The hierarchy of cytoadhesion molecules involved in hematopoietic/stem progenitor cell mobilization has not yet been delineated. Previous studies have suggested an important role for α4β1 integrin in this process. To test whether mobilization involves dynamic interactions of α4β1 with other integrins on hematopoietic cells, especially the β2 integrins, mice and primates were treated with anti-β1 or anti-β2 antibodies alone or in combination. A single injection of anti-α4β1 antibody elicited reproducible mobilization in contrast to other antibodies, and 3 injections yielded higher mobilization efficiency than each of the other antibodies. When the anti-β2 (anti-CD11a or anti-CD18) or anti-α5/β1 integrin antibody was combined with anti-α4, an augmentation in mobilization was seen that was either additive or synergistic, depending on the potency of the antibody used. Synergy between anti-α4 and anti-CD18 (β2) antibody blockade was seen in primates and confirmed in anti-α4–treated CD18-deficient mice. In the latter, there was a 49-fold increase in mobilization with anti-α4, much higher than in littermate control animals, in CD18 hypomorphic mice, or in other strains of mice tested. Data from both the antibody blockade and gene-targeted mice suggest that the cooperativity of α4β1 with β2 integrins becomes evident when they are concurrently inhibited. It is unclear whether this cooperativity is exerted at the stage of reversible adhesion versus migration, and enhancement of and whether the 2 integrins work in a sequential or parallel manner. Whatever the mechanism, the data provide a novel example of β1 and β2 integrin crosstalk in stem/progenitor cell mobilization.
Introduction
The physiologic egress of mature leukocytes from bone marrow to peripheral blood, as well as the escape of a small number of stem/progenitor cells from the normal bone marrow environment to the circulation, are poorly understood phenomena. The movement of cells from the extravascular spaces of bone marrow to circulation may require a coordinated sequence of reversible adhesion and migration steps. The repertoire of adhesion molecules expressed by stem/progenitor cells or by stromal cells in bone marrow is crucial in this process. Alterations in the adhesion and/or migration of progenitor cells triggered by diverse stimuli would likely result in their dislodgment or redistribution between bone marrow and peripheral blood. It has been shown over the last 3 decades that several empiric in vivo treatments1 can evoke the release of stem/progenitor cells from bone marrow into the circulation, although the mechanisms involved were not uncovered. Because integrins appear to be important regulators of adhesion and migration events in many cellular systems,2 it has been speculated that this family of molecules, which has a broad expression pattern in hemopoietic cells, also plays an important role in the mobilization of stem/progenitor cells. Several in vitro3-5 as well as in vivo6-11 observations support this contention. Specifically, it was shown that in vivo use of function-blocking antibodies against the α4 integrin or its major ligand vascular cell adhesion molecule-1 (VCAM-1) can promote mobilization of progenitor cells in mice8-10,12 or monkeys,11imparting a major role for α4β1 integrins in mobilization.
Because mobilization is a complex, multistep process, it is likely that several classes of cytoadhesion molecules are participating in this process in a sequential and/or overlapping manner, as is the case with mature leukocytes in well-studied models of inflammation.13 In these latter studies the interplay of selectins with β2 and β1 integrins on activated endothelial surfaces is instrumental for the migration of leukocytes to sites of inflammation. Among these molecules, the essential contribution of β2 integrins in polymorphonuclear leukocyte migration is illustrated in patients with a virtual absence of β2 integrins.14However, monocytes, eosinophils, and lymphocytes may also use α4β1 integrin for their migration.15
Unlike mature leukocytes migrating through activated endothelial surfaces, stem/progenitor cells migrate through normal endothelia with a reverse directionality of movement (from tissues to circulation rather than the opposite), and the hierarchy of molecules involved in these movements has not been delineated. Despite these differences, many of the same molecules, especially of the integrin family (because of their wide distribution in hemopoietic cells) may work cooperatively in this process as well. To test the contribution of β2 integrins and their cooperation with β1 integrins in mobilization, we used function-blocking antibodies against β1 and β2 integrins alone or in combination and measured their mobilization efficiency in vivo. In addition to antibody treatments, β2 integrin–targeted mice were used to test the potential cooperation of these 2 integrin families. The data from both approaches suggest that abrogation of β2 integrin function contributes to significant mobilization when α4β1 integrin is concurrently inhibited.
Materials and methods
Mice
Mice used in these experiments were housed under specific pathogen–free conditions in an American Association for the Accreditation of Laboratory Animal Care accredited facility at the University of Washington (Seattle, WA). BDF1 mice, B6.129 mice, and C57Bl6 CD18 hypomorphic mice16 were purchased from the Jackson Laboratory (Bar Harbor, ME). B6.129 CD18 knockout17mice were generated and generously provided by Arthur L. Beaudet, Baylor University (Houston, TX), and were bred in our facility.
Monkeys
Healthy juvenile macaques (Macaca nemestrina andM. fascicularis) of either sex weighing between 5 and 6 kg were employed in our study. They were housed at the University of Washington Regional Primate Center under approved conditions. Blood samples and intravenous injections were done under anesthesia as previously described.11 All animals were provided with food and water ad libitum throughout the study.
Monoclonal antibodies
Directly conjugated antimouse CD49d/α4 (clone PS/2) was purchased from Southern Biotechnology (Birmingham, AL). An unconjugated form of this antibody as well as another anti-α4 antibody (R1/2) both low in endotoxin and free of azide were kindly provided by Roy Lobb (Biogen, Cambridge, MA). Other fluorescence-conjugated antibodies against mouse cell surface epitopes, as well as low-endotoxin, azide-free forms used for injection were all purchased from Pharmingen (San Diego, CA). These included the following: CD11a/LFA-1 (clone M17/4), CD11b/Mac-1 (clone M1/70), CD18/β2 (clones C71/16, M18/2, and GAME-46), CD49e/α5 (clone 5H10-27), and CD117/Kit (clone 2B8). An additional antimouse CD18 (2E6) was purified from hybridoma cells purchased from American Type Culture Collection (Manassas, VA) and was provided by R. Winn (Seattle, WA). For depletion of lineage-committed cells, a cocktail of antibodies was used: GR-1, Mac-1 (CD11b), L3T4 (CD5), B220 (CD45R), and Ter-119, all rat antimouse immunoglobulin G (IgG) purchased from Pharmingen. Magnetic beads labeled with goat antirat IgG from Miltenyi Biotec (Auburn, CA) were used to collect lineage depleted (Lin−) cells according to the manufacturer's protocols.
Monoclonal antibody 60.3 (IgG2a)18 recognizes a functional epitope on the common β chain of the CD11/CD18 complex in man and in primates and was provided by R. Winn and J. Harlan (University of Washington, Seattle, WA). Monoclonal antibody HP1/2 (IgG1) recognizes the α chain of human very late activation antigen (VLA)4 (CD49d) and blocks VLA4-dependent adhesion in vitro.19 A humanized version of this antibody was provided by Roy Lobb (Biogen).8 According to enzyme-linked immunosorbent assays (ELISA) (performed by Dr Lobb), the half-life of this antibody in circulation is quite prolonged, with detectable levels circulating for about 7 days after cessation of treatment (unpublished data,1993).
Fluorescence-activated cell sorter analysis and sorting
Confirmation of genotype in CD18 knockout and CD18 hypomorphic animals was performed on peripheral blood white blood cells (WBCs) stained with anti-CD18 fluorescein isothiocynate (FITC) antibody and analyzed using a FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA). Cell sorting of Lin− samples was performed on a FACSVantage (Becton Dickinson).
Genotyping
Progeny of CD18+/− mice were genotyped at 3 weeks of age by polymerase chain reaction amplification of tail DNA samples. Presence of the wild-type allele was detected by the primers 5′-CTGGACTGTTCTTCCTGGGATC-3′ (forward) and 5′-GTACTTGGTGCATTCCTGGGAC-3′ (reverse), whereas presence of the targeted allele was detected by the primers 5′-CTGGACTGTTCTTCCTGGGATC-3′ (forward) and 5′-CTCGATGCGATGTTTCGGTTGGTG-3′ (reverse). Amplifications were performed on a Stratagene Robocycler (La Jolla, CA) for 35 cycles with an oligonucleotide annealing temperature of 54°C. Of the 123 animals tested in this manner, 16% were CD18−/−, a frequency consistent with previous reports.16 Genotypes of the knockout mice were routinely confirmed by fluorescence-activated cell sorter (FACS) analysis of peripheral blood samples with an anti-CD18 antibody as described above.
Clonogenic progenitor cell assays
Blood from mice was collected in preservative-free heparin. The WBC count was measured with a Coulter (Hialeah, FL) particle counter using a Zap-oglobin II–lysed sample, which was then corrected for heparin volume. The remainder was washed, lysed with hemolytic buffer, washed, and cultured in methylcellulose (MC) as previously described.20 Cells from the femoral bone marrow and spleen were similarly washed and cultured, without lysis. Blood from primates was collected in ethylenediaminetetraacetic acid for complete blood counts or preservative-free heparin for cultures.11Cultures were incubated at 37°C in 5% CO2 and saturating humidity for 7 days (murine cultures) or 14 days (primate cultures), and colonies were counted based on morphologic criteria under a dissecting microscope. Colonies were classified as burst-forming units–erythroid (BFU-E), colony-forming units–granulocyte macrophage (CFU-GM), or CFU–granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM) or were totaled and reported as CFU-culture (CFU-C).
Assays for chemokines and cytokines
Chemokine and cytokine levels in primate plasma were analyzed by the Cytokine Analysis Laboratory, Fred Hutchinson Cancer Center, Seattle, WA.
Statistics
Statistical analysis was performed using Student ttest as found in Microsoft Excel software, using 2-tailed analysis assuming unequal sample variance.
Results
Treatment of mice with anti-integrin antibodies
Groups of at least 5 mice received intravenous treatments with various purified (endotoxin-free) anti-integrin antibodies using the same dose and schedule, ie, 3 injections over 3 days (1 injection daily of 2 mg/kg of body weight, Figure 1). The animals were bled the following day for determining total leukocyte count and for inoculation into clonogenic MC cultures for quantitation of circulating progenitors. Doses of antibodies used were derived from our previous experience with anti-VLA48,9,20 or from data by other investigators.10 Although a single injection of anti-α4 (clone PS/2) induced a significant mobilization, single injections of each of 2 anti-CD18 antibodies (GAME-46 and 2E6) were ineffective (Figure 2 and data not shown). Also, a single injection of anti-CD11a (LFA-1) was previously reported to be ineffective.21 Results with groups of mice injected 3 times with anti-CD11a, anti-CD18 (GAME-46), anti-α5, or anti-α4 are presented in Figure 1. Although differences before and after treatment seen with all tested antibodies were statistically significant (P < .002), anti-α4 treatment, as seen in Figure 1, was superior to the others in mobilization (P < .0001). A different anti-α4 antibody (clone R1/2) in an independent experiment gave results similar to PS/2: pretreatment, 101.7 ± 58.8 CFU-C/mL; posttreatment (2 mice), 375 and 1709 CFU-C/mL. Most CFU-C mobilized by PS/2 were of GM-type (92%), similar to the pattern seen in baseline circulating CFU-C (95% are CFU-GM). However, in these mice, BFU-E increased from 5.3 ± 1.8/mL of blood at baseline to 35 ± 8.2/mL, and CFU-GEMM increased from 2.5 ± 1.0/mL to 19.4 ± 6.2/mL, N = 15-17. The increase of BFU-E in mice after anti-α4 is consistent with preferential BFU-E increase in primates reported earlier.11 During anti-CD11a treatment, there was also a notable increase in circulating BFU-E (from 2.0 ± 0.8 to 52.2 ± 7.7 BFU-E/mL, N = 5 mice). To test whether BFU-E or other progenitors were indeed expressing LFA-1, we sorted Lin−/kit+/LFA-1+ or kit+/LFA-1− cells and inoculated these in clonogenic MC media. The data (Table1) show that although the frequency of progenitors is higher (2-fold) in the Lin−/kit++/LFA-1− fraction, the total number of progenitors present in the kit++/LFA-1+ fraction is higher because more kit++ cells were LFA-1+ than LFA-1−. Of interest, all BFU-E were present in the kit++/LFA-1+ population. In addition to the anti-CD18 antibody used in Figure 1 (GAME-46), a 3-day treatment with another anti-CD18 (M18/2) yielded a smaller increase in CFU-C from 102 ± 15/mL at baseline to 158 ± 60/mL of blood. This antibody was reported to inhibit metastasis22 when used in vivo. However, when tested in vitro,23 it did not inhibit adhesion of LFA-1+ cells to intercellular adhesion molecule (ICAM)-1, thus questioning its mode of action in vivo. In all anti-CD18 treatments, no increase in BFU-E or CFU-GEMM was seen. Collectively, the data from all 3 anti-CD18 treatments would support the concept that if there is an increase in CFU-C, it is likely modest and is not accompanied by a concurrent increase in WBCs, questioning their in vivo effectiveness.
Mobilization by single and combined anti-integrin antibody treatments.
Mice were injected with antibodies at 2 mg/kg of body weight daily for 3 days and bled on the fourth day. Dark columns in the front row show mice treated with anti-CD11a (M17/4), anti-CD18 (GAME-46), and anti-CD49e (5H10-27). Data from these mice were compared with control mice (left, dark column) treated with phosphate-buffered saline plus bovine serum albumin. Lighter columns in the back row show data from combined (with anti-CD49d [PS/2]) antibody treatments compared with mice treated with anti-CD49d alone (left lighter column in back row). Anti-CD18 plus anti-CD49d was not statistically significant (P > .1) in contrast to the other 2 combinations (P < .005). The bars on top of the columns show standard error of the mean.
Mobilization by single and combined anti-integrin antibody treatments.
Mice were injected with antibodies at 2 mg/kg of body weight daily for 3 days and bled on the fourth day. Dark columns in the front row show mice treated with anti-CD11a (M17/4), anti-CD18 (GAME-46), and anti-CD49e (5H10-27). Data from these mice were compared with control mice (left, dark column) treated with phosphate-buffered saline plus bovine serum albumin. Lighter columns in the back row show data from combined (with anti-CD49d [PS/2]) antibody treatments compared with mice treated with anti-CD49d alone (left lighter column in back row). Anti-CD18 plus anti-CD49d was not statistically significant (P > .1) in contrast to the other 2 combinations (P < .005). The bars on top of the columns show standard error of the mean.
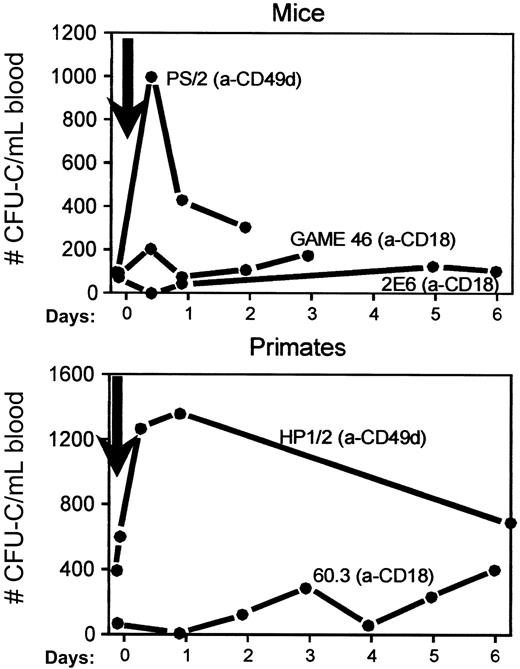
Mobilization kinetics after single anti-integrin antibodies in mice and primates.
Mice were treated with 1 injection alone of anti-α4 (a-CD49d, clone PS/2) or a single injection of 2 different clones of anti-β2 (anti-CD18, clones GAME 46 and 2E6, upper panel). The monkeys were given 1 injection alone of anti-α4 (anti-CD49d, clone HP1/2 humanized) or anti-β2 (anti-CD18, clone 60.3, lower panel).
Mobilization kinetics after single anti-integrin antibodies in mice and primates.
Mice were treated with 1 injection alone of anti-α4 (a-CD49d, clone PS/2) or a single injection of 2 different clones of anti-β2 (anti-CD18, clones GAME 46 and 2E6, upper panel). The monkeys were given 1 injection alone of anti-α4 (anti-CD49d, clone HP1/2 humanized) or anti-β2 (anti-CD18, clone 60.3, lower panel).
Concentration of colony-forming units–culture in bone marrow Lin−/kit++/LFA-1+ and Lin−/kit++/LFA-1− cells
| . | Bone marrow (Lin−) . | |
|---|---|---|
| Lin−/kit++/LFA-1+(17.3%)* . | Lin−/kit++/LFA-1−(6.3%) . | |
| CFU-C, % | 24.7 ± 1.03 | 45.7 ± 5.45 |
| BFU-E, % | 3.2 | 0 |
| Total CFU-C fraction in Lin− cells† | 0.6 | 0.4 |
| . | Bone marrow (Lin−) . | |
|---|---|---|
| Lin−/kit++/LFA-1+(17.3%)* . | Lin−/kit++/LFA-1−(6.3%) . | |
| CFU-C, % | 24.7 ± 1.03 | 45.7 ± 5.45 |
| BFU-E, % | 3.2 | 0 |
| Total CFU-C fraction in Lin− cells† | 0.6 | 0.4 |
CFU-C indicates colony-forming units–culture; BFU-E, burst-forming units–erythroid; FACS, fluorescence-activated cell sorter; MC, methylcellulose.
Bone marrow mononuclear cells were depleted of lineage+cells to derive Lin− populations. These were subsequently labeled with directly conjugated anti-kit and anti–LFA-1 antibodies. Lin−/kit++/LFA-1+ cells (17.3% of Lin− cells) and Lin−/kit++/LFA-1− cells (6.3%) were FACS-sorted and cultured in MC media.
CFU-C fraction was estimated from percentage of CFU-C in each fraction and percentage of total cells in that fraction.
Combined anti-α4 and anti-β2 antibody treatments augment mobilization in mice
To test whether anti-α4 administered in combination with the other antibodies had an additive or synergistic effect on mobilization, mice (at least 5 each) were treated with the same schedule and doses as above but either with or without the anti-α4 (ie, 3 injections, once daily) and bled the day after the third injection. In all treatments with coadministered anti-α4, augmentations in mobilization were seen (Figure 1). Interestingly, the combined anti-α4 and anti-CD11a treatment was highly effective in increasing further BFU-E mobilization from 52.2 ± 7.7 BFU-E/mL with anti-CD11a alone to 292.2 ± 43 BFU-E/mL after the combined treatment (also see Table 2).
Cytokine levels (ng/mL) in monkey plasma before and after anti-CD18 treatment
| Animal no. . | Hours post . | SDF1 . | IL-8 . | MCP-1 . | IL-6 . | G-CSF . | M-CSF . | SCF . | sTNFR1 . |
|---|---|---|---|---|---|---|---|---|---|
| 93232 | 0 | — | 0.05 | 0.42 | — | — | — | 1.20 | 0.45 |
| +24 | — | 0.07 | 1.15 | 0.07 | 0.20 | 0.04 | 1.58 | 0.70 | |
| +72 | — | 0.05 | 0.50 | — | — | — | 1.49 | 0.53 | |
| +144 | — | 0.04 | 0.48 | — | — | — | 1.05 | 0.41 | |
| 97160 | 0 | — | 0.07 | 0.19 | — | — | — | 0.83 | 0.17 |
| +24 | — | 0.13 | 0.59 | 0.29 | 0.33 | — | 0.67 | 0.51 | |
| +48 | 0.23 | 0.11 | — | ||||||
| +72 | 0.40 | 0.33 | 0.32 | 0.25 | — | 0.02 | 0.47 | 0.62 | |
| +168 | — | 0.21 | 0.28 | — | — | — | 0.87 | 0.22 |
| Animal no. . | Hours post . | SDF1 . | IL-8 . | MCP-1 . | IL-6 . | G-CSF . | M-CSF . | SCF . | sTNFR1 . |
|---|---|---|---|---|---|---|---|---|---|
| 93232 | 0 | — | 0.05 | 0.42 | — | — | — | 1.20 | 0.45 |
| +24 | — | 0.07 | 1.15 | 0.07 | 0.20 | 0.04 | 1.58 | 0.70 | |
| +72 | — | 0.05 | 0.50 | — | — | — | 1.49 | 0.53 | |
| +144 | — | 0.04 | 0.48 | — | — | — | 1.05 | 0.41 | |
| 97160 | 0 | — | 0.07 | 0.19 | — | — | — | 0.83 | 0.17 |
| +24 | — | 0.13 | 0.59 | 0.29 | 0.33 | — | 0.67 | 0.51 | |
| +48 | 0.23 | 0.11 | — | ||||||
| +72 | 0.40 | 0.33 | 0.32 | 0.25 | — | 0.02 | 0.47 | 0.62 | |
| +168 | — | 0.21 | 0.28 | — | — | — | 0.87 | 0.22 |
SCF indicates stem cell factor; sTNRF1, soluble tumor necrosis factor receptor-1.
Treatment of monkeys with anti-CD18, either alone or in combination with anti-α4
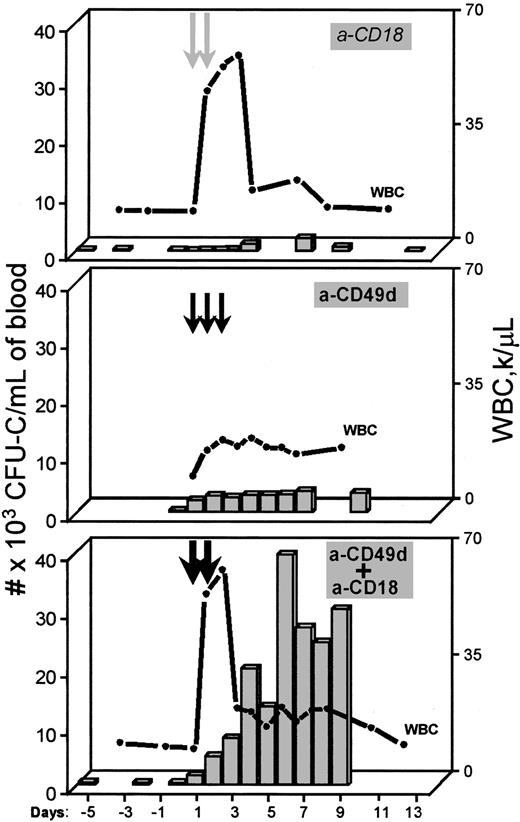
To test whether the results obtained in mice were largely dependent on the antibodies used and whether conclusions drawn from the murine data were applicable to other species, we treated monkeys with antihuman CD18 (clone 60.311,24-26) or antihuman α4 (clone HP1/2) antibodies of known efficacies. Either 1, 2, or 3 injections of anti-CD18 alone were given. Observations before, during, and several days after treatment were made. Treatment of 2 monkeys with either 1 (Figure 2, lower panel) or 2 (Figure3, upper panel) injections of anti-CD18 antibody did not elicit significant mobilization during the first 3 to 4 days despite a prominent increase in WBC count, confirming our earlier observations.11 Assays of blood continued for several days after treatment revealed a significant elevation in mobilized CFU-C between days 5 and 8 posttreatment in the animal given 2 injections. This increase was not as evident after only 1 injection (data not shown). At the time the CFU-C peak was noted, the WBC count had returned to normal and circulating antibody was no longer detectable in these animals, as indicated by specific ELISA performed in R. Winn's laboratory.26 (These 2 monkeys had plasma levels of circulating 60.3 antibody of 0.96 μg/mL and 3.1 μg/mL 24 hours after a single injection.) One of these 2 monkeys was given a second injection of 60.3 at 2 mg/kg (Figure 3, upper panel), which then resulted in a plasma level of 5.0 μg/mL 24 hours later. (The data from another monkey given 3 injections of a-CD18 antibody at 2 mg/kg [data not shown] did not differ significantly from this animal.) When anti-CD18 was combined with anti-VLA4 in another animal (one daily injection at 2 mg/kg each for 2 days), a dramatic synergy was seen with a delayed mobilization peak (Figure 3, lower panel). Because the anti-α4 antibody used in this animal was the humanized version with a long half-life of over 10 days8 27 (unpublished data, 1993), its influence on mobilization was prolonged (Figure 3, middle panel). Whatever the mechanism for the delayed peak observed with anti-CD18, it worked synergistically with the continued inhibition of VLA4 function to augment mobilization.
Mobilization in monkeys with anti-CD18 alone, anti-VLA4 alone, or anti-CD18 in combination with anti-VLA4 at standard doses of 2 mg/kg.
The top panel shows WBCs and CFU-C per milliliter of blood from a monkey treated with 2 injections (arrows) of anti-CD18. The middle panel shows results representing the mean of 3 experiments with monkeys given 3 injections of the humanized anti-α4, and the monkey in the lower panel was given 2 injections of both the anti-β2 and humanized anti-α4 antibodies concurrently (dark arrows).
Mobilization in monkeys with anti-CD18 alone, anti-VLA4 alone, or anti-CD18 in combination with anti-VLA4 at standard doses of 2 mg/kg.
The top panel shows WBCs and CFU-C per milliliter of blood from a monkey treated with 2 injections (arrows) of anti-CD18. The middle panel shows results representing the mean of 3 experiments with monkeys given 3 injections of the humanized anti-α4, and the monkey in the lower panel was given 2 injections of both the anti-β2 and humanized anti-α4 antibodies concurrently (dark arrows).
Cytokine/chemokine levels in monkeys following anti-CD18 treatment
To test whether the delayed peak observed in monkeys after 2 injections of anti-CD18 was due to the potential release of cytokines or chemokines in circulation, we performed ELISAs to test for the presence of interleukin (IL)-8, macrophage inflammatory protein (MIP)-1α, monocyte chemoattractant protein (MCP)-1, IL-1α, and stromal cell–derived factor (SDF)1—chemokines that have been previously implicated in mobilization—or for cytokines like granulocyte colony-stimulating factor (G-CSF), kit ligand, or macrophage colony-stimulating factor (M-CSF). The results are shown in Table 2. No consistent or significant increases were noted from this study to allow firm conclusions.
Treatment of CD18-hypomorphic or CD18-deficient mice
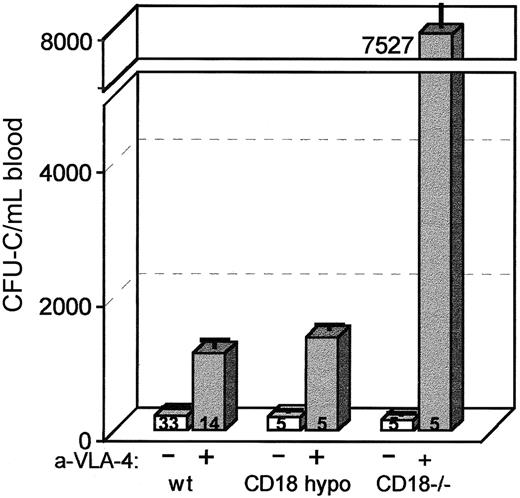
Because antibody treatments in vivo are difficult to interpret and may differ on occasion from results in gene-targeted mice,28 we tested whether synergy in mobilization between β1 and β2 integrins could be seen in CD18-hypomorphic or in CD18−/− mice. CD18 expression in the peripheral blood cells of the CD18-hypomorphic or CD18−/− mice was tested by cell surface staining with anti-CD18 antibody (C71/16). As expected from previous studies, it was approximately 15% in hypomorphic mice,16 whereas no expression of CD18 was seen in white cells of CD18-deficient mice17 (Figure4). The bone marrow cellularity of the latter showed a mild increase with a predominance of myeloid elements (data not shown). Baseline WBC counts were higher, similar to previously reported values in CD18 null mice17 (6 mice: WBC/μL 33 831 ± 3677) compared with CD18 hypomorphic mice (16 animals: WBC 11 480 ± 522). Also, circulating CFU-C were higher in CD18−/− mice (6 mice: 399 ± 24 vs 227 ± 18.9/mL of blood in hypomorphic mice). However, in our antibiotic-treated colony of CD18 null mice, both the WBCs and the CFU-C were lower (5 mice: WBC = 17 050 ± 1261 and CFU-C = 154 ± 47/mL of blood). Treatment of these mice with anti-α4 antibody elicited a significant mobilization that was several-fold higher (Figure 5) than that observed in control mice. Treatment of 14 B6.129+/+ mice with anti-α4 alone elicits an increase of an average of 5-fold above baseline (range 3- to 12-fold). In 5 anti-α4–treated CD18 hypomorphic mice, the increase was about 7-fold (201 ± 34 to 1387 ± 107 CFU-C/mL of blood), not significantly different from controls. However, in 5 similarly treated CD18−/− mice, CFU-C increased 49-fold (from 154 ± 47 to 7527 ± 2411 CFU-C/mL [Figure 5]; WBC: from 17 050 ± 1261 to 74 694 ± 11 003). This difference was unlike any increase in other strains of mice that we have treated thus far (25 mice of 6 different strains gave an average of 5.4-fold increase with a range of 3.2- to 12-fold). To test whether the synergy in mobilization was delayed or extended in CD18−/− mice treated with anti-α4 (as observed in primates), a single injection of anti-α4 was given with assessment of circulating CFU-C daily. Anti-α4 alone given to +/+ littermates showed a 12- to 24-hour mobilization peak (Figure 2). By contrast, mobilization in CD18−/− mice after a single anti-α4 injection was maintained at peak levels over the first 3 days postinjection (2 animals, data not shown). Collectively, the data in gene-targeted mice and those with antibody blockade are consistent with the cooperativity between β1 and β2 integrins and suggest that both β1 and β2 integrins are likely responsible for progenitor anchoring within the bone marrow in vivo and that abrogation of their function leads to enhanced migration out of the bone marrow.
Peripheral blood WBCs from CD18 hypomorphic mice, CD18 knockout mice, and wild-type mice stained with anti-CD18 FITC.
Staining of wild-type mouse WBCs is shown with dashed lines (92% positive), hypomorphic mouse WBCs by dotted lines (14.65% positive), and knockout mouse WBCs by the bold line (< 1% positive). FITC isotype control WBCs are shown in the gray shaded histogram.
Peripheral blood WBCs from CD18 hypomorphic mice, CD18 knockout mice, and wild-type mice stained with anti-CD18 FITC.
Staining of wild-type mouse WBCs is shown with dashed lines (92% positive), hypomorphic mouse WBCs by dotted lines (14.65% positive), and knockout mouse WBCs by the bold line (< 1% positive). FITC isotype control WBCs are shown in the gray shaded histogram.
Mobilization in CD18 hypomorphic, CD18 knockout mice, and wild-type control mice treated with anti-CD49d (VLA4).
Mice were given 3 daily injections of antibody (PS/2) at 2 mg/kg per day and bled on the fourth day. Anti-α4 treatment of CD18 hypomorphic mice increased the circulating CFU-C level about 7-fold above baseline, similar to wild-type controls (about 5-fold), whereas the same treatment of CD18 knockout mice raised the CFU-C per milliliter of blood about 49-fold above baseline.
Mobilization in CD18 hypomorphic, CD18 knockout mice, and wild-type control mice treated with anti-CD49d (VLA4).
Mice were given 3 daily injections of antibody (PS/2) at 2 mg/kg per day and bled on the fourth day. Anti-α4 treatment of CD18 hypomorphic mice increased the circulating CFU-C level about 7-fold above baseline, similar to wild-type controls (about 5-fold), whereas the same treatment of CD18 knockout mice raised the CFU-C per milliliter of blood about 49-fold above baseline.
Discussion
In the present study, we have documented that, in addition to α4β1 integrin,11 the functional inhibition of β2 integrins plays a role in mobilization. Importantly, the main impact of β2 integrin's contribution in mobilization becomes evident only when α4β1 is concurrently inhibited. The fact that single antibody treatments against β2 integrins were without effect may indicate an overlapping or a redundant function. When a dominant molecule like α4 is inhibited at the same time, the β2 integrin function is unmasked. Alternatively, the abrogation of both β1 and β2 integrin functions may have an impact on additional molecules affecting the migration of stem/progenitor cells or may differentially affect cells with different motilities.
Although a synergistic effect in mobilization was seen with inhibition of both β1 and β2 integrins in our experiments, it is unclear at what stage this cooperativity was exerted: at the stage of reversible adhesion or by the enhancement of migration. The contribution of both of these integrins to events downstream of selectin influence, ie, to firm adhesion and migration of mature leukocytes to inflammatory tissues, is well known.29 However, the molecular mechanics underlying the migration of hemopoietic stem/progenitor cells out of bone marrow have not been elucidated and may involve kinetic differences in integrin usage as compared with more mature cells. For mobilization to occur, the stem/progenitor cells need first to be released from the extravascular bone marrow space (extracellular matrix and/or stromal cells), to migrate across sinusoidal endothelia and, finally, to be released from the luminal surface of endothelial cells. During these steps, β1 and β2 integrins may act in sequence or in parallel. Because VCAM-1 is expressed both by endothelial cells30 and also by stromal/reticular cells in bone marrow,30 its interaction with α4β1 within the bone marrow matrix could be an early event in migration with a contribution of the β2 integrins later. Alternatively, CD11a or CD18/ICAM-1 interactions could complement the effect of α4β1 both in early and late events in mobilization. For example, inhibition of α4β1 may decrease the strength of β2-dependent adhesion and potentially increase the rate of cell migration.31
Are stem/progenitor cells the targets of the combined anti-β1– and anti-β2–induced mobilization? Previous experience with in vivo anti–LFA-1 antibody treatments in mice suggested that its role in inhibiting IL-8–induced mobilization was only indirect, acting on neutrophils and not on progenitor cells, most of which do not express LFA-1 (CD11a).32 Therefore, we repeated these experiments and tested the expression of LFA-1 in lineage−/kit+ bone marrow cells. As the data in Table 1 indicate, all BFU-E and a proportion of other progenitors were present in LFA-1+ fractions; this finding may be relevant to the increase in BFU-E mobilization seen with anti–LFA-1 and supports a direct rather than indirect effect on these progenitors. Furthermore, because CD18 is present in a significant proportion of adult Lin−/kit++ progenitor cells,33 our data do not exclude the possibility that some CFU-C are direct targets of anti-CD18 treatment.
Studies in monkeys were particularly instructive. The synergistic effect of β1 and β2 integrins using the combination of antibodies was exaggerated in this model as compared with mice. This could be the result of differences in the antibodies used as judged by their ability to increase WBCs in vivo (in mice, no increase in WBCs was seen). Furthermore, although no changes in CFU-C were seen in the first 3 or 4 days with anti-β2 antibody treatments (Figure 3), confirming our earlier observations,11 there was a small mobilization peak after day 5, and it was this peak that was enhanced by the combination of anti-α4 and anti-β2 treatments. When levels of circulating antibody were measured, it became evident that the effects on mature cells (WBCs) were dissociated from the effects on progenitor cells; the former were totally dependent on the presence of anti-CD18, but the peak in progenitors was during the time that no circulating antibody could be detected. The mechanism of this delayed peak in monkeys remains obscure.
Because of the difficulties in pinpointing the in vivo effects of antibodies,28 we sought to complement our data using mice deficient in CD18 integrins. If abrogation of CD18 function is important for the synergy with anti-α4, it should become evident in these mice. CD18 hypomorphic mice16 with significant reduction of CD18 expression (Figure 4) and mice with total ablation of CD18 expression were used.17 The CD18−/−mice used in the present experiments exhibited only a moderate increase in WBCs and no increased circulating levels of CFU-C. In these particular mice, there was an exaggerated response in the mobilizing effect of anti-VLA4 (49-fold compared to about 5-fold in +/+ mice). It is unlikely that this response is due to synergy of anti-VLA4 treatment with cytokines (like IL-6 or IL-3) found to be increased in CD18−/− mice,17 because it is outside the expected range with concurrent administration of anti-VLA4 and cytokines.8 Furthermore, because the same synergy was observed with the combined antibody treatment in primates without any increment in circulating chemokines/cytokines (Table 2), we attribute the synergy to the abrogation of both CD49d and CD18 function.
Several examples on the cooperativity between β1 and β2 integrins in vivo can be cited from the literature. The unexpected role of the α4β1/VCAM-1 pathway in homing to peripheral lymph node, but not to the spleen, was uncovered only in LFA-1–deficient mice.32These data suggest that alternative mechanisms can be employed in the absence of one dominant molecule (LFA-1 in this case) and only uncovered by specific testing. Likewise, when anti-α4 and anti–LFA-1 monoclonal antibodies were coinjected into host BALB/C mice, entry of lymphocytes into peripheral lymph nodes was totally prevented. Of interest, the combination of anti-LFA-1 and α4 monoclonal antibodies also completely abolished lymphocyte trafficking through bone marrow, whereas LFA-1+/+ and LFA-1−/− cells did not differ in their trafficking through the bone marrow.34 Interaction with a constitutively expressed VCAM-1 in both bone marrow endothelial cells30 and in high endothelial venules34 could explain these events. Other examples of cooperation between β2 (CD18) and α4β1 integrins can be cited, including the migration of neutrophils to the inflamed peritoneum in rabbits, where the very same antibodies (HP1/2 and 60.3) used in our monkey studies were employed.24 In addition, VLA4 engagement can induce or enhance the β2 integrin–dependent adhesion on endothelial cells35 in vitro. In other studies, both anti-VLA4 and LFA-1 were required for maximal in vivo blocking in a rat model of skin delayed-type hypersensitivity.36Notably, however, these 2 integrins do not always work in cooperation. The selective release of eosinophils from bone marrow is regulated by β2 and α4β1 integrins in opposite directions, because anti-β2 antibody decreased eosinophil mobilization induced by IL-5, whereas anti-α4 antibody increased mobilization by IL-5.37 Also, the ligation of LFA-1 (CD11a) integrin in T cells may lead to a decrease of α4β1 or α5β1 function.38 The above-mentioned examples are all illustrative of β1 and β2 integrin crosstalk, but details of how this crosstalk is achieved in all cases, including ours, are lacking.
We kindly thank Arthur L. Beaudet for providing the CD18 knockout mice and Margaret Oppenheimer for her expert secretarial assistance.
Supported by National Institutes of Health Grants AI32177, DK46557, and RR00166.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thalia Papayannopoulou, Division of Hematology, University of Washington, Box 357710, Seattle, WA 98195-7710; e-mail: thalp@u.washington.edu.

![Fig. 1. Mobilization by single and combined anti-integrin antibody treatments. / Mice were injected with antibodies at 2 mg/kg of body weight daily for 3 days and bled on the fourth day. Dark columns in the front row show mice treated with anti-CD11a (M17/4), anti-CD18 (GAME-46), and anti-CD49e (5H10-27). Data from these mice were compared with control mice (left, dark column) treated with phosphate-buffered saline plus bovine serum albumin. Lighter columns in the back row show data from combined (with anti-CD49d [PS/2]) antibody treatments compared with mice treated with anti-CD49d alone (left lighter column in back row). Anti-CD18 plus anti-CD49d was not statistically significant (P > .1) in contrast to the other 2 combinations (P < .005). The bars on top of the columns show standard error of the mean.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/5/10.1182_blood.v97.5.1282/6/m_h80510753001.jpeg?Expires=1769084255&Signature=1aiXYJ6LrBLCHijBE-Tw6Rx8kJVu3DggsHeAuieyQM1Ge39deAMFzy3RoKbWxg5h~2vuZOh0tUMPT~7Jaqzdij~FtsuppF5BSn~VMBHynfJPX~Ciw6JzR-QbN7fjhMQQB~BU2iscFMYIBI43lLw2bVWdLK~3Sv3D5TxyKVED5M2C8ZoYXIATBm3EmMWs1Xrye0yBPgHrLQuB9KONIt5sUeNsDorixoNi5FcxubEJAf4aEZydr7aFiMpq11s4tVpU-K6rLDaPbI~avEPc0qcdc3KGWUBr-ktqFQCeSPbzqhAnNMs5gVe4lmPT4PTVdE4avneB8uBXVyCZg1h9PKCELQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal