Abstract
Umbilical cord blood (UCB) is being increasingly used for hematopoietic stem cell transplantation and has been associated with a reduced incidence of severe graft-versus-host disease (GVHD). To further investigate the relative merits of unrelated donor UCB versus bone marrow (BM), a matched-pair analysis comparing the outcomes of recipients of 0 to 3 human leukocyte antigen (HLA)–mismatched UCB and HLA-A, B, DRB1-matched BM was performed. UCB patients, who received cyclosporine (CSA) and methylprednisolone (MP), were matched for age, diagnosis, and disease stage with BM patients, who received either methotrexate (MTX) and CSA (26 pairs) or T-cell depletion (TCD) and CSA/MP (31 pairs). Patients were predominantly children (median age, 5 years) undergoing transplantation for malignancy, storage diseases, BM failure, and immunodeficiency syndromes between 1991 and 1999. Although neutrophil recovery was significantly slower after UCB transplantation, the probability of donor-derived engraftment at day 45 was 88% in UCB versus 96% in BM-MTX recipients (P = .41) and 85% in UCB versus 90% in BM-TCD recipients (P = .32), respectively. Platelet recovery was similar in UCB versus BM pairs. Furthermore, incidences of acute and chronic GVHD were similar in UCB and BM recipients, with 53% of UCB versus 41% of BM-MTX recipients alive (P = .40) and 52% of UCB versus 56% of BM-TCD recipients alive at 2 years (P > .80), respectively. These data suggest that despite increased HLA disparity, probabilities of engraftment, GVHD, and survival after UCB transplantation are comparable to those observed after HLA-matched BM transplantation. Therefore, UCB should be considered an acceptable alternative to HLA-matched BM for pediatric patients.
Introduction
Hematopoietic stem cell (HSC) transplantation, using human leukocyte antigen (HLA)–matched sibling or unrelated bone marrow (BM) donors, has been used successfully to treat patients with high-risk or relapsed hematologic malignancies, BM failure syndromes, and hereditary immunodeficiency and metabolic disorders.1However, use of this therapy has been limited by availability of fully HLA-matched donors, despite the increasing size of unrelated donor registries.2 For those transplanted with unrelated donor BM, increased HLA disparity adversely affects survival due to increased risks of severe acute and chronic graft-versus-host disease (GVHD) and opportunistic infection. Only young recipients are able to tolerate a single HLA-A, B, DRB1 mismatch in this setting.3-5 To potentially extend the donor pool, umbilical cord blood (UCB) has been used as an alternative source of HSC. Since the first unrelated donor UCB transplant in 1993, approximately 1500 UCB transplants have been performed worldwide. It has been shown that cryopreserved unrelated UCB from 0 to 3 HLA-A, B, DRB1-mismatched donors contains sufficient HSC to engraft most pediatric patients.6-10 In addition to rapid availability and very low rate of contamination with herpes group viruses, UCB transplantation (UCBT) results in a low incidence of both severe acute GVHD and extensive chronic GVHD, despite the use of grafts with substantial donor-recipient HLA disparity.7-11
For many patients, both unrelated donor BM and UCB are available as potential options for transplantation. However, to date there have been no reported comparisons of the outcomes of UCBT versus BM transplantation (BMT) in the unrelated setting. To formally compare the outcomes of patients undergoing transplantation using these different HSC sources, a matched-pair analysis of patients who have undergone unrelated donor UCBT or BMT at a single institution was performed. The results include comparisons of hematopoietic recovery, acute and chronic GVHD, mortality at day 100, survival, and causes of death.
Patients, materials, and methods
Patients
All patients who had undergone unrelated donor UCBT at the University of Minnesota were eligible for analysis. Patients were eligible for unrelated UCBT if HLA-compatible related or unrelated BM donors were not available within 3 months of search initiation. The UCB patients underwent transplantation between 1994 and 1999 with 0 to 3 HLA-A, B, DRB1-mismatched UCB units obtained from the Placental Blood Programs at the New York Blood Center and the St Louis Cord Blood Bank, and through Netcord. Recipients of HLA-A, B, DRB1-matched unrelated donor BM used for comparison had a transplantation performed between 1991 and 1999 using BM donors identified by the National Marrow Donor Program. Patients who had a previous allogeneic HSC transplantation, or an immunodeficiency state not requiring myeloablative therapy, or who were alive but with fewer than 100 days of follow-up were excluded.
Sixty-three recipients of unrelated donor UCBT were considered for potential matching with recipients of BMT. Patient diagnoses included acute leukemia, myelodysplasia, chronic myelogenous leukemia (CML), BM failure syndromes (Fanconi anemia or severe aplastic anemia), immunodeficiency disorders, or inborn errors of metabolism. Patients with malignancy qualified as standard risk if they were in first or second complete remission (CR1 or CR2), or chronic phase CML, or had no high-risk cytogenetics (eg, acute lymphoblastic leukemia with t(4;11) or t(9;22)). Patients in CR3, relapse, CML beyond chronic phase, or who had myelodysplasia or high-risk cytogenetics were classified as high risk.
For all patients, HLA-A and -B typing was determined by serology, whereas HLA-DRB1 allele level typing was determined by high-resolution molecular techniques. Treatment protocols for myeloablative therapy and the use of unrelated HSC from UCB or BM were reviewed and approved by the Institutional Review Board of the University of Minnesota. Written informed consent was obtained from the patients or their parents before transplantation.
Conditioning and GVHD prophylaxis
All patients, regardless of HSC source, were conditioned with cyclophosphamide (CY), 120 mg/kg, fractionated total body irradiation (TBI), 1320 to 1375 cGy, except for a minority in whom TBI was contraindicated who received CY, 200 mg/kg, and busulphan, 16 mg/kg (3 UCB and 6 BM patients). Patients with Fanconi anemia received a dose-reduced preparative regimen as previously described.12 All UCB recipients received antithymocyte globulin (ATG), 15 mg/kg, on days −3 to −1 every 12 hours for 6 doses, and 71% received granulocyte colony-stimulating factor (G-CSF), 5 μg/kg daily from day 0 until neutrophil engraftment. GVHD prophylaxis for UCBT consisted of cyclosporine (CSA) for 6 months, maintaining a trough blood level of more than 200 μg/L, and methylprednisone (MP) 1 mg/kg every 12 hours days 5 to 19 after transplantation.
Two different approaches to GVHD prophylaxis were used for patients undergoing unrelated donor BMT: methotrexate (MTX) and CSA for recipients of unprocessed BM (designated BM-MTX) or T-cell depletion (TCD) followed by CSA and MP (designated BM-TCD). Selection of patients for either method of unrelated donor BMT was determined by the current institutional study available for which all patients were eligible. This choice was not determined by disease or disease risk. The method of BM processing was counterflow elutriation for all TCD recipients.13 Those in the BM-MTX arm received methotrexate, 15 mg/m2 on day +1 and 10 mg/m2on days +3, +6, and +11. The BM-TCD recipients received ATG before transplantation, and MP days 5 to 19 after transplantation, identical to that given to UCB recipients. Both BMT groups received the same CSA prophylaxis as for UCB recipients. Since January 1997, G-CSF was administered to BMT patients at 5 μg/kg per day from day 7 until neutrophil engraftment.
Selection of matched pairs
Recipients of HLA-matched unrelated donor BM were divided into 2 cohorts for comparison: those who received BM-MTX and those who received BM-TCD. Thus, 2 data sets were derived for comparison to recipients of 0 to 3 HLA antigen-mismatched unrelated donor UCB. Matching criteria were recipient age (± 3 years), diagnosis, and risk status (malignancy patients only). If multiple BM recipients matched with a UCB recipient, preference was given to the BM recipient with the closest age followed by the closest year of transplantation. The 2 cohorts were compared for recipient weight, gender, conditioning regimen, recipient cytomegalovirus (CMV) seropositivity, graft cell dose, year of transplantation, and median follow-up.
Statistical analysis
The major study end points were neutrophil and platelet engraftment, acute GVHD (grades II-IV and III-IV), chronic GVHD, and survival. Other outcomes evaluated were 100-day mortality and causes of death. Event time for neutrophil engraftment was the date of transplantation to the first of 3 consecutive days with an absolute neutrophil count (ANC) above 5 × 108/L. The cumulative incidence of neutrophil engraftment was calculated by treating patients with very slow engraftment (ie, achieved an ANC > 5 × 108/L after day 45) or patients who failed to have marrow reconstitution of donor origin as graft failures. Patients were censored if they died prior to day 28 after transplantation or had a diagnosis of persistent disease.14 The cumulative incidence of platelet independence (defined as 14 consecutive days without a platelet transfusion) was calculated by treating deaths from other causes as competing risks. The estimate of the overall proportion of engraftment, without regard to the time to engraftment, was perfomed by a McNemar test.15
The cumulative incidence of acute and chronic GVHD was also calculated by treating deaths from other causes as competing risks. Diagnosis of GVHD was based on standard clinical criteria with histopathologic confirmation where possible.16-18 The statistical end point of survival was estimated by the Kaplan-Meier method.15 Event times for survival were measured from date of transplantation to date of death or date of last contact.
Statistical comparisons for the end points were completed with the Prentice-Wilcoxon test for matched pairs.19 To compare patient and transplant characteristics between UCB and BM recipients, the Pearson χ2 test was performed for categorical factors, and the Wilcoxon test was used for continuous variables.15
Results
Patient characteristics
The demographics of the UCB and BM patients matched for age, diagnosis, and disease risk are shown in Table1. There were no significant differences in recipient age, weight, diagnosis in either UCB versus BM-MTX or UCB versus BM-TCD matched pairs. Race, gender, months from diagnosis to transplantation, and conditioning regimens were also similar (data not shown). The vast majority of UCB grafts were 1 to 2 HLA mismatched (77% in the UCB recipients matched with BM-MTX patients and 87% in UCB recipients paired with BM-TCD patients). In contrast, all BM patients received HLA-A, B, DRB1-matched grafts. In the UCB versus BM-MTX matched pairs, the majority of patients had hematologic malignancy (65%), whereas in the UCB versus BM-TCD data set, the majority of patients had a metabolic storage disease (52%). Year of transplantation also differed somewhat between the recipients of UCB and BM because all UCB transplantations were performed since 1994 (Table 1). Differences in the cell dose between UCB and BM grafts in recipients of MTX reflect the different nature of the HSC sources, with TCD of BM yielding similar nucleated cell doses to UCB grafts. Fewer UCB recipients were CMV positive as compared with those receiving BM-TCD (26% versus 52%, P = .04), but there was no difference in recipient CMV seropositivity within the UCB versus BM-MTX data set (31% versus 38%, P = .56).
Demographics of unrelated donor umbilical cord blood versus bone marrow–methotrexate recipients and umbilical cord blood versus bone marrow–T-cell depletion recipients
| . | UCB . | BM-MTX . | P . | UCB . | BM-TCD . | P . |
|---|---|---|---|---|---|---|
| N | 26 | 26 | 31 | 31 | ||
| Age (y)* | NS | NS | ||||
| Median (range) | 4.5 (0.2-17.9) | 4.7 (0.6-17.7) | 5.8 (0.2-17.9) | 6.8 (0.5-1.7) | ||
| Weight (kg) | NS | NS | ||||
| Median (range) | 19.6 (5-78) | 19.7 (5.9-80) | 20.2 (5-91) | 21.0 (5.7-91) | ||
| Transplant year | < .05 | NS | ||||
| 1991-1993 | 0 (0%) | 5 (19%) | 0 (0%) | 1 (3%) | ||
| 1994-1999 | 26 (100%) | 21 (81%) | 31 (100%) | 30 (97%) | ||
| HLA disparity | ||||||
| 3/6 | 1 (4%) | 0 | 0 | 0 | ||
| 4/6 | 8 (31%) | 0 | 5 (16%) | 0 | ||
| 5/6 | 12 (46%) | 0 | 22 (71%) | 0 | ||
| 6/6 | 5 (19%) | 26 (100%) | 4 (13%) | 31 (100%) | ||
| Diagnosis* | NS | NS | ||||
| ALL | 10 (38%) | 10 (38%) | 8 (26%) | 8 (26%) | ||
| AML | 5 (19%) | 5 (19%) | 3 (10%) | 3 (10%) | ||
| MDS | 1 (4%) | 1 (4%) | 1 (3%) | 1 (3%) | ||
| CML | 1 (4%) | 1 (4%) | 0% | 0% | ||
| FA/SAA | 3 (12%) | 3 (12%) | 3 (10%) | 3 (10%) | ||
| Immunodeficiency | 3 (12%) | 3 (12%) | 0% | 0% | ||
| Storage disease | 3 (12%) | 3 (12%) | 16 (52%) | 16 (52%) | ||
| Risk* | NS | NS | ||||
| High | 15 (58%) | 15 (58%) | 24 (77%) | 24 (77%) | ||
| Standard | 11 (42%) | 11 (42%) | 7 (23%) | 7 (23%) | ||
| Cell dose × 108/kg | < .05 | NS | ||||
| Median (range) | 0.3 (0.1-2.8) | 2.0 (1.9-4.0) | 0.3 (0.1-2.8) | 0.5 (0.2-2) |
| . | UCB . | BM-MTX . | P . | UCB . | BM-TCD . | P . |
|---|---|---|---|---|---|---|
| N | 26 | 26 | 31 | 31 | ||
| Age (y)* | NS | NS | ||||
| Median (range) | 4.5 (0.2-17.9) | 4.7 (0.6-17.7) | 5.8 (0.2-17.9) | 6.8 (0.5-1.7) | ||
| Weight (kg) | NS | NS | ||||
| Median (range) | 19.6 (5-78) | 19.7 (5.9-80) | 20.2 (5-91) | 21.0 (5.7-91) | ||
| Transplant year | < .05 | NS | ||||
| 1991-1993 | 0 (0%) | 5 (19%) | 0 (0%) | 1 (3%) | ||
| 1994-1999 | 26 (100%) | 21 (81%) | 31 (100%) | 30 (97%) | ||
| HLA disparity | ||||||
| 3/6 | 1 (4%) | 0 | 0 | 0 | ||
| 4/6 | 8 (31%) | 0 | 5 (16%) | 0 | ||
| 5/6 | 12 (46%) | 0 | 22 (71%) | 0 | ||
| 6/6 | 5 (19%) | 26 (100%) | 4 (13%) | 31 (100%) | ||
| Diagnosis* | NS | NS | ||||
| ALL | 10 (38%) | 10 (38%) | 8 (26%) | 8 (26%) | ||
| AML | 5 (19%) | 5 (19%) | 3 (10%) | 3 (10%) | ||
| MDS | 1 (4%) | 1 (4%) | 1 (3%) | 1 (3%) | ||
| CML | 1 (4%) | 1 (4%) | 0% | 0% | ||
| FA/SAA | 3 (12%) | 3 (12%) | 3 (10%) | 3 (10%) | ||
| Immunodeficiency | 3 (12%) | 3 (12%) | 0% | 0% | ||
| Storage disease | 3 (12%) | 3 (12%) | 16 (52%) | 16 (52%) | ||
| Risk* | NS | NS | ||||
| High | 15 (58%) | 15 (58%) | 24 (77%) | 24 (77%) | ||
| Standard | 11 (42%) | 11 (42%) | 7 (23%) | 7 (23%) | ||
| Cell dose × 108/kg | < .05 | NS | ||||
| Median (range) | 0.3 (0.1-2.8) | 2.0 (1.9-4.0) | 0.3 (0.1-2.8) | 0.5 (0.2-2) |
UCB indicates umbilical cord blood; BM-MTX, bone marrow–methotrexate; BM-TCD, BM–T-cell depletion; NS, not significant; HLA, human leukocyte antigen; ALL, acute lymphoblastic leukemia; AML, acute myeloblastic leukemia; MDS, myelodysplasia; CML, chronic myelogenous leukemia; FA, Fanconi anemia; SAA, severe aplastic anemia.
Indicates matching criteria.
Engraftment
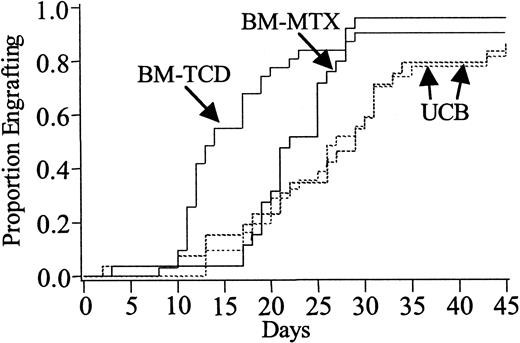
Neutrophil recovery was significantly delayed in UCB recipients when compared with those receiving either BM-MTX (29 days [range, 2-54] versus 22 days [range, 3-29]; P = .03) or BM-TCD (27 days [range, 13-54] versus 14 days [range, 8-29];P < .01) (Figure 1). However, by day 45 the overall engraftment rate of 88% versus 96% in UCB versus BM-MTX recipients (P = .41) and 85% versus 90% in UCB versus BM-TCD recipients (P = .32), respectively, were not statistically different (Table2).
Neutrophil recovery.
Cumulative incidence of neutrophil recovery in recipients of unrelated donor UCB versus HLA-matched BM who received GVHD prophylaxis with MTX (BM-MTX) and UCB versus HLA-matched BM who received GVHD prophylaxis with TCD (BM-TCD). UCB versus BM-MTX: engraftment rate,P = .03; engraftment at day 45, P = .41. UCB versus BM-TCD: engraftment rate, P < .01; engraftment at day 45, P = .32.
Neutrophil recovery.
Cumulative incidence of neutrophil recovery in recipients of unrelated donor UCB versus HLA-matched BM who received GVHD prophylaxis with MTX (BM-MTX) and UCB versus HLA-matched BM who received GVHD prophylaxis with TCD (BM-TCD). UCB versus BM-MTX: engraftment rate,P = .03; engraftment at day 45, P = .41. UCB versus BM-TCD: engraftment rate, P < .01; engraftment at day 45, P = .32.
Analysis of the neutrophil and platelet recovery, acute graft-versus-host disease at day 100, chronic graft-versus-host disease at 1 year, and 2-year survival of recipients of 0-3 human leukocyte antigen–mismatched umbilical cord blood versus human leukocyte antigen–matched BM
| HSC source . | N . | Neutrophil recovery d 45 . | Platelet recovery d 180 . | Grades II-IV acute GVHD . | Chronic GVHD . | 2-y survival . |
|---|---|---|---|---|---|---|
| UCB | 26 | 88% (75-100) | 72% (50-94) | 42% (23-61) | 5% (0-13) | 53% (31-75) |
| BM-MTX | 26 | 96% (89-100) | 76% (54-98) | 35% (17-53) | 20% (5-35) | 41% (22-60) |
| UCB | 31 | 85% (72-98) | 84% (64-100) | 36% (19-53) | 7% (0-16) | 52% (30-73) |
| BM-TCD | 31 | 90% (80-100) | 84% (64-100) | 35% (18-52) | 13% (1-25) | 56% (38-79) |
| HSC source . | N . | Neutrophil recovery d 45 . | Platelet recovery d 180 . | Grades II-IV acute GVHD . | Chronic GVHD . | 2-y survival . |
|---|---|---|---|---|---|---|
| UCB | 26 | 88% (75-100) | 72% (50-94) | 42% (23-61) | 5% (0-13) | 53% (31-75) |
| BM-MTX | 26 | 96% (89-100) | 76% (54-98) | 35% (17-53) | 20% (5-35) | 41% (22-60) |
| UCB | 31 | 85% (72-98) | 84% (64-100) | 36% (19-53) | 7% (0-16) | 52% (30-73) |
| BM-TCD | 31 | 90% (80-100) | 84% (64-100) | 35% (18-52) | 13% (1-25) | 56% (38-79) |
95% confidence intervals are in parentheses. All outcomes were equivalent (P > .05).
HSC indicates hematopoietic stem cell; GVHD, graft-versus-host disease; for other abbreviations, see Table 1.
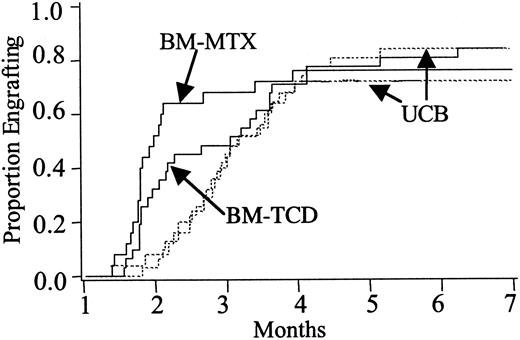
The difference in time to achieve platelet independence in the UCB versus BM-MTX recipients was not statistically significant, taking 66 days (range 0-255) versus 30 days (range 12-89) (P = .12). UCB versus BM-TCD recipients were similar at 61 days (range 19-255) versus 59 days (range 11-162) (P = .74) (Figure2). At 6 months platelet engraftment was similar, being 72% in UCB versus 76% in BM-MTX recipients (P > .80) and 84% in UCB versus 84% in BM-TCD recipients (P > .80), respectively (Table 2).
Platelet recovery.
Cumulative incidence of platelet recovery in recipients of unrelated donor UCB versus HLA-matched BM who received GVHD prophylaxis with MTX (BM-MTX) and UCB versus HLA-matched BM who received GVHD prophylaxis with TCD (BM-TCD). UCB versus BM-MTX: engraftment rate,P = .12; engraftment at day 45, P > .80. UCB versus BM-TCD: engraftment rate, P = .74; engraftment at day 45, P > .80.
Platelet recovery.
Cumulative incidence of platelet recovery in recipients of unrelated donor UCB versus HLA-matched BM who received GVHD prophylaxis with MTX (BM-MTX) and UCB versus HLA-matched BM who received GVHD prophylaxis with TCD (BM-TCD). UCB versus BM-MTX: engraftment rate,P = .12; engraftment at day 45, P > .80. UCB versus BM-TCD: engraftment rate, P = .74; engraftment at day 45, P > .80.
Notably, there were no marked differences in the incidence of graft failure in the UCB and BM recipients (2 UCB versus 1 BM-MTX and 5 UCB versus 3 BM-TCD recipients, respectively). All episodes of graft failure were early (before day 60) except for one BM-MTX and one UCB recipient.
Acute and chronic GVHD
Grades II, III, and IV acute GVHD developed in 42% of UCB recipients versus 35% of those receiving BM-MTX (P = .80) and 36% in UCB versus 35% in BM-TCD recipients (P > .80) (Table 2). In addition, rates of severe grade III to IV GVHD in the UCB and BM pairs were similar: 19% (4%-34%) versus 8% (0%-18%) in UCB versus BM-MTX recipients (P = .32) and 10% (0%-20%) versus 13% (1%-25%) in UCB versus BM-TCD recipients (P = .78). Although there was a trend toward less chronic GVHD in recipients of UCB, particularly when compared to recipients of BM-MTX, the differences did not reach statistical significance (UCB versus BM-MTX, P = .12 and UCB versus BM-TCD, P = .32) (Table 2).
Survival
Survival at 100 days and 2 years was comparable between UCB and BM pairs. At 100 days there was a 27% (95% CI 10-44) mortality in UCB versus 15% (95% CI 1-29) in BM-MTX recipients (P = .35) and 23% (95% CI 8-38) in UCB versus 16% (95% CI 3-29%) in BM-TCD recipients (P = .60). Notably, survival at 2 years after transplantation was 53% (CI 31%-75%) in UCB versus 41% (CI 22%-60%) in BM-MTX recipients (P = .40) and 52% (CI 30%-73%) in UCB versus 56% (CI 38%-79%) in those receiving BM-TCD (P > .80) (Table 2, Figure3). Causes of death are summarized in Table 3, and although trends may be suggested, the patient numbers were too few for meaningful statistical comparison.
Estimates of survival.
Kaplan-Meier estimates of survival in recipients of unrelated donor UCB versus HLA-matched BM who received GVHD prophylaxis with MTX (BM-MTX) and UCB versus HLA-matched BM who received GVHD prophylaxis with TCD (BM-TCD). UCB versus BM-MTX, P = .40. UCB versus BM-TCD,P > .80.
Estimates of survival.
Kaplan-Meier estimates of survival in recipients of unrelated donor UCB versus HLA-matched BM who received GVHD prophylaxis with MTX (BM-MTX) and UCB versus HLA-matched BM who received GVHD prophylaxis with TCD (BM-TCD). UCB versus BM-MTX, P = .40. UCB versus BM-TCD,P > .80.
Comparison of causes of death for recipients of 0-3 HLA–mismatched umbilical cord blood versus human leukocyte antigen–matched bone marrow
| Cause of death . | UCB n = 11 . | BM-MTX n = 15 . | UCB n = 13 . | BM-TCD n = 14 . |
|---|---|---|---|---|
| GVHD +/− infection | 4 (36%) | 5 (33%) | 3 (23%) | 7 (50%) |
| Relapse/refractory leukemia | 5 (45%) | 4 (27%) | 3 (23%) | 1 (7%) |
| Graft failure | 0 (0%) | 0 (0%) | 2 (15%) | 2 (14%) |
| Organ failure +/− infection | 1 (9%) | 4 (27%) | 1 (8%) | 0 (0%) |
| Other | 1 (9%) | 2 (13%) | 4 (31%) | 4 (29%) |
| Cause of death . | UCB n = 11 . | BM-MTX n = 15 . | UCB n = 13 . | BM-TCD n = 14 . |
|---|---|---|---|---|
| GVHD +/− infection | 4 (36%) | 5 (33%) | 3 (23%) | 7 (50%) |
| Relapse/refractory leukemia | 5 (45%) | 4 (27%) | 3 (23%) | 1 (7%) |
| Graft failure | 0 (0%) | 0 (0%) | 2 (15%) | 2 (14%) |
| Organ failure +/− infection | 1 (9%) | 4 (27%) | 1 (8%) | 0 (0%) |
| Other | 1 (9%) | 2 (13%) | 4 (31%) | 4 (29%) |
Discussion
Experience in related and unrelated donor UCBT has indicated that UCB is associated with a slower rate of hematopoietic recovery, with a clear association between cell dose and both engraftment and survival.9-11,20,21 Also, the low incidence of severe acute and extensive chronic GVHD is remarkable in view of the increased use of HLA-disparate grafts.6-11 20-22 However, no randomized trials exist that allow direct comparison between UCB and BM from unrelated donors. Therefore, we performed a matched-pair analysis comparing the outcomes of pediatric recipients of 0 to 3 HLA-antigen mismatched unrelated donor UCB grafts with those receiving HLA-matched BM. This study has the advantage of being from a single institution in which patients have been exposed to similar and consistent clinical practice in regard to the conditioning and GVHD prophylaxis, with identical supportive care and a uniform grading system for GVHD.
Similar to the findings of Rocha and colleagues,22 who compared the outcomes of sibling donor UCBT and BMT, unrelated donor UCBT was associated with delayed neutrophil recovery compared to unrelated donor BMT. However, unlike the Rocha study, this analysis found that the overall neutrophil engraftment rates were comparable. Nonetheless, improving the speed of and overall engraftment rate in UCBT remain ongoing challenges. Therefore, a major focus in UCBT is to augment UCB cell dose by optimizing collection techniques,23 ex vivo expansion, or the transplantation of UCB units from multiple donors. Interestingly, platelet recovery was similar in both UCB and BM groups. This is in keeping with recent studies that have documented delayed and incomplete platelet recovery in recipients of unrelated donor BM.24
Case series have shown low incidences of acute and chronic GVHD in unrelated donor UCB recipients, suggesting that these may be lower than those seen in unrelated donor BMT. However, in this study the rates of acute and chronic GVHD in UCBT and BMT recipients were similar. Although these findings may appear to contrast to those reported by Rocha and coworkers, in which HLA-identical sibling UCBT was associated with a lower risk of acute and chronic GVHD as compared to sibling BMT,22 it should be noted that the vast majority of UCB recipients in this paper received an HLA-disparate graft as compared to BM recipients who received HLA-matched grafts. In unrelated donor BMT, HLA disparity has been associated with a very high risk of GVHD. Balduzzi and associates found incidences of grades II to IV acute GVHD of 98% and chronic GVHD of 69% in pediatric recipients of HLA-mismatched BM.3 Thus, this study supports the contention that HLA-mismatch does not have a major impact on the outcome of unrelated donor UCBT11 as it does in unrelated donor BMT, such that outcomes with mismatched UCB are comparable to those of HLA-matched BMT. Although the reasons for this relatively low risk of GVHD in UCBT despite HLA disparity are not known, it may be attributable to a relatively low T-cell dose or the functional immaturity of the neonatal immune system.25 26
Most importantly, like the study by Rocha and coworkers,22which demonstrated similar survival in recipients of sibling donor UCB and BM, in this analysis unrelated donor UCBT compares favorably with HLA-matched BMT in that 100-day mortality, and 1- and 2-year survival are similar. Despite the slower rate of engraftment in the UCB recipients, there was no impact on the early transplantation-related mortality as compared to that seen in BMT. Therefore, this study demonstrates that the UCB cell dose is sufficient to achieve similar survival outcomes as seen with unrelated donor BM. This is in contrast to the findings in unrelated donor BMT in which HLA disparity is associated with a significantly increased risk of transplantation-related mortality.3 5
These data will need to be confirmed in the future with larger patient numbers. Also, it should be emphasized that the majority of UCB recipients in this analysis received UCB grafts that were either 1 or 2 HLA-antigen mismatched, and these results cannot be generalized to recipients of 3 antigen-mismatched grafts. Furthermore, it should be noted this was an analysis of the outcomes in children and therefore cannot be generalized to adults. Interestingly, a recent analysis of UCBT in 68 adults demonstrated similar engraftment results to those seen in pediatric series.21 Nonetheless, the limitation of UCB cell dose may potentially have an impact on the relative merits of UCB and BM as HSC sources when providing transplants for adults and will require additional study.
In conclusion, this study is of importance because it suggests that, based on the data currently available, UCBT is a reasonable alternative to HLA-matched BMT in pediatric recipients. This is of particular relevance to patients requiring urgent transplantation for whom an unrelated donor UCB unit can be obtained substantially more quickly than BM.
Supported in part by grants from the National Cancer Institute (PO1-CA65493), National Heart, Lung and Blood Institute (NO1-HB-67139), and the Children's Cancer Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Juliet Barker, Department of Medicine, Box 480, 420 Delaware St, SE, Minneapolis, MN 55455; e-mail:barke014@tc.umn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal