Selectins are carbohydrate-binding adhesion molecules that play important roles in control of leukocyte traffic. Glycosyltransferases involved in selectin ligand biosynthesis include the α1,3-fucosyltransferases FucT-VII and FucT-IV, one or more sialyltransferases, and at least one O-linked branching enzyme. Previous studies have shown that core 2 β1-6-N-glucosaminyltransferase (C2GlcNAcT-I; EC 2.4.1.102) is required for functional modification of PSGL-1, the leukocyte P-selectin ligand, but have been ambiguous on whether this enzyme is involved in E-selectin ligand formation. Using an attachment and rolling assay under defined shear flow in vitro, this study shows that C2GlcNAcT-I− lymphoid cells stably transfected with FucT-VII complementary DNA attach and roll well on E-selectin at 1.5 dynes/cm.2 Further, attachment and rolling on P-selectin of neutrophils is sharply reduced and that of short- term polarized Th1 cells is virtually abolished, with leukocytes from C2GlcNAcT-I−/− mice. In contrast, both neutrophils and Th1 cells from C2GlcNAcT-I−/− mice attach and roll as well as wild-type cells on E-selectin. These results show that C2GlcNAcT-I is selectively required for biosynthesis of ligands for P-selectin, but is not essential for at least some E-selectin ligands. Distinct requirements for C2GlcNAcT-I in the formation of ligands for E-selectin versus P-selectin represents a novel level of regulation of expression of selectin ligands and lymphocyte traffic.
Introduction
Selectins are a family of carbohydrate-binding adhesion molecules that mediate the earliest steps of leukocyte interaction with the vessel wall.1 Although the carbohydrate ligands for selectins have not been definitively characterized at the structural level, some of the glycosyltransferases responsible for selectin ligand biosynthesis have been identified. Among these is FucT-VII, an α1,3-fucosyltransferase established as essential for ligands for all 3 selectins.2-6 In addition, several studies have indicated that the O-linked branching enzyme core 2 β1,6-N-glucosaminyltransferase or C2GlcNAcT-I,7 which forms a β1,6 linkage from the N-acetylgalactosamine of a core 1 structure, is required for modification of PSGL-1 for effective interaction with P-selectin.8-10 Thus, cells lines that fail to express C2GlcNAcT-I bound quite poorly to P-selectin, even when cotransfected with complementary (cDNA) for both PSGL-1 and FucT-VII, and binding to P-selectin was conferred by expression of C2GlcNAcT-I.8,9 However, divergent results have been obtained in different assays with respect to a role for C2GlcNAcT-I in binding to E-selectin.8-10
More recently, mice deficient in C2GlcNAcT-I (C2GlcNAcT-I−/−) have been described.10These mice have strong defects in acute leukocyte recruitment to the peritoneum in response to chemical irritants. This defect is greater than that seen with mice deficient in only a single selectin and is comparable to mice deficient in FucT-VII or in both endothelial selectins.2,11,12 In addition, using a controlled detachment assay on plate-bound selectin chimeras, neutrophils from C2GlcNAcT-I−/− mice exhibited a strong but incomplete loss of ligands for both E- and P-selectins, whereas binding of soluble selectin/IgM chimeras was abolished for P-selectin and sharply reduced for E-selectin.10 These more recent results, in a different experimental system and with different cellular reagents, appeared to be at odds with previous data, which appeared to show either no role or an important role for C2GlcNAcT-I in E-selectin ligand formation.8 9 Further, the role of C2GlcNAcT-I has thus far not been directly examined in a standard attachment and rolling assay under defined shear flow in vitro. Thus, the precise role of C2GlcNAcT-I in biosynthesis of ligands for P- and E-selectin has remained unclear.
We have therefore analyzed C2GlcNAcT-I− cell lines, neutrophils, and polarized Th1 cells in a well-characterized attachment and rolling assay under physiologic conditions of defined shear flow in vitro for their ability to interact with cells expressing either P-selectin or E-selectin. The results clearly indicate that C2GlcNAcT-I is essential for attachment and rolling on P-selectin of all cell types studied. Expression of C2GlcNAcT-I was dispensable for attachment and rolling on E-selectin, but led to decreased rolling velocities of cell lines coexpressing FucT-VII. The accompanying paper (Sperandio et al, page 3812)28 offers corroborating data for neutrophil rolling in vivo. Collectively, these results identify C2GlcNAcT-I as the first glycosyltransferase selectively required for binding to P-selectin but not essential for E-selectin, and document important differences in the requirements for this enzyme in biosynthesis of ligands for the 2 endothelial selectins. These results have important implications for the control of lymphocyte traffic.
Materials and methods
Mice
Mice with a null mutation in C2GlcNAcT-I have been described,10 and were back-crossed more than 6 generations onto a C57BL6/J background. Wild-type (WT) C57BL6/J mice were initially obtained from Jackson Laboratories (Bar Harbor, ME) and were bred in our colony at Northwestern University Medical School. All procedures and experiments were approved by the Northwestern University Animal Care and Use Committee.
Cell lines and transfectants
The 300.19 cells13 were stably transfected with cDNA encoding human FucT-VII, with approaches exactly as previously described for other cell lines and stable transfectants,5 6 cloned by limiting dilution, and analyzed by reverse transcription–polymerase chain reaction (RT-PCR), fluorescence-activated cell sorter (FACS), or in rolling assays as described below. The 300.19 cells coexpressing both human FucT-VII and human C2GlcNAcT-1(L) were produced by transfection of cloned 300.19/FucT-VII cells.
Isolation of neutrophils and generation of Th1 cells
Neutrophils were isolated from bone marrow of both C2GlcNAcT-I−/− and WT mice by double discontinuous gradients (density of 1.083 and 1.119 g/L; Sigma Chemical, St Louis, MO). Cells recovered from the lower interface were more than 80% Gr-1+ and exhibited high side scatter. For CD4 T-cell activation, CD4 cells were isolated from the spleens of mice by magnetic bead technology (Miltenyi, Auburn, CA) according to the manufacturer's instructions. The 24-well tissue culture plates were precoated with 1 μg/mL each anti-CD3 and anti-CD28. Purified CD4 cells were incubated at 2 × 106 cells/mL on antibody-coated plates for about 40 hours in the presence or absence of interleukin-12 (IL-12) (10 ng/mL, Biosource, Camarillo, CA) plus anti–IL-4 (10 μg/mL 11B11, from American Tissue Culture Collection, prepared by standard methods), harvested, and replated on fresh plates with 20 ng/mL IL-2 (Biosource) in the continued presence or absence of IL-12 plus anti–IL-4. Cells were maintained at about 106 cells/mL, and fresh media and cytokines were added every 2 days. Except where indicated otherwise, cells were analyzed by FACS and in rolling assays on day 8.
RT-PCR
Semiquantitative RT-PCR for human and murine FucT-VII, C2GlcNAcT-I, or dihydrofolate reductase (DHFR) were carried out exactly as previously described.5,6 14 Primers specific for either human or mouse FucT-VII, primers that are identical for both human and mouse C2GlcNAcT-I, and primers that are identical for mouse and human DHFR were used.
Parallel plate attachment and rolling assay
The interaction of cells with E-selectin and P-selectin was assayed in a parallel plate flow chamber (Glycotech, Rockville, MD). Monolayers of Chinese hamster ovary (CHO) cells stably transfected with either E-selectin (CHO/E) or P-selectin (CHO/P) were grown to confluence in 35-mm tissue culture plates and served as the rolling substrate. CHO/E cells expressed E-selectin at levels similar to tumor necrosis factor (TNF)–activated human umbilical vein endothelial cells (HUVEC), whereas these CHO/P cells expressed P-selectin at levels 2- to 3-fold higher (data not shown). Neutrophils and activated CD4 cells, prepared as described above, at a concentration of 1 × 106 cells/mL, and transfectants and cell lines at a concentration of 0.5 × 106 cells/mL, were introduced into the flow chamber in a buffer of Dulbecco modified Eagle medium (DMEM) supplemented with 0.1% serum. Shear stress in the flow chamber was controlled by a syringe pump (Harvard Apparatus, Holliston, MA), and except for those shown in Figure 6, were maintained constant at 1.5 dynes/cm.2 Images were obtained using a Nikon Eclipse TE300 inverted microscope. Data analysis was performed using Celltrak software developed by Compix (Cranberry Township, PA), as previously described.3 Briefly, a rolling event is defined as a rolling cell that can be tracked between sequential images separated by a defined time delay, here 2 seconds. The total number of rolling events was collected for 50 sequential images. Data are presented as mean ± SD rolling events or rolling velocity from multiple analyses of the indicated cells.
FACS
Cells were stained with directly conjugated monoclonal antibody (mAb) 1B11 (glycosylation-dependent epitope of CD43; Pharmingen, San Diego, CA), S11 (CD43, kindly supplied by Dr Tom Waldschmidt, University of Iowa, Iowa City), or Gr-1 (neutrophils/Ly6G) (Pharmingen). For one-color staining with E- or P-selectin chimeras,2 cells were stained with purified E- or P-selectin/IgM chimeras2 (E-RIgM or P-RIgM) (kindly supplied by Dr Lloyd Stoolman, University of Michigan, Ann Arbor) followed by Cy5-conjugated, mouse absorbed goat antihuman IgM (Jackson Immunoresearch, West Grove, PA). For 2-color staining of activated T cells, cells were stained with purified E- or P-selectin/IgM chimeras as above, followed by 1B11-fluorescein isothiocyanate (FITC). All staining was carried out in the presence of 2.4G2/Fc block (kindly supplied by Dr Tom Waldschmidt). Analysis was carried out on a FACScalibur (Becton Dickinson, Mountain View, CA) equipped with Cellquest software.
Results and discussion
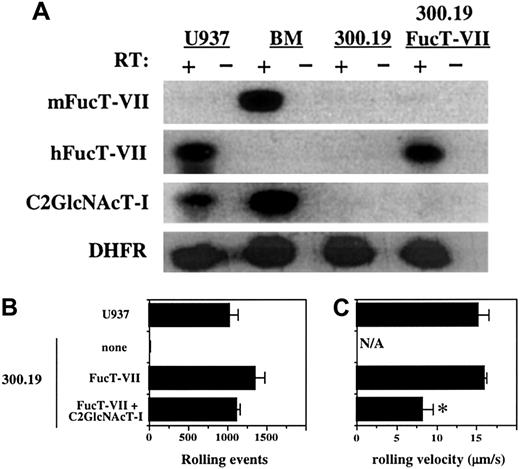
A survey of multiple hematopoietic cell lines revealed only one that failed to express detectable C2GlcNAcT-I, as assessed by RT-PCR (data not shown). This cell line, a murine pre-B-cell line called 300.19,13 also fails to express detectable FucT-VII or FucT-IV. We and others have previously shown that enforced expression of FucT-VII in any cell line examined confers the ability to attach and roll on E-selectin.3 6 The 300.19 cells were therefore stably transfected with human FucT-VII cDNA, and clones were selected that expressed levels of FucT-VII messenger RNA (mRNA) approximately equivalent to myeloid cells (Figure 1A and data not shown). Analysis of representative clones is shown in Figure 1. The 300.19/FucT-VII cells rolled well on E-selectin, with the number of rolling events approaching that of the myeloid cell line U937 (Figure 1B). Importantly, the rolling velocity of 300.19/FucT-VII cells was also quite similar to that of U937 cells (Figure 1C). The 300.19 cells stably transfected with C2GlcNAcT-I alone did not exhibit any interactions with either E-selectin or P-selectin (data not shown). When 300.19/FucT-VII cells were stably transfected with C2GlcNAcT-I cDNA, the number of rolling events did not change (Figure 1B), but the velocity of these rolling cells was lower (Figure 1C). These data demonstrate that lymphoid cells can attach and roll on E-selectin in the absence of C2GlcNAcT-I, and that C2GlcNAcT-I can, in the presence of FucT-VII, create new ligands, modify existing ligands, or both.
Enforced expression of FucT-VII in a C2GlcNAcT-I− lymphoid cell line confers binding to E-selectin.
(A) Expression of FucT-VII and C2GlcNAcT-I in 300.19 cells and 300.19/FucT-VII cells. RT-PCR analysis was performed as described in “Materials and methods.” Murine bone marrow and U937 cells were used as positive controls. Levels of FucT-VII mRNA in 300.19/FucT-VII/C2GlcNAcT-1(L) cells were similar to those of FucT-VII in 300.19/FucT-VII cells (data not shown). (B) Rolling of 300.19, 300.19/FucT-VII, and 300.19/FucT-VII/C2GlcNAcT-I cells on CHO/E-selectin. Data are presented as mean ± SD of total rolling events. (C) Rolling velocity of 300.19, 300.19/FucT-VII, and 300.19/FucT-VII/C2GlcNAcT-I cells on E-selectin. Rolling velocity (± SD) of cells from panel B are presented. For both panels B and C, U937 cells are included for comparison and as a positive control. Data represent one of 4 similar experiments. Asterisk indicates statistically different (P < .05) from 300.19/FucT-VII.
Enforced expression of FucT-VII in a C2GlcNAcT-I− lymphoid cell line confers binding to E-selectin.
(A) Expression of FucT-VII and C2GlcNAcT-I in 300.19 cells and 300.19/FucT-VII cells. RT-PCR analysis was performed as described in “Materials and methods.” Murine bone marrow and U937 cells were used as positive controls. Levels of FucT-VII mRNA in 300.19/FucT-VII/C2GlcNAcT-1(L) cells were similar to those of FucT-VII in 300.19/FucT-VII cells (data not shown). (B) Rolling of 300.19, 300.19/FucT-VII, and 300.19/FucT-VII/C2GlcNAcT-I cells on CHO/E-selectin. Data are presented as mean ± SD of total rolling events. (C) Rolling velocity of 300.19, 300.19/FucT-VII, and 300.19/FucT-VII/C2GlcNAcT-I cells on E-selectin. Rolling velocity (± SD) of cells from panel B are presented. For both panels B and C, U937 cells are included for comparison and as a positive control. Data represent one of 4 similar experiments. Asterisk indicates statistically different (P < .05) from 300.19/FucT-VII.
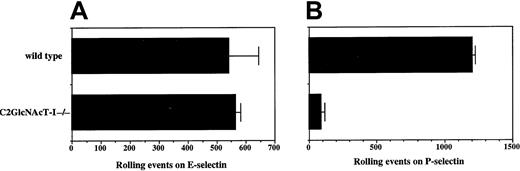
We therefore examined the rolling of neutrophils from C2GlcNAcT-I−/− and WT mice on both E- and P-selectin. The number of rolling cells for WT and C2GlcNAcT-I−/−neutrophils was nearly identical for E-selectin (Figure2A), and there was no significant difference in rolling velocity (data not shown). In contrast, rolling of C2GlcNAcT-I−/− neutrophils on P-selectin was nearly abolished, with less than 10% remaining, compared to WT (Figure 2B). Therefore, expression of C2GlcNAcT-I in normal neutrophils is essential for attachment and rolling on P-selectin, but is not required for attachment and rolling on E-selectin, at least under these conditions of ligand density and shear.
Rolling of neutrophils from C2GlcNAcT-I−/−and WT mice on E-selectin and P-selectin.
Rolling of purified (80% Gr-1+) bone marrow neutrophils on CHO cells expressing either E-selectin (A) or P-selectin (B) at 1.5 dynes/cm2 was analyzed as described above. Data are presented as mean ± SD of total rolling events. One of 8 experiments.
Rolling of neutrophils from C2GlcNAcT-I−/−and WT mice on E-selectin and P-selectin.
Rolling of purified (80% Gr-1+) bone marrow neutrophils on CHO cells expressing either E-selectin (A) or P-selectin (B) at 1.5 dynes/cm2 was analyzed as described above. Data are presented as mean ± SD of total rolling events. One of 8 experiments.
These results, which were obtained in a standard attachment and rolling assay at 1.5 dynes/cm2, contrast with those obtained by FACS analysis of the binding of selectin/IgM chimeras.10We therefore re-examined the binding of E-RIgM and P-RIgM to neutrophils from C2GlcNAcT-I−/− and WT mice. As previously shown,10 the binding of P-RIgM was abolished, and the binding of E-RIgM was significantly diminished, on neutrophils from C2GlcNAcT-I−/− mice, as compared to WT (data not shown). We have also observed that certain transfected cell lines that roll well on P-selectin do not detectably bind P-RIgM (data not shown). Collectively, these data show clearly that binding of selectin/Ig chimeric proteins does not necessarily predict rolling behavior. This discordance between selectin chimera binding and bona fide rolling assays likely reflects differences inherent in the binding of a soluble protein to a cell surface in the absence of shear, compared to interactions between cell surface bound molecules under shear.
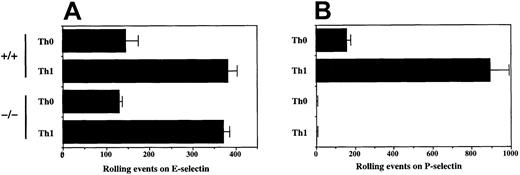
The results obtained with neutrophils were extended to analysis of activated T cells. Previous work has shown that Th1 cells, generated by activation in the presence of IL-12, have high levels of ligands for both E- and P-selectin, as assessed both by standard rolling assays and by staining with E-RIgM and P-RIgM.14 15 CD4 cells were isolated from WT and C2GlcNAcT-I−/− mice, activated in the presence (Th1) or absence (Th0) of IL-12 plus anti–IL-4, and analyzed for their ability to roll on the endothelial selectins. As expected, Th1 cells from WT mice rolled well on both E- and P-selectin. In contrast, Th1 cells from C2GlcNAcT-I−/− mice did not exhibit detectable rolling on P-selectin, whereas they rolled as well as WT on E-selectin (Figure 3). Th0 cells from both WT and C2GlcNAcT-I−/− mice showed similarly low levels of rolling on E-selectin (Figure 3). These results parallel those obtained in neutrophils and indicate that C2GlcNAcT-I is absolutely required for P-selectin ligands in T cells, but is dispensable for at least some E-selectin ligands, under these assay conditions.
Rolling of activated T cells from C2GlcNAcT-I−/− and WT mice on E-selectin and P-selectin.
CD4 cells were purified and activated in the presence (Th1) or absence (Th0) of IL-12 plus anti–IL-4 as described in “Materials and methods,” and analyzed for rolling on (A) CHO/E-selectin or (B) CHO/P-selectin as described above. Data are presented as mean ± SD of total rolling events. One of 7 experiments.
Rolling of activated T cells from C2GlcNAcT-I−/− and WT mice on E-selectin and P-selectin.
CD4 cells were purified and activated in the presence (Th1) or absence (Th0) of IL-12 plus anti–IL-4 as described in “Materials and methods,” and analyzed for rolling on (A) CHO/E-selectin or (B) CHO/P-selectin as described above. Data are presented as mean ± SD of total rolling events. One of 7 experiments.
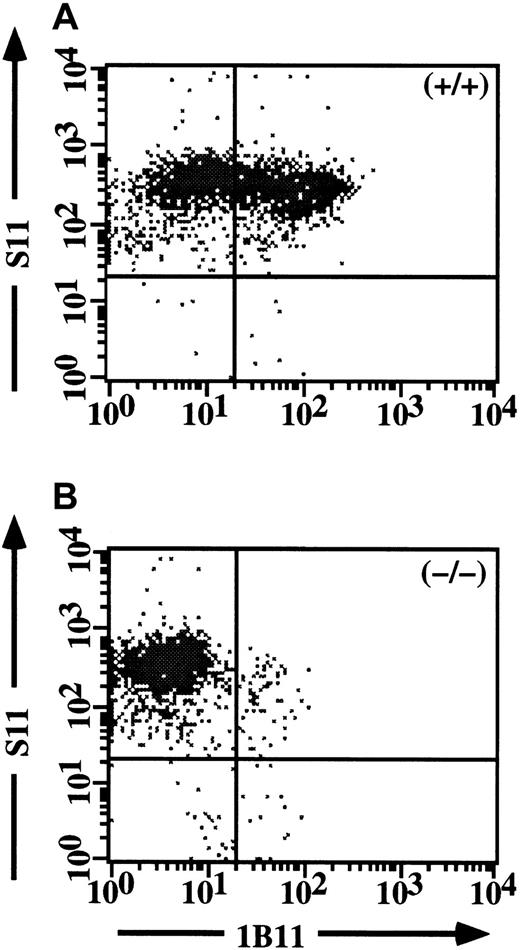
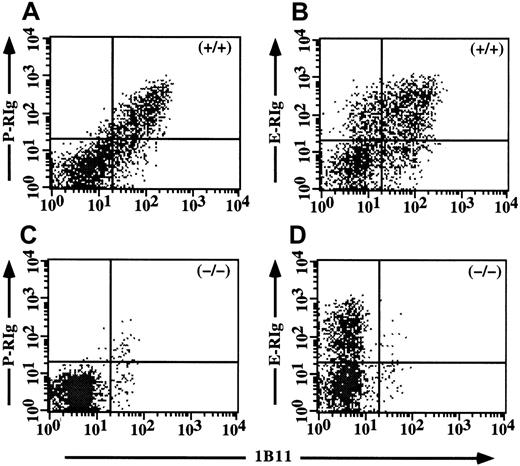
As an independent approach toward confirming the crucial role of C2GlcNAcT-I in P-selectin ligands but not in E-selectin ligands in activated CD4 cells, we first determined whether the 1B11 mAb, which has been shown to identify C2GlcNAcT-I-dependent epitopes on myeloid cells,10 also did so in CD4 cells. Th1 cells from WT and C2GlcNAcT-I−/− mice were analyzed in 2 colors with 1B11 versus S11, which recognizes all protein isoforms of CD43, on which most of the 1B11 epitopes are expressed.16 17 These experiments showed that 1B11 staining identified a clear subset of Th1 cells from WT mice (Figure 4A), but virtually no cells from C2GlcNAcT-I−/− mice (Figure 4B), with no difference in the levels of CD43 protein. Expression of C2GlcNAcT-I in CD4+ T cells can therefore be assessed by 1B11 staining in CD43+/+ mice.
Expression of the 1B11 epitope in CD4 cells requires C2GlcNAcT-I.
Staining of CD43 (S11) versus 1B11 for WT Th1 cells (A) and C2GlcNAcT-I−/− Th1 cells (B). 1B11 staining is abrogated in Th1 cells from the C2GlcNAcT-I−/− mice, but identifies a discrete subset of Th1 cells from WT mice.
Expression of the 1B11 epitope in CD4 cells requires C2GlcNAcT-I.
Staining of CD43 (S11) versus 1B11 for WT Th1 cells (A) and C2GlcNAcT-I−/− Th1 cells (B). 1B11 staining is abrogated in Th1 cells from the C2GlcNAcT-I−/− mice, but identifies a discrete subset of Th1 cells from WT mice.
We therefore used 1B11 staining as a reporter of C2GlcNAcT-I activity in 2-color FACS analysis of selectin ligand expression, measured using E-RIgM and P-RIgM binding. In contrast to myeloid cells and cell lines, staining with E-RIgM or P-RIgM on primary T cells accurately predicts rolling behavior in this assay4,14 15 (and see below). Two-color FACS analysis of Th1 cells from WT mice with 1B11 versus P-RIgM showed concordant staining, across the entire range of 1B11 and P-RIgM staining (Figure 5A). In contrast, 2-color staining of WT Th1 cells with 1B11 versus E-RIgM displayed a distinct pattern, with a significant 1B11+/E-RIgM−/lo population and a 1B11-/E-RIgM+ population evident, in addition to the 1B11+/E-RIgM+ subset (Figure 5B). An analysis of C2GlcNAcT-I−/− Th1 cells carried out in parallel showed very few P-RIgM+ or 1B11+ cells (Figure5C), but disclosed an E-RIgM+/1B11− subset (Figure 5D). These data reinforce the relationship between C2GlcNAcT-I expression and expression of P-selectin ligands on activated T cells, and further indicate that no direct relationship exists between C2GlcNAcT-I expression and expression of E-selectin ligands, at least in primary T cells. These data independently support the lack of an absolute requirement for C2GlcNAcT-I in generation of E-selectin ligands in activated CD4+ T cells.
Expression of C2GlcNAcT-I correlates with P-selectin ligands but not with E-selectin ligands on activated T cells.
Two-color analysis of 1B11 versus P-RIgM (A) or E-RIgM (B) in WT Th1 cells. Concordant staining is seen with P-RIgM, but not with E-RIgM, where 1B11+/E-RIgM− and 1B11−/E-RIgM+ subsets are also seen. (C, D) Analysis of C2GlcNAcT-I−/− Th1 cells as for panels A and B. As expected, no staining is seen for 1B11 or P-RIgM (C), but a clear subset of cells is E-RIgM+ but 1B11−(D).
Expression of C2GlcNAcT-I correlates with P-selectin ligands but not with E-selectin ligands on activated T cells.
Two-color analysis of 1B11 versus P-RIgM (A) or E-RIgM (B) in WT Th1 cells. Concordant staining is seen with P-RIgM, but not with E-RIgM, where 1B11+/E-RIgM− and 1B11−/E-RIgM+ subsets are also seen. (C, D) Analysis of C2GlcNAcT-I−/− Th1 cells as for panels A and B. As expected, no staining is seen for 1B11 or P-RIgM (C), but a clear subset of cells is E-RIgM+ but 1B11−(D).
The above data also suggest that the induction of P-selectin ligands in developing Th1 cells involves up-regulation of C2GlcNAcT-I in response to IL-12. In studies to be reported elsewhere, we show that this IL-12–induced up-regulation of C2GlcNAcT-I, but not the constitutive low level present in naive CD4 cells, is dependent on expression of Stat4, whereas FucT-VII induction is largely independent of Stat4 (S.J. White and colleagues, manuscript submitted). If C2GlcNAcT-I is not required for formation of E-selectin ligands, independent control of C2GlcNAcT-I and FucT-VII should permit independent control of expression of ligands for E-selectin or P-selectin. Consistent with this idea, and with the data above demonstrating a lack of a requirement for C2GlcNAcT-I in E-selectin ligand formation, we have recently shown that normal IgG plasma cells express high levels of FucT-VII but little or no C2GlcNAcT-I, and roll well on E-selectin but poorly or not at all on P-selectin (Underhill GH, Kansas GS. IgG plasma cells display a unique spectrum of leukocyte adhesion and homing molecules, submitted for publication). The E-selectin binding/P-selectin nonbinding phenotype of these plasma cells is strikingly similar to that of the C2GlcNAcT-I−/−CD4 cells described in the present report. These data further reinforce the lack of an absolute requirement for C2GlcNAcT-I in E-selectin ligand formation, at least in lymphocytes.
Taken together, these results suggest a new model in which the independent control of expression of ligands for E-selectin versus P-selectin in activated CD4+ T cells is accomplished through independent control of glycosyltransferases that are differentially required for E-selectin ligands versus P-selectin ligands. FucT-VII is absolutely required for selectin ligand formation in T cells, but lower levels are needed for maximal P-selectin ligands than for maximal E-selectin ligands.4 Quantitative control of FucT-VII levels will therefore contribute to determining whether activated T cells express ligands for E-selectin, P-selectin, or both. The data in the present report indicate that down-regulation or inhibition of C2GlcNAcT-I would allow for expression of E-selectin ligands but not P-selectin ligands. Other enzymes such as members of the ST3Gal family of α2,3-sialyltransferases may also be regulated in activated CD4 cells or differentially required for ligands for E-selectin versus P-selectin. Independent control of these different enzymes, coupled with differential requirements for individual enzymes in formation of ligands for E-selectin versus P-selectin, represents a novel level of regulation of lymphocyte traffic.
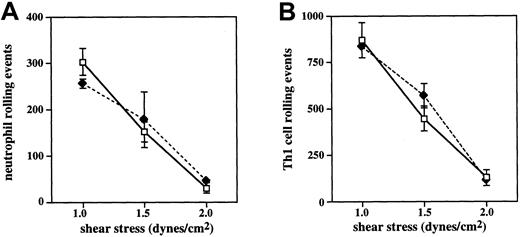
The present results appear to differ somewhat from observations obtained in vivo (see accompanying report by Sperandio and coworkers28). It is possible that a requirement for C2GlcNAcT-I exists for rolling on E-selectin only at shear stresses above the 1.5 dynes/cm2 used in the present studies. To address this possibility, we analyzed the rolling of WT and C2GlcNAcT-I−/− Th1 cells and neutrophils on E-selectin across a range of shear values. The results show that, for both Th1 cells and neutrophils, no difference is detectable between WT and C2GlcNAcT-I−/− cells up to 2 dynes/cm2(Figure 6). At 2 dynes/cm,2attachment and therefore rolling on E-selectin is largely lost, preventing analysis at higher shear rates. The absence of significant levels of attachment to E-selectin at this shear level reflects the comparatively lower efficiency of E-selectin for attachment, as well as the known differences between the in vitro situation and that in vivo, including the different geometries and hemodynamics and the presence of red blood cells, all of which make attachment in vivo much more efficient than in vitro. However, data presented in the accompanying report28 show that, in vivo, little if any difference exists between rolling flux values of C2GlcNAcT-I−/− and WT neutrophils at shear stress levels less than 3.0 dynes/cm2, a shear stress level at which most initial attachment of leukocytes occurs, whereas a role for C2GlcNAcT-I is revealed at shear rates above 3 dynes/cm2. Our data are therefore consistent with the hypothesis that a role for C2GlcNAcT-I in E-selectin ligand biosynthesis is in part a function of the shear level. Under conditions of low stringency, such as with low shear or high ligand density, C2GlcNAcT-I is not required for efficient interaction with E-selectin, whereas under more stringent conditions of high shear or low ligand density, modification of E-selectin ligands or creation of new ligands by C2GlcNAcT-I is required. This shear dependence of a requirement for C2GlcNAcT-I in E-selectin ligand biosynthesis represents a novel relationship between enzymatic requirement for selectin ligands and shear, one not evident with FucT-VII.
Shear dependence of attachment and rolling on E-selectin.
Neutrophils (A) and Th1 cells (B) from WT (open symbols) and C2GlcNAcT-I−/− (closed symbols) mice were analyzed as above for rolling across a range of shear values. For both cell types, no difference was detected between WT and C2GlcNAcT-I−/−cells up to 2 dynes/cm.2
Shear dependence of attachment and rolling on E-selectin.
Neutrophils (A) and Th1 cells (B) from WT (open symbols) and C2GlcNAcT-I−/− (closed symbols) mice were analyzed as above for rolling across a range of shear values. For both cell types, no difference was detected between WT and C2GlcNAcT-I−/−cells up to 2 dynes/cm.2
Regarding differences between our results and those of Ellies and coworkers,10 several distinct factors likely play a role. The controlled detachment assay used by Ellies and colleagues bypasses the initial attachment step, and consequently allows for significant interactions with E-selectin at levels of shear considerably higher than in our attachment and rolling assay. We also note that coexpression of C2GlcNAcT-I in stably transfected 300.19/FucT-VII cells decreased the rolling velocity of those cells, without increasing the number of rolling cells. Because a slower velocity indicates a stronger interaction, these observations are consistent with the decrement in rolling on E-selectin seen in the controlled detachment assay in the study by Ellies and associates.10 It is also possible that differences in the nature, presentation, or density of the ligand (E-selectin chimera versus native E-selectin) contribute to the observed differences between the present data and those obtained in a controlled detachment assay. In this regard, the previous studies by Ellies and coworkers10 were performed on E-selectin chimera that we estimate to represent significantly lower densities than is expressed on the stably transfected CHO cells used here, even assuming appropriate orientation of the plate-bound protein. Ligand density is well known to affect both the attachment rate and the number and velocity of rolling cells in these types of in vitro assays. Additionally, presentation of E-selectin in the context of the plasma membrane of living cells may be advantageous compared with molecules immobilized to plastic, particularly those immobilized at low density, such as in the study by Ellies and colleagues. Taken together, the differences inherent in the 2 types of assays, along with the lower densities and different presentations of E-selectin, appear to be sufficient to account for the apparent differences between our results and those of Ellies and coworkers.10
The combined results indicate that formation of at least some E-selectin ligands does not absolutely require C2GlcNAcT-I, but also that this enzyme can either modify existing ligands or contribute to the generation of distinct ligands that are specialized to operate under conditions of limiting ligand or high shear. The present results do not allow us to distinguish between the modification of existing ligands and the generation of novel ones, particularly as the molecular identity of the relevant E-selectin ligands has not been firmly established. It is possible that those ligands for E-selectin that exist in C2GlcNAcT-I−/− mice are expressed on N-linked glycans or on unbranched core 1 O-linked structures that reside on distinct molecular scaffolds. However, our results do not rule out the possibility that core 2 or related structures are involved in E-selectin ligand biosynthesis. At least 2 other enzymes capable of forming core 2 linkages have been identified and cloned, and one of them is expressed in leukocytes,18-20 making it possible that this enzyme is involved in E-selectin ligand formation. However, the present results indicate that the C2GlcNAcT-I enzyme studied here is essential only for P-selectin ligand synthesis and plays a comparatively less important role in E-selectin ligand construction.
If C2GlcNAcT-I is not absolutely required for E-selectin ligand formation, then why do C2GlcNAcT-I−/− mice exhibit a defect in neutrophil recruitment to the inflamed peritoneum similar to that in E-selectin/P-selectin−/− or FucT-VII−/− mice? Evidence presented by Ellies and colleagues10 shows a greater loss of L-selectin ligands on neutrophils than of ligands for either E-selectin or P-selectin. We speculate that L-selectin ligands on endothelium at sites of inflammation21,22 are also sharply reduced or absent in C2GlcNAcT-I−/− mice. The cumulative effect of the loss of both of these classes of L-selectin ligands, in addition to the loss of P-selectin ligands, may explain the severe defect in neutrophil recruitment in C2GlcNAcT-I−/− mice. Consistent with this, both L-selectin−/− and P-selectin−/− mice have significant, albeit milder, defects in this assay, and L-selectin/P-selectin−/− mice exhibit defects similar to C2GlcNAcT-I−/− mice.23-25 Moreover, the combination of L-selectin and P-selectin mAb virtually eliminates neutrophil recruitment in this model, and E-selectin−/−mice exhibit no defect unless treated with anti–P-selectin mAb.26 27 Taken together with all of the present data, these findings make it likely that the defect in neutrophil recruitment in the acute peritonitis model is due to the complete or nearly complete loss of ligands for both L- and P-selectin, with a lesser effect on E-selectin ligands.
In summary, we have used a well-characterized attachment and rolling assay to show that in transfected cell lines, normal neutrophils and primary T cells, expression of C2GlcNAcT-I is essential for generation of ligands for P-selectin, but is not absolutely required for generation of ligands for E-selectin. These results, in combination with the accompanying report,28 clarify previously discrepant results, and identify C2GlcNAcT-I as the first enzyme selectively required for biosynthesis of only a subset of selectin ligands. Independent control of expression of C2GlcNAcT-I and other enzymes required for selectin ligand formation represents a novel mechanism of regulation of leukocyte traffic.
The authors thank Dr Thomas Waldschmidt, University of Iowa, for provision of reagents and helpful discussions.
Supported by National Institutes of Health grants HL55647 (to G.S.K.), DK48247 (to J.D.M.), and F32CA79130 (to L.G.E.), and by grant 003003N from the American Heart Association (to K.R.S.). G.S.K. is an Established Investigator of the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Geoffrey S. Kansas, Department of Microbiology-Immunology, Northwestern University Medical School, 303 E Chicago Ave, Chicago, IL 60611; e-mail: gsk@northwestern.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal