Protein kinase C (PKC)–potentiated inhibitory phosphoprotein of myosin phosphatase (CPI) was detected in human platelets. Like smooth muscle CPI-17, in vitro phosphorylation of platelet CPI by PKC inhibited the activity of myosin phosphatase containing the PP1δ catalytic subunit and the 130-kd myosin-binding subunit (MBS). Treatment of intact platelets with thrombin or the stable thromboxane A2 analog STA2 resulted in increased phosphorylation of both CPI and MBS at Thr-696, whereas phorbol myristate acetate (PMA) and the Ca++ ionophore ionomycin only induced CPI phosphorylation. PMA induced slow adenosine triphosphate (ATP) secretion of fura 2–loaded platelets with no change in cytosolic Ca++. The PMA-induced increase in CPI phosphorylation preceded phosphorylation of 20-kd myosin light chain (MLC20) at Ser-19 and ATP secretion. The PKC inhibitor, GF109203X, inhibited PMA-induced phosphorylation of CPI and MLC20 with similar IC50 values. These findings suggest that the activation of PKC by PMA induces MLC20phosphorylation by inhibiting myosin phosphatase through phosphorylation of CPI. STA2-induced MLC20phosphorylation was also diminished but not abolished by GF109203X, even at high concentrations that completely inhibited STA2-induced CPI phosphorylation. A combination of the Rho-kinase inhibitor Y-27632 and GF109203X led to a further decrease in STA2-induced MLC20 phosphorylation, mainly because of a significant inhibition of MBS phosphorylation at Thr-696. Inhibition of STA2-induced ATP release by Y-27632, GF109203X, or both appeared to correlate with the extent of MLC20 phosphorylation. Thus, CPI phosphorylation by PKC may participate in inhibiting myosin phosphatase, in addition to the Rho-kinase–mediated regulation of myosin phosphatase, during agonist-induced platelet secretion.
Introduction
Phosphorylation of the 20-kd myosin light chain (MLC20) at Ser-19 is thought to be one of the primary steps in the activation of actomyosin contractile events, which involve platelet shape change and secretion.1-7 The extent of MLC20 phosphorylation is regulated not only by protein kinases, such as Ca++-dependent MLC kinase8and Ca++-independent Rho-kinase,9 but also by myosin phosphatase.10,11 Platelet shape change, the earliest response induced by agonists, is a prerequisite for full platelet activation, including secretion and aggregation.1,2 Platelet shape change is regulated by MLC20 phosphorylation either through RhoA/Rho-kinase activation or through Ca++-dependent MLC kinase activation.4 Activation of protein kinase C (PKC) is not likely to be involved in platelet shape change.1Inhibition of myosin phosphatase activity appears to be an important mechanism for increasing the Ca++ sensitivity of MLC20 phosphorylation and platelet secretion, as is the case for smooth muscle.10,11 Myosin phosphatase is composed of 3 subunits—a catalytic subunit of the type 1 protein phosphatase δ isoform (PP1δ) and 2 noncatalytic subunits (a 130-kd myosin-binding subunit (MBS) and a 20-kd subunit).12-14Different mechanisms have been proposed to account for the inhibition of myosin phosphatase,10,11 including dissociation of subunits of the holoenzyme by arachidonate,15,16phosphorylation of MBS by RhoA/Rho-kinase,17,18 or phosphorylation of CPI-17 through PKC activation.19-23 The regulation of myosin phosphatase occurs through phosphorylation of its regulatory subunit, the 130-kd MBS, by Rho-kinase,18 a downstream target of the small GTPase RhoA.24Phosphorylation of MBS by Rho-kinase inhibits myosin phosphatase activity,18 and we reported that Thr-695 on chicken MBS (Thr-696 in human MBS) is the major Rho-kinase phosphorylation site involved in the inhibition of phosphatase activity.25 With use of an antibody that specifically recognizes MBS phosphorylated at Thr-695, we have shown that phosphorylation of Thr-695 is increased in Swiss 3T3 cells after stimulation by lysophosphatidic acid, which is thought to activate the RhoA pathway.25 Treatment of intact platelets with agonists, including thrombin, thromboxane A2 (TXA2) mimetic STA2, and epinephrine, leads to rapid phosphorylation of MBS and to inactivation of myosin phosphatase.26 27 However, it remains to be clarified whether phosphorylation of MBS at Thr-696 is increased in intact human platelets in response to various agonists.
Another possible candidate for the inhibition of myosin phosphatase in platelets is PKC, an event that may be independent of the RhoA/Rho-kinase pathway. In permeabilized platelets, GTPγS and phorbol ester enhanced Ca++ sensitivity of secretion without inositol phospholipid hydrolysis,7 suggesting that PKC activation contributes to Ca++ sensitivity of platelet secretion. It has been suggested that PKC inhibits myosin phosphatase in smooth muscle by the phosphorylation of CPI-17, a 17-kd PKC-potentiated inhibitory protein of myosin phosphatase. CPI-17 is a phosphorylation-dependent inhibitory protein for myosin phosphatase selectively expressed in smooth muscle.19-21Phosphorylation of CPI-17 at Thr-38 by PKC enhances the inhibitory potency with respect to myosin phosphatase, approximately 1000-fold in vitro,20,21 and the phosphorylated CPI-17 induces contraction in permeabilized smooth muscle.22,23,28 There has been no evidence that CPI-17 or its homologous protein is present and is involved in the in vivo regulation of myosin phosphatase in human platelets. Moreover, the relative importance of the Rho-kinase–mediated pathway and the PKC-mediated pathway for regulating myosin phosphatase activity in platelet secretion has remained to be elucidated. We report here that platelet CPI has a slightly higher apparent molecular mass than does smooth muscle CPI-17.19 We also provide evidence that phosphorylation of platelet CPI by PKC regulates myosin phosphatase activity in vitro and in vivo; this is in addition to the inhibition that results from MBS phosphorylation at Thr-696 by Rho-kinase.
Materials and methods
Materials
Y-27632 [(+)-(R)-trans-4-(1-aminoethyl)-N-(4-pynidyl) cyclohexanecarboxamide dihydrochloride, monohydrade] was a kind gift from WelFide (Osaka, Japan). Other drugs and suppliers were as follows: GF109203X (2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)-maleimide) and ionomycin from Calbiochem-Novabiochem (La Jolla, CA); STA2 [9, 11-epithio-11, 12-methano-thromboxane A2] from Ono Pharmaceutical (Osaka, Japan); fura 2-am from Dojindo Laboratory (Kumamoto, Japan); phorbol 12-myristate 13-acetate (PMA) from Sigma Chemical (St Louis, MO); and adenosine triphosphate (ATP)γS from Boehringer Mannheim GmbH (Mannheim, Germany); [γ-32P]ATP (111 τBq/mmol) from NEN Life Science Products (Boston, MA). Polyclonal antibodies against the PP1δ catalytic subunit and 130 kd MBS were raised with synthesized fragments of respective proteins and used after purification.26,29 Polyclonal anti–CPI-17 antibody raised against recombinant CPI-17 was affinity-purified.30Polyclonal antimyosin light chain (anti-MLC) antibody was raised against mixed light chains of chicken gizzard myosin. These anti-MLC antibodies recognized both the 20-kd and the 17-kd light chains of human platelet myosin. A monoclonal antibody against MLC phosphorylated at Ser-19 (anti–pSer19-MLC20)31 was kindly provided by Y. Sasaki (Kitasato University, Tokyo, Japan). Polyclonal rabbit antibodies against MBS phosphorylated at Thr 695 (anti–pThr695-MBS)25 and CPI-17 phosphorylated at Thr-38 (anti–pThr38–CPI-17)30 were generated, absorbed onto the respective unphosphorylated peptide resins, and affinity-purified. PKC (a mixture of α- and β-isoforms) was prepared to apparent homogeneity from fresh platelets, as described.32 CPI-17 was purified from the porcine aorta, as described.30
Measurement of PKC-potentiated inhibitory phosphoprotein of myosin phosphatase in human platelets
We estimated the amount of PKC-potentiated inhibitory phosphoprotein of myosin phosphatase (CPI) in human platelets by immunoblot analysis, using an anti–CPI-17 antibody. Immunoblot analysis was carried out as described.27,28 Quantitative estimation of the levels of CPI in human platelets was performed densitometrically, using a Personal Scanning Imager (Amersham Pharmacia Biotech AB, Uppsala, Sweden) in conjugation with the Imagequant program run on a Dell Personal Computer (Austin, TX) by scanning the immunoreactive band after immediately photographing the visualized band.29 The signal was then compared with that of known amounts of purified porcine smooth muscle CPI-17.
Immunoprecipitation of platelet CPI
Platelet suspension (109/mL) was dissolved in ice-cold lysis buffer (1% NP-40, 20 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 2 mM EDTA, 2 mM sodium vanadate, and an appropriate amount of protease inhibitor cocktail tablets [Boehringer Mannheim GmbH]). Immunoprecipitation of platelet lysates was performed after clarification of the samples by centrifugation at 15 000gfor 10 minutes, and the soluble fraction, precleared with protein A–Sepharose CL-4B (Amersham Pharmacia Biotech AB), was incubated with 30 μL anti–CPI-17 antibody at 4°C for 1 hour.27 28The immune complex was precipitated by adding protein A–Sepharose CL-4B (Amersham Pharmacia Biotech AB), incubating it for an additional 1 hour at 4°C, and washing the beads 3 times with lysis buffer. These preparations were used to detect the in vitro phosphorylation of CPI and for assay of the phosphatase activity.
In vitro CPI phosphorylation by PKC and its effect on myosin phosphatase activity
Phosphorylation of the anti–CPI-17 immunoprecipitate was carried out using purified platelet PKC in the presence of 50 μM ATP with 10 mM MgCl2, 0.5 mM CaCl2, 50 μg/mL phosphatidylserine, 12.5 μg/mL diolein, 0.5 μM okadaic acid (Wako Pure Chemicals, Osaka, Japan), and 20 mM Tris-HCl, pH 7.5, at 30°C for 2 minutes.32 The kinase reaction was initiated by adding ATP and terminated by adding sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Samples were subjected to SDS-PAGE, and CPI phosphorylation was analyzed by immunoblotting, using an anti–pThr38–CPI-17 antibody.
To analyze phosphatase activities, thiophosphorylation of the anti–CPI-17 immunoprecipitate was carried out using platelet PKC in the presence of 1 mM ATPγS with 10 mM MgCl2, 0.5 mM CaCl2, 50 μg/mL phosphatidylserine, 12.5 μg/mL diolein, and 20 mM Tris-HCl, pH 7.5, at 30°C for 60 minutes. The kinase reaction initiated by adding the ATPγS solution was terminated by adding 12 mM ethyleneglycotetraacetic acid (EGTA) and 2 mM EDTA; then phosphatase activity was immediately measured. Activities of PP1, PP2A, PP2B, and PP2C were determined using [32P]-phosphorylated MLC of chicken gizzards as a substrate, as described elsewhere.26 33-35 PP1 and PP2A activities were measured in a reaction mixture (50μL) containing 20 mM Tris-HCl, pH 7.4, 50 mM NaCl, 0.1 mM EGTA, 2 mM sodium vanadate ± 10 nM okadaic acid ± heat-stable phosphatase inhibitor-2, 4 mM phenylmethylsulfonyl fluoride, and 200 μg/mL leupeptin. PP2A was completely inhibited with 10 nM okadaic acid, whereas PP1 at this concentration was little affected. PP1 can be taken as the activity that is sensitive to the inhibitor-2, and PP2A can be taken as the activity that is blocked by 10 nM okadaic acid. PP2B and PP2C activities were determined as activity in the presence of 0.5 mM CaCl2, 5 mM MgCl2, 0.5 μM okadaic acid, 1 μM calmodulin, and ± 0.15 mM trifluoperazine. PP1 and PP2A are completely inhibited by 0.5 μM okadaic acid; therefore, this activity was taken as the total activity of PP2B and PP2C. Trifluoperazine (0.15 mM) was added to completely inhibit PP2B, but PP2C was unaffected by trifluoperazine, even at 0.15 mM. PP2B activity was determined by subtracting PP2C (the activity in the presence of 0.15 mM trifluoperazine) from the total activity. Assays were made in duplicate, and the phosphatase activity was indicated as pmol 32Pi released per minute in cell samples.
Detection of agonist-induced phosphorylation of MLC20 at Ser-19, MBS at Thr-696, and CPI in human platelets
Venous blood was freshly drawn from a healthy donor who had not taken any drugs for at least 2 weeks. Human platelets (109/mL) were resuspended in Ca++-free Tyrode-HEPES buffer that contained a final concentration of 0.14 M NaCl, 2.7 mM KCl, 1 mM MgCl2, 0.1% d-glucose, 3.75 mM NaH2PO4, and 15 mM HEPES, pH 7.4. Washed platelets (300 μL) were stimulated with various agonists, without stirring, at 37°C in an PA-200 aggregometer (Kowa, Tokyo, Japan), as described.5 29 Precipitates treated with 10% trichloroacetic acid were subjected to SDS-PAGE for determination of the extent of MLC20, MBS, and CPI phosphorylation. Quantitative estimation of the level of phosphorylation was made densitometrically by scanning the immunoreactive bands after immediately photographing the visualized band. The area of an individual peak was measured above the background of the densitometric tracing and was estimated as an arbitrary unit.
Measurement of ATP secretion and cytosolic Ca++concentration in platelets.
ATP secretion was measured, using a Lumi-aggregometer (Chrono-Log, Havertown, PA), as described.5 The platelet reaction mixture consisted of 400μL washed platelet suspension (5 × 109/mL) in Ca++-free HEPES-Tyrode buffer, 50 μL Chrono-Lumi luciferase luciferin reagent, 50μL inhibitor (or vehicle), and STA2 or PMA. Washed platelet suspensions pretreated with or without the inhibitor at 37°C for 3 minutes were activated by 0.5 μM STA2 or 100 nM PMA for 3 minutes, under conditions of nonstirring. For measurement of cytosolic Ca++ concentrations, platelets loaded with fura 2-am (1 μM) were resuspended in Ca++-free HEPES-saline buffer (145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM HEPES, pH 7.4, 5 mM glucose).36 Fluorescence and ATP secretion were monitored simultaneously, in parallel samples. Fluorescence of fura 2 was measured at 340- and 380-nm excitation and at 550-nm emission using a Hitachi F-4000 fluorescence spectrometer, according to the method of Tsien et al.37 Two-milliliter aliquots of fura 2-loaded platelet suspensions (1 × 108/mL) were transferred to quartz cuvettes, maintained at 37°C in a circulating water bath, continually stirred with a small magnetic stirrer for 2 minutes, and stimulated with STA2 or PMA under conditions of non-stirring. In all experiments, the fluorescence of untreated and unloaded cells was used for subtraction of the autofluorescence. To minimize the time-dependent effects on platelet responsiveness and leakage of fura 2, experiments were designed to be completed within 1 hour.
Statistical analysis
Data were analyzed using the StatView statistical software package (Version 5; SAS Institute, Cary, NC). Data are shown as mean ± SE and were compared statistically by Student ttest (unpaired). P < .05 was considered significant.
Results
Detection and level of CPI, PKC-potentiated inhibitory phosphoprotein of myosin phosphatase, in human platelets
Recent reports have suggested that CPI-17 is almost exclusively expressed in smooth muscle tissues.19-21 We determined whether CPI is present in human platelets by immunoblot analysis using an anti–CPI-17 antibody.30 As shown in Figure1A, the anti–CPI-17 antibody detected a major immunoreactive band with an apparent molecular mass of 22 kd, higher than that in smooth muscle CPI-17 with an apparent molecular mass of 20 kd in SDS-PAGE.19 To determine whether the immunoreactive protein at 22 kd was the CPI present in human platelets, we analyzed immunoprecipitates with anti-CPI antibody using platelet extracts (Figure 1B). The procedure was to subject the protein A–Sepharose precipitates to SDS-PAGE and follow that by immunoblotting with polyclonal antibodies against the PP1δ catalytic subunit and MBS of myosin phosphatase. PP1 δ isoform and MBS of myosin phosphatase were detected at 38 kd and 130 kd, respectively, in the anti–CPI-17 immunoprecipitate of human platelets (Figure 1B). We further examined phosphatase activity of the immunoprecipitates using [γ-32P]-phosphorylated MLC as a substrate. Approximately 85% of the total phosphatase activity in the precipitate was attributed to PP1 activity (Figure 1C). We then asked whether the 22-kd immunoreactive band was phosphorylated by PKC, using the anti–CPI-17 immunoprecipitate. CPI phosphorylation was analyzed by immunoblot using an antibody that specifically recognizes CPI-17 phosphorylated at Thr-38 (anti–pThr38–CPI-17). As shown in Figure 1D, CPI phosphorylation at a Thr residue corresponding to Thr-38 in CPI-17 was increased approximately 4-fold with the addition of PKC, and this phosphorylation was inhibited by a non-isoform–selective PKC inhibitor, GF109203X.38 We then investigated the effect of CPI phosphorylation on myosin phosphatase activity in the immunoprecipitates. Because thiophosphorylation of proteins, unlike regular phosphorylation, is highly resistant to protein phosphatases,39 we used the thiophosphorylation form of CPI to determine the effect on myosin phosphatase activity. Myosin phosphatase activity was decreased by CPI thiophosphorylation and was recovered by the inhibition of CPI thiophosphorylation with GF109203X (Figure 1E). These results indicate that the immunoreactive protein at 22 kd is human platelet CPI.
Detection of CPI, an inhibitory phosphoprotein of myosin phosphatase, in human platelets.
(A) Immunoblot analysis of CPI in human platelets. Platelet lysates were applied to SDS-PAGE, and this was followed by immunoblotting using an anti–CPI-17 antibody. Lane 1, smooth muscle CPI-17 purified from porcine aorta. Lane 2, platelet CPI. Molecular weight markers are indicated on the left end. (B) Co-immunoprecipitation of platelet myosin phosphatase with anti–CPI-17 antibody. Immunoprecipitates of platelet lysates with anti–CPI-17 antibody (lane 1) or with the preimmune serum (lane 2) were immunoblotted with anti–CPI-17 antibody. Immunoprecipitates with anti–CPI-17 antibody were immunoblotted using antibodies against the PP1δ catalytic subunit and MBS, respectively. (C) Immunoprecipitates with anti–CPI-17 antibody were assayed for phosphatase activity, using [γ-32P]-phosphorylated MLC as a substrate. Data represent the mean ± SE of 3 experiments. (D) In vitro platelet CPI phosphorylation by PKC. Anti–CPI-17 immnoprecipitates from platelet lysates were incubated for 2 minutes, without or with GF109203X, in the presence of purified platelet PKC. Protein phosphorylation was analyzed by SDS-PAGE, and this was followed by immunoblot analysis using an anti–pThr38–CPI-17 antibody. Results were expressed as fold increase in the phosphorylation relative to the activity measured in the absence of PKC. Data represent the mean ± SE of 3 experiments. (E) Effect of CPI thiophosphorylation by PKC on platelet myosin phosphatase activity. Platelet anti–CPI-17 immunoprecipitates were incubated, with or without purified platelet PKC, in the presence or absence of 5 μM GF109203X for 60 minutes at 30°C, as described in “Materials and methods.” Aliquots of the reaction mixture were quenched by adding a solution that resulted in a final concentration of 12 mM EGTA and 2 mM EDTA to stop the reaction, then were kept on ice. Myosin phosphatase activity was immediately determined. Data represent the mean ± SE of 4 experiments. IP, immunoprecipitation antibody used; IB, immunobotting antibody used; Ig, cross-reacting immunoglobulin.
Detection of CPI, an inhibitory phosphoprotein of myosin phosphatase, in human platelets.
(A) Immunoblot analysis of CPI in human platelets. Platelet lysates were applied to SDS-PAGE, and this was followed by immunoblotting using an anti–CPI-17 antibody. Lane 1, smooth muscle CPI-17 purified from porcine aorta. Lane 2, platelet CPI. Molecular weight markers are indicated on the left end. (B) Co-immunoprecipitation of platelet myosin phosphatase with anti–CPI-17 antibody. Immunoprecipitates of platelet lysates with anti–CPI-17 antibody (lane 1) or with the preimmune serum (lane 2) were immunoblotted with anti–CPI-17 antibody. Immunoprecipitates with anti–CPI-17 antibody were immunoblotted using antibodies against the PP1δ catalytic subunit and MBS, respectively. (C) Immunoprecipitates with anti–CPI-17 antibody were assayed for phosphatase activity, using [γ-32P]-phosphorylated MLC as a substrate. Data represent the mean ± SE of 3 experiments. (D) In vitro platelet CPI phosphorylation by PKC. Anti–CPI-17 immnoprecipitates from platelet lysates were incubated for 2 minutes, without or with GF109203X, in the presence of purified platelet PKC. Protein phosphorylation was analyzed by SDS-PAGE, and this was followed by immunoblot analysis using an anti–pThr38–CPI-17 antibody. Results were expressed as fold increase in the phosphorylation relative to the activity measured in the absence of PKC. Data represent the mean ± SE of 3 experiments. (E) Effect of CPI thiophosphorylation by PKC on platelet myosin phosphatase activity. Platelet anti–CPI-17 immunoprecipitates were incubated, with or without purified platelet PKC, in the presence or absence of 5 μM GF109203X for 60 minutes at 30°C, as described in “Materials and methods.” Aliquots of the reaction mixture were quenched by adding a solution that resulted in a final concentration of 12 mM EGTA and 2 mM EDTA to stop the reaction, then were kept on ice. Myosin phosphatase activity was immediately determined. Data represent the mean ± SE of 4 experiments. IP, immunoprecipitation antibody used; IB, immunobotting antibody used; Ig, cross-reacting immunoglobulin.
We determined the amount of CPI in whole platelets by immunoblot analysis using purified CPI-17 from aorta smooth muscle as standard. The amount of CPI in platelets was 0.33 ± 0.04 μM (mean ± SE), which is almost the same as that in smooth muscle.21
Agonist-induced phosphorylation of CPI and MBS in intact platelets
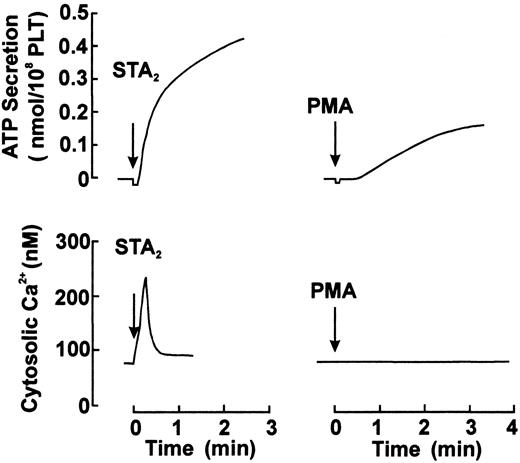
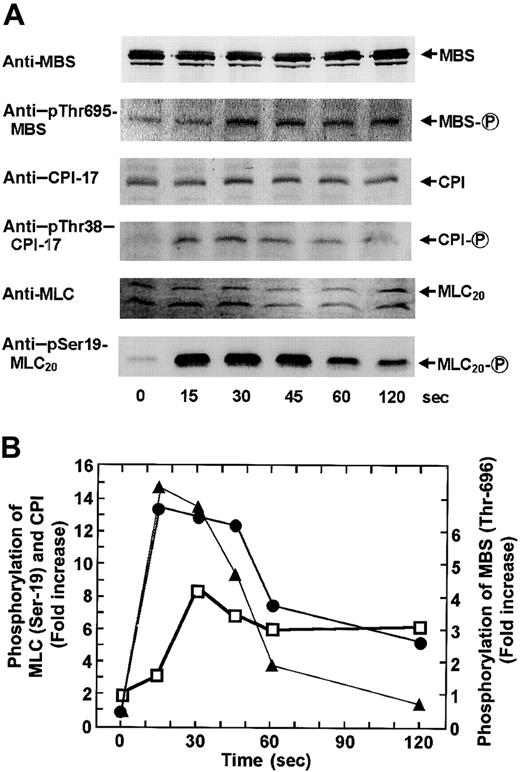
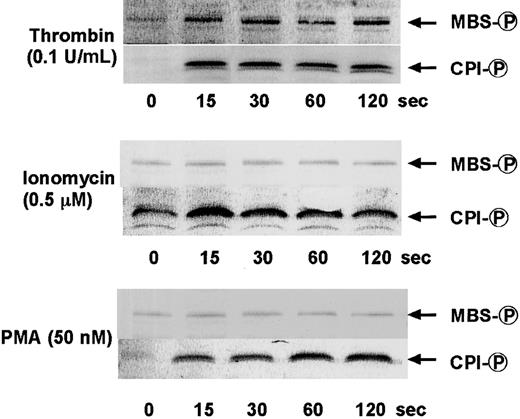
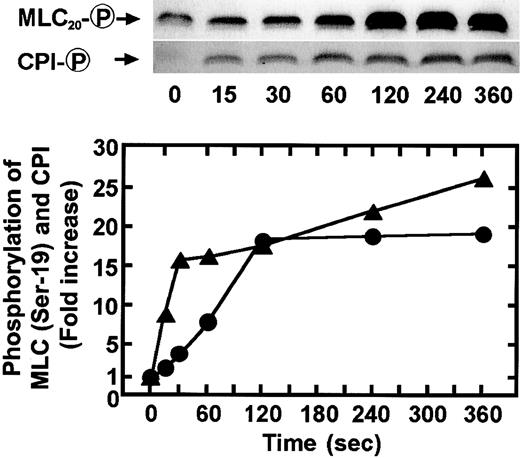
To determine whether the agonist-induced phosphorylation of CPI occurs in intact platelets, human platelets were stimulated with various agonists under conditions of nonstirring, and CPI phosphorylation was analyzed by immunoblot analysis using an anti–pThr38–CPI-17 antibody. In parallel, we also examined the phosphorylation of MLC20 at Ser-19 and MBS at Thr-696 by immunoblot analysis using the respective phosphospecific antibodies. When platelets were activated with agonists in the absence of stirring, they changed shape and secreted ATP from dense bodies, without aggregation.5,29 Figure 2shows ATP secretion and cytosolic Ca++ elevation response to STA2 and PMA in a nominally Ca++-free medium and under conditions of nonstirring. STA2 (0.5 μM) treatment led to ATP secretion and a rapid increase in the cytosolic Ca++ level. This Ca++ increase reached a peak level within 15 to 20 seconds and was followed by a rapid decrease. As expected from the cytosolic Ca++ response to STA2, the maximum extent of MLC20phosphorylation at Ser-19 was obtained within 20 seconds of STA2 (0.5 μM) stimulation, followed by dephosphorylation, as shown in Figure 3. CPI was rapidly phosphorylated and then dephosphorylated after STA2stimulation, similar to the phosphorylation of MLC20 at Ser-19, whereas STA2-induced MBS phosphorylation was slightly delayed. Thus, treatment of intact platelets with STA2 led to a rapid and transient increase in phosphorylation of CPI at a Thr residue (corresponding to Thr-38 on CPI-17), in addition to MBS phosphorylation at Thr-696. To determine whether phosphorylation of CPI can be induced by agonists other than STA2 in platelets, CPI phosphorylation was examined after stimulation with thrombin, PMA, and ionomycin. As shown in Figure4, treatment with 0.1 U/mL thrombin led to a rapid increase in phosphorylation of CPI at a Thr residue and of MBS at Thr-696. On the other hand, though the Ca++ionophore ionomycin or PMA, which activates PKC, could induce the phosphorylation of CPI, these artificial agonists failed to increase MBS phosphorylation. Ionomycin-induced CPI phosphorylation was almost completely inhibited by 0.1 mg/mL aspirin, suggesting that most of the CPI phosphorylation resulted from the potential of ionomycin to promote cyclooxygenase products.40 It is now apparent that PMA and the Ca++ ionophore cannot activate the RhoA signaling pathway. Incubation of fura 2-loaded platelets with PMA led to ATP secretion, with no change in cytosolic Ca++ concentrations (Figure 2), as reported,41 and PMA (100 nM)–induced ATP release was slower and less than that induced by STA2 (0.5 μM). We then examined the time course of phosphorylation of CPI and MLC20 at Ser-19 during PMA-induced platelet activation. As shown in Figure 5, after stimulation with 100 nM PMA, CPI was rapidly phosphorylated, and this phosphorylation preceded MLC20 phosphorylation at Ser-19 and ATP secretion.
ATP secretion and cytosolic Ca++ elevation in response to STA2 and PMA.
ATP secretion and cytosolic Ca++ levels induced by STA2 (0.5 μM) or PMA (100 nM) were measured in parallel in the same preparation of fura 2-loaded platelets suspended in Ca++-free HEPES–saline buffer, under conditions of nonstirring. Shown are representative traces from 4 independent experiments. PLT indicates platelets.
ATP secretion and cytosolic Ca++ elevation in response to STA2 and PMA.
ATP secretion and cytosolic Ca++ levels induced by STA2 (0.5 μM) or PMA (100 nM) were measured in parallel in the same preparation of fura 2-loaded platelets suspended in Ca++-free HEPES–saline buffer, under conditions of nonstirring. Shown are representative traces from 4 independent experiments. PLT indicates platelets.
STA2-induced phosphorylation of CPI, MBS at Thr-696, and MLC20 at Ser-19 in intact platelets.
Human platelets were stimulated with 0.5 μM STA2 at 37°C without stirring for 0, 15, 30, 45, 60, and 120 seconds, as indicated, and whole platelet lysates originating from 30 μL of platelet suspension were resolved by SDS-PAGE. This was followed by immunoblotting with respective antibodies, including anti-MBS, anti–pThr695-MBS, anti–CPI-17, anti–pThr38–CPI-17, anti-MLC and anti–pSer19-MLC20. (A) Paired sets of representative immunoblots. (B) The extent of STA2-induced phosphorylation of CPI (▴), MBS at Thr-696 (■), or MLC20 at Ser-19 (●). Results are expressed as fold increase in phosphorylation of each protein relative to the level at 0 second. Densities of the immunoreactive bands determined with each antiphosphoprotein antibody were normalized according to that of each total protein. Similar results were obtained in 3 other experiments using different donor platelets.
STA2-induced phosphorylation of CPI, MBS at Thr-696, and MLC20 at Ser-19 in intact platelets.
Human platelets were stimulated with 0.5 μM STA2 at 37°C without stirring for 0, 15, 30, 45, 60, and 120 seconds, as indicated, and whole platelet lysates originating from 30 μL of platelet suspension were resolved by SDS-PAGE. This was followed by immunoblotting with respective antibodies, including anti-MBS, anti–pThr695-MBS, anti–CPI-17, anti–pThr38–CPI-17, anti-MLC and anti–pSer19-MLC20. (A) Paired sets of representative immunoblots. (B) The extent of STA2-induced phosphorylation of CPI (▴), MBS at Thr-696 (■), or MLC20 at Ser-19 (●). Results are expressed as fold increase in phosphorylation of each protein relative to the level at 0 second. Densities of the immunoreactive bands determined with each antiphosphoprotein antibody were normalized according to that of each total protein. Similar results were obtained in 3 other experiments using different donor platelets.
Phosphorylation of CPI and MBS at Thr-696 after stimulation of intact platelets with thrombin, ionomycin, or PMA.
Human platelets were stimulated with 0.1 U/mL thrombin, 0.5 μM ionomycin, or 50 nM PMA for the indicated time at 37°C under conditions of nonstirring, and whole platelet lysates originating from 30 μL of platelet suspension were resolved by SDS-PAGE. This was followed by immunoblotting with anti–pSer19-MLC20 and anti–pThr38–CPI-17 antibodies. Similar results were obtained in 3 other experiments using different donor platelets.
Phosphorylation of CPI and MBS at Thr-696 after stimulation of intact platelets with thrombin, ionomycin, or PMA.
Human platelets were stimulated with 0.1 U/mL thrombin, 0.5 μM ionomycin, or 50 nM PMA for the indicated time at 37°C under conditions of nonstirring, and whole platelet lysates originating from 30 μL of platelet suspension were resolved by SDS-PAGE. This was followed by immunoblotting with anti–pSer19-MLC20 and anti–pThr38–CPI-17 antibodies. Similar results were obtained in 3 other experiments using different donor platelets.
PMA-induced phosphorylation of CPI and MLC20at Ser-19 in intact platelets.
Human platelets were stimulated with 100 nM PMA for the indicated time at 37°C under conditions of nonstirring, and whole platelet lysates originating from 30 μL of platelet suspension were resolved by SDS-PAGE. This was followed by immunoblotting with anti–pSer19-MLC20 and anti–pThr38–CPI-17 antibodies. Results were expressed as fold increase in phosphorylation of each protein relative to the level at 0 second. Densities of the immunoreactive bands determined with each antiphosphoprotein antibody were normalized according to total of each protein. Similar results were obtained in 3 other experiments using different donor platelets.
PMA-induced phosphorylation of CPI and MLC20at Ser-19 in intact platelets.
Human platelets were stimulated with 100 nM PMA for the indicated time at 37°C under conditions of nonstirring, and whole platelet lysates originating from 30 μL of platelet suspension were resolved by SDS-PAGE. This was followed by immunoblotting with anti–pSer19-MLC20 and anti–pThr38–CPI-17 antibodies. Results were expressed as fold increase in phosphorylation of each protein relative to the level at 0 second. Densities of the immunoreactive bands determined with each antiphosphoprotein antibody were normalized according to total of each protein. Similar results were obtained in 3 other experiments using different donor platelets.
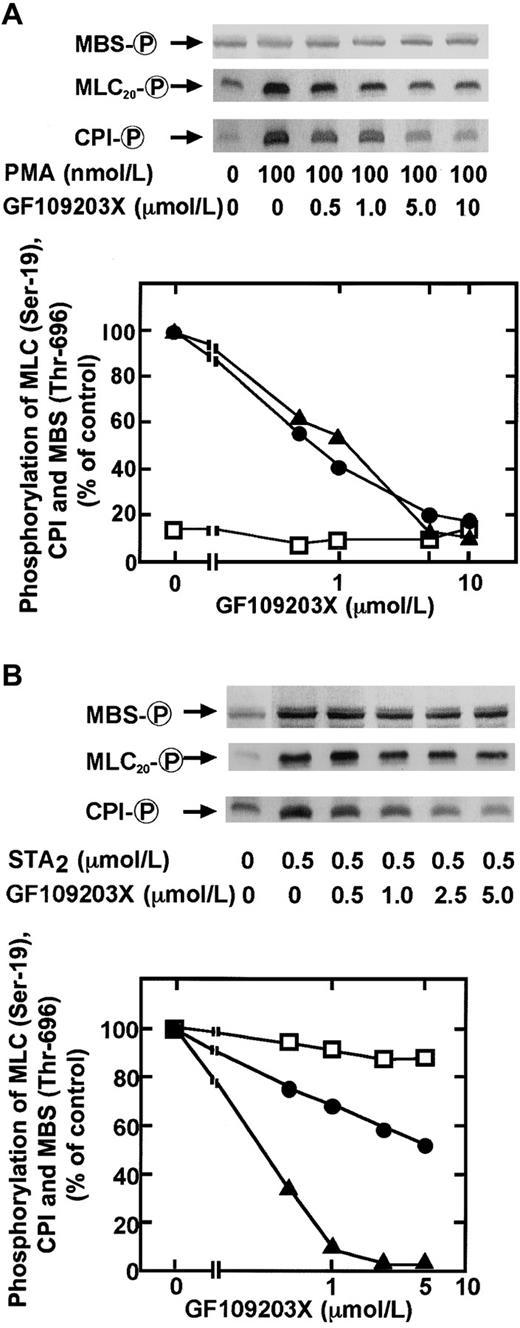
Inhibitory effects of GF109203X, Y-27632 alone and in combination on PMA- and STA2-induced phosphorylation of CPI, MBS, and MLC20
PKC cannot directly phosphorylate MLC20 at Ser-19, the MLC kinase site8,42,43; therefore, the phosphorylation of this site induced by PMA in intact platelets appeared to result from the inhibition of myosin phosphatase through phosphorylation of CPI at a Thr residue. In the next set of experiments, we determined the participation of PKC in PMA- or STA2-induced MLC20 phosphorylation of intact platelets, using a specific PKC inhibitor, GF109203X (Figure 6). As shown in Figure 6A, preincubation of platelets with GF109203X attenuated the PMA-induced phosphorylation of CPI and MLC20at Ser-19, with similar IC50 values (1.52 ± 0.33 μM, n = 3, for CPI phosphorylation; 1.17 ± 0.21 μM, n = 3, for MLC20 phosphorylation, respectively). Inhibition of PMA-induced MLC20 phosphorylation by GF109203X correlated well with CPI phosphorylation. GF109203X at 2 μM completely abolished PMA-induced ATP secretion, as reported.38 The Rho-kinase inhibitor Y-2763244 diminished neither PMA-induced phosphorylation of MLC20 nor CPI (data not shown). GF109203X also dose dependently inhibited the STA2-induced phosphorylation of CPI and MLC20. However, GF109203X caused a nearly complete inhibition of STA2-induced CPI phosphorylation, whereas the drug did not inhibit STA2-induced MLC20 phosphorylation to the same extent seen with the inhibition of CPI phosphorylation (Figure 6B). GF109203X decreased MLC20 phosphorylation by approximately 60%, even at 2 μM, a concentration at which the drug abolished STA2-induced CPI phosphorylation. GF109203X pretreatment resulted in a marginal decrease in STA2-induced phosphorylation of MBS at Thr-696.
Inhibitory effects by a PKC inhibitor GF109203X on PMA- or STA2-induced phosphorylation of MBS at Thr-696, CPI, and MLC20 phosphorylation at Ser-19 in intact platelets.
Human platelets pretreated with various concentrations of GF109203X for 2 minutes were stimulated with 100 nM PMA (A) or 0.5 μM STA2 (B) for 30 seconds at 37°C under conditions of nonstirring, and whole platelet lysates originating from 30 μL of platelet suspension were resolved by SDS-PAGE. This was followed by immunoblotting with anti–pThr695-MBS, anti–pThr38–CPI-17, and anti–pSer19-MLC20 antibodies. Inhibitory effects by GF109203X on agonist-induced phosphorylation of CPI (▴), MBS at Thr-696 (■), and MLC20 at Ser-19 (●) were expressed as a percentage of the phosphorylation level of platelets induced by each agonist. Inhibition of PMA-induced MBS phosphorylation by GF109203X was expressed as a percentage of the phosphorylation level found in STA2-stimulated platelets without GF109203X. Values represent the average of 3 experiments (SD < 10%).
Inhibitory effects by a PKC inhibitor GF109203X on PMA- or STA2-induced phosphorylation of MBS at Thr-696, CPI, and MLC20 phosphorylation at Ser-19 in intact platelets.
Human platelets pretreated with various concentrations of GF109203X for 2 minutes were stimulated with 100 nM PMA (A) or 0.5 μM STA2 (B) for 30 seconds at 37°C under conditions of nonstirring, and whole platelet lysates originating from 30 μL of platelet suspension were resolved by SDS-PAGE. This was followed by immunoblotting with anti–pThr695-MBS, anti–pThr38–CPI-17, and anti–pSer19-MLC20 antibodies. Inhibitory effects by GF109203X on agonist-induced phosphorylation of CPI (▴), MBS at Thr-696 (■), and MLC20 at Ser-19 (●) were expressed as a percentage of the phosphorylation level of platelets induced by each agonist. Inhibition of PMA-induced MBS phosphorylation by GF109203X was expressed as a percentage of the phosphorylation level found in STA2-stimulated platelets without GF109203X. Values represent the average of 3 experiments (SD < 10%).
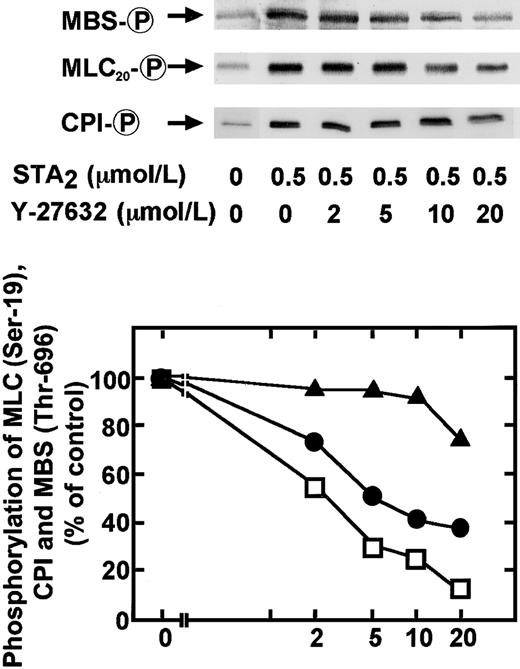
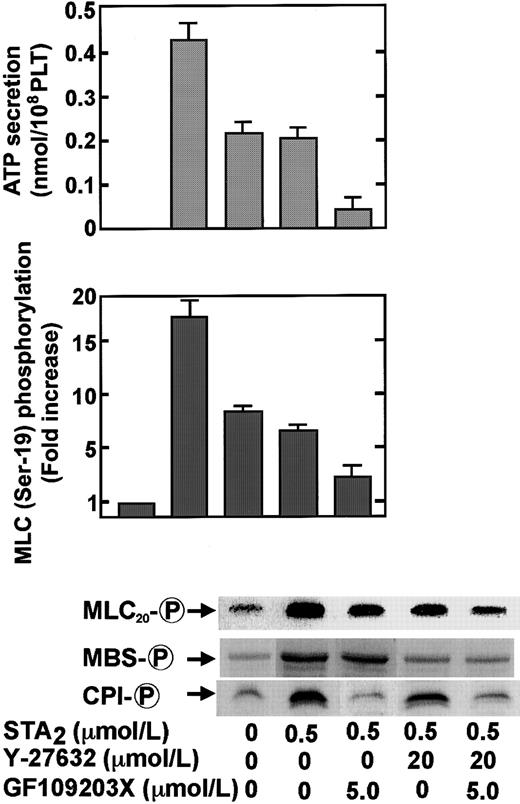
We reported that the Rho-kinase inhibitor Y-27632 could inhibit the STA2-induced MBS phosphorylation dose dependently (1-20 μM) in intact platelets.27 Smooth muscle CPI-17 was shown to be phosphorylated in a cell-free system not only by PKC but also by the downstream kinases RhoA, Rho-kinase,30 and protein kinase N.45 We also examined the effect of the Y-27632 on the STA2-induced phosphorylation of CPI, MBS, and MLC20 in intact platelets. As shown in Figure7, Y-27632 almost completely inhibited the STA2-induced phosphorylation of MBS at Thr-696, whereas the drug had only a minor inhibitory effect on the STA2-induced CPI phosphorylation, which suggests that Rho-kinase preferentially phosphorylates MBS, compared to CPI, in intact platelets. Y-27632 partly inhibited the STA2-induced MLC20 phosphorylation at Ser-19 in intact platelets. As shown in Figure 8, Y-27632 inhibited STA2-induced MLC20 phosphorylation at Ser-19 to approximately 40% of the level seen without Y-27632, an inhibition likely because of the inhibition of MBS phosphorylation, though slight inhibition of CPI phosphorylation was evident at 20 μM Y-27632. The addition of GF109203X (5 μM) to Y-27632 (20 μM) led to a significant inhibition of CPI phosphorylation and further suppression of STA2-induced MLC20 phosphorylation. This additional inhibition of MLC20 phosphorylation by GF109203X appeared to be mainly due to the inhibition of CPI phosphorylation because the additional inhibition of MBS phosphorylation by GF109203X was slight. STA2-induced ATP secretion was partially inhibited by either Y-27632 (20 μM) or GF109203X (5 μM) treatment, and treatment with Y-27632 or GF109203X led to a further decrease in ATP secretion (Figure 8). Inhibition of STA2-induced ATP release by these compounds appeared to correlate with the extent of MLC20 phosphorylation at Ser-19.
Inhibitory effects by a Rho-kinase inhibitor Y-27632 on STA2-induced phosphorylation of MBS at Thr-696, CPI, and MLC20 phosphorylation at Ser-19 in intact platelets.
Human platelets pretreated with various concentrations of Y-27632 for 2 minutes were stimulated with 0.5 μM STA2 for 30 seconds at 37°C under conditions of nonstirring, and whole platelet lysates originating from 30 μL of platelet suspension were resolved by SDS-PAGE. This was followed by immunoblotting with anti–pThr695-MBS, anti–pThr38–CPI-17, and anti–pSer19-MLC20antibodies. Inhibitory effects by Y-27632 on STA2-induced phosphorylation of CPI (▴), MBS at Thr-696 (■), and MLC20 at Ser-19 (●) were expressed as a percentage of the phosphorylation level found in STA2-stimulated platelets. Values represent the average of 3 experiments (SD < 10%).
Inhibitory effects by a Rho-kinase inhibitor Y-27632 on STA2-induced phosphorylation of MBS at Thr-696, CPI, and MLC20 phosphorylation at Ser-19 in intact platelets.
Human platelets pretreated with various concentrations of Y-27632 for 2 minutes were stimulated with 0.5 μM STA2 for 30 seconds at 37°C under conditions of nonstirring, and whole platelet lysates originating from 30 μL of platelet suspension were resolved by SDS-PAGE. This was followed by immunoblotting with anti–pThr695-MBS, anti–pThr38–CPI-17, and anti–pSer19-MLC20antibodies. Inhibitory effects by Y-27632 on STA2-induced phosphorylation of CPI (▴), MBS at Thr-696 (■), and MLC20 at Ser-19 (●) were expressed as a percentage of the phosphorylation level found in STA2-stimulated platelets. Values represent the average of 3 experiments (SD < 10%).
Inhibition by Y-27632, GF109203X, or both of STA2-induced ATP secretion and phosphorylation of MLC20 at Ser-19, MBS at Thr-696, and CPI in intact platelets.
Human platelets pretreated without or with 20 μM Y-27632, 5 μM GF109203X, or both for 3 minutes were stimulated with 0.5 μM STA2 under conditions of nonstirring. Incubation was carried out for 30 seconds at 37°C to measure protein phosphorylation and for 2 minutes at 37°C to measure ATP release. Whole platelet lysates originating from 30 μL of platelet suspension were resolved by SDS-PAGE. This was followed by immunoblotting with anti–pThr695-MBS, anti–pThr38–CPI-17, and anti–pSer19-MLC20 antibodies. MLC20phosphorylation at Ser-19 was expressed as fold increase in the basal value, without the addition of inhibitors. Similar results were obtained in 3 other experiments using different donor platelets.
Inhibition by Y-27632, GF109203X, or both of STA2-induced ATP secretion and phosphorylation of MLC20 at Ser-19, MBS at Thr-696, and CPI in intact platelets.
Human platelets pretreated without or with 20 μM Y-27632, 5 μM GF109203X, or both for 3 minutes were stimulated with 0.5 μM STA2 under conditions of nonstirring. Incubation was carried out for 30 seconds at 37°C to measure protein phosphorylation and for 2 minutes at 37°C to measure ATP release. Whole platelet lysates originating from 30 μL of platelet suspension were resolved by SDS-PAGE. This was followed by immunoblotting with anti–pThr695-MBS, anti–pThr38–CPI-17, and anti–pSer19-MLC20 antibodies. MLC20phosphorylation at Ser-19 was expressed as fold increase in the basal value, without the addition of inhibitors. Similar results were obtained in 3 other experiments using different donor platelets.
Discussion
Our experiments revealed the existence of a CPI-17–like protein in human platelets and its functional role in regulating MLC20 phosphorylation at Ser-19 in case of agonist-induced platelet secretion. CPI-17 was originally reported to be exclusively expressed in smooth muscle tissues,19-21 but we detected a single major immunoreactive band with an apparent molecular mass of 22 kd, a slightly higher molecular mass than noted in smooth muscle CPI-17 (molecular mass, 20 kd),19 in human platelets. Myosin phosphatase co-immunoprecipitated with CPI from human platelets, suggesting that platelet CPI interacts with myosin phosphatase. Phosphorylation by PKC of smooth muscle CPI-17 at a Thr residue (Thr-38) is sufficient to induce the inhibition of myosin phosphatase activity.20,28 We showed that in vitro phosphorylation of platelet CPI by PKC at a Thr residue, corresponding to Thr-38 on CPI-17, resulted in a decrease in myosin phosphatase activity in the anti–CPI-17 immunoprecipitate of platelets. Inhibition by the PKC inhibitor GF109203X of PKC-induced CPI-17 phosphorylation reduced inactivation of the phosphatase activity. Thus, similar to smooth muscle CPI-17, platelet CPI appears to be a phosphorylation-dependent inhibitor of myosin phosphatase. Concentration of CPI in human platelets was estimated to be approximately 0.3 μM, similar to the concentration in porcine aorta smooth muscle.21
Stimulus-induced phosphorylation of CPI at a Thr residue appears to be one mechanism by which PKC enhances the intracellular pathway of regulated platelet secretion. PMA can induce ATP secretion in intact platelets without producing any elevation in cytosolic Ca++concentration, as determined using the fluorescent Ca++probe fura-2,41 albeit the secretion is slower and of lesser amount than the extent of secretion stimulated by agonists such as thrombin and STA2. Our study shows that PMA increases both CPI phosphorylation at a Thr residue and MLC20phosphorylation at the same site (Ser-19) phosphorylated by MLC kinase8 and Rho-kinase.9 This activating MLC kinase site is not directly phosphorylated by PKC, but phosphorylation of MLC20 on PKC sites in vitro inhibits smooth muscle ATPase activity.42 43 The mechanism of Ca++sensitization of platelet secretion by PMA and by GTPγS appears to be mediated by the increased phosphorylation of MLC20, mainly through the inhibition of myosin phosphatase. This notion is strengthened by our findings that the PKC inhibitor GF109203X inhibits the PMA-induced increases in phosphorylation of both CPI and MLC20 at Ser-19, with similar IC50 values. GF109203X completely inhibited PMA-induced ATP secretion. Moreover, PMA or ionomycin did not induce MBS phosphorylation at Thr-696, thus indicating that these nonphysiological agonists do not activate the RhoA/Rho-kinase signaling pathway in human platelets. If PMA stimulation does not activate MLC kinase, MLC20phosphorylation at Ser-19 may slowly increase because of the PKC-mediated inhibition of myosin phosphatase. This possibility is consistent with our findings that the PMA-induced increase in CPI phosphorylation precedes MLC20 phosphorylation and ATP secretion. Therefore, it is likely that in intact platelets, PMA-induced MLC20 phosphorylation at Ser-19 results from the inhibition of myosin phosphatase by the phosphorylation of CPI rather than by the direct phosphorylation of MLC20 by PKC.
Platelet agonists such as TXA2 and thrombin function through heterotrimeric G protein-coupled receptors, which activate Gq-type,46G12/G13-type,47,48 and Gi-type49 G proteins. Gq-mediated phospholipase C activation, stimulating the synthesis of inositol triphosphate and diacylglycerol, is thought to represent the main signal pathway leading to full platelet activation such as secretion, whereas G12/G13 couple receptors to the RhoA/Rho-kinase signaling pathways.2,47,48TXA2 mimetic STA2 and thrombin induce an elevation of intracellular Ca++concentrations36,41 and most likely lead to Ca++/calmodulin-dependent MLC kinase-mediated MLC20 phosphorylation at Ser-19 of platelets.1,2,5,8 In addition to MLC kinase-dependent MLC20 phosphorylation, Rho-kinase–mediated MLC20 phosphorylation (both directly and indirectly through MBS phosphorylation) contributes to the platelet secretion induced by these agonists.27 Our findings suggest that PKC-mediated CPI phosphorylation and the resultant inhibition of myosin phosphatase is also functionally involved in platelet secretion. STA2and thrombin, at doses sufficient to elicit platelet secretion, led to the phosphorylation not only of CPI but also of MBS at Thr-696 in intact platelets; these findings differ from results with PMA or ionomycin bypassing the receptor-operated signaling pathway. STA2-induced MLC20 phosphorylation at Ser-19 was diminished, but not abolished, by pretreatment of GF109203X, even at the high concentrations with which STA2-induced CPI phosphorylation was completely inhibited, in parallel samples. These data suggest that PKC-induced CPI phosphorylation is an important, but not the only, mechanism of the STA2-induced increase in MLC20 phosphorylation at Ser-19. We reported that G protein–coupled receptor agonists such as epinephrine, STA2, and thrombin induce MBS phosphorylation and inhibit myosin phosphatase activity and that both reactions are inhibited by a prior treatment with Y-27632.27 We also reported that myosin phosphatase activity is inhibited when chicken gizzard MBS is phosphorylated by Rho-kinase at Thr-695, which corresponds to Thr-696 of human MBS,25 although this site of MBS can be phosphorylated by other, as yet unidentified, kinases.17Phosphorylation of Thr-696 occurred under in vivo conditions in which RhoA/Rho-kinase was thought to be activated after G protein–coupled receptor activation of human platelets. Our data support the hypothesis that Thr-696 is a major functional site of phosphorylation by Rho-kinase in human platelets. The Rho-kinase inhibitor Y-27632 almost completely attenuated the STA2-induced MBS phosphorylation at Thr-696, whereas this inhibitor slightly inhibited STA2-induced CPI phosphorylation. Smooth muscle CPI-17 was reported to be an in vitro substrate not only for PKC but also for downstream kinases of RhoA Rho-kinase30 and protein kinase N,45 both of which are sensitive to Y-27632. However, our present findings suggest that platelet CPI may be a substrate, but not a preferential one, for Rho-kinase, compared with MBS in intact human platelets. Y-27632 alone partially inhibited STA2-induced MLC20 phosphorylation at Ser-19, as was the case with GF109203X. The Y-27632–insensitive component of STA2-induced MLC20 phosphorylation was further suppressed by the addition of GF109203X, an event mainly attributed to a significant inhibition of CPI phosphorylation and myosin phosphatase suppression. Inhibition of STA2-induced ATP secretion by Y-27632 or GF109203X apparently correlated with the extent of MLC20 phosphorylation at Ser-19. Combined treatment with Y-27632 and GF109203X substantially inhibited STA2 (0.5 μM)–induced MLC20 phosphorylation and ATP secretion, suggesting that Ca++-dependent activation of MLC kinase contributes to a lesser extent to MLC20 phosphorylation at Ser-19 and to platelet secretion under these conditions. The extent of MLC kinase activation appears to depend on the type and concentration of agonist,27 whereas activation of Rho-kinase and PKC can lead to an increase in Ca++ sensitivity of platelet secretion through myosin phosphatase inhibition.
In summary, these pharmacological studies suggest that Rho and PKC participate in parallel pathways to increase MLC20phosphorylation mainly through the inhibition of myosin phosphatase following heterotrimeric G protein–coupled receptor activation, as illustrated in Figure 9. RhoA activates Rho-kinase, which phosphorylates MBS and inhibits myosin phosphatase activity. Activation of PKC can also inhibit myosin phosphatase by phosphorylating CPI. These mechanisms operate independently of the activation of Ca2+-dependent MLC kinase. The dual activation of MLC20 phosphorylation by kinase activation and phosphatase inhibition may be important for a rapid and potent platelet response to agonist.
Model depicting the dual regulation by MLC kinase and myosin phosphatase of myosin phosphorylation in platelet secretion.
[Ca2+]i indicates intracellular Ca++ concentration; CPI- P○, phosphorylated CPI; DG, diacylglycerol; Ins(1,4,5)P3, inositol 1,4,5-triphosphate; GF109203X, inhibitor of PKC; MBS- P○, phosphorylated MBS at Thr696; MLC20- P○, phosphorylated MLC20 at Ser19; PKN, protein kinase N; PLC, phospholipase C; GEF, Rho-specific guanine nucleotide exchange factor; Y-27632, inhibitor of Rho-kinase.
Model depicting the dual regulation by MLC kinase and myosin phosphatase of myosin phosphorylation in platelet secretion.
[Ca2+]i indicates intracellular Ca++ concentration; CPI- P○, phosphorylated CPI; DG, diacylglycerol; Ins(1,4,5)P3, inositol 1,4,5-triphosphate; GF109203X, inhibitor of PKC; MBS- P○, phosphorylated MBS at Thr696; MLC20- P○, phosphorylated MLC20 at Ser19; PKN, protein kinase N; PLC, phospholipase C; GEF, Rho-specific guanine nucleotide exchange factor; Y-27632, inhibitor of Rho-kinase.
We thank Dr R. S. Adelstein (National Instititutes of Health) for helpful discussions, and we thank M. Ohara for language assistance.
Supported in part by grants for research from the Ministry of Education, Science, Technology, Sports and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masakatsu Nishikawa, 2nd Department of Internal Medicine, Mie University School of Medicine, 2-174 Edobashi, Tsu, Mie 514-8507, Japan; e-mail: nisikawa@clin.medic.mie-u.ac.jp.

![Fig. 1. Detection of CPI, an inhibitory phosphoprotein of myosin phosphatase, in human platelets. / (A) Immunoblot analysis of CPI in human platelets. Platelet lysates were applied to SDS-PAGE, and this was followed by immunoblotting using an anti–CPI-17 antibody. Lane 1, smooth muscle CPI-17 purified from porcine aorta. Lane 2, platelet CPI. Molecular weight markers are indicated on the left end. (B) Co-immunoprecipitation of platelet myosin phosphatase with anti–CPI-17 antibody. Immunoprecipitates of platelet lysates with anti–CPI-17 antibody (lane 1) or with the preimmune serum (lane 2) were immunoblotted with anti–CPI-17 antibody. Immunoprecipitates with anti–CPI-17 antibody were immunoblotted using antibodies against the PP1δ catalytic subunit and MBS, respectively. (C) Immunoprecipitates with anti–CPI-17 antibody were assayed for phosphatase activity, using [γ-32P]-phosphorylated MLC as a substrate. Data represent the mean ± SE of 3 experiments. (D) In vitro platelet CPI phosphorylation by PKC. Anti–CPI-17 immnoprecipitates from platelet lysates were incubated for 2 minutes, without or with GF109203X, in the presence of purified platelet PKC. Protein phosphorylation was analyzed by SDS-PAGE, and this was followed by immunoblot analysis using an anti–pThr38–CPI-17 antibody. Results were expressed as fold increase in the phosphorylation relative to the activity measured in the absence of PKC. Data represent the mean ± SE of 3 experiments. (E) Effect of CPI thiophosphorylation by PKC on platelet myosin phosphatase activity. Platelet anti–CPI-17 immunoprecipitates were incubated, with or without purified platelet PKC, in the presence or absence of 5 μM GF109203X for 60 minutes at 30°C, as described in “Materials and methods.” Aliquots of the reaction mixture were quenched by adding a solution that resulted in a final concentration of 12 mM EGTA and 2 mM EDTA to stop the reaction, then were kept on ice. Myosin phosphatase activity was immediately determined. Data represent the mean ± SE of 4 experiments. IP, immunoprecipitation antibody used; IB, immunobotting antibody used; Ig, cross-reacting immunoglobulin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/12/10.1182_blood.v97.12.3798/6/m_h81211168001.jpeg?Expires=1767726138&Signature=tEgK5Rmic7rXcQ-4r5Zq7Jp28cTUQ4REFgejV~Nmp-4h~D4ke~ipVdsNhV1Hxld1zInLT6dXvArIOgcyMMtl6wSqLLkXSewBjy5xw8wucBRtk1Xxj78TVv3depdL8F762UNJ11ejo9KPvkIyc-4tKedmdK3w0I4Z7fg~KuqDudA7CmFJuMdRBNA4h~PvvJNl~ByRd2yFH0pCuETfN4QWmhd3hvkJdRz8quR0-2d8xbBc~IFbk2j3hLIepqvk6MIpbCU~A085gQbJ5K0WZrU6D0foIUea2m3~r~gZXiUeyKe24PkLFoXXJIwSDMe7MqUPSC8hmJmMEbsGXVBcnz-oHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 9. Model depicting the dual regulation by MLC kinase and myosin phosphatase of myosin phosphorylation in platelet secretion. / [Ca2+]i indicates intracellular Ca++ concentration; CPI- P○, phosphorylated CPI; DG, diacylglycerol; Ins(1,4,5)P3, inositol 1,4,5-triphosphate; GF109203X, inhibitor of PKC; MBS- P○, phosphorylated MBS at Thr696; MLC20- P○, phosphorylated MLC20 at Ser19; PKN, protein kinase N; PLC, phospholipase C; GEF, Rho-specific guanine nucleotide exchange factor; Y-27632, inhibitor of Rho-kinase.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/12/10.1182_blood.v97.12.3798/6/m_h81211168009.jpeg?Expires=1767726138&Signature=I7zlwB3OOP8jj-~lJN03TTKjsU--x6pJhpxMcbCai0mUMVSaw3dEhzKxVeQuLhumXUeOZcllFs~dby69U28i1WueGGUmPxiqIp77Z4bAtP12QsiBaVcy9ac6QhPwqjDL4jeVYPNKbjDROpBwjL1xhPXrpfsOuV-mYMPL2rXLvCkW86scW1-~5i6~ag~tnn1XNy2sJ13mLWN6j3IalBziFHz~Qr~QkE6~TIxeCZ00jaX3qzpIOIVPEqAmaksP5hubBBcH8hysqDKEgIiJ2-S8mmIpJJA4pkKTwFVsRFEgTkTmEcDm65BbvLyMYK2-5GnNhONaeKTdwZlHD4RHOnwyxQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal