Abstract
Recent reports have suggested an association betweenHelicobacter pylori infection and idiopathic thrombocytopenic purpura (ITP). The prevalence of H pyloriinfection and the effect of its eradication in a series of 30 ITP patients were investigated. H pylori infection has been documented in 13 patients (43.33%) by 13C urea breath test and confirmed by histologic examination. Bacterium eradication with antibiotics, obtained in 12 of 13 infected patients (92.3%), led to a complete response in 4 (33.33%) and to a partial response (platelets 90 × 109/L-120 × 109/L) in 2 (16.66%). The response was maintained for a median of 8.33 months, but 1 patient relapsed 7 months after eradication. Search for H pyloriinfection seems appropriate in ITP patients at diagnosis. Bacterium eradication provides a new good option for a nonimmunosuppressive treatment in some ITP patients.
Introduction
Helicobacter pylori has been clearly recognized as the main cause of gastritis and most cases of peptic ulcer,1 and it has also been implicated in the pathogenesis of gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma.2 It has been hypothesized that the host immunologic response against H pylori plays a main role in determining gastric mucosal injury, through the release of cytokines and the action of autoantibodies against H+/K+-adenosine triphosphatase of gastric epithelial cells.3,4 Moreover, there is increasing evidence that H pylori strains are highly diverse and that strain diversity associated with variability in host immune response may contribute to the clinical outcome in infected patients.5 In the last few years, H pylori has been implicated in the pathogenesis of some autoimmune diseases, such as rheumatoid arthritis,6 autoimmune thyroid diseases,7 and idiopathic thrombocytopenic purpura (ITP).8 Relevant to this, Gasbarrini et al8recently showed a high prevalence of H pylori infection in patients with ITP and reported a good response to bacterium eradication in most of them. This finding has been confirmed in a further anecdotal ITP case.9 Thus, we performed a prospective open study to verify the prevalence of H pylori infection and the effects of its eradication in a larger proportion of patients with chronic ITP.
Study design
Thirty patients with ITP were evaluated, including 13 males and 17 females; median age was 50.3 years (range 17-89). ITP was defined by idiopathic thrombocytopenia (platelets < 100 × 109/L) when other causes had been excluded, megakaryocytic hyperplasia in the bone marrow, shortened platelet survival (measured using 111In-labeled platelets), and increased autoantibodies against platelets (platelet-associated immunoglobulin G [PAIgG]) in the serum.
H pylori infection was assessed by 13C urea breath test (Helicobacter test, Infai, Bochum, Germany), by the detection of serum antibodies (indirect immunofluorescence) and, whenever possible, by histologic examination (Giemsa stain) of specimens obtained by an upper gastrointestinal endoscopy.
The presence of serum antibodies against hepatitis C virus was also evaluated by the enzyme-linked immunosorbent assay and confirmed by the recombinant-based immunoblot assay. All immunosuppressive treatments were withdrawn 1 month before eradication except in 1 patient.H pylori eradication was performed with amoxicillin (1000 mg twice daily), clarithromycin (250 mg 3 times daily), and pantoprazole (40 mg twice daily) for 7 days. Eradication was assessed by urea breath test 4 weeks after treatment withdrawal. Platelet counts were monitored every 2 weeks and assessed at 3 and 6 months, after the end of treatment. In statistical analysis, data were expressed as the mean (SD) or median (range) as appropriate and were analyzed by using the t test. Percentages were compared by χ2 test (Fisher exact test for values ≤ 5). AP value < .05 was considered statistically significant.
Approval for this study was obtained from the Institutional Review Board of the University of Modena, and informed consent was provided according to the Declaration of Helsinki.
Results and discussion
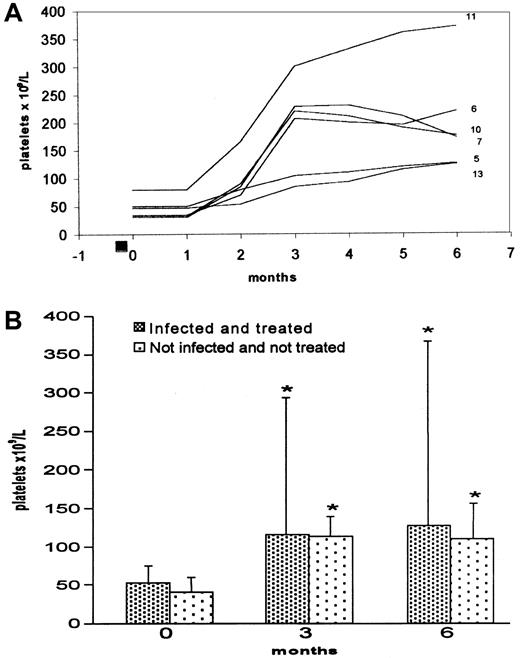
H pylori infection was found in 13 of 30 ITP patients (43.3%) by 13C urea breath test. A prevalence of infected males was noted (8 infected vs 5 uninfected; 5 infected females vs 12 uninfected); median age was higher in infected patients (63.2 [SD 14.6] vs 47 [SD 20.8] years, P < .02). Platelet count was similar in infected and uninfected patients (53.1 × 109/L [SD 28.5] vs 41.7 × 109/L [SD 14.8], P < .16). Eight of 13 H pylori–infected patients showed, at diagnosis, a severe form of ITP (platelets < 30 × 109/L) and needed immunosuppressive treatment (one also needed splenectomy) before eradication. All patients showed a shortened platelet survival (2-4 days vs 8-9 days, as normal value), except for the patients no. 9 and 21 (Table 1). In 10 patients, the diagnosis of H pylori infection was also confirmed by histologic examination, while in 9 the presence of serum antibodies against the bacterium was also detected. No patient was found to be positive for serum antibodies against hepatitis C virus. The bacterium eradication was obtained in 12 of 13 H pylori–positive patients (92.3%). The follow-up, performed for a median of 8.33 months (range 6-12), showed that 6 of the 12 eradicated patients (50%) had a significant increase in platelet count after 3 and 6 months (P < .007) (Table 1); 4 patients (33.3%) achieved a complete response (CR) (P < .03), while 2 patients showed a partial response (platelet count not higher than 120 × 109/L) (P < .16) (Figure1A). The outcome over time of platelet counts of H pylori–infected and treated patients compared with noninfected patients looked similar (Figure 1B). Four of 30 ITP patients were PAIgG-positive; 2 of these were infected withH pylori. Among the responsive patients, 2 who achieved CR were PAIgG-positive, while the remaining 4 were PAIgG-negative. The only patient in whom H pylori eradication failed (no. 1) has been unresponsive. Only 1 patient (no. 7), with a platelet count of 25 × 109/L, required steroid treatment for 1 month after eradication; subsequently, the platelet count increased and maintained in normal range without therapy. In 5 of the 6 responsive patients followed for more than 6 months, the platelet count did not change after H pylori eradication. In 1 patient (no. 10), the ITP relapsed 7 months after eradication, although the breath test remained negative.
Clinical and laboratory characteristics in 30 idiopathic thrombocytopenic purpura patients; H pylori eradication in 12
| Patient, age, sex . | Disease duration, mo . | Previous treatment; duration, mo . | H pylori infection . | Platelets × 109/L . | 13C urea breath test (delta) . | ||
|---|---|---|---|---|---|---|---|
| Before . | After (at 6 mo) . | Before . | After . | ||||
| 1. CM, 52, m | 16 | None | Yes | 80 | 67* | 11.20 | 14.20 |
| 2. DR, 58, f | 15 | None | Yes | 80 | 111 | 12.40 | 1.20 |
| 3. BG, 63, m | 14 | None | Yes | 52 | 68 | 17.60 | 0.68 |
| 4. MC, 60, f | 16 | P, 16 | Yes | 30 | 31 | 17.30 | 1.14 |
| 5. MA, 67, m | 46 | None | Yes | 50 | 126† | 21.60 | 0.88 |
| 6. GR, 79, f | 70 | P, 63; V, 2 | Yes | 33 | 220† | 19.20 | 2.50 |
| 7. ME, 60, m | 15 | P, 15 | Yes | 25 | 176† | 20.30 | 1.90 |
| 8. IC, 61, m | 39 | P, 25 | Yes | 80 | 76 | 25.57 | 0.48 |
| 9. RV, 75, m | 20 | None | Yes | 80 | 84 | 10.57 | 1.13 |
| 10. GL, 77, f | 21 | P, 2 | Yes | 27 | 172† | 18.50 | 3.10 |
| 11. DR, 33, m | 35 | P, 15; Sple | Yes | 80 | 371† | 16.20 | 1.80 |
| 12. LD, 89, m | 13 | P, 12 | Yes | 15 | 35 | 18.70 | 2.10 |
| 13. MA, 48, f | 53 | P, 40 | Yes | 50 | 125† | 22.60 | 0.95 |
| 14. BC, 70, f | 110 | Ig, 6; Cy, 24 | No | 37 | 80 | 0.80 | |
| 15. SP, 79, m | 65 | P, 3 | No | 41 | 130 | 1.13 | |
| 16. SA, 36, m | 52 | P, 6; V, 4 | No | 30 | 110 | 2.70 | |
| 17. BJ, 19, f | 19 | None | No | 62 | 58 | 3.10 | |
| 18. BM, 18, f | 18 | None | No | 58 | 70 | 0.38 | |
| 19. CL, 20, f | 29 | P, 1 | No | 38 | 120 | 1.10 | |
| 20. CD, 53, f | 64 | P, 2; Ig, 2; C, 3 | No | 35 | 80 | 0.90 | |
| 21. RA, 55, f | 84 | P, 2; C, 2; V, 2 | No | 32 | 90 | 1.80 | |
| 22. GA, 39, f | 34 | P, 1 | No | 43 | 100 | 2.10 | |
| 23. PM, 26, f | 15 | P, 6 | No | 65 | 150 | 0.76 | |
| 24. BG, 64, m | 79 | Ig, 2; P, 4 | No | 45 | 130 | 2.30 | |
| 25. NR, 64, m | 31 | P, 2; C, 4; Sple | No | 2 | 120 | 1.83 | |
| 26. SL, 66, m | 23 | P, 22 | No | 40 | 140 | 1.26 | |
| 27. VR, 49, f | 32 | Ig, 1; Sple | No | 35 | 130 | 0.89 | |
| 28. TA, 26, f | 21 | P, 20 | No | 40 | 140 | 0.55 | |
| 29. BD, 35, f | 18 | P, 17 | No | 42 | 130 | 0.80 | |
| 30. DV, 68, f | 120 | P, 24; Sple; Cy, 13 | No | 40 | 115 | 1.45 | |
| Patient, age, sex . | Disease duration, mo . | Previous treatment; duration, mo . | H pylori infection . | Platelets × 109/L . | 13C urea breath test (delta) . | ||
|---|---|---|---|---|---|---|---|
| Before . | After (at 6 mo) . | Before . | After . | ||||
| 1. CM, 52, m | 16 | None | Yes | 80 | 67* | 11.20 | 14.20 |
| 2. DR, 58, f | 15 | None | Yes | 80 | 111 | 12.40 | 1.20 |
| 3. BG, 63, m | 14 | None | Yes | 52 | 68 | 17.60 | 0.68 |
| 4. MC, 60, f | 16 | P, 16 | Yes | 30 | 31 | 17.30 | 1.14 |
| 5. MA, 67, m | 46 | None | Yes | 50 | 126† | 21.60 | 0.88 |
| 6. GR, 79, f | 70 | P, 63; V, 2 | Yes | 33 | 220† | 19.20 | 2.50 |
| 7. ME, 60, m | 15 | P, 15 | Yes | 25 | 176† | 20.30 | 1.90 |
| 8. IC, 61, m | 39 | P, 25 | Yes | 80 | 76 | 25.57 | 0.48 |
| 9. RV, 75, m | 20 | None | Yes | 80 | 84 | 10.57 | 1.13 |
| 10. GL, 77, f | 21 | P, 2 | Yes | 27 | 172† | 18.50 | 3.10 |
| 11. DR, 33, m | 35 | P, 15; Sple | Yes | 80 | 371† | 16.20 | 1.80 |
| 12. LD, 89, m | 13 | P, 12 | Yes | 15 | 35 | 18.70 | 2.10 |
| 13. MA, 48, f | 53 | P, 40 | Yes | 50 | 125† | 22.60 | 0.95 |
| 14. BC, 70, f | 110 | Ig, 6; Cy, 24 | No | 37 | 80 | 0.80 | |
| 15. SP, 79, m | 65 | P, 3 | No | 41 | 130 | 1.13 | |
| 16. SA, 36, m | 52 | P, 6; V, 4 | No | 30 | 110 | 2.70 | |
| 17. BJ, 19, f | 19 | None | No | 62 | 58 | 3.10 | |
| 18. BM, 18, f | 18 | None | No | 58 | 70 | 0.38 | |
| 19. CL, 20, f | 29 | P, 1 | No | 38 | 120 | 1.10 | |
| 20. CD, 53, f | 64 | P, 2; Ig, 2; C, 3 | No | 35 | 80 | 0.90 | |
| 21. RA, 55, f | 84 | P, 2; C, 2; V, 2 | No | 32 | 90 | 1.80 | |
| 22. GA, 39, f | 34 | P, 1 | No | 43 | 100 | 2.10 | |
| 23. PM, 26, f | 15 | P, 6 | No | 65 | 150 | 0.76 | |
| 24. BG, 64, m | 79 | Ig, 2; P, 4 | No | 45 | 130 | 2.30 | |
| 25. NR, 64, m | 31 | P, 2; C, 4; Sple | No | 2 | 120 | 1.83 | |
| 26. SL, 66, m | 23 | P, 22 | No | 40 | 140 | 1.26 | |
| 27. VR, 49, f | 32 | Ig, 1; Sple | No | 35 | 130 | 0.89 | |
| 28. TA, 26, f | 21 | P, 20 | No | 40 | 140 | 0.55 | |
| 29. BD, 35, f | 18 | P, 17 | No | 42 | 130 | 0.80 | |
| 30. DV, 68, f | 120 | P, 24; Sple; Cy, 13 | No | 40 | 115 | 1.45 | |
For the uninfected patients, platelet values before and after immunosuppressive treatment. For the infected patients, immunosuppression withdrawal 1 month before H pylorieradication treatment. Delta represents the difference between the13C value on breath at 0 minutes and at 30 minutes; positive = greater than 5. Prior immunosuppressive treatments were successful, except in patients No. 4, 6, 7, 10, and 12. No other treatments were performed in the intervening time, since H pylori eradication.
P indicates prednisone; C, cyclophosphamide; V, vincristine; Cy, cyclosporin A; Ig, intravenous immunoglobulin; Sple, splenectomy.
H pylori eradication failed.
Eradication-responsive patients.
The course of platelet counts over time.
(A) Platelet counts in 6 responsive patients. Each line represents values from a single patient identified by number (Table 1). ■ = H pylori eradication treatment (7 days). (B) Platelet counts in all 30 ITP patients. Most uninfected patients are under immunosuppressive therapy. Data are mean (SD). *P < .012 compared with initial count.
The course of platelet counts over time.
(A) Platelet counts in 6 responsive patients. Each line represents values from a single patient identified by number (Table 1). ■ = H pylori eradication treatment (7 days). (B) Platelet counts in all 30 ITP patients. Most uninfected patients are under immunosuppressive therapy. Data are mean (SD). *P < .012 compared with initial count.
Our data lend further support to a relationship betweenH pylori infection and ITP. The bacterium might induce, at least in some cases, the formation of platelet autoantibodies by means of a chronic immunologic stimulus or a cross mimicry between platelets and itself.8,9 In respect to the only nonanecdotal report of the literature by Gasbarrini et al,8 we observed, in our series of ITP patients, a lower prevalence of H pyloriinfection (43.33% vs 61.11%, P < .37) and a lower frequency of platelet response to the same eradication treatment (46.14% vs 72.72%, P < .40). Moreover, only 33.33% of our patients achieved a CR, versus 100% of eradicated patients in the above-mentioned report (P < .27), even though eradication has been obtained in a similar percentage of patients (92.3% vs 100%). We followed our patients for a median of 8.33 months—versus a maximum of 4 months in the study by Gasbarrini et al.8This allowed us to document a relapse of ITP 7 months after H pylori eradication in 1 PAIgG-negative patient. Finally, variety in the frequency of H pylori–associated ITP and in the rate of platelet response to bacterium eradication may be related either to the variability of host immune response to H pylori or to the bacterium's high genetic diversity, ie, to the existence of different H pylori strains with possibly different pathogenic potential.5
In conclusion, even though the pathogenetic mechanisms of H pylori–induced thrombocytopenia remain obscure, the search forH pylori infection and an attempt to eradicate bacterium in positive cases seem appropriate in ITP patients at diagnosis. This approach may be a new good option for a nonimmunosuppressive treatment, at least in some ITP patients. Further investigations on a larger number of patients, with a long follow-up, might allow a better definition of the true prevalence of H pylori infection and the duration of the effect of its eradication in ITP patients.
Supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy (M.L.) and Associazione Italiana contro le Leucemie (AIL), section of Modena, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giovanni Emilia, Department of Medical Sciences, Section of Internal Medicine, Policlinico, via del Pozzo 71, 41100 Modena, Italy; e-mail: emilia.giovanni@unimo.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal