Splenic extramedullary hematopoiesis is an integral component of myelofibrosis with myeloid metaplasia (MMM) and may be classified into 3 distinct histologic patterns of infiltration by myeloid precursors: diffuse, nodular, and a predominance of immature granulocytes. These 3 histologic patterns occurred in 121 (56.8%), 75 (35.2%), and 17 (8%), respectively, of 213 patients with MMM who underwent splenectomy at a single institution. In general, karyotypic findings in splenic tissue (n = 92) were similar to those seen in the bone marrow. The histologic pattern of immature granulocyte predominance, the presence of microscopic splenic infarcts (26 patients), or the detection of an abnormal splenic karyotype (52 patients) was significantly associated with decreased postsplenectomy survival. These adverse features were also associated with characteristics of advanced disease. These observations support the bone marrow origin of the myeloid progenitor pool in the spleen of patients with MMM and suggest a prognostic value for splenic histopathology and karyotype.
Introduction
Specific extramedullary hematopoiesis (EMH) in myelofibrosis with myeloid metaplasia (MMM) was originally believed to arise from reactivation of fetal hematopoietic elements.1Current evidence suggests that splenic EMH in MMM results from sequestration, accumulation, and proliferation of circulating myeloid progenitors in splenic cords.2 Immunohistochemical analysis of splenic tissue has revealed that EMH in MMM is primarily granulocytic3 as compared with the fetal spleen, which is mainly a site for erythroid differentiation.4 The bone marrow origin of EMH precursors in MMM has further been suggested by immunohistologic and morphometric studies of megakaryocytes5 and the in vitro demonstration of committed, but not pluripotent, myeloid progenitors in splenic tissue.6 Splenectomy may be necessary to palliate symptoms and improve the quality of life in patients with MMM.7 It was recently reported that splenic pathology in MMM may undergo a prognostically relevant progression from an erythroid to a pan-myeloid composition.8 Accordingly, and to provide complementary information in a recently described series of patients with MMM who underwent splenectomy,7 we investigated the prognostic value of splenic histopathology and karyotype.
Study design
Archived splenic tissue obtained from splenectomized patients with MMM (n = 213) was stained with hematoxylin and eosin and examined under light microscopy by one of the authors (C.-Y.L.), who was blinded to the clinical characteristics and the outcomes of the study patients. All cases were histologically categorized according to the pattern of myeloid precursor infiltration in the spleen. The 3 categories recognized were diffuse (diffuse pattern of EMH with trilineage myeloid involvement), nodular (macronodular proliferation of EMH), and a predominance of immature granulocytes (immature granulocyte predominance) (Figure 1). In addition, the presence or absence of microscopic splenic infarctions was noted (Figure 1), and the results of karyotypic studies in the splenic tissue and bone marrow were recorded.
Splenic histologic findings in patients with myelofibrosis with myeloid metaplasia.
(A) Normal spleen (× 128). (B) Diffuse splenic EMH (× 128). (C) Nodular splenic EMH (× 80). (D) Immature granulocytic predominant EMH (× 128). (E) Splenic microinfarction (× 51).
Splenic histologic findings in patients with myelofibrosis with myeloid metaplasia.
(A) Normal spleen (× 128). (B) Diffuse splenic EMH (× 128). (C) Nodular splenic EMH (× 80). (D) Immature granulocytic predominant EMH (× 128). (E) Splenic microinfarction (× 51).
Correlations among clinical, histologic, and cytogenetic parameters were studied by nonparametric statistical techniques. The relations between categorical variables were studied with the Fisher exact test. When a continuous variable was divided into 2 categories, the Wilcoxon rank sum test was used to compare the medians of the continuous variable between the 2 categories. When a continuous variable was divided into 3 or more categories, medians of the continuous variable in each of the 3 or more categories were compared by means of a Kruskal-Wallis test. Kaplan-Meier9 methodology was used to estimate the distributions of survival from diagnosis and survival from splenectomy. The log-rank test was used to assess whether survival from diagnosis and survival from splenectomy differed between various categories. Multivariate analysis was performed using logistic regression.
Results and discussion
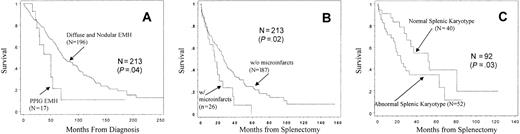
One hundred twenty-one (56.8%) of the 213 splenectomized patients with MMM had a diffuse infiltrative pattern of EMH that was composed of granulocytic, erythroid, and megakaryocytic precursors (diffuse). In another 75 patients (35.2%), this trilineage infiltration of precursors formed a macronodular pattern (nodular). The histologic pattern in the remaining 17 patients (8.0%) consisted almost exclusively of immature granulocyte precursors (immature granulocyte predominance). In none of the patients was the EMH composed strictly of erythroid elements. Results of our histologic review of MMM splenic tissue are consistent with those of previous reports.3,10Patients with immature granulocyte predominance had unfavorable prognostic scores11 (P = .04) and a higher incidence of cytopenias (erythrocyte transfusion dependence,P < .01; and platelet count less than 50 × 109/L, P < .01) compared with those with more balanced trilineage EMH (nodular or diffuse) (Table1). In addition, independent of blastic transformation, the particular histologic pattern was associated with decreased overall survival (Figure 2A). The respective median survival times from diagnosis were 49.4, 64.5, and 90.8 months for immature granulocyte predominance, diffuse, and nodular EMH histology. Although direct comparison of the 2 most common histologic patterns revealed no survival difference, patients with diffuse EMH had significantly worse prognostic scores11and a higher incidence of cytopenias (Table 1). Collectively, these observations suggest that splenic EMH in MMM may initially follow a nodular pattern and then undergo a prognostically relevant histologic transformation into a diffuse pattern first and granulocyte predominance second.
Results of statistical analysis among splenic histologic subgroups in 213 splenectomized patients with myelofibrosis with myeloid metaplasia
| Parameter . | Pathology category (n = 213) . | Diffuse vs nodular (n = 196) . | Splenic infarct (n = 26) . |
|---|---|---|---|
| General parameters | |||
| Number of patients | D = 121 (56.8%) | D = 121 (61.7%) | 26 (12.2%) |
| N = 75 (35.2%) | N = 75 (38.3%) | ||
| PPIG = 17 (8.0%) | |||
| Survival | |||
| From diagnosis | .04 | .17 | .01 |
| From splenectomy | .29 | .82 | .02 |
| Cause of death | .39 | .14 | .85 |
| Dupriez score* | |||
| At diagnosis | .09 | .02† | < .01 |
| At splenectomy | .04 | .01 | .20 |
| Time to splenectomy (from diagnosis) | .82 | .16 | .18 |
| Parameters at the time of splenectomy | |||
| Age | .99 | .53 | .38 |
| Laboratory values | |||
| Hemoglobin < 9 g/dL | .20 | .10 | .09 |
| RBC transfusion dependent (yes/no) | < .01 | .02 | .09 |
| Leukocyte count | .27 | .49 | .16 |
| Platelet count < 50 × 109/L | < .01 | < .01† | < .01† |
| Circulating blasts | .44 | .44 | .04 |
| Spleen mass | .70 | .10 | .63 |
| Splenic infarct (yes/no) | .90 | .56 | — |
| Perioperative complications | |||
| Bleeding | .87 | 1.0 | .02 |
| Thrombosis | 1.0 | 1.0 | .23 |
| Long-term complications | |||
| Leukemia | .65 | .75 | .08 |
| Thrombocytosis | .27 | 1.0 | .62 |
| Hepatomegaly | .70 | .43 | .77 |
| Parameter . | Pathology category (n = 213) . | Diffuse vs nodular (n = 196) . | Splenic infarct (n = 26) . |
|---|---|---|---|
| General parameters | |||
| Number of patients | D = 121 (56.8%) | D = 121 (61.7%) | 26 (12.2%) |
| N = 75 (35.2%) | N = 75 (38.3%) | ||
| PPIG = 17 (8.0%) | |||
| Survival | |||
| From diagnosis | .04 | .17 | .01 |
| From splenectomy | .29 | .82 | .02 |
| Cause of death | .39 | .14 | .85 |
| Dupriez score* | |||
| At diagnosis | .09 | .02† | < .01 |
| At splenectomy | .04 | .01 | .20 |
| Time to splenectomy (from diagnosis) | .82 | .16 | .18 |
| Parameters at the time of splenectomy | |||
| Age | .99 | .53 | .38 |
| Laboratory values | |||
| Hemoglobin < 9 g/dL | .20 | .10 | .09 |
| RBC transfusion dependent (yes/no) | < .01 | .02 | .09 |
| Leukocyte count | .27 | .49 | .16 |
| Platelet count < 50 × 109/L | < .01 | < .01† | < .01† |
| Circulating blasts | .44 | .44 | .04 |
| Spleen mass | .70 | .10 | .63 |
| Splenic infarct (yes/no) | .90 | .56 | — |
| Perioperative complications | |||
| Bleeding | .87 | 1.0 | .02 |
| Thrombosis | 1.0 | 1.0 | .23 |
| Long-term complications | |||
| Leukemia | .65 | .75 | .08 |
| Thrombocytosis | .27 | 1.0 | .62 |
| Hepatomegaly | .70 | .43 | .77 |
All results (except those for distribution) reflect aP value from univariate analysis. Column 1, D vs N vs PPIG; column 2, D vs N only.
D indicates diffuse extramedullary hematopoiesis pattern; N, nodular extramedullary hematopoiesis pattern; PPIG, predominant presence of immature granulocytes in splenic extramedullary hematopoiesis; RBC, red blood cell.
Dupriez prognostic score for myelofibrosis with myeloid metaplasia.11
Significant in multivariate analysis using logistic regression.
Histologic prognostic factors from splenic tissue in 213 patients with myelofibrosis with myeloid metaplasia.
(A) Survival from diagnosis according to splenic EMH. (B) Survival from splenectomy according to the presence of microinfarctions. (C) Survival from splenectomy according to splenic karyotype. PPIG indicates predominance of immature granulocytes.
Histologic prognostic factors from splenic tissue in 213 patients with myelofibrosis with myeloid metaplasia.
(A) Survival from diagnosis according to splenic EMH. (B) Survival from splenectomy according to the presence of microinfarctions. (C) Survival from splenectomy according to splenic karyotype. PPIG indicates predominance of immature granulocytes.
Microscopic splenic infarcts were observed in 26 patients and were not associated with a particular histologic pattern or with the occurrence of postsplenectomy thrombocytosis or vascular events. In contrast, their presence was significantly associated with an adverse prognostic score,11 thrombocytopenia, and the presence of circulating blasts. In addition, patients with splenic infarcts weremore likely to have their disease transform into acute leukemia (P = .08) and to have worse overall and postsplenectomy survival (Figure 2). Splenic cytogenetic studies were performed in 92 patients, and 52 (56.5%) had an abnormal karyotype (29 single and 23 multiple karyotypic lesions). Specific abnormalities included 20q− (n = 15), 13q− (n = 11), +9 (n = 5), abnormalities of chromosome 5 or 7 (n = 5), 12p− (n = 4), abnormal chromosome 1 (n = 4), isochromosome 17q (n = 3), and +8 (n = 2). Of the 92 patients who had splenic karyotype analysis, 68 had information on bone marrow karyotype that was performed either before (n = 60) or after (n = 8) splenectomy. The karyotypic findings in the 2 tissues were concordant in more than 85% of cases. Among 9 of the 10 patients who had karyotype discordance between spleen and bone marrow, the splenic karyotype showed the same clone as in the bone marrow but with additional chromosomal lesions. Only one patient had an abnormal karyotype that was found in the bone marrow but not in the spleen. The presence of an abnormal splenic karyotype was associated with decreased postsplenectomy survival (P = .03) (Figure 2), but not with other clinicopathologic variables.
The excellent concordance between bone marrow and splenic cytogenetic clones, as well as our histologic observations, strengthens the hypothesis12 that splenic EMH in MMM arises from filtration of the clonally involved circulating progenitor cells. Splenic EMH arising in other conditions, such as myelophthisis from metastatic cancer or marrow stimulation by granulocyte colony-stimulating factor,13 has also been shown to arise from filtration of circulating progenitors.14,15 The peripheral blood progenitor pool in MMM is markedly elevated,16 and its preferential localization and proliferation in the spleen and liver suggest that these organs provide an environment conducive to progenitor growth and differentiation. Our observation concerning the detrimental prognostic significance of immature granulocyte predominance of splenic EMH may therefore reflect a changing circulating progenitor pool in advanced MMM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ayalew Tefferi, Division of Hematology and Internal Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal