at M12). For patients with successful therapy, there was a decrease in average CD8 repertoire perturbation (black bars). Note for patient T19 the transient decrease in perturbation that parallels an initial efficient control of HIV replication. Data for seronegative controls C4 and C5 are also presented.
Evolution of CD8 repertoires on resumption of HIV replication
Two patients experienced early (T19) or late (T18) resumption of viral replication, probably attributable to withdrawal of the antiprotease in the first (Ritonavir was removed from the therapeutic regimen of T19 because of hepatic side effects) and to poor compliance in the second. Both patients experienced, during their first 6 months of treatment, substantial decreases in HIV viremia and repertoire stabilization. The expansion of clones that were not dominant, or even detectable, at baseline was mostly responsible for the rebound in repertoire perturbation observed. Four and 3 “new” clones are found at M28 in patient T18 and at M20 in patient T19, respectively. However, in patient T19, several baseline expanded clones were still present at M20 (Figure 5).
Repertoire analysis in a patient with viral relapse.
Longitudinal repertoire analysis of CD8+ PBMCs for patient T19. Treatment with the HIV protease inhibitor was interrupted after M6, and a viral rebound occurred. Between M0 and M20, a clear shift in repertoire usage is observed. Gray areas represent CD8 clonal expansions present at the onset of treatment. Black areas represent new dominant clonal expansions (not every overrepresented peak size is shaded; Figure 2 shows the count of the latter).
Repertoire analysis in a patient with viral relapse.
Longitudinal repertoire analysis of CD8+ PBMCs for patient T19. Treatment with the HIV protease inhibitor was interrupted after M6, and a viral rebound occurred. Between M0 and M20, a clear shift in repertoire usage is observed. Gray areas represent CD8 clonal expansions present at the onset of treatment. Black areas represent new dominant clonal expansions (not every overrepresented peak size is shaded; Figure 2 shows the count of the latter).
DNA sequencing confirmed that the repertoire bias was shifted in favor of previously undetected clones that expanded gradually during the time of analysis (eg, BV2, BV7, and BV8 for patient T19; Figure 5). As expected from the Immunoscope profile of BV2 obtained on day 0, TCR transcripts with 30-bp long CDR3 regions (CDR3 loop size 10) were predominantly characterized (8 of 18). Among those, a dominant clone (RDLKGLNTEA) was found (4 of 18). The same BV2 clone was still found 12 months later, but, at a greatly reduced frequency (1 of 9), it was no longer detectable at M20 (Table 4). Instead, a previously undetected TCR clone with an 8-residue-long CDR3 became progressively dominant (6 of 9, then 2 of 21). In the BV7 subset, M3, M6, M12, and M20 CDR3 sequences were completely unrelated to the pretreatment dominant clones (Table 4). Interestingly, the repertoire shift occurred very early because the baseline BV7 clone did not dominate the subset after only 1 month of evolution. A different situation occurred in the BV8 subset, in which the same dominant clones were detected in the 20 months of follow up.
Discussion
We have attempted a global and nevertheless quantitative analysis of CD8 T-cell repertoires in patients with HIV. Immunoscope analysis has revealed, before antiviral treatment, dominant clones in virtually every BV subset analyzed, in all cases studied. A high proportion of individual clonal expansions was remarkably stable for extended periods of time. The disrupted CDR3 length profiles are obviously not the result of multiple clonal deletions but correspond to single clonal expansions among a particular BV subset, as confirmed by DNA sequencing. Indeed, if we assume that the TCR-β chains characterized are pairing with one single TCR-α chain, it is the presence of massive CD8 T-cell clonal expansions (up to 21% of all CD8 cells in one patient) that accounts for the CD8 repertoire perturbations observed in HIV patients.
As reported previously, clonal CD8 T cell expansions can frequently be found in healthy young adults,4,30; however, only a few clones reach detection levels, and they typically become stable with time.30 In contrast, CD8 repertoires of HIV patients are predominantly oligoclonal and are susceptible to extensive reorganization under treatment. In the 4 patients in whom HIV RNA levels were maintained below 200 copies/mL for prolonged periods (T8/M30, T17/M12, T18/M12, T20/M12), CD8 repertoires were significantly stabilized. In one of them (patient T8), a significant level of perturbation was nevertheless maintained, though HIV viremia remained undetectable for at least 20 months. Because the repertoire bias documented in patients with HIV can presumably be modulated by many pathogens other than HIV, such an observation is not surprising. Of note, patient T8 had a recurrence of herpes zoster during the last year of follow-up (see Table 1). In patients T11, T13, and T16, HIV replication was less actively suppressed, indicating poor efficacy of the antivirals or poor patient compliance. No significant reduction in the global levels of CD8 repertoire perturbation developed after, respectively, 31, 10, and 30 months of treatment.
The antigenic specificity of the CD8 clonal expansions we detected before and during treatment is unknown. Nevertheless, the fact that their frequency is dramatically reduced only when HIV replication is efficiently suppressed, whereas insignificant changes in TCR repertoire perturbations occurred when HIV antigens remained expressed, strongly suggests that the latter repertoire perturbations are HIV-related. Indeed, in a longitudinal study of HIV patients receiving HAART, a strong inverse correlation between the in vivo frequency of HIV-specific clones and the levels of HIV viremia was evident.9 Furthermore, tetrameric major histocompatibility complex–peptide complexes were recently used to show that during the asymptomatic stage of the infection, chronically expanded CD8+ T cells found in the circulating blood are HIV-specific.11 In a previous work, 14 we detected, with the use of Immunoscope, oligoclonal subsets of CD8 T cells in the skin of HIV patients, and the corresponding T-cell lines were also shown to be HIV-specific.
In the 2 patients in whom the analysis was performed during resumption of HIV replication after a period of efficient viral control, an expected rebound in repertoire perturbation was observed. However, clones that were not found at baseline most clearly expanded during that period. That situation contrasts with the stability of the repertoire bias observed during chronic infection. In patient T18, we detected 4 significant clonal expansions at baseline and 1 year later, but they were all different. In the peripheral blood of patient T19, several clonal expansions were observed at baseline and at 20 months of follow-up, but only 2 clones remained dominant throughout that period (corresponding to peak sizes 9 and 6 in BV8 and BV15, respectively; Figure 5). DNA sequencing confirmed that a new clone progressively overshadowed a previously dominant one in BV2 and BV7 and that only the baseline BV2 clone could still be detected 1 year later (Table 4). In patient T19, the BV7 clone found expanded after the relapse of viral replication is CD45RO−, CD45RA+, CD62L−, CD57+, and it lacks the expression of the costimulation molecule CD28 (data not shown). These cells fulfill the criteria of cytotoxic T-lymphocyte effector-type cells.31 Of note, the latter clone was already detectable after the first month of treatment, but, as shown in Table 2, it greatly expanded during the last 6 months of follow-up. Additional studies, in particular a concomitant analysis of the viral quasi-species, is required to determine whether the repertoire shift is secondary to the emergence of new dominant HIV-1 variants (possibly originating from sites in which viral replication would be only partially suppressed under HAART). The resumption of viral replication in patients T18 and T19 was clearly secondary to a lack of adherence to a previously active therapeutic regimen. In such instances, it is not surprising to detect the amplification of baseline CD8 clones. The superimposed drift in repertoire bias was less expected and could reflect a response to viral variants, or even to pathogens other than HIV. In any case, our data support the view that persistent antigen stimulation is required for the maintenance of T-cell responses,10 32 a different response being generated upon viral relapse after an extended period of treatment.
In summary, the removal of HIV antigens is associated with a progressive decline in baseline CD8 T-cell expansion. The latter clones and novel CD8 amplifications are detected on HIV reactivation. Taken together, the high prevalence of thymic tissue in adults with HIV-1,33 the observed transient renewal of thymopoiesis in infected thymic implants during HAART,34 the slow peripheral increase in CD4 and CD8 cell counts under treatment,15,35 and the progressive repertoire reorganization we describe during treatment and after relapse show that restoration of the immune system is possible in chronically infected patients, if HIV replication is kept under control. Our results also indicate that the antigenic pressure responsible for repertoire skewing is lifted long before HIV can actually be eradicated from the body.36-38 If HIV-specific cellular surveillance has been significantly alleviated in successfully treated patients, specific boosting of the cellular response may be needed, in association with antiviral therapy, to promote the eradication of residual disease and to reduce the risks for viral relapse on drug removal. Indeed, it was recently suggested that strong virus-specific immune responses could be associated with the rare patient in whom HIV replication remains contained after the discontinuation of HAART.39 It was also proposed that a broad T-cell response would be more efficient at containing HIV spread than a response focused on few HIV epitopes.40 We, therefore, suggest that in future studies, Immunoscope analysis should be coupled to functional tests to further characterize the amplitude and the qualitative nature of the T-cell responses elicited by immunization.
We thank C. Pannetier and P. Kourilsky for providing the Immunoscope software package, V. Calvez for the measurement of some viral loads, and B. Autran, M. Karmochkine, and G. Raguin for providing clinical samples.
Supported by the Fondation pour la Recherche Medicale, Paris (Sidaction), the Agence Nationale de la Recherche contre le Sida (ANRS, Paris), the Bettencourt-Schueller Foundation, the Gonda-Goldschmied Medical Diagnostic Center, the Committee for the Advancement of Research of Bar-Ilan University, and an Arc-en-Ciel French-Israel collaboration grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Guy Gorochov, Laboratoire d'Immunologie Cellulaire, CERVI, CNRS UMR 7627, Hopital de la Pitié-Salpétrière, 83 Bvd de l'Hopital, 75013 Paris, France; e-mail: guy.gorochov@psl.ap-hop-paris.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal