Abstract
Relapse is the major cause of death after allogeneic bone marrow transplantation (BMT). This study tested the hypothesis that the numbers of donor mononuclear cells, lymphocytes, and CD34+cells influence relapse and event-free survival (EFS) after BMT. The study population consisted of 113 consecutive patients with hematologic malignancies who underwent non–T-cell–depleted BMT from HLA-matched siblings. Sixty-four patients had low-risk diagnoses (ALL/AML CR1, MDS RA/RARS, and CML CP1); 49 patients had high-risk diagnoses (all others). CD34+ cells, T cells, B cells, natural killer cells, monocytes, and a rare population of CD3−, CD4bright cells in the allografts were measured by flow cytometry. The CD3−, CD4bright cells in bone marrow had the same frequency and phenotype as CD123brighttype 2 dendritic cell (DC) progenitors, and they differentiated into typical DCs after short-term culture. Cox regression analyses evaluated risk strata, age, gender, and the numbers of nucleated cells, CD3+ T cells, CD34+ hematopoietic cells, and CD4bright cells as covariates for EFS, relapse, and nonrelapse mortality. Recipients of larger numbers of CD4bright cells had significantly lower EFS, a lower incidence of chronic graft-versus-host disease (cGVHD), and an increased incidence of relapse. Recipients of larger numbers of CD34+ cells had improved EFS; recipients of fewer CD34+ cells had delayed hematopoietic engraftment and increased death from infections. In conclusion, the content of donor CD4bright cells was associated with decreased cGVHD and graft-versus-leukemia effects in recipients of allogeneic bone marrow transplantation, consistent with a role for donor DCs in determining immune responses after allogeneic BMT.
Introduction
Despite significant improvements in supportive care in the past 30 years, aggregate 5-year survival for all patients undergoing allogeneic transplantation from human leukocyte antigen (HLA)-matched siblings remains approximately 50%.1-7Numerous factors, including the patient's disease stratum, age, conditioning regimen, and type of graft-versus-host disease (GVHD) prophylaxis, influence the probability of survival after allogeneic transplantation.1,8 9
Optimal numbers for each cell type in the bone marrow (BM) or peripheral blood progenitor cell allograft are unknown. Recipients of T-cell–depleted allografts have decreased risk for GVHD10,11 but experience delayed immune reconstitution and have increased risk for infection, graft failure, and relapse.12-14 Larger numbers of colony-forming unit (CFU)–granulocyte macrophage colonies in bone marrow allografts have been associated with improved survival after transplantation and faster hematopoietic engraftment.15 Larger numbers of transplanted CD34+ cells have been correlated with faster hematopoietic reconstitution after allogeneic transplantation16-20 and increased risk for acute GVHD after transplantation.21 Transplantation with fewer than 1 × 106 CD34+ cells/kg from T-cell–depleted allogeneic bone marrow and peripheral stem cell grafts have been associated with increased relapse and treatment-related death.16,22 In one study, transplantation of more than 3 × 106 CD34+ cells/kg resulted in a 14% relapse rate versus a 48% rate for recipients of lower CD34+ cell doses.22
The role of type 1 and type 2 dendritic cells (DCs) in regulating immune reconstitution, GVHD, and GvT effects after allogeneic transplantation has become an area of increasing interest. Type 1 DC (DC1) promotes Th1 immune responses in responding CD4+ T cells characterized by enhanced interferon-γ (IFN-γ), tumor necrosis factor (TNF), and IL-12 synthesis. Type 2 DC (DC2) promotes Th2 responses in CD4+ T cells characterized by IL-4 and IL-10 synthesis and the inhibition of IFN-γ and TNF production in cognate T cells.23 Bone marrow contains monocytes and CD86+, CD34+ progenitor cells that can differentiate into DC1 in the presence of TNF and granulocyte macrophage–colony-stimulating factor (GM-CSF),24-26 and it contains CD123bright DC progenitors that can differentiate into DC2 in the presence of IL-3.27 Most studies involving hematopoietic progenitor cell transplantation to date have examined the role of DC1 as a method of adoptive immunotherapy for cancer after autologous hematopoietic progenitor cell (HPC) transplantation.28 One recent report, using a murine transplantation model, indicated that only host CD11c+ DC (DC1) was necessary for the development of acute GVHD.29Donor DC did not appear to efficiently present host antigens by cross-priming in a way that led to the initiation of GVHD.29 In this study, the possible effect of donor DCs on inhibiting GVHD was not examined. The potential role of increased numbers of donor DC2 in regulating GVHD after G-CSF mobilized allogeneic peripheral stem cell transplants has recently been recognized.30
We undertook a prospective analysis of the cellular constituents of the bone marrow allograft to help identify those factors that might predict relapse, GVHD, and event-free survival (EFS) among patients undergoing transplantation for hematologic malignancy. The CD3−, CD4bright, CD8−, low side-scatter phenotype was used as a surrogate marker for one population of DCs in bone marrow.27 31 The most significant cellular factors in predicting overall survival and EFS after transplantation were the content of donor hematopoietic CD34+ progenitor cells and the numbers of donor CD3−, CD4bright, CD8− cells in the allograft. We herein present data that the donor CD3−, CD4bright, CD8−cells in bone marrow are equivalent to CD123bright DC2 progenitors in bone marrow. Patients who underwent transplantation with larger numbers of CD4bright cells had more relapses and decreased chronic GVHD, supporting an important association of donor CD4bright DCs with graft-versus-leukemia effects after transplantation.
Patients, materials, and methods
Patient characteristics
One hundred nineteen consecutive patients underwent allogeneic non–T-cell–depleted bone marrow transplantation from HLA-matched sibling donors between April 1995 and September 1999. All had hematologic malignancies or preleukemic (myelodysplastic) syndromes. Data on the numbers of transplanted stem cells and lymphoid subsets were unavailable for 5 patients, and 1 patient died of pulmonary embolism during transplantation, leaving a subset of 113 patients who constituted the study group. Patients with acute lymphoblastic leukemia (ALL) in first remission, acute myelogenous leukemia (AML) in first remission, chronic myelogenous leukemia (CML) in first chronic phase, myelodysplastic syndrome (MDS) with refractory anemia (RA), and MDS with RA and ringed sideroblasts (RARS) were considered to be at low risk for transplantation-related complications or death after transplantation. Patients with all other diagnoses—including chronic lymphocytic leukemia (CLL), Hodgkin disease (HD), other MDS, non-Hodgkin lymphoma (NHL), and multiple myeloma (MM) and with more advanced ALL, AML, and CML—were considered to be at high risk for death after transplantation.
Bone marrow harvest and processing
Bone marrow cells were aspirated from the posterior iliac crests of donors under general anesthesia. The target number of marrow cells harvested was a minimum transplantation dose of 2 × 108nucleated cells per kilogram patient weight, within the limit of a maximum marrow volume of 20 mL/kg donor weight. Marrow was anticoagulated with heparin to a final concentration of 10 U/mL. Red cell or plasma depletion was performed if donor and recipient were incompatible by ABO blood group typing. Fresh marrow grafts were infused into recipients on the same day through an indwelling central venous catheter, without T-cell depletion or CD34+ cell enrichment.
T-cell proliferation assays
Subpopulations of bone marrow mononuclear cells were isolated by fluorescence-activated cell sorter (FACS) to more than 95% purity and tested as stimulators in a one-way mixed lymphocyte reduction (MLR) assay. Purified bone marrow cells irradiated to 1500 cGy and serial dilutions were prepared starting at 50 000 cells/well to 400 cells/well in 96-well culture plates containing RPMI media with 50 ng/mL IL-3, 100 ng/mL GM-CSF, and 10% AB− human plasma. Then 50 000 allogeneic peripheral blood mononuclear cells from a normal donor were added to each well. Cultures were maintained in a humidified atmosphere at 37°C in 5% CO2 for 5 days. Cultures were pulsed with 1 μCi/well of 3H-thymidine for 18 hours before they were harvested and counted on a beta-counter on the fifth day of culture.
Analysis of cellular constituents of the graft
Three- and 4-color flow cytometry was used to enumerate the frequency of T cells, B cells, monocytes, natural killer (NK) cells, dendritic cells, CD34+ cells, and the subset of CD34+, CD38− cells in the graft using Paint-A-Gate (Becton Dickinson, San Jose, CA).32The number of transplanted cells in each subset was determined by multiplying the percentage of nucleated (CD45+) cells with each phenotype by the number of nucleated cells in the graft. All cell transplantation data are expressed as the number of cells transplanted per kilogram body weight of the recipient. Aliquots of the transplant product were cultured in methyl cellulose according to standard methods for a subset of 77 patients, and the number of CFU of various types were scored at 10 to 12 days according to standard definitions.33
Conditioning regimens
Patients with acute lymphoblastic leukemia (ALL) and patients with advanced AML and NHL underwent a conditioning regimen consisting of total body irradiation (TBI) at a dose of 200 cGy twice daily for 3 days in combination with cyclophosphamide (Cy/TBI) at a dose of 60 mg/kg daily for 2 days. Patients with CML, low-risk MDS, and AML in first remission typically received busulfan at a dose of 1 mg/kg every 6 hours for a total of 16 doses, in combination with cyclophosphamide at a dose of 60 mg/kg daily for 2 days (Bu/Cy), or Bu/Cy plus a single infusion of cytosine arabinoside at a dose of 2 gm/m2(Bu/Cy/Ara-C). Patients with chronic lymphocytic leukemia (CLL), multiple myeloma (MM), NHL, and Hodgkin disease (HD) received the combination of Bu/Cy and etoposide at 10 mg/kg daily for 3 days (Bu/Cy/VP16).34
GVHD prophylaxis and management
GVHD prophylaxis and management consisted of a short course of methotrexate at doses of 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11. Leucovorin rescue was administered for 4 doses every 6 hours beginning 24 hours after the second, third and fourth doses. Patients received tacrolimus starting on the day before transplantation and continuing through day 180.35 Acute GVHD was initially managed with prednisone or methylprednisolone at a dose of 2 mg/kg; patients whose GVHD failed to respond to a combination of prednisone with Prograf were treated with equine ATG at a dose of 30 mg/kg daily for 4 days. Chronic GVHD was typically managed with combinations of Prograf, prednisone, and mycophenolate.
Prophylactic and empiric antibiotic therapy
All patients received prophylactic antibiotics by mouth. These consisted of fluconazole (400 mg, 4 times a day), acyclovir (400 mg, 3 times a day) penicillin VK (500 mg, twice a day), and ciprofloxacin (500 mg, twice a day) starting on the day before transplantation and continuing until an absolute neutrophil count greater than 500 was reached. Empiric treatment of febrile neutropenia included ceftazidime, vancomycin, and amphotericin B as clinically indicated. Fluconazole was continued until day 75 after transplantation. Trimethoprim–sulfamethoxazole was prescribed at a dose of 1 double-strength tablet 3 times a week starting on day 30 and continuing until day 180, or until all immunosuppressive medication was discontinued for the patient. Blood samples were assessed weekly by qualitative polymerase chain reaction (PCR) amplification of cytomegalovirus (CMV) DNA for the presence of CMV after transplantation; patients with positive PCR findings underwent pre-emptive treatment with ganciclovir for at least 3 weeks.
Outcome measurements
Analyses were performed on the reference date of September 1, 2000. Median follow-up for patients alive on the reference date was 3 years (range, 1-5 years). Overall survival was defined as the time elapsed from bone marrow transplantation (BMT) to death (from whatever cause), or to last-documented contact with the patient. EFS was defined as survival with no evidence of relapse. Engraftment of granulocytes was defined as the first of 3 consecutive days after BMT on which the absolute neutrophil count was greater than 500/μL. Engraftment of platelets was defined as the first day after BMT on which the platelet count was greater than 20 000/μL and the patient had not received a platelet transfusion for the 7 preceding days. Acute GVHD (aGVHD) was defined as occurring before day 100 and was graded using standard IBMTR criteria (one patient had the first documented occurrence of aGVHD on day 104).35 Chronic GVHD (cGVHD) was assessed at 100 days, 6 months after transplantation, and annually thereafter. The time to develop GVHD was defined as the time from BMT to the first occurrence of either aGVHD or cGVHD. Patients who had relapses were censored for the subsequent occurrence of GVHD because management of relapse usually included maneuvers that could induce GVHD. Causes of death were classified according to IBMTR criteria.36 Relapse was listed as the primary cause of death for any patient who died after the diagnosis of relapse or disease progression. GVHD was considered the primary cause of death if patients died of infection during active treatment for aGVHD or cGVHD.
Statistical methods
Baseline characteristics between groups of patients were compared using the Student t test. Clinical outcomes (death, relapse, infection, GVHD) were correlated with patient characteristics (diagnosis, age, conditioning regimens) and donor characteristics (age, sex, graft constituents) in univariate analyses using Cox regression and SPSS software version 5.1. Multivariate regression analysis performed according to the Cox proportional hazards regression model37 used EFS as the dependent variable and the factors thought to be associated with post-transplant EFS—patient age, risk classification, and numbers of nucleated cells, T cells, and CD34+ cells transplanted—as covariates. In addition, other factors that were correlated with EFS at the significance level ofP < .05 in the univariate analyses were also included. Multivariate Cox regression models for the entire group of patients and subsets of patients at low risk and high risk were performed using all individual factors as covariates, without pre-set inclusion or exclusion criteria for each factor. The entire study population of 113 patients was subdivided based on the number of nucleated cells, CD34+ cells, CD4bright cells, or CD3+ T cells in the graft. Relative quantity (low, intermediate, or high) was used as a factor for survival analyses. Level of significance assumed to be significant in comparisons of survival curves by the log-rank test was P < .05.
Results
Clinical characteristics that affected relapse-free survival after transplantation
Diagnoses, median ages, and genders of the 64 patients in the low-risk group and of the 49 patients in the high-risk group are shown in Table 1. The most frequent indications for transplantation were CML (40 patients) followed by AML (28 patients), MDS (16 patients), NHL (12 patients), and ALL (8 patients). Median ages of patients in the low-risk group (45 years) and the high-risk group (42 years) were similar. Male patients were more prevalent in the high-risk group. Actuarial EFS rates in the low-risk group were 84%, 68%, and 58% at 100 days, 1 year, and 3 years after transplantation, respectively, versus 57%, 27%, and 14%, respectively, for patients in the high-risk group. Analysis of overall survival showed a similar pattern (data not shown). Univariate analysis of clinical factors associated with relapse or death after transplantation showed that high-risk status was the most significant factor associated with worse EFS (P < .0001; Table2). Other patient-related factors significantly associated with increased death or relapse after transplantation included male gender (relative risk [RR], 1.9; 95% confidence interval [CI], 1.1-3.8; P = .017) and the use of TBI in the conditioning regime (RR, 2.3; 95% CI, 1.1-4.8;P = .03). Of note, TBI was used only in a subset of 10 patients with AML, ALL, and NHL.
Clinical characteristics of patients in the study group
| Diagnosis . | Number . | Median age (range) . | Male gender . |
|---|---|---|---|
| Low risk | 64 | 45 (19-60) | 33 |
| ALL CR1 | 5 | 33 (30-51) | 2 |
| AML CR1 | 16 | 46 (19-60) | 10 |
| CML CP1 | 37 | 45 (21-58) | 19 |
| MDS RA, RARS | 6 | 45 (21-58) | 2 |
| High risk | 49 | 42 (22-64) | 36 |
| ALL not CR1 | 3 | 25 (24-64) | 3 |
| AML not CR1 | 12 | 50 (34-62) | 8 |
| CLL | 2 | 46 (45-46) | 2 |
| CML not CP1 | 3 | 33 (24-36) | 3 |
| HD | 5 | 29 (24-35) | 3 |
| NHL | 12 | 40 (22-57) | 8 |
| MDS not RA, RARS | 10 | 45 (39-53) | 8 |
| MM | 2 | 50 (49-51) | 1 |
| Diagnosis . | Number . | Median age (range) . | Male gender . |
|---|---|---|---|
| Low risk | 64 | 45 (19-60) | 33 |
| ALL CR1 | 5 | 33 (30-51) | 2 |
| AML CR1 | 16 | 46 (19-60) | 10 |
| CML CP1 | 37 | 45 (21-58) | 19 |
| MDS RA, RARS | 6 | 45 (21-58) | 2 |
| High risk | 49 | 42 (22-64) | 36 |
| ALL not CR1 | 3 | 25 (24-64) | 3 |
| AML not CR1 | 12 | 50 (34-62) | 8 |
| CLL | 2 | 46 (45-46) | 2 |
| CML not CP1 | 3 | 33 (24-36) | 3 |
| HD | 5 | 29 (24-35) | 3 |
| NHL | 12 | 40 (22-57) | 8 |
| MDS not RA, RARS | 10 | 45 (39-53) | 8 |
| MM | 2 | 50 (49-51) | 1 |
Univariate analyses of the clinical factors that influenced EFS
| . | Mean ± SD . | Relative risk . | (95% CI) . | P . |
|---|---|---|---|---|
| Risk strata | 49/113 high risk | 3.6 | (2.2-5.9) | < .0001 |
| Patient age | 43 ± 11 | 1.00 | (0.98-1.03) | .74 |
| Donor age | 42 ± 11 | 1.00 | (0.98-1.02) | .83 |
| Male gender | 69/113 | 1.90 | (1.12-3.20) | .017 |
| TBI | 10/113 | 2.26 | (1.08-4.76) | .031 |
| G-CSF or GM-CSF after transplantation | 42/113 | 1.6 | (0.98-2.6) | .058 |
| . | Mean ± SD . | Relative risk . | (95% CI) . | P . |
|---|---|---|---|---|
| Risk strata | 49/113 high risk | 3.6 | (2.2-5.9) | < .0001 |
| Patient age | 43 ± 11 | 1.00 | (0.98-1.03) | .74 |
| Donor age | 42 ± 11 | 1.00 | (0.98-1.02) | .83 |
| Male gender | 69/113 | 1.90 | (1.12-3.20) | .017 |
| TBI | 10/113 | 2.26 | (1.08-4.76) | .031 |
| G-CSF or GM-CSF after transplantation | 42/113 | 1.6 | (0.98-2.6) | .058 |
Separate regression analyses were performed for the entire group of patients (n = 113) using each covariate listed. Significant (P < .05) associations of covariates with EFS are in bold.
Graft characteristics that affected EFS after transplantation
Patients who received more CD34+ cells with the allograft had improved EFS after transplantation (RR, 0.79, associated with an increase of 1 × 106 donor CD34+cells/kg in the graft; 95% CI, 0.64-0.97; P = .028; Table3). In contrast, the numbers of transplanted CD34+, CD38− cells were not significantly associated with EFS after BMT. Larger numbers of donor cells with the phenotype CD3−, CD4bright, CD8−, low side-scatter were associated with increased deaths after transplantation (RR, 2.2, associated with an increase of 1 × 106 cells/kg; 95% CI, 1.2-3.8;P = .0072). Total numbers of CFU and nucleated cells transplanted and numbers of donor T cells, B cells, NK cells, and monocytes were not significantly associated with the risk for relapse or with death after transplantation (Table 3).
Univariate analyses of the cellular composition of the graft on EFS
| . | Mean ± SD . | Relative risk . | (95% CI) . | P . |
|---|---|---|---|---|
| Total cells (×108/kg) | 2.9 ± 0.73 | .84 | (0.60-1.2) | .29 |
| CFU-GM (×104/kg) | 6.1 ± 5.5 | .99 | (0.94-1.0) | .77 |
| CFU-GEMM (×104/kg) | 1.8 ± 1.6 | .90 | (0.76-1.1) | .27 |
| CFU-TOT (×104/kg) | 70.0 ± 43 | .99 | (0.99-1.0) | .12 |
| CD34+(×106/kg) | 3.1 ± 1.3 | .79 | (0.64-0.97) | .028 |
| CD34+, CD38−(×106/kg) | 0.13 ± 0.1 | .074 | (0.004-1.3) | .077 |
| CD3+(×106/kg) | 25 ± 9.7 | .99 | (0.96-1.0) | .33 |
| CD3+, CD4+, CD8−(×106/kg) | 13 ± 5.4 | .98 | (0.94-1.0) | .26 |
| CD3+, CD4−, CD8+(×106/kg) | 8.8 ± 3.8 | .99 | (0.93-1.1) | .86 |
| CD3+, CD4−, CD8−(×106/kg) | 0.77 ± 0.5 | .92 | (0.56-1.5) | .73 |
| CD3+, CD56+(×106/kg) | 1.7 ± 2.3 | .89 | (0.75-1.0) | .14 |
| CD19+(×106/kg) | 7.8 ± 4.1 | .95 | (0.89-1.0) | .10 |
| CD3−, CD56+(×106/kg) | 2.2 ± 1.3 | .97 | (0.80-1.2) | .77 |
| CD3−, CD4−, CD8lo(×106/kg) | 1.0 ± 0.8 | 1.1 | (0.85-1.5) | .39 |
| CD3−, CD4lo, CD8−(×106/kg) | 12.1 ± 5.5 | .99 | (0.95-1.0) | .65 |
| CD3−, CD4+, CD8−(×106/kg) | 0.80 ± 0.4 | 2.2 | (1.2-3.8) | .0072 |
| . | Mean ± SD . | Relative risk . | (95% CI) . | P . |
|---|---|---|---|---|
| Total cells (×108/kg) | 2.9 ± 0.73 | .84 | (0.60-1.2) | .29 |
| CFU-GM (×104/kg) | 6.1 ± 5.5 | .99 | (0.94-1.0) | .77 |
| CFU-GEMM (×104/kg) | 1.8 ± 1.6 | .90 | (0.76-1.1) | .27 |
| CFU-TOT (×104/kg) | 70.0 ± 43 | .99 | (0.99-1.0) | .12 |
| CD34+(×106/kg) | 3.1 ± 1.3 | .79 | (0.64-0.97) | .028 |
| CD34+, CD38−(×106/kg) | 0.13 ± 0.1 | .074 | (0.004-1.3) | .077 |
| CD3+(×106/kg) | 25 ± 9.7 | .99 | (0.96-1.0) | .33 |
| CD3+, CD4+, CD8−(×106/kg) | 13 ± 5.4 | .98 | (0.94-1.0) | .26 |
| CD3+, CD4−, CD8+(×106/kg) | 8.8 ± 3.8 | .99 | (0.93-1.1) | .86 |
| CD3+, CD4−, CD8−(×106/kg) | 0.77 ± 0.5 | .92 | (0.56-1.5) | .73 |
| CD3+, CD56+(×106/kg) | 1.7 ± 2.3 | .89 | (0.75-1.0) | .14 |
| CD19+(×106/kg) | 7.8 ± 4.1 | .95 | (0.89-1.0) | .10 |
| CD3−, CD56+(×106/kg) | 2.2 ± 1.3 | .97 | (0.80-1.2) | .77 |
| CD3−, CD4−, CD8lo(×106/kg) | 1.0 ± 0.8 | 1.1 | (0.85-1.5) | .39 |
| CD3−, CD4lo, CD8−(×106/kg) | 12.1 ± 5.5 | .99 | (0.95-1.0) | .65 |
| CD3−, CD4+, CD8−(×106/kg) | 0.80 ± 0.4 | 2.2 | (1.2-3.8) | .0072 |
Separate regression analyses were performed for the entire group of patients (n = 113) using each covariate listed. Significant (P < .05) associations of covariates with EFS are in bold.
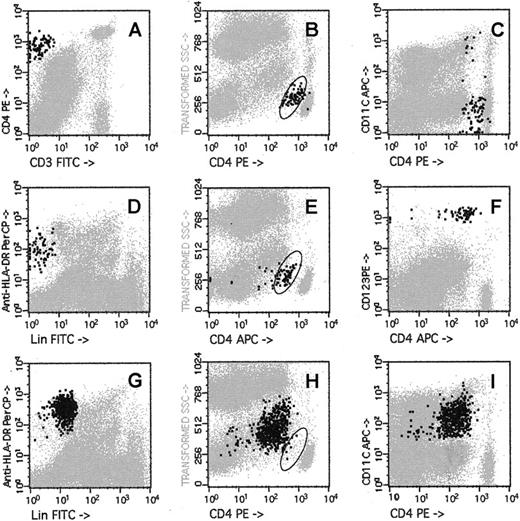
CD4bright, CD3−, low side-scatter cells measured in BM are DC2
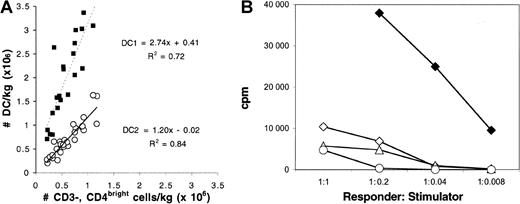
CD4bright, CD3− low side-scatter cells had the same phenotype as the “plasmacytoid T cell” (Figure1A-B)31 and the lineage-negative (Lin−), HLADR+, CD123+ CD11c− dendritic cell progenitor (DC2p).27 Ninety percent of the CD123+, Lin−, HLADR+ low side-scatter cells (Figure 1D-E) expressed relatively bright levels of CD4 (Figure1F).27 Only 10% of the CD3−, CD4bright, CD8−, low side-scatter cells expressed CD11c (Figure 1C), indicating that they are distinct from the Lin−, HLADR+, CD123dimCD11c+ (DC1). In particular, the Lin−, HLADR+, CD123dim CD11c+ (DC1) (Figure 1G, 1I) populations had higher side-scatter and lower CD4 expression than CD4bright or CD123brightpopulations (compare Figures 1B, 1E with 1H). Twenty-nine paired samples of bone marrow were separately analyzed for the content of (1) CD3−, CD4bright, CD8−, low side-scatter cells, (2) Lin− HLADR+, CD123bright CD11c− cells (DC2p), and (3) Lin− HLADR+, CD123dimCD11c+ cells (DC1). The number of CD4brightdendritic cell progenitors in the grafts was nearly identical to the number of Lin−, HLADR+, CD123+, CD11c− cells (ratio of CD123bright to CD4bright cells, 1.2; r = 0.92, Figure2A). In contrast, the number of DC1 was more than twice the number of CD3−, CD4bright, CD8−, low side-scatter cells (Figure 2A). FACS-isolated CD4bright, CD123bright, Lin− BM cells cultured in the presence of IL-3 and GM-CSF had the typical appearance of “veiled” DC (cells with a ruffled extension of their cytoplasm, observed under light microscopy) and efficiently elicited an MLR from HLA-mismatched allogeneic lymphocytes (Figure 2B).
Flow cytometric analysis of DCs in the bone marrow aspirate.
A sample of bone marrow harvested from a healthy donor was stained separately with 3 panels of monoclonal antibodies—a combination of CD3– fluorescein isothiocyanate (FITC), CD4-PE, CD8-PerCP, and CD45-APC (A-C); a combination of “lineage” cocktail (CD3, CD14, CD20, CD56)-FITC, CD123-PE, HLA-DR-PerCP, and CD4-APC (D-F); or Lin cocktail-FITC, CD4-PE, HLA-DR-PerCP, and CD11c-APC (G-I). Location of the CD3−, CD4bright, low side-scatter cells is shown by the oval drawn in panels B, E, and H. CD3−, CD4bright, low side-scatter cells are shown as bold black dots (0.14% of nucleated cells) in panels A to C; Lin−, CD123bright, HLADR+ cells are shown as bold black dots (0.15% of nucleated cells) in panels D to F; Lin−, HLADR+, CD11c+ cells are shown as bold black dots (1.1% of nucleated cells) in panels G to I.
Flow cytometric analysis of DCs in the bone marrow aspirate.
A sample of bone marrow harvested from a healthy donor was stained separately with 3 panels of monoclonal antibodies—a combination of CD3– fluorescein isothiocyanate (FITC), CD4-PE, CD8-PerCP, and CD45-APC (A-C); a combination of “lineage” cocktail (CD3, CD14, CD20, CD56)-FITC, CD123-PE, HLA-DR-PerCP, and CD4-APC (D-F); or Lin cocktail-FITC, CD4-PE, HLA-DR-PerCP, and CD11c-APC (G-I). Location of the CD3−, CD4bright, low side-scatter cells is shown by the oval drawn in panels B, E, and H. CD3−, CD4bright, low side-scatter cells are shown as bold black dots (0.14% of nucleated cells) in panels A to C; Lin−, CD123bright, HLADR+ cells are shown as bold black dots (0.15% of nucleated cells) in panels D to F; Lin−, HLADR+, CD11c+ cells are shown as bold black dots (1.1% of nucleated cells) in panels G to I.
Characterization of DCs in bone marrow allografts.
(A) Frequencies of cells with phenotypes (1) CD3−, CD4bright, CD8−, low side-scatter, (2) Lin−, CD123bright, HLADR+, CD11c−, and (3) Lin−, CD11c+, HLADR+ were determined by FACS analysis of 29 samples of bone marrow harvested from healthy allogeneic donors. The number of donor cells/kg (×106) in the allograft with phenotype 1 was compared to the numbers of cells with phenotype 2 (○) or 3 (▪). Equations for the best-fit lines comparing the relation between populations 1 and 2 (slope, 1.2; r = 0.92) and between populations 1 and 3 (slope, 2.7; r = 0.85) are shown. (B) Ability of unfractionated bone marrow cells (○) and FACS-isolated CD4+ T-cells (⋄). FACS-isolated CD123− cells (▵) and FACS-isolated Lin−, CD4bright, CD123bright cells (♦) to stimulate proliferation of allogeneic lymphocytes in an MLR was assessed after 5 day co-culture in media containing 10% AB− human serum, 50 ng/mL IL-3, and 100 ng/mL GM-CSF.
Characterization of DCs in bone marrow allografts.
(A) Frequencies of cells with phenotypes (1) CD3−, CD4bright, CD8−, low side-scatter, (2) Lin−, CD123bright, HLADR+, CD11c−, and (3) Lin−, CD11c+, HLADR+ were determined by FACS analysis of 29 samples of bone marrow harvested from healthy allogeneic donors. The number of donor cells/kg (×106) in the allograft with phenotype 1 was compared to the numbers of cells with phenotype 2 (○) or 3 (▪). Equations for the best-fit lines comparing the relation between populations 1 and 2 (slope, 1.2; r = 0.92) and between populations 1 and 3 (slope, 2.7; r = 0.85) are shown. (B) Ability of unfractionated bone marrow cells (○) and FACS-isolated CD4+ T-cells (⋄). FACS-isolated CD123− cells (▵) and FACS-isolated Lin−, CD4bright, CD123bright cells (♦) to stimulate proliferation of allogeneic lymphocytes in an MLR was assessed after 5 day co-culture in media containing 10% AB− human serum, 50 ng/mL IL-3, and 100 ng/mL GM-CSF.
Patients who underwent transplantation with fewer CD4bright DCs had improved EFS after allogeneic BMT
A striking effect on enhanced EFS was seen among patients at low risk who received fewer CD4bright DCs in their grafts (Figure 3A). Patients at low risk in group 1(DC) (less than 0.6 × 106CD4bright DCs/kg) had a 3-year actuarial EFS of 80%, whereas those in group 3(DC) (more than 0.9 × 106 CD4bright DCs/kg) had a 3-year actuarial EFS of only 48% (Figure 3A; P = .009 comparing group 1(DC) versus group 3(DC)). Analysis of the 49 patients at high risk divided according to the content of CD4bright DC showed a trend toward better survival among recipients of fewer CD4bright DC (P = .09; Figure 3B). In contrast, patients at low risk who received larger numbers of CD34+ cells had significantly improved EFS (Figure 3C). Transplantation with larger numbers of total nucleated cells was also associated with improved EFS among patients at low risk only (not shown), whereas the content of CD3+cells in the graft did not affect EFS in either low-risk or high-risk subsets (not shown).
Kaplan-Meier estimates of EFS for patients undergoing allogeneic bone marrow transplantation.
(A, C) Low-risk patients (n = 64) and (B) high-risk patients (n = 49) were divided into subgroups based on the numbers of CD4bright DC cells (A, B) and CD34+ cells (C) in the graft. Integer values of cells/kg (×105) were chosen as boundaries between subgroups to yield similar-sized populations for each parameter. Subgroups based on CD4bright DC content were defined as less than 0.6 × 106 cells/kg (group 1(DC), n = 35); 0.6 to 0.9 × 106 cells/kg (group 2(DC), n = 38); and more than 0.9 × 106 cells/kg (group 3(DC), n = 40). Subgroups based on CD34+ cell content were defined as less than 2.5 × 106 cells/kg (group 1(CD34), n = 41); 2.5 to 3.5 × 106cells/kg (group 2(CD34), n = 33); and more than 3.5 × 106 cells/kg (group 3(CD34), n = 39). In each panel, Kaplan-Meier curves for actuarial EFS are shown according to subgroups, with surviving patients represented as symbols: group 1 (▿), group 2 (■), and group 3 (○).
Kaplan-Meier estimates of EFS for patients undergoing allogeneic bone marrow transplantation.
(A, C) Low-risk patients (n = 64) and (B) high-risk patients (n = 49) were divided into subgroups based on the numbers of CD4bright DC cells (A, B) and CD34+ cells (C) in the graft. Integer values of cells/kg (×105) were chosen as boundaries between subgroups to yield similar-sized populations for each parameter. Subgroups based on CD4bright DC content were defined as less than 0.6 × 106 cells/kg (group 1(DC), n = 35); 0.6 to 0.9 × 106 cells/kg (group 2(DC), n = 38); and more than 0.9 × 106 cells/kg (group 3(DC), n = 40). Subgroups based on CD34+ cell content were defined as less than 2.5 × 106 cells/kg (group 1(CD34), n = 41); 2.5 to 3.5 × 106cells/kg (group 2(CD34), n = 33); and more than 3.5 × 106 cells/kg (group 3(CD34), n = 39). In each panel, Kaplan-Meier curves for actuarial EFS are shown according to subgroups, with surviving patients represented as symbols: group 1 (▿), group 2 (■), and group 3 (○).
Multivariate model of EFS
A multivariate Cox regression model was built using factors significantly associated (P < .05) with EFS in the univariate analyses (Tables 2, 3). In addition, patient and graft characteristics hypothesized as potentially significant with respect to EFS were also included (age, number of nucleated cells transplanted, numbers of donor T cells transplanted). Seven individual factors were entered into the multivariate models without P value thresholds for inclusion or exclusion. The multivariate model was tested on the entire patient population (n = 113) and on the subsets of patients at low risk (n = 64) and high risk (n = 49). TBI was not included in the multivariate model because only 10 patients underwent TBI-based conditioning (1 ALL, 6 AML, 2 NHL, 2 MM), and TBI was not a significant factor in univariate or multivariate analyses of EFS when the patient population was stratified by disease diagnosis (data not shown). Covariates independently associated with increased risk for death or relapse in all 113 patients included risk strata (RR, 2.8; P = .0002), male gender (RR, 1.8;P = .04), and number of donor CD4bright DC (RR, 2.0; P = .03) in the bone marrow allograft (Table4). In contrast, larger numbers of donor CD34+ cells (RR, 0.76; P = .027) were associated with improved EFS in the group of 113 patients (Table 4). Separate analysis of the subset of 64 patients at low risk showed that CD4bright DC content remained a highly significant factor for worse EFS (RR, 21; P = .0008). Transplantation with larger numbers of donor-nucleated cells (RR, 0.41;P = .05) and larger numbers of donor CD34+cells (RR, 0.71; P = .1) were also associated with improved EFS among patients at low risk (Table 4). In the subset of 49 patients at high risk, no covariate was significantly associated with EFS in the multivariate analysis (Table 4). The use of conditional entry criteria for inclusion of covariates in the multivariate model (P < .05 or .1) did not alter the role of gender, risk, CD34+ cell content, and CD4bright DC cell content as independently significant factors (data not shown). Multivariate analyses based on overall survival as the clinical end-point gave similar results in multivariate analyses (data not shown).
Multivariate Cox regression analyses for EFS
| . | All patients (n = 113) . | Low risk (n = 64) . | High risk (n = 49) . | |||
|---|---|---|---|---|---|---|
| RR (95% CI) . | P . | RR (95% CI) . | P . | RR (95% CI) . | P . | |
| Risk strata | 2.8 (1.7-4.9) | .0002 | ||||
| Patient age | 1 (0.98-1.0) | .83 | 0.99 (0.95-1.0) | .67 | 1 (0.97-1.0) | .94 |
| Gender (male) | 1.8 (1.0-3.2) | .04 | 0.82 (0.33-2.1) | .67 | 2 (0.90-4.4) | .091 |
| Total cells (×108/kg) | 0.82 (0.52-1.3) | .43 | 0.41 (0.17-1) | .05 | 1.2 (0.6-2.2) | .66 |
| CD34+ (×106/kg) | 0.76 (0.59-0.97) | .027 | 0.71 (0.47-1.1) | .10 | 0.84 (0.61-1.2) | .3 |
| CD3+(×106/kg) | 1 (0.97-1.0) | .84 | 0.98 (0.93-1.0) | .61 | 1.0 (0.96-1.1) | .62 |
| CD3−, CD4+, CD8−(×106/kg) | 2.0 (1.1-4.2) | .03 | 21 (3.5-121) | .0008 | 1.2 (0.51-2.9) | .66 |
| . | All patients (n = 113) . | Low risk (n = 64) . | High risk (n = 49) . | |||
|---|---|---|---|---|---|---|
| RR (95% CI) . | P . | RR (95% CI) . | P . | RR (95% CI) . | P . | |
| Risk strata | 2.8 (1.7-4.9) | .0002 | ||||
| Patient age | 1 (0.98-1.0) | .83 | 0.99 (0.95-1.0) | .67 | 1 (0.97-1.0) | .94 |
| Gender (male) | 1.8 (1.0-3.2) | .04 | 0.82 (0.33-2.1) | .67 | 2 (0.90-4.4) | .091 |
| Total cells (×108/kg) | 0.82 (0.52-1.3) | .43 | 0.41 (0.17-1) | .05 | 1.2 (0.6-2.2) | .66 |
| CD34+ (×106/kg) | 0.76 (0.59-0.97) | .027 | 0.71 (0.47-1.1) | .10 | 0.84 (0.61-1.2) | .3 |
| CD3+(×106/kg) | 1 (0.97-1.0) | .84 | 0.98 (0.93-1.0) | .61 | 1.0 (0.96-1.1) | .62 |
| CD3−, CD4+, CD8−(×106/kg) | 2.0 (1.1-4.2) | .03 | 21 (3.5-121) | .0008 | 1.2 (0.51-2.9) | .66 |
Separate analyses were performed for the entire group of patients (n = 113) and the subsets of low-risk (n = 64) and high-risk (n = 49) patients. All covariates listed were entered into the multivariate Cox model. Significant (P < .05) associations of covariates with EFS are in bold.
Increased numbers of CD4brightDCs in the graft were associated with increased relapse after transplantation
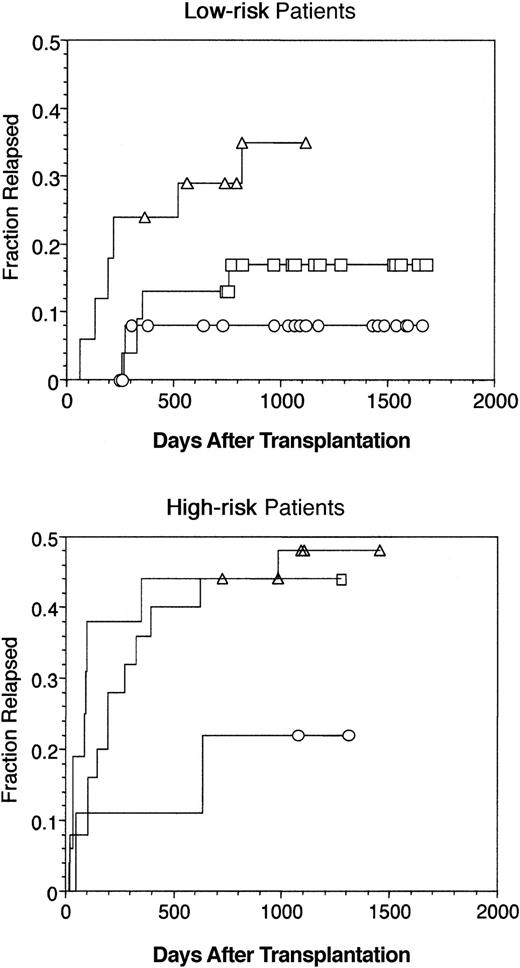
Overall, 60 of 113 patients died by the reference date; 21 of 64 (33%) were from the low-risk group, and 39 of 49 (80%) were from the high-risk group. Relapse was the most frequent primary cause of death, and fatal relapse occurred in 7 of 64 (11%) patients at low risk and 20 of 49 (41%) patients at high risk at a median of 331 days after transplantation. Thirteen deaths (3 relapse, 10 nonrelapse) occurred among patients who underwent transplantation with less than 0.6 × 106 CD4bright DC cells/kg (group 1(DC)). Twenty-one deaths (11 relapse, 10 nonrelapse) occurred in the group 2(DC) patients, and 26 deaths (13 relapse, 13 nonrelapse) occurred in the group 3(DC)patients (Table 5). Cumulative incidences of relapse in groups 1(DC), 2(DC), and 3(DC) were compared after stratification on risk. The incidence of relapse was 33% among low-risk patients who received the largest number of CD4bright DC versus 8% among low-risk patients who received the fewest DC (Figure4A; P = .01; group 1(DC) vs group 3(DC)). The incidence of relapse was 48% among high-risk patients who received the highest and the intermediate numbers of CD4bright DC versus only 22% among high-risk patients who received the fewest CD4bright DC (Figure 4B; P = .01; group 1(DC) vs group 2(DC) or group 3(DC)). A multivariate model using the same covariates as in Table 4 evaluated relapse as a single clinical end-point (Table 6). Risk strata (RR, 3.8; P = .0023) and age (RR, 0.94;P = .003) were independently associated with relapse in the entire group of 113 patients. Among patients at low risk, relapses were fewer among recipients of a larger number of nucleated cells (RR, 0.2; P = .03) and were more frequent among recipients of more CD4bright DCs (RR, 114; P = .0074). Among patients at high risk, the only covariate significantly associated with relapse was age (RR 0.92; P = .0028).
Causes of death among patients divided according to content of CD4bright DCs in bone marrow graft
| . | Low DC (n = 35) < 0.6 × 106/kg . | Interim DC (n = 38) 0.6-0.9 × 106/kg . | High DC (n = 40) > 0.9 × 106/kg . | Median day of death . |
|---|---|---|---|---|
| Graft rejection | 1 | 0 | 2 | 88 |
| Infection | 5 | 5 | 4 | 38 |
| Interstitial pneumonia | 0 | 0 | 2 | 66 |
| Acute GVHD | 0 | 1 | 0 | 50 |
| Chronic GVHD | 1 | 1 | 2 | 440 |
| Relapse | 3 | 11 | 13 | 331 |
| Organ failure | 0 | 2 | 3 | 24 |
| Hemorrhage | 2 | 1 | 0 | 75 |
| Other | 1 | 0 | 0 | 195 |
| Total | 13 (37%) | 21 (55%) | 26 (65%) | 128 |
| . | Low DC (n = 35) < 0.6 × 106/kg . | Interim DC (n = 38) 0.6-0.9 × 106/kg . | High DC (n = 40) > 0.9 × 106/kg . | Median day of death . |
|---|---|---|---|---|
| Graft rejection | 1 | 0 | 2 | 88 |
| Infection | 5 | 5 | 4 | 38 |
| Interstitial pneumonia | 0 | 0 | 2 | 66 |
| Acute GVHD | 0 | 1 | 0 | 50 |
| Chronic GVHD | 1 | 1 | 2 | 440 |
| Relapse | 3 | 11 | 13 | 331 |
| Organ failure | 0 | 2 | 3 | 24 |
| Hemorrhage | 2 | 1 | 0 | 75 |
| Other | 1 | 0 | 0 | 195 |
| Total | 13 (37%) | 21 (55%) | 26 (65%) | 128 |
Incidence of relapse after allogeneic BMT.
(Top) Low-risk patients (n = 64) and (bottom) high-risk patients (n = 49) were divided into 3 groups according to the numbers of CD4bright DCs they received in the bone marrow allograft (Figure 3). Relapse increased proportionally to the content of CD4bright DCs in the graft. Log-rank statistic comparing the incidence of relapse between low-risk recipients of smaller (○)(group 1(DC), n = 26) versus larger numbers of DC (▵)(group 3(DC), n = 15) was 6.5 (P = .01). Log-rank statistic comparing the incidence of relapse between high-risk recipients of smaller versus intermediate numbers of DC (■)(group 1(DC), n = 9 vs group 2(DC), n = 25) was 2.5 (P = .1).
Incidence of relapse after allogeneic BMT.
(Top) Low-risk patients (n = 64) and (bottom) high-risk patients (n = 49) were divided into 3 groups according to the numbers of CD4bright DCs they received in the bone marrow allograft (Figure 3). Relapse increased proportionally to the content of CD4bright DCs in the graft. Log-rank statistic comparing the incidence of relapse between low-risk recipients of smaller (○)(group 1(DC), n = 26) versus larger numbers of DC (▵)(group 3(DC), n = 15) was 6.5 (P = .01). Log-rank statistic comparing the incidence of relapse between high-risk recipients of smaller versus intermediate numbers of DC (■)(group 1(DC), n = 9 vs group 2(DC), n = 25) was 2.5 (P = .1).
Multivariate Cox regression analyses for relapse
| . | All patients (n = 113) . | Low risk (n = 64) . | High risk (n = 49) . | |||
|---|---|---|---|---|---|---|
| RR (95% CI) . | P . | RR (95% CI) . | P . | RR (95% CI) . | P . | |
| Risk strata | 3.8 (1.6-8.8) | .0023 | ||||
| Patient age | 0.94 (0.91-0.98) | .003 | 0.94 (0.87-1.01) | .07 | 0.92 (0.86-0.97) | .0028 |
| Gender (male) | 1.5 (0.67-3.2) | .33 | 1.2 (0.22-6) | .87 | 1.4 (0.51-3.9) | .51 |
| Total cells (×108/kg) | 0.70 (0.38-1.3) | .26 | 0.2 (0.04-0.86) | .03 | 1.0 (0.43-2.3) | .99 |
| CD34+ (×106/kg) | 0.99 (0.71-1.4) | .94 | 0.79 (0.43-1.5) | .45 | 1.3 (0.83-2.1) | .24 |
| CD3+ (×106/kg) | 1 (0.97-1.1) | .44 | 1.0 (0.92-1.1) | .90 | 1.0 (0.96-3.9) | .31 |
| CD3−, CD4+, CD8−(×106/kg) | 1.8 (0.7-4.9) | .21 | 114 (3.6-3700) | .0074 | 0.67 (0.21-2.9) | .50 |
| . | All patients (n = 113) . | Low risk (n = 64) . | High risk (n = 49) . | |||
|---|---|---|---|---|---|---|
| RR (95% CI) . | P . | RR (95% CI) . | P . | RR (95% CI) . | P . | |
| Risk strata | 3.8 (1.6-8.8) | .0023 | ||||
| Patient age | 0.94 (0.91-0.98) | .003 | 0.94 (0.87-1.01) | .07 | 0.92 (0.86-0.97) | .0028 |
| Gender (male) | 1.5 (0.67-3.2) | .33 | 1.2 (0.22-6) | .87 | 1.4 (0.51-3.9) | .51 |
| Total cells (×108/kg) | 0.70 (0.38-1.3) | .26 | 0.2 (0.04-0.86) | .03 | 1.0 (0.43-2.3) | .99 |
| CD34+ (×106/kg) | 0.99 (0.71-1.4) | .94 | 0.79 (0.43-1.5) | .45 | 1.3 (0.83-2.1) | .24 |
| CD3+ (×106/kg) | 1 (0.97-1.1) | .44 | 1.0 (0.92-1.1) | .90 | 1.0 (0.96-3.9) | .31 |
| CD3−, CD4+, CD8−(×106/kg) | 1.8 (0.7-4.9) | .21 | 114 (3.6-3700) | .0074 | 0.67 (0.21-2.9) | .50 |
Separate analyses were performed for the entire group of patients (n = 113) and the subsets of low-isk (n = 64) and high-isk (n = 49) patients. All covariates listed were entered into the multivariate Cox model. Significant (P < .05) associations of covariates with EFS are in bold.
Incidence of acute GVHD was not associated with any graft constituent
Among the entire study population, aGVHD developed in 24 of 113 (21%) patients a median of 38 days after transplantation. Twenty of 113 (18%) patients experienced grade 2 to 4 aGVHD, and 8 of 113 (7%) patients experienced grade 3 or 4 aGVHD. Twenty-one patients with aGVHD were treated with systemic steroids. No single donor factor (Tables 2,3) or combination of factors (Table 4) was significantly associated (P < .05) with the risk for aGVHD in univariate or multivariate analyses (data not shown). In particular, the median number of CD4bright DCs among patients in whom aGVHD developed (0.83 ± 0.33 × 106/kg) was not significantly different than the corresponding value of those patients in whom aGVHD did not develop (0.7 ± 0.4 × 106/kg;P = .2).
Incidence of cGVHD varied according to the content of CD4bright DCs in the graft
cGVHD developed in 34 of 113 (30%) patients at a median of 182 days after transplantation. Nine patients had limited-grade and 25 patients had extensive-grade cGVHD. Thirty-one patients received systemic corticosteroids for the treatment of cGVHD. Among 82 patients who survived to day 100 and were evaluable for cGVHD, a higher content of CD4bright DC was associated with a lower risk for cGVHD (RR, 0.42; 95% CI, 0.17-1.05; P = .06). Larger numbers of CD34+ cells in the graft were also associated with lower risk for cGVHD (RR, 0.74; 95% CI, 0.56-1.0; P = .05). cGVHD was observed more frequently in men (RR, 2.3; 95% CI, 1.1-4.8;P = .03) and in recipients of larger numbers of CD56+ “NK-like” T cells (RR, 1.1; 95% CI, 1.0-1.3;P = .03). Patient age and content of total numbers of nucleated cells, CD3+ T cells, or other T-cell subsets were not significantly associated with the development of cGVHD in univariate analyses (data not shown). The mean number of CD4bright DC among patients in whom cGVHD developed was significantly lower than the corresponding value for those patients in whom aGVHD did not develop (0.68 ± 0.37 × 106/kg versus 0.85 ± 0.4 × 106/kg, respectively;P = .03). Figure 5 shows that the cumulative incidence of cGVHD was lower among recipients of more than 0.9 × 106/kg CD4bright DC/kg compared with recipients of less than 0.6 × 106 and 0.6 to 0.9 × 106 CD4bright DC/kg (22% vs 52% and 50%, respectively; P = .04). Multivariate analysis using the same covariates shown in Table 4 revealed that gender was the only factor independently associated with increased risk for cGVHD (RR for males, 2.7; 95% CI, 1.1-6.7; P = .03).
Incidence of cGVHD after allogeneic BMT.
All patients who survived until day 100 (n = 82) were divided into 3 groups according to the numbers of CD4bright DCs they received in the bone marrow allograft (Figure 3). Log-rank statistic comparing the incidence of cGVHD between recipients of smallest numbers of CD4bright DCs (group 1(DC), n = 29) versus the recipients of intermediate numbers of CD4bright DCs (Group 2(DC), n = 26) or the largest numbers of CD4bright DCs (group 3(DC), n = 27) was 4.2 (P = .04).
Incidence of cGVHD after allogeneic BMT.
All patients who survived until day 100 (n = 82) were divided into 3 groups according to the numbers of CD4bright DCs they received in the bone marrow allograft (Figure 3). Log-rank statistic comparing the incidence of cGVHD between recipients of smallest numbers of CD4bright DCs (group 1(DC), n = 29) versus the recipients of intermediate numbers of CD4bright DCs (Group 2(DC), n = 26) or the largest numbers of CD4bright DCs (group 3(DC), n = 27) was 4.2 (P = .04).
Patients transplanted with more CD34+cells had faster engraftment and decreased incidence of death from infection after transplantation
Univariate and multivariate analyses demonstrated the CD34+ cell dose was the single significant covariate associated with the time to achieve granulocyte engraftment (RR, 1.2;P = .02) and platelet engraftment (RR, 1.3;P = .003). Patients who received grafts containing more than 3.5 × 106 CD34+ cells/kg (group 3(CD34)) had granulocyte and platelet engraftment after 18 and 26 days, respectively. Patients who received less than 2.5 × 106 CD34+ cells/kg (group 1(CD34)) had significantly delayed granulocyte engraftment (22 days; P = .02) and platelet engraftment (37 days;P = .008). Death from infectious causes (other than interstitial pneumonia) occurred in 14 patients at a median of 38 days after transplantation. Comparing causes of death according to the CD34+ cell dose in the bone marrow graft, 29 of 41 (71%) patients in group 1(CD34) died by the reference date, with 10 of 29 deaths attributable to infections (Table7). Within group 2(CD34), 13 of 33 (39%) patients died, with 3 deaths attributable to infection. Within group 3(CD34), 18 of 39 (46%) patients died, with 1 death attributable to infection. Multivariate analysis of covariates associated with nonrelapse mortality confirmed the significance of the CD34+ cell dose (Table 8). Among the entire group of 113 patients, risk strata (RR, 2.3;P = .02), age (RR, 1.1; P = .002), and CD34+ cell dose (RR, 0.58; P = .0042) were associated with nonrelapse mortality in the multivariate model. No covariates were significantly associated with nonrelapse mortality among patients at low risk. Among patients at high risk, nonrelapse death was more frequent among older patients (RR, 1.1;P = .0018) and less frequent among recipients of larger numbers of CD34+ cells (RR, 0.51; P = .012; Table 8).
Causes of death among patients grouped according to content of CD34+ cells in bone marrow graft
| . | Low CD34 (n = 41) < 2.5 × 106/kg . | Interim CD34 (n = 33) 2.5-3.5 × 106/kg . | High CD34 (n = 39) > 3.5 × 106/kg . | Median day of death . |
|---|---|---|---|---|
| Graft rejection | 0 | 2 | 1 | 88 |
| Infection | 10 | 3 | 1 | 38 |
| Interstitial pneumonia | 1 | 1 | 0 | 66 |
| Acute GVHD | 0 | 0 | 1 | 50 |
| Chronic GVHD | 2 | 0 | 2 | 440 |
| Relapse | 9 | 6 | 12 | 331 |
| Organ failure | 4 | 0 | 1 | 24 |
| Hemorrhage | 2 | 1 | 0 | 75 |
| Other | 1 | 0 | 0 | 195 |
| Total | 29 (71%) | 13 (39%) | 18 (46%) | 128 |
| . | Low CD34 (n = 41) < 2.5 × 106/kg . | Interim CD34 (n = 33) 2.5-3.5 × 106/kg . | High CD34 (n = 39) > 3.5 × 106/kg . | Median day of death . |
|---|---|---|---|---|
| Graft rejection | 0 | 2 | 1 | 88 |
| Infection | 10 | 3 | 1 | 38 |
| Interstitial pneumonia | 1 | 1 | 0 | 66 |
| Acute GVHD | 0 | 0 | 1 | 50 |
| Chronic GVHD | 2 | 0 | 2 | 440 |
| Relapse | 9 | 6 | 12 | 331 |
| Organ failure | 4 | 0 | 1 | 24 |
| Hemorrhage | 2 | 1 | 0 | 75 |
| Other | 1 | 0 | 0 | 195 |
| Total | 29 (71%) | 13 (39%) | 18 (46%) | 128 |
Multivariate Cox regression analyses of nonrelapse deaths
| . | All patients (n = 113) . | Low risk (n = 64) . | High risk (n = 49) . | |||
|---|---|---|---|---|---|---|
| RR (95% CI) . | P . | RR (95% CI) . | P . | RR (95% CI) . | P . | |
| Risk strata | 2.3 (1.1-4.7) | .02 | ||||
| Patient age | 1.1 (1.0-1.1) | .002 | 1.03 (0.97-1.1) | .32 | 1.1 (1.0-1.1) | .0018 |
| Gender (male) | 1.6 (0.69-3.8) | .26 | 0.77 (0.25-2.4) | .65 | 2 (0.78-16.6) | .10 |
| Total cells (×108/kg) | 1.1 (0.54-2.1) | .84 | 0.62 (0.19-2) | .42 | 1.2 (0.66-4.2) | .28 |
| CD34+(×106/kg) | 0.58 (0.39-0.84) | .0042 | 0.63 (0.36-1.1) | .10 | 0.51 (0.29-0.89) | .012 |
| CD3+ (×106/kg) | 1 (0.95-1.1) | .97 | 0.98 (0.91-1.1) | .64 | 1.0 (0.93-1.1) | .90 |
| CD3−, CD4+, CD8− (×106/kg) | 2.2 (0.83-5.6) | .11 | 7.3 (0.89-60) | .065 | 2.1 (0.65-7) | .21 |
| . | All patients (n = 113) . | Low risk (n = 64) . | High risk (n = 49) . | |||
|---|---|---|---|---|---|---|
| RR (95% CI) . | P . | RR (95% CI) . | P . | RR (95% CI) . | P . | |
| Risk strata | 2.3 (1.1-4.7) | .02 | ||||
| Patient age | 1.1 (1.0-1.1) | .002 | 1.03 (0.97-1.1) | .32 | 1.1 (1.0-1.1) | .0018 |
| Gender (male) | 1.6 (0.69-3.8) | .26 | 0.77 (0.25-2.4) | .65 | 2 (0.78-16.6) | .10 |
| Total cells (×108/kg) | 1.1 (0.54-2.1) | .84 | 0.62 (0.19-2) | .42 | 1.2 (0.66-4.2) | .28 |
| CD34+(×106/kg) | 0.58 (0.39-0.84) | .0042 | 0.63 (0.36-1.1) | .10 | 0.51 (0.29-0.89) | .012 |
| CD3+ (×106/kg) | 1 (0.95-1.1) | .97 | 0.98 (0.91-1.1) | .64 | 1.0 (0.93-1.1) | .90 |
| CD3−, CD4+, CD8− (×106/kg) | 2.2 (0.83-5.6) | .11 | 7.3 (0.89-60) | .065 | 2.1 (0.65-7) | .21 |
Separate analyses were performed for the entire group of patients (n = 113) and the subsets of low-risk (n = 64) and high-risk (n = 49) patients. All covariates listed were entered into the multivariate Cox model. Significant (P < .05) associations of covariates with EFS are in bold.
Discussion
Our hypothesis was that significant interdonor variability in the cellular constituents of the graft would lead to quantitative and qualitative differences in hematopoietic engraftment, GVHD, and relapse. The novel finding in this study was that larger numbers of CD4bright DCs had adverse effects on survival and were associated with increased post-transplantation relapse and decreased cGVHD. In contrast to previous reports of increased relapse associated with T-cell–depleted allografts, the number of transplanted T cells or various T-cell subsets was not significantly correlated with either survival or relapse.38
As predicted, the CD34+ cell dose positively affected engraftment kinetics and survival after transplantation (Table3).16,22,39 Barrett et al22 recently described increased relapse among recipients of T-cell–depleted allografts transplanted with fewer numbers of CD34+ donor cells. In contrast, patients in the present study who received fewer CD34+ donor cells in the context of a T-cell–replete allograft did not experience a significantly increased incidence of relapse (Tables 6, 7). A lower dose of donor CD34+ cells was associated with a higher incidence of death from infection (Table7) and increased nonrelapse-related death (Table 8). Donor CD34+ myeloid and lymphoid progenitors may differentiate rapidly into donor-derived granulocytes and T cells and B cell, thus accelerating hematopoietic and immune reconstitution after transplantation.40 In contrast to a previous report, the number of CFU in the allograft was not significantly correlated (P < .05) with the kinetics of hematopoietic reconstitution, EFS, or overall survival after transplantation.15 As has been previously reported,18 larger numbers of total nucleated cells transplanted were associated with improved EFS, though the association was limited to the subset of low-risk patients in the present study (Tables 4, 6).
The CD4bright DC2p measured in this study (Figure1) have an overlapping phenotype with the previously described dendritic cell progenitor (Lin−, HLADR+, CD123+, CD11c−)27 and the thymic “plasmacytoid T cell.”31 A criticism of the present study is that a DC population cannot be measured by flow cytometry using only the expression of CD3, CD4, CD8, and side-scatter. When this study began in 1995, additional surface markers were unavailable to enumerate accurately the true number of CD123bright DC2p in the bone marrow. Direct comparison of the 2 methods of enumerating DC2 (CD123bright vs CD4bright) showed overlapping phenotypes and a high degree of correlation (Figures 1, 2A). In contrast, there were more than twice as many Lin−, HLADR+, CD123dim, CD11c+ cells (DC1) than CD4bright DC (Figure 2A), making it unlikely that measurement of the CD4bright DC actually represented the number of DC1 in the graft. The numbers of CD4bright, CD123bright, and CD11c+ DC cells in the graft (Figure 2A) represent the products of the percentages of nucleated bone marrow cells with each phenotype times the total numbers of cells in the graft. Direct comparison of the respective percentages of cells with the CD4bright DC versus the CD11c+ DC phenotype yielded a correlation coefficient of only 0.68. In contrast, the correlation coefficient between the percentage of CD4bright DC versus the CD123bright DCp was 0.85. These data support the identity of the CD4bright DC with the Lin−, CD123bright, HLADR+, CD11c−, and DC2p and the distinction between CD4bright DC and CD11c+ DC1 (Figure 1).
Overall, recipients of more CD34+ cells and fewer CD4bright DCs had superior EFS than patients who received fewer CD34+ cells or more DC. The independent association of CD4bright DCs with EFS is supported by the lack of correlation between the numbers of transplanted CD34+ cells and CD4bright DCs (r = 0.22) and the independent significance of nucleated cells, CD34+ cells, and CD4bright DCs as covariates in multivariate analysis. A number of hypotheses may explain the role of donor CD4bright DCs in influencing relapse and cGVHD after transplantation. Larger numbers of the CD4bright DCs in allogeneic bone marrow grafts may shift the developing donor immune system toward a type 2 response characterized by decreased Th1 cellular immune responses, and a decreased graft-versus-leukemia effect.30,41,42 Donor DCs may capture allo-antigen through apoptotic bodies of residual host hematopoietic cells and induce tolerance to minor histocompatability antigens in donor T cells (indirect antigen presentation or “cross-priming”), thereby inhibiting the graft-versus-leukemia effect.43-45 Such an antigen-specific effect could occur as one of the initial events in the early period after transplantation or during subsequent de novo T-cell development of donor stem cells in the host thymic microenvironment.46 In addition, donor DC2p may function as immunoregulatory cells that synthesize IFN-α and promote the survival of mature DC2,47 thus potentiating the effect of relatively small numbers of donor DC2 on immune responses of donor T cells.23 Finally, the association of higher numbers of CD4bright DC with less graft-versus-leukemia and cGVHD may reflect the pre-existing polarization of donor T cells toward Th2/Tc2 independent of any direct effect of donor DC on post-transplantation immunity.48 The net effect of DC in regulating immune reconstitution after transplantation likely represents the competing effects of donor DC1 versus DC2 in the graft and the donor-derived dendritic cells that differentiate from CD34+cells under the influence of either GM-CSF/TNF (DC1) or IL-3 ligand (DC2).24,25 49
The effect of larger or smaller numbers of transplanted donor dendritic cell subsets with the DC1 phenotype (Lin−, HLA-DR+, CD123−, CD11c+) on clinical outcomes after allogeneic bone marrow and peripheral stem cell transplantation are being studied. Optimal engineering of the allograft may include strategies to polarize donor T cells toward effective graft-versus-leukemia responses by cytokine priming30 or relative enrichment or depletion of subset(s) of donor DC or DCp.
We greatly acknowledge Dr William J. Eley for his helpful discussions. We thank Sylvia Ennis for assistance in preparing the manuscript.
Supported (E.K.W.) by the Doris Duke Charitable Foundation, the Cancer Research Institute, and the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Edmund K. Waller, Bone Marrow and Stem Cell Transplant Center, Emory University, Suite 1003, 1639 Pierce Dr, Atlanta, GA 30322; e-mail: ewaller@emory.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal