Leukocyte capture and rolling are mediated by selectins expressed on leukocytes (L-selectin) and the vascular endothelium (P- and E-selectin). To investigate the role of core 2 β1-6-N-glucosaminyltransferase (C2GlcNAcT-I) for synthesis of functional selectin ligands in vivo, leukocyte rolling flux and velocity were studied in venules of untreated and tumor necrosis factor-α (TNFα)–pretreated autoperfused cremaster muscles of C2GlcNAcT-I–deficient (core 2−/−) and littermate control mice. In untreated core 2−/− mice, leukocyte rolling was dramatically reduced with markedly increased rolling velocities (81 ± 4 μm/s vs 44 ± 3 μm/s). The reduced rolling in core 2−/− mice was due mainly to severely impaired binding of P-selectin to P-selectin glycoprotein ligand-1 (PSGL-1). Some rolling remained after blocking PSGL-1 in controls but not in core 2−/− mice. In TNFα-pretreated mice, rolling was markedly reduced in core 2−/− mice owing to impaired P-selectin– and E-selectin–mediated rolling. Rolling velocities in core 2−/− mice treated with an E-selectin–blocking monoclonal antibody (59 ± 4 μm/s) were significantly higher than in controls (14 ± 1 μm/s), which provides further evidence for the severe impairment in P-selectin–mediated rolling. In conclusion, P-selectin ligands including PSGL-1 are largely C2GlcNAcT-I dependent. In addition, E-selectin–mediated rolling in vivo is partially dependent on the targeted C2GlcNAcT-I.
Introduction
Leukocyte recruitment to sites of inflammation requires a multistep adhesion cascade beginning with leukocyte capture and rolling leading to firm adhesion and transmigration.1,2 Capture and rolling are mediated largely by the selectin family of adhesion molecules. Intravital microscopy studies on inflamed tissue of the cremaster muscle conducted in gene-targeted mice deficient in either E-, P-, or L-selectin or various combinations have revealed both overlapping and unique functions of the selectins.3,4 The overlapping function of selectins is in particular evident in E-selectin–deficient mice. These mice do not show obvious inflammatory deficits in certain models unless P-selectin is also blocked,5 which then leads to a dramatic reduction in the number of rolling leukocytes.6Characteristic properties of individual selectins become evident in their ability to mediate leukocyte rolling at distinct velocities. Untreated wild-type mice show P-selectin–dependent rolling with typical rolling velocities in venules of the cremaster muscle of below 50 μm/s.7 In tumor necrosis factor-α (TNFα)–treated mice, E-selectin preferentially mediates slow rolling (10 μm/s or less),6 and P-selectin mediates rolling at higher velocities.3
Selectin ligands carry sialylated and fucosylated oligosaccharides found at the nonreducing termini of core 2-dependent O-linked glycans.8-10 Core 2 structures are formed by a family of core 2 β1-6-N-glucosaminyltransferase branching enzymes that play a key role in controlling O-glycan structural diversity.11The major ligand for P-selectin, P-selectin glycoprotein ligand-1 (PSGL-1), is a glycoprotein with at least one sulfated tyrosine near the N-terminus and with sialylated and fucosylated O-glycans.12,13 Previous studies on Chinese hamster ovary (CHO) cells transfected with different fucosyltransferases and PSGL-1 have shown that transfection of these cells with C2GlcNAcT-I, a core 2 β1-6-N-glucosaminyltransferase, leads to a dramatic increase in binding to P-selectin.14 These and other findings demonstrate that PSGL-1 requires C2GlcNAcT-I for optimal binding to P-selectin.15 Recent work by Ellies et al16 provides evidence that selectin ligand function is impaired but not absent in neutrophils from mice deficient in C2GlcNAcT-I. These conclusions are based on results from binding of soluble recombinant selectins to neutrophils and from parallel-plate flow chamber–controlled detachment assays showing impaired leukocyte adhesion on all 3 selectins. Leukocyte recruitment in a thioglycollate-induced peritonitis model showed an 80% reduction in polymorphonuclear cells, which is comparable to the defects seen in E/P-selectin–deficient or fucosyltransferase VII–deficient mice.17-19
The present study was designed to test whether P- and/or E-selectin ligand functions are impaired under physiological conditions in vivo by the absence of C2GlcNAcT-I. To this end, we investigated leukocyte rolling in vivo in C2GlcNAcT-I–deficient mice in 2 models of inflammation (untreated and TNFα-treated mice). Mild trauma caused by the exteriorization of the cremaster muscle leads to a rapid induction of P-selectin–dependent leukocyte rolling7,20; E-selectin expression is absent or very low in this model. Short-term (2-hour) TNFα treatment leads to the expression of P- and E-selectin on the surface of venular endothelium,21 which coincides with the induction of slow leukocyte rolling in these microvessels.6
Materials and methods
Animals
Mice lacking a functional gene encoding core 2 β1-6-N-glucosaminyl-transferase (C2GlcNAcT-I, EC 2.4.1.102) were derived from a colony described previously.16C2GlcNAcT-I alleles were analyzed by Southern blotting and polymerase chain reaction (PCR) as described earlier.16The wild-type C2GlcNAcT-I allele was detected by means of PCR primers located adjacent to the deleted region (W5′: 5′-GGGTTACGGATGAGCTCTGTGTC; and W3′: 5′-CCCTGGAAGCAGGACAATTCTG-3′), resulting in a 304–base pair (bp) fragment, and the mutant allele was detected by means of W5′ and a loxP primer (M3′: 5′-CTCGAATTGATCCCCGGGTAC-3′), yielding a 200-bp fragment. Control experiments were performed in heterozygous (+/−) and homozygous (+/+) littermates. All experiments were performed on healthy mice that were at least 8 weeks of age. Mice were housed in a barrier facility under specific-pathogen–free conditions, and animal experiments were approved by the animal care and use committee of the University of Virginia.
Antibodies and cytokines
The rat antimouse E-selectin monoclonal antibody (mAb) 9A9 (rat immunoglobulin G1 [IgG1], 30 μg per mouse) has previously been shown to specifically block E-selectin function in vitro22and E-selectin–dependent rolling in vivo.6 The mAb 9A9 was a gift from Dr B. Wolitzky (Hoffman- La Roche, Nutley, NJ). The P-selectin mAb RB40.34 (rat IgG1, 30 μg per mouse) blocks P-selectin–dependent adhesion in vitro,23P-selectin–dependent leukocyte rolling in vivo,7 and P-selectin–dependent leukocyte recruitment in vivo.23 The rat antimouse mAb to PSGL-1, 4RA10 (rat IgG1, 30 μg per mouse), has been shown to specifically block PSGL-1 in vitro and in vivo.24 The mAbs RB40.34 and 4RA10 were gifts from Dr Dietmar Vestweber at the University of Münster (Germany). The L-selectin mAb MEL14 (rat IgG2a; 50 μg per mouse) was purified from hybridoma supernatant (American Type Culture Collection, Manassas, VA) and blocks L-selectin–dependent leukocyte rolling in vivo.6 7 Recombinant murine TNFα (Genzyme, Cambridge, MA) was injected intrascrotally at a dose of 500 ng per mouse in a volume of 0.3 mL sterile saline 2 hours before the beginning of the intravital microscopic experiment.
Flow cytometry
Flow cytometry was used to find the saturating dose of PSGL-1–blocking mAb 4RA10 after systemic injection into mice. Four anesthetized control mice were treated with increasing doses of mAb 4RA10 (1 μg, 3 μg, 10 μg, and 100 μg per mouse) or a rat antimouse IgG control antibody (100 μg) (Pharmingen, San Diego, CA). At 20 minutes after injection of the PSGL-1–blocking mAb 4RA10 or the isotype control, peripheral blood was collected from the carotid artery. Red blood cells were lysed in Pharm-Lyse-10X solution (Pharmingen). After centrifugation and removal of the supernatant, blood cells (1 × 106 cells) were suspended in phosphate-buffered saline (PBS)–1% bovine saline solution and incubated with a mouse antirat secondary antibody conjugated with flourescein isothiocyanate (FITC) (Pharmingen) for 30 minutes on ice (1 μg/106 cells). In addition, mAb Ly-6G against the neutrophilic surface antigen GR-1 conjugated with phycoerythrin (Pharmingen) was added to gate for neutrophilic granulocytes. PSGL-1 expression was determined on 10 000 leukocytes per mouse by means of a 4-decade FACScan with Cell Quest software package (Becton Dickinson, San Jose, CA).
Western blotting
Bone marrow cells were flushed from the femurs and tibias of control and core 2−/− mice by means of cold RPMI 1640 (Gibco BRL, Grand Island, NY) and immediately centrifuged at 1000 rpm for 5 minutes. The pellet was washed once with cold PBS and extracted with lysis buffer (108 cells/mL) containing 1% Triton X-100 (Fisher Scientific, Pittsburgh, PA) along with 1 mM phenylmethyl sulfonyl fluoride, 1 mM egtazic acid, and 1% protease inhibitor cocktail (all Sigma Chemical, St Louis, MO) in PBS, pH 7.4. Extraction was carried out at 4°C for 30 minutes on an orbital shaker. The samples were clarified by centrifugation at 14 000g for 30 minutes at 4°C, and the supernatant was transferred to fresh tubes. Samples were boiled for 5 minutes in sodium dodecyl sulfate–polymerase chain reaction sample buffer with β-mercaptoethanol, electrophoresed on a 10% polyacrylamide gel, and transferred to nitrocellulose. After blocking with 5% nonfat milk in PBS for 1 hour at room temperature, the membrane was probed with PSGL-1 mAb 4RA10 or rat isotype control (5 μg/mL, BD Pharmingen) in 2.5% nonfat milk and 0.05% Tween-20 (Fisher Scientific) in PBS for 1 hour at room temperature, washed 3 times with PBS-Tween 20 (0.05%), and then incubated with goat antirat IgG conjugated to horseradish peroxidase (Pierce, Rockford, IL) for 1 hour at room temperature. After 3 washes in PBS–Tween 20 (0.05%) followed by 1 wash in PBS, the blots were developed by enzyme chemiluminescence by means of the substrate kit (Pierce) and were exposed to X-omat AR5 film (Eastman Kodak, Rochester, NY).
Intravital microscopy
Mice were anesthetized with an intraperitoneal injection of ketamine (125 mg/kg body weight) (Ketalar) (Parke-Davis, Morris Plains, NJ); xylazine (12.5 mg/kg body weight) (Phoenix Scientific, St Joseph, MO); and atropin sulfate (0.025 mg/kg body weight) (Elkins-Sinn, Cherry Hill, NJ) and placed on a heating pad to maintain body temperature. Some mice were pretreated with an intrascrotal injection of recombinant murine TNFα as described.6
Microscopic observations were made on an intravital microscope (Axioskop) (Zeiss, Thornwood, NY) with a saline immersion objective (SW 40/0.75 numerical aperture). The trachea was intubated, and one jugular vein was cannulated for administration of anesthetic throughout the intravital microscopic experiment. One carotid artery was cannulated for blood pressure monitoring, blood samples, and systemic mAb injections. Blood pressure was monitored intermittently during the experiment (model BPMT-2) (Stemtech, Menomonee Falls, WI). During the experiment, mice received 0.2 mL/h diluted pentobarbital in saline to maintain anesthesia and a neutral fluid balance.
Cremaster muscle preparation
The cremaster muscle was prepared for intravital microscopy as described.6 The epididymis and testis were gently pinned to the side, exposing the well-perfused cremaster microcirculation. The cremaster muscle was superfused with thermocontrolled (35°C) bicarbonate-buffered saline. To detect changes in systemic white blood cell count after injection of the various antibodies, systemic blood samples (10 μL) were taken after each mAb injection. Blood samples were diluted 1:10 with Kimura (11 mL of 5% [wt/wt] toluidine blue. 0.8 mL of 0.03% light green SF yellowish, 0.5 mL saturated saponin, and 5 mL of 0.07 M phosphate buffer, pH 6.4) (all from Sigma) and were analyzed for leukocyte concentration (expressed as number of leukocytes per microliter of whole blood). Differential leukocyte counts were taken from blood smears by means of the Hema 3 Kit (Biochemical Sciences, Swedesboro, NJ). To differentiate intravascular and interstitial leukocytes into neutrophils, eosinophils, and mononuclear cells, whole mounts of cremaster muscle were prepared as described elsewhere.25 Both rolling and firmly adhered leukocytes are visible as intravascular cells in whole-mount histological preparations.26
Data analysis
Microvessel diameters, lengths, and rolling leukocyte velocities were measured by means of a digital image processing system.27 Each rolling leukocyte passing a line perpendicular to the vessel axis was counted, and leukocyte rolling flux was expressed as leukocytes per minute. Rolling flux fraction was calculated as described7 by dividing leukocyte rolling flux by total leukocyte flux estimated as [WBC] × vb × π × (d/2)2where [WBC] is the systemic leukocyte count, vb is the blood flow velocity, and d is the venular diameter. Leukocyte rolling velocities were measured as averages over a 2-second time window. Rolling velocities of 5 leukocytes were measured in each venule. Centerline red blood cell velocity in the cremaster muscle preparation was measured by means of a dual photodiode and a digital on-line cross-correlation program (Circusoft Instrumentation, Hockessin, DE). Centerline velocities were converted to mean blood flow velocities by multiplying with an empirical factor of 0.625.28 Wall shear rates (γw) were estimated as 2.12 (8 vb/d), where vb is the mean blood flow velocity, d is the diameter of the vessel, and 2.12 is a median empirical correction factor obtained from velocity profiles measured in microvessels in vivo.29
To specifically address the shear rate dependence of leukocyte rolling through E-selectin, rolling flux fractions were stratified and grouped by wall shear rate and used to construct Figure 5.
Statistics
Statistical analysis was performed with the Sigma-Stat 2.0 software package (SPSS Science, Chicago, IL). Average vessel diameter, leukocyte rolling flux fractions, leukocyte rolling velocities, and shear rates between groups and treatments were compared with the one-way analysis of variance on ranks (Kruskal-Wallis) and with a multiple pairwise comparison test (Dunn test). Leukocyte counts and differentials were compared with the Student t test or by the Wilcoxon rank-sum test as appropriate. Statistical significance was set at P < .05.
Results
All mice used in this work appeared healthy, active, and of normal size and weight for their ages. The systemic leukocyte counts were significantly higher in C2GlcNAcT-I–deficient mice than in wild-type mice. This was true for both the group without pretreatment and the TNFα-pretreated group (P < .05) (Table1). The observed increase in white blood cell count in core 2−/− mice was due mainly to the marked elevation in the number of neutrophils. Lymphocyte counts were only slightly elevated in core 2−/− mice compared with control mice, and eosinophils and mononuclear cells showed no difference. In the TNFα pretreatment group, the significantly elevated systemic white blood cell count showed similar increases in neutrophils and lymphocytes for core 2−/− mice and control mice.
Systemic leukocyte counts and differentials for trauma- and tumor necrosis factor-α–induced inflammation
| Mouse genotype . | Systemic white blood cell count (cells/μL) . | PMN cells (%) . | Lymph cells (%) . | Mononuclear cells (%) . | Eosinophils (%) . |
|---|---|---|---|---|---|
| Untreated | |||||
| Core 2−/− | 5200 ± 800* | 46 ± 3* | 46 ± 2* | 4 ± 1 | 1 ± 0.3 |
| Control | 3000 ± 360* | 27 ± 4* | 69 ± 3* | 2 ± 1 | 1 ± 0.4 |
| TNF-treated | |||||
| Core 2−/− | 5000 ± 150* | 47 ± 4 | 45 ± 4 | 4 ± 2 | 2 ± 1 |
| Control | 2700 ± 290* | 48 ± 8 | 49 ± 8 | 2 ± 0.4 | 1 ± 0.3 |
| Mouse genotype . | Systemic white blood cell count (cells/μL) . | PMN cells (%) . | Lymph cells (%) . | Mononuclear cells (%) . | Eosinophils (%) . |
|---|---|---|---|---|---|
| Untreated | |||||
| Core 2−/− | 5200 ± 800* | 46 ± 3* | 46 ± 2* | 4 ± 1 | 1 ± 0.3 |
| Control | 3000 ± 360* | 27 ± 4* | 69 ± 3* | 2 ± 1 | 1 ± 0.4 |
| TNF-treated | |||||
| Core 2−/− | 5000 ± 150* | 47 ± 4 | 45 ± 4 | 4 ± 2 | 2 ± 1 |
| Control | 2700 ± 290* | 48 ± 8 | 49 ± 8 | 2 ± 0.4 | 1 ± 0.3 |
All values are presented as mean ± SEM.
PMN indicates polymorphonuclear; TNF, tumor necrosis factor.
Indicates significant differences (P < .05) between core 2−/− and control mice.
Leukocyte rolling in untreated venules
Leukocyte rolling was analyzed in 62 venules of 11 core 2−/− mice and compared with rolling in 25 venules of 6 littermate controls. Hemodynamic parameters for both groups are presented in Table 2 and show no significant differences in diameter, blood flow velocity, shear rate, or systemic blood pressure. Leukocyte rolling was assessed as leukocyte rolling flux fraction, which is defined as the number of rolling leukocytes divided by the total number of leukocytes passing through the same vessel.30 During the first hour after exteriorization of the cremaster muscle, rolling flux fraction was only 4% in C2GlcNAcT-I–deficient mice, compared with 27% in control mice (P < .05) (Figure1A). Leukocyte rolling during that time was almost exclusively P-selectin dependent, because it was blocked by the P-selectin mAb RB40.34 (Figure 1A). The lower leukocyte rolling flux fraction in core 2−/− mice can therefore be attributed to a severe impairment in P-selectin–mediated rolling.
Hemodynamic and microvascular parameters in trauma- and tumor necrosis factor-α–induced inflammation
| Mouse genotype . | Mice (n) . | Venules (n) . | Diameter (μm) . | Centerline velocity (μm/s) . | Wall shear rate (s−1) . | Systemic blood pressure (mmHg) . |
|---|---|---|---|---|---|---|
| Untreated | ||||||
| Core 2−/− | 11 | 62 | 35 ± 1 | 3600 ± 300 | 1200 ± 100 | 95 ± 6 |
| Control | 6 | 25 | 32 ± 1 | 4000 ± 400 | 1500 ± 150 | 100 ± 4 |
| TNF-treated | ||||||
| Core 2−/− | 11 | 55 | 37 ± 1 | 2900 ± 310 | 830 ± 100 | 81 ± 4 |
| Control | 11 | 43 | 37 ± 1 | 3500 ± 410 | 1020 ± 120 | 87 ± 7 |
| Mouse genotype . | Mice (n) . | Venules (n) . | Diameter (μm) . | Centerline velocity (μm/s) . | Wall shear rate (s−1) . | Systemic blood pressure (mmHg) . |
|---|---|---|---|---|---|---|
| Untreated | ||||||
| Core 2−/− | 11 | 62 | 35 ± 1 | 3600 ± 300 | 1200 ± 100 | 95 ± 6 |
| Control | 6 | 25 | 32 ± 1 | 4000 ± 400 | 1500 ± 150 | 100 ± 4 |
| TNF-treated | ||||||
| Core 2−/− | 11 | 55 | 37 ± 1 | 2900 ± 310 | 830 ± 100 | 81 ± 4 |
| Control | 11 | 43 | 37 ± 1 | 3500 ± 410 | 1020 ± 120 | 87 ± 7 |
Diameters, centerline velocity, wall shear rate, and systolic blood pressure are presented as mean ± SEM of all investigated venules.
TNF indicates tumor necrosis factor.
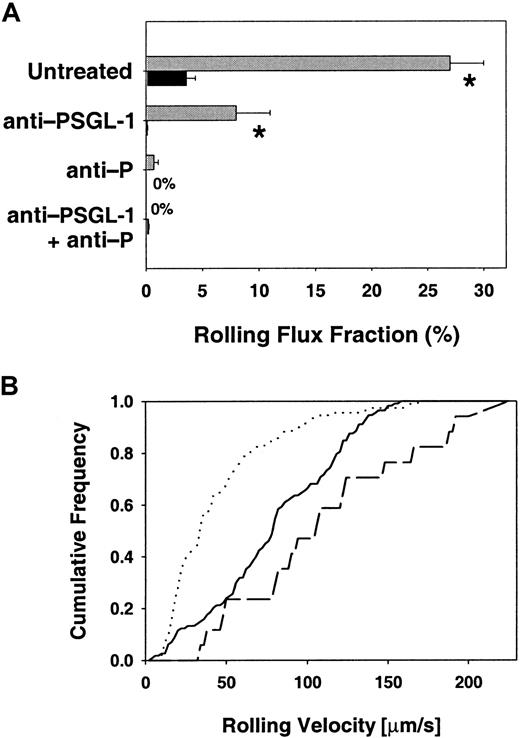
Leukocyte rolling flux fraction and rolling velocities.
(A) Leukocyte rolling flux fraction in untreated cremaster venules. Effect of function-blocking mAbs on leukocyte rolling flux fraction (mean ± SEM) in venules of the cremaster muscle of C2GlcNAcT-I–deficient mice (▪) and control mice (░). * indicates significant differences (P < .05) in leukocyte rolling flux fraction between core 2−/− and control mice. (B) Leukocyte rolling velocities in venules of untreated core 2−/− and control mice. Data represent cumulative histograms of leukocyte rolling velocities measured during the first hour after exteriorization of the cremaster muscle. Velocity distribution for untreated core 2−/− mice (solid line, 113 cells), untreated control mice (dotted line, 44 cells), and control mice treated with PSGL-1–blocking mAb 4RA10 (dashed line, 114 cells). Core2−/− mice treated with 4RA10 showed no rolling.
Leukocyte rolling flux fraction and rolling velocities.
(A) Leukocyte rolling flux fraction in untreated cremaster venules. Effect of function-blocking mAbs on leukocyte rolling flux fraction (mean ± SEM) in venules of the cremaster muscle of C2GlcNAcT-I–deficient mice (▪) and control mice (░). * indicates significant differences (P < .05) in leukocyte rolling flux fraction between core 2−/− and control mice. (B) Leukocyte rolling velocities in venules of untreated core 2−/− and control mice. Data represent cumulative histograms of leukocyte rolling velocities measured during the first hour after exteriorization of the cremaster muscle. Velocity distribution for untreated core 2−/− mice (solid line, 113 cells), untreated control mice (dotted line, 44 cells), and control mice treated with PSGL-1–blocking mAb 4RA10 (dashed line, 114 cells). Core2−/− mice treated with 4RA10 showed no rolling.
To investigate whether PSGL-1 was necessary for P-selectin–dependent rolling in core 2−/− mice in vivo, we treated C2GlcNAcT-I–deficient mice with a PSGL-1–blocking mAb, 4RA10. Injection of 4RA10 into core 2−/− mice removed all rolling leukocytes (Figure 1A). Injection of PSGL-1 mAb 4RA10 into control animals led to a significant reduction in rolling flux fraction from 27% to 8%, which is similar to previous findings in rat mesenteric venules.31 Rolling in control mice treated with mAb 4RA10 was completely blocked by additional injection of the P-selectin–blocking antibody RB40.34 (Figure 1A), confirming that rolling under these conditions was completely P-selectin dependent.
Average rolling velocity (Vavg) in core 2−/−mice was 81 ± 4 μm/s (Figure 1B) and almost 2-fold higher than in control mice (Vavg = 44 ± 3 μm/s) (P < .05). Control mice pretreated with mAb 4RA10 showed an increase in rolling velocity with Vavg of 110 ± 13 μm/s (Figure 1B), similar to the rolling velocity seen in core 2−/− mice. The increased rolling velocity at reduced flux suggests that the PSGL-1–P-selectin interaction may have increased off-rates when PSGL-1 is not decorated by core 2.
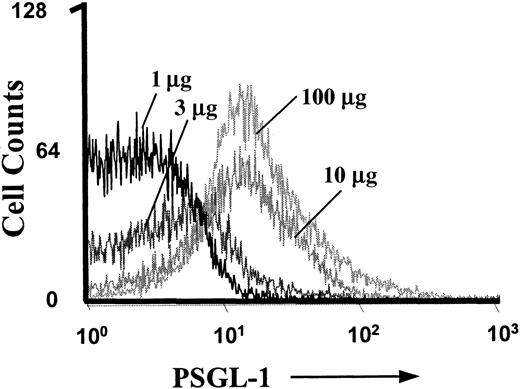
To show that the PSGL-1–blocking mAb 4RA10 (30 μg) was injected at a saturating dose, 4 control mice were treated with increasing doses (1 μg, 3 μg, 10 μg, and 100 μg) of the PSGL-1–blocking mAb 4RA10. Fluorescence-activated cell sorter (FACS) analysis of blood obtained 20 minutes after injection showed that 10 μg per mouse saturated all binding sites on neutrophils, and no further increase was seen at 100 μg 4RA10 (Figure 2). In addition, rolling flux fraction was assessed before and after injection of 30 μg 4RA10 followed by additional injection of 70 μg 4RA10, for a total dose of 100 μg 4RA10. The additional injection led to no further drop in rolling flux fraction. Subsequent injection of mAb RB40.34 abolished rolling completely. This confirms that the dose of 30 μg 4RA10 per mouse was also functionally saturating.
FACS analysis of PSGL-1 on neutrophils.
Control mice were treated with increasing doses of PSGL-1–blocking mAb 4RA10 (1 μg, 3 μg, 10 μg, and 100 μg) for more than 20 minutes. Peripheral blood was collected and processed for FACS analysis of GR-1+ cells.
FACS analysis of PSGL-1 on neutrophils.
Control mice were treated with increasing doses of PSGL-1–blocking mAb 4RA10 (1 μg, 3 μg, 10 μg, and 100 μg) for more than 20 minutes. Peripheral blood was collected and processed for FACS analysis of GR-1+ cells.
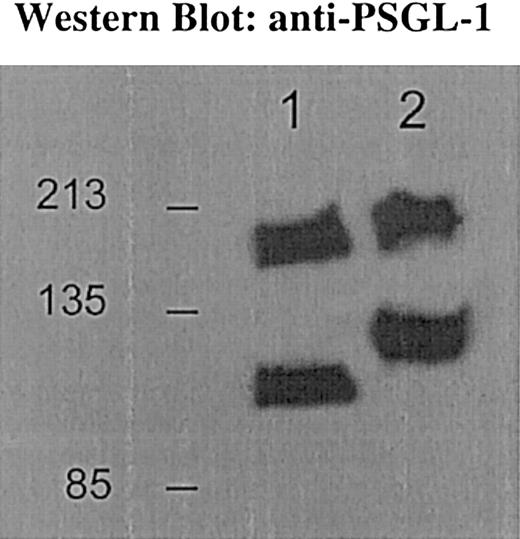
To verify that PSGL-1 was indeed significantly modified by the C2GlcNAcT-I enzyme, we probed bone marrow neutrophils and their precursors for PSGL-1 protein. We found an increased mobility of PSGL-1 from core 2−/− mice compared with littermate control mice (Figure 3). The calculated molecular weights were 117 kd and 220 kD for the control PSGL-1 monomer and dimer and 98 kd and 188 kd for the core 2−/− PSGL-1 monomer and dimer, respectively.
Western blot for PSGL-1.
Bone marrow cells from control mice express PSGL-1 at 117 kd and 220 kd (lane 2); core 2−/− mice express PSGL-1 at 98 kd and 188 kd (lane 1) for the monomeric and dimeric form, respectively. PSGL-1 from core 2−/− neutrophils shows faster mobility compared with the control bands, consistent with absence of core 2 decoration on PSGL-1.
Western blot for PSGL-1.
Bone marrow cells from control mice express PSGL-1 at 117 kd and 220 kd (lane 2); core 2−/− mice express PSGL-1 at 98 kd and 188 kd (lane 1) for the monomeric and dimeric form, respectively. PSGL-1 from core 2−/− neutrophils shows faster mobility compared with the control bands, consistent with absence of core 2 decoration on PSGL-1.
Short-term (2-hour) TNFα-induced inflammation
Treatment with TNFα for 2 hours induces the expression of E-selectin and enhances the expression of P-selectin on venules of the cremaster muscle.21 We assessed leukocyte rolling in 55 venules of 11 TNFα-treated mice lacking C2GlcNAcT-I and compared the results with rolling in 43 venules of 11 control animals. Hemodynamic parameters for both groups are presented in Table 2 and show similar vessel diameters, centerline velocities, wall shear rates, and systemic blood pressures. Leukocyte rolling flux fraction after TNFα treatment was reduced to 8% in core 2−/−mice compared with 21% in control mice (P < .05) (Figure4), suggesting a severe defect in selectin ligand function in C2GlcNAcT-I–deficient mice.
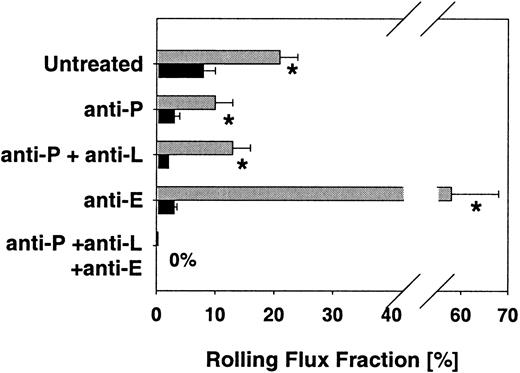
Leukocyte rolling flux fraction (mean ± SEM) in TNFα-treated venules.
Effect of function-blocking mAbs on leukocyte rolling flux fraction of C2GlcNAcT-I–deficient mice (▪) and control mice (░) 2 hours after TNFα injection. * indicates significant differences (P < .05) in leukocyte rolling flux fraction between core 2−/− and control mice.
Leukocyte rolling flux fraction (mean ± SEM) in TNFα-treated venules.
Effect of function-blocking mAbs on leukocyte rolling flux fraction of C2GlcNAcT-I–deficient mice (▪) and control mice (░) 2 hours after TNFα injection. * indicates significant differences (P < .05) in leukocyte rolling flux fraction between core 2−/− and control mice.
To investigate E-selectin–mediated rolling in core 2−/−mice, we injected the P-selectin–blocking antibody RB40.34 into C2GlcNAcT-I–deficient mice, which led to a decrease in rolling flux fraction from 8% to 3% (P < .05). Treating control animals with mAb RB40.34 led to a significant decrease in rolling flux fraction, from 21% to 10%, which is similar to earlier findings in P-selectin–deficient mice,6 and significantly higher than in RB40.34-treated core 2−/− mice (P < .05). L-selectin–blocking mAb MEL-14 had no additional effect on leukocyte rolling fluxes in core 2−/− and control mice (Figure 4). Adding the E-selectin–blocking mAb 9A9 to mice treated with P-selectin–blocking mAb RB40.34 and L-selectin–blocking mAb MEL-14 completely abolished leukocyte rolling in both core 2−/− mice and control mice (Figure 4). This confirms that the residual rolling seen in core 2−/− mice and control mice treated with P-selectin–blocking mAb RB40.34 and L-selectin–blocking mAb MEL-14 is completely E-selectin dependent. In addition, it shows a significant impairment of E-selectin–mediated rolling in core 2−/− mice compared with controls.
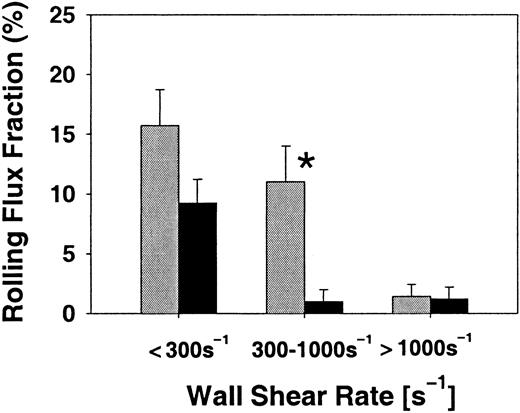
Since the defect for E-selectin–mediated rolling in core 2−/− mice was not complete, we investigated whether rolling was differentially affected in venules with different wall shear rates. Indeed, rolling was almost not affected in venules of core 2−/− mice treated with blocking mAbs to L- and P-selectin at wall shear rates below 300 s−1, but was significantly reduced at wall shear rates between 300 and 1000 s−1(Figure 5). At very high shear rates (greater than 1000 s−1), rolling flux fraction also decreased in littermate controls (Figure 5). This analysis shows that E-selectin–dependent rolling is not significantly affected in core 2−/− mice at low shear rates, which is consistent with the in vitro analysis presented by Snapp et al (page 3806).32
E-selectin–mediated rolling in venules with different wall shear rates.
Rolling flux fraction (mean ± SEM) in cremaster muscle venules of TNFα-pretreated core 2−/− mice (▪) and littermate control mice (░) treated with blocking mAbs against P- and L-selectin. Data for low (less than 300 s−1), normal (300 to 1000 s−1), and high (greater than 1000 s−1) wall shear rates. Results from 24 control venules and 43 core 2−/− venules. * indicates significant differences (P < .05) between core 2−/− mice and control mice.
E-selectin–mediated rolling in venules with different wall shear rates.
Rolling flux fraction (mean ± SEM) in cremaster muscle venules of TNFα-pretreated core 2−/− mice (▪) and littermate control mice (░) treated with blocking mAbs against P- and L-selectin. Data for low (less than 300 s−1), normal (300 to 1000 s−1), and high (greater than 1000 s−1) wall shear rates. Results from 24 control venules and 43 core 2−/− venules. * indicates significant differences (P < .05) between core 2−/− mice and control mice.
To study P-selectin–dependent rolling in core 2−/− mice, we injected the E-selectin–blocking mAb 9A9 into core 2−/− mice, which resulted in a decrease in rolling flux fraction to 3% (Figure 4). This is in sharp contrast to control animals, where we observed a dramatic increase in rolling flux fraction to 58% after injection of 9A9 (P < .05) (Figure 4), consistent with previous results.6
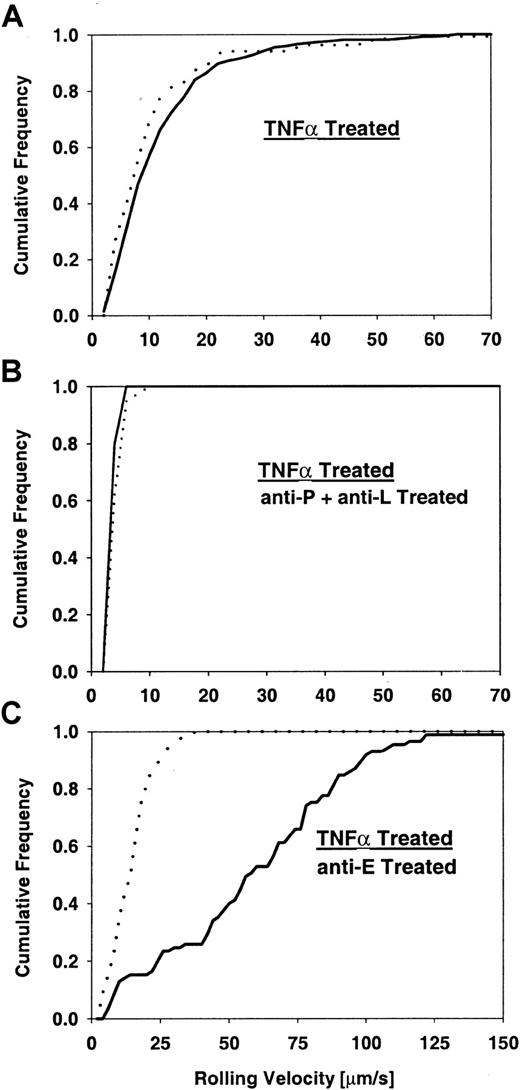
Next, we investigated leukocyte rolling velocities and velocity distributions in TNFα-treated cremaster muscle venules. TNFα-treated core 2−/− mice showed a rolling velocity distribution (Vavg = 11.5 ± 0.6 μm/s) similar to the distribution in control mice (Vavg = 10.7 ± 0.8 μm/s) (Figure 6A). Injection of RB40.34 led to a comparable decrease in rolling velocity in both C2GlcNAcT-I–deficient (Vavg = 5.8 ± 0.4 μm/s) and control mice (Vavg = 5.5 ± 0.4 μm/s) (distributions not shown). Similarly, injection of both RB40.34 and MEL14 resulted in a comparable decrease in rolling velocity in both C2GlcNAcT-I–deficient (Vavg = 3.5 ± 0.1 μm/s) and control mice (Vavg = 3.8 ± 0.2 μm/s) (Figure 6B), suggesting that E-selectin–mediated slow rolling still operates in C2GlcNAcT-I–deficient mice. In contrast, we found a different velocity distribution with a dramatic increase in rolling velocities in core 2−/− mice after injection of the E-selectin–blocking antibody 9A9 (Vavg = 59 ± 4 μm/s) and only a mild increase in rolling velocity in control mice (Vavg = 14 ± 1 μm/s) (P < .05) (Figure 6C). Leukocyte rolling velocities after injecting mAb 9A9 into control mice were similar to results from previous studies6 and reflect largely P-selectin–mediated rolling. The much higher rolling velocities found in core 2−/− mice point toward impairment in P-selectin–mediated leukocyte rolling.
Leukocyte rolling velocities in TNFα-treated mice.
Cumulative velocity distribution for core 2−/− mice (solid line) and control mice (dotted line) with no treatment (A), P-selectin–blocking mAb RB40.34 and L-selectin–blocking mAb MEL14 (B), and E-selectin–blocking mAb 9A9 (C). Significant velocity differences (P < .05) between control and core 2−/− mice were detected when E-selectin was blocked (C). Rolling flux fraction for controls was 21%, 13%, and 58% for panels A, B, and C, respectively, and for core 2−/− mice, 8%, 2%, and 3%.
Leukocyte rolling velocities in TNFα-treated mice.
Cumulative velocity distribution for core 2−/− mice (solid line) and control mice (dotted line) with no treatment (A), P-selectin–blocking mAb RB40.34 and L-selectin–blocking mAb MEL14 (B), and E-selectin–blocking mAb 9A9 (C). Significant velocity differences (P < .05) between control and core 2−/− mice were detected when E-selectin was blocked (C). Rolling flux fraction for controls was 21%, 13%, and 58% for panels A, B, and C, respectively, and for core 2−/− mice, 8%, 2%, and 3%.
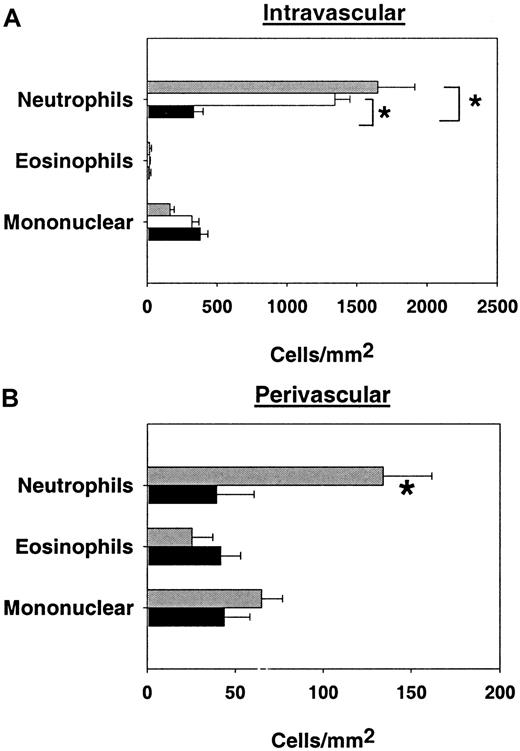
Leukocyte differentials in cremaster muscle venules
To determine the effect of eliminating C2GlcNAcT-I on the composition of cells in venules (intravascular) and recruited into cremaster tissue (perivascular), we used Giemsa-stained whole-mount mouse cremaster muscles to differentiate leukocytes in TNFα-treated mice. The number of intravascular neutrophils was significantly reduced by about 90% in core 2−/− mice compared with control mice (Figure 7A). By contrast, adhesion of eosinophils or mononuclear cells appeared to be unaffected by the absence of C2GlcNAcT-I. To directly address the contribution of E-selectin to leukocyte accumulation in venules, we investigated the number of intravascular leukocytes in cremaster muscle of mice treated with mAbs to L- and P-selectin shortly before injection of TNFα. This treatment did not alter leukocyte adhesion, suggesting that the absence of C2GlcNAcT-I causes a severe reduction in neutrophil adhesion that cannot be achieved by blocking P- and L-selectin. Similar to the findings on intravascular leukocytes, absence of C2GlcNAcT-I also reduced the number of extravascular neutrophils, but not eosinophils or mononuclear cells (Figure 7B).
Adherent and rolling leukocytes per square millimeter of venular surface area and perivascular space.
(A) Number of adherent and rolling leukocytes per square millimeter of venular surface area (mean ± SEM). Data are presented for control mice (░), control mice treated with mAbs against P- (anti-P) and L-selectin (anti-L) (■) and core 2−/− mice (▪). * indicates significant differences (P < .05). (B) Number of adherent and rolling leukocytes per square millimeter of perivascular space (mean ± SEM). Data are presented for control mice (░) and core 2−/− mice (▪). * indicates significant differences from control (P < .05).
Adherent and rolling leukocytes per square millimeter of venular surface area and perivascular space.
(A) Number of adherent and rolling leukocytes per square millimeter of venular surface area (mean ± SEM). Data are presented for control mice (░), control mice treated with mAbs against P- (anti-P) and L-selectin (anti-L) (■) and core 2−/− mice (▪). * indicates significant differences (P < .05). (B) Number of adherent and rolling leukocytes per square millimeter of perivascular space (mean ± SEM). Data are presented for control mice (░) and core 2−/− mice (▪). * indicates significant differences from control (P < .05).
Discussion
In this study, we have used the mouse cremaster muscle to investigate the role of C2GlcNAcT-I in the biosynthesis and physiological function of selectin ligands in vivo. We found that in cremaster muscle venules of C2GlcNAcT-I–deficient mice, P- and E-selectin–dependent rolling is sharply reduced. In addition, we show that the observed defect in E-selectin–mediated rolling becomes evident only at wall shear rates above 300 s−1.
In untreated cremaster muscle venules of C2GlcNAcT-I–deficient but not control mice, P-selectin–mediated rolling is completely blocked by a mAb to PSGL-1. This is a novel finding, demonstrating that C2GlcNAcT-I is required for optimal PSGL-1 function, but that PSGL-1 lacking core 2 oligosaccharides can still bind to P-selectin at a rate and affinity that allow some leukocyte rolling. The significant size difference in PSGL-1 monomer and dimer expressed on leukocytes of core 2−/− and control mice, which has not been described before, is consistent with a loss of core 2-containing glycans, suggesting that the targeted enzyme is indeed responsible for all or most of the core 2 decoration of PSGL-1. Consistent with our findings, flow cytometry showed that binding of P-selectin IgM to neutrophils from core 2−/− mice was reduced and that neutrophils from these mice bound poorly to immobilized P-selectin, but that binding activity was not completely abolished.16 In the accompanying report by Snapp et al,32 who used a rolling assay in a parallel-plate flow chamber, the number of rolling neutrophils from core 2−/− mice was reduced to about 10% of control.
Previous in vitro work had suggested that PSGL-1 requires the core 2 modification to bind to P-selectin.15 CHO cells transfected with PSGL-1 and fucosyltransferase IV required C2GlcNAcT-I to avidly bind fluid-phase P-selectin. Kumar et al14 found some residual binding of soluble P-selectin to PSGL-1 when cotransfected with fucosyltransferase III but without C2GlcNAcT-I into CHO cells. Binding was much improved by adding C2GlcNAcT-I. Ramachandran et al33 showed that CHO cells transfected with fucosyltransferase VII and PSGL-1 did not support rolling of a pre-B cell line expressing P-selectin in a flow chamber system. However, rolling was found after cotransfection with C2GlcNAcT-I. This flow chamber assay is reversed in orientation compared with the in vivo situation (P-selectin on the rolling cell, PSGL-1 on the substrate), which could be of significance, since PSGL-1 is known to be preferentially localized to the tips of microvilli on leukocytes.34 Taken together, our findings confirm and extend previous in vitro findings to physiologically relevant wall shear rates. Interestingly, the velocity of rolling leukocytes after blocking PSGL-1 was increased to a degree similar to that in C2GlcNAcT-I–deficient mice. This shows that PSGL-1 in the absence of C2GlcNAcT-I supports rolling only at a higher velocity, suggesting an increased off-rate of the P-selectin–PSGL-1 bond in core 2−/− mice.
The present data also show that other ligands for P-selectin may exist and mediate some leukocyte rolling in control mice, consistent with previous findings31 and recent findings in PSGL-1–deficient mice.35 However, there is a quantitative difference between the degree of rolling after blocking PSGL-1. Under conditions where a mAb to P-selectin blocked almost all leukocyte rolling, a mAb to PSGL-1 blocked only 73% in control mice, although P-selectin–dependent rolling was reduced by 95% in PSGL-1–deficient mice.35 Therefore, we cannot formally exclude the possibility that mAb 4RA10, even at saturating concentrations, may not completely block PSGL-1 function in wild-type mice. Since PSGL-1–blocking mAb 4RA10 completely blocked rolling in core 2−/− mice, our findings suggest that P-selectin uses PSGL-1 and other ligands of unknown molecular identity that require C2GlcNAcT-I.
Requirements for E-selectin–dependent rolling in vivo are not well understood. Therefore, we addressed whether E-selectin ligands require modification by C2GlcNAcT-I. It is clear that E-selectin ligands must be modified by fucosyltransferase VII or IV or another fucosyltransferase in order to be functional.36 In fact, transfection of fucosyltransferase VII into all cell lines tested conferred the ability to bind to E-selectin in a flow chamber assay,37,38 suggesting that the glycoprotein requirements for E-selectin binding may be more relaxed than for P-selectin. Although PSGL-1 has been shown to bind to E-selectin,15,39,40 recent studies in PSGL-1–deficient mice show that PSGL-1 is not required for neutrophil rolling through E-selectin.35 Another candidate ligand for E-selectin, ESL-1,41 awaits confirmation of significance in vivo. ESL-1 is decorated with N-linked but not O-linked carbohydrates. Therefore, ESL-1 glycosylation should not be affected in C2GlcNAcT-I–deficient mice.
The present data show that E-selectin–dependent rolling operates in core 2−/− mice at low shear rates, but, when compared with control mice, the number of leukocytes rolling by binding to E-selectin is markedly reduced in venules with shear rates above 300 s−1. This translates into a significant recruitment defect for neutrophils in core 2−/− mice. Interestingly, this defect could not be recapitulated by blocking L- and P-selectin in control mice (Figure 7A), suggesting that C2GlcNAcT-I activity is indeed necessary for a functionally important E-selectin ligand on neutrophils. It is possible that other, C2GlcNAcT-I–independent E-selectin ligands exist on eosinophils and mononuclear cells. However, the present data do not unequivocally show this, because eosinophils and mononuclear cells also use selectin-independent pathways for rolling and adhesion.42 Our result are in agreement with previous findings of reduced core 2−/− neutrophil binding to E-selectin IgG chimeras in a flow chamber detachment assay and reduced binding of neutrophils to E-selectin IgM chimeras in a flow cytometric assay.16 However, the accompanying report did not find an impairment of E-selectin ligand function in a parallel-plate flow chamber attachment and rolling assay coated with E-selectin transfectants.32 In this assay, low shear stress (1.5 dyne/cm2) was used to allow for neutrophil attachment under in vitro conditions. In vitro, neutrophil attachment is reduced because of geometric differences to in vivo conditions43 and the absence of red blood cells.44 Under in vivo conditions, we observed shear rates in the range of 150 to 1500 s−1, which approximately correspond to wall shear stress levels of 1.5 to 15 dyne/cm2. In agreement with the accompanying results from the in vitro assay,32 we found very little decrease in E-selectin–dependent rolling at wall shear rates below 300 s−1, when the rolling flux fraction almost reached control values. This finding suggests an explanation for the difference in E-selectin–dependent rolling between the accompanying report32 and the flow chamber detachment assay presented by Ellies et al.16 We conclude that E-selectin–dependent rolling under low shear conditions is similar in the presence or absence of C2GlcNAcT-I, whereas E-selectin–dependent rolling is impaired in the absence of C2GlcNAcT-I at normal shear rates, resulting in reduced neutrophil accumulation in TNFα-treated venules. Interestingly, the ratio of perivascular neutrophils to intravascular neutrophils is very similar for both core 2−/− mice and control mice. This suggests that the rate of transmigration is not affected by the absence of C2GlcNAcT-I. Rather, the defect in neutrophil migration can most likely be attributed to the dramatic decrease in neutrophil rolling flux.
In conclusion, the present study has identified some of the mechanisms by which the absence of C2GlcNAcT-I impairs neutrophil recruitment to sites of inflammation.16 We conclusively show (1) that P-selectin–dependent rolling via PSGL-1 is severely impaired, (2) that rolling through other P-selectin ligands is completely absent in core 2−/− mice, and (3) that, on the basis of direct evidence, E-selectin–dependent rolling under physiological flow conditions is impaired in core 2−/− mice at higher wall shear rates. It is possible that other defects exist in core 2−/− mice that might interfere with normal signaling, adhesion, or transmigration of neutrophils. However, the severe functional deficits in leukocyte rolling appear to be sufficient to explain the severe decrease of neutrophil recruitment found in core 2−/− mice.
We thank Drs Ruth Eytner, Martin Wild, and Dietmar Vestweber, Universität Münster, Germany, for providing mAbs RB40.34 and 4RA10, and Dr Barry Wolitzky for providing mAb 9A9.
Supported by National Institutes of Health (NIH) grants HL-64381 and HL-54136 (K.L.); by NIH grant DK 48247 (J.D.M.); by a stipend from the German Research Foundation (DFG) SP 621/1–1 (M.S.); and by National Cancer Institute Fellowship F32CA79130 (L.G.E.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Klaus Ley, Department of Biomedical Engineering and Cardiovascular Research Center, University of Virginia, Health Sciences Center, Box 800759, Charlottesville, VA 22908; e-mail: klausley@virginia.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal