Abstract
T cells with natural killer cell phenotype and function (NKT cells) have been described in both human and murine tissues. In this study, culture conditions were developed that resulted in the expansion of CD8+ NKT cells from bone marrow, thymus, and spleen by the timed addition of interferon-γ (IFN-γ), interleukin 2 (IL-2), and anti-CD3 monoclonal antibody. After 14 to 21 days in culture, dramatic expansion of CD3+, CD8+, αβT-cell receptor+ T cells resulted with approximately 20% to 50% of the cells also expressing the NK markers NK1.1 and DX5. The CD8+ NKT cells demonstrated lytic activity against several tumor target cells with more than 90% lysis by day 14 to day 21 of culture. Cytotoxicity was observed against both syngeneic and allogeneic tumor cell targets with the greatest lytic activity by the cells expressing either NK1.1 or DX5. The expanded CD8+ NKT cells produce TH1-type cytokines with high levels of IFN-γ and tumor necrosis factor α. Expansion of the CD8+ NKT cells was independent of CD1d. Ly49 molecules were expressed on only a minority of cells. A single injection of expanded CD8+ NKT cells was capable of protecting syngeneic animals from an otherwise lethal dose of Bcl1 leukemia cells. Expanded CD8+ NKT cells produced far less graft-versus-host disease (GVHD) than splenocytes across major histocompatibility barriers, even when 10 times the number of CD8+ NKT cells as compared to splenocytes were injected. This reduction in GVHD was related to IFN-γ production since cells expanded from IFN-γ knock-out animals caused acute lethal GVHD, whereas cells expanded from animals defective in fas ligand, fas, IL-2, and perforin did not. These data indicate that CD8+ NKT cells expanded in this fashion could be useful for preserving graft-versus-leukemia activity without causing GVHD.
Introduction
T cells with natural killer (NK) cell activity have been identified in both murine and human tissues.1-4 Murine NKT cells typically express phenotypic markers found on T cells such as CD3 and the αβT-cell receptor (TCR), as well as the NK markers NK1.1 and DX5.5Two populations of murine NKT cells have been described. One population either does not express the co-receptors CD4 or CD8 or are CD4+. These NKT cells express the invariant Vα14-Jα281 TCR and have been found in the liver, thymus, bone marrow (BM), and spleen with approximately 0.5 to 1.5 million cells per organ.6-8 Functionally, CD4+NK1.1+ NKT cells produce large amounts of interleukin 4 (IL-4) on activation.8,9 Therefore, this population of IL-4–producing NKT cells are thought to play an important role in the regulation of immune responses, especially those of the TH2 type.10 CD4+ NKT cells are positively selected by the major histocompatibility complex (MHC) class I-like molecule CD1d and associated β-2 microglobulin since these cells are markedly reduced in mice deficient in these molecules.9 11-13
Recently, a second population of NKT cells has been described that expresses a variable TCR repertoire and is not dependent on CD1d for maturation and development.14 In addition, these CD1d-independent splenic NKT cells expressed mainly CD8 or were double negative with respect to CD4 and CD8 expression. Approximately 1% to 2% of splenocytes from C57BL/6 mice are αβTCR+NK1.1+, and, of these cells, approximately 20% are CD8+.14 Little is known about the function of this later population of CD8+ NKT cells.
T cells are readily expandable following mitogenic stimulation with monoclonal antibodies (MAbs) directed against the TCR complex.15 The combination of anti-CD3 and IL-2 results in the expansion of murine and human T cells capable of lysing a variety of different tumor cell lines, some of which are resistant to NK cells.16-18 The expanded cells also have in vivo antitumor cell activity against murine tumors16,19 and human tumors transplanted into immunodeficient mice.18,20 It has been demonstrated that T cells activated with anti-CD3 and IL-2 and cultured for 6 to 8 days have a decreased capacity for inducing graft-versus-host disease (GVHD). Both CD4+ and CD8+ cells were less likely to result in GVHD following activation with anti-CD3 and IL-2.21
In this report we have used culture conditions that allow for the growth of large numbers of T cells that share functional and phenotypic properties with NK cells. Following activation and culture, αβTCR+, CD8+, and NK1.1+ T cells with NK cell function are readily expandable. We characterize the phenotype, cytokine production, and in vitro and in vivo biological activity of this population of CD8+ NKT cells that have antitumor activity yet limited capacity to cause GVHD.
Materials and methods
Animals
Balb/c (H-2d), C57BL/6 (H-2b), B10.D2/nSnJ (H-2d), FVB/J (H-2q), and the following mice of C57BL/6 background, fas deficient, fas ligand deficient, CD1d−/−, interferon-γ (IFN-γ)−/−, IL-2−/−, and perforin−/− were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in a pathogen-free environment in the Department of Laboratory Medicine. All animal protocols were approved by the Institutional Animal Care and Use Committee of Stanford University.
Expansion of NKT cells
Single cell suspensions were prepared from BM, thymus, and spleen. Cells were cultured in cRPMI (10% fetal calf serum [FCS], 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μM 2-mercaptoethanol) with 1000 U/mL mouse IFN-γ (Genetics Institute, Cambridge, MA) added on the first day of culture. After 24 hours, the cells were transferred to flasks coated with 50 ng/mL of the anti-CD3 MAb clone 145-2C11 (Pharmingen, San Diego, CA) and cultured in the presence of 300 IU/mL human rIL-2 (Chiron, Emeryville, CA). After 3 days, cells were divided into flasks with new media and not re-stimulated with anti-CD3 MAb. Every 2 to 3 days thereafter, cRPMI was added supplemented with IL-2 (300 U/mL).
Flow cytometry
The following MAbs were used in this study. MAbs against CD3, CD4, αβTCR, γδTCR, CD8, CD25, CD69, Ly49D, and Ly49G2 were conjugated to fluorescein isothiocyanate (FITC). MAbs against CD3, CD4, CD8, NK1.1, DX5, Ly49A, and Ly49C were conjugated to phycoerythrin (PE; Pharmingen, San Diego, CA). PE-conjugated mouse immunoglobulin (Ig)G2aκ, rat IgG2aκ, hamster IgG, and FITC-conjugated mouse IgG2aκ, rat IgG2a, and rat IgM were used as negative isotype controls. Approximately 106 cells were incubated with Fc Block (antimouse CD16/CD32 MAb; Pharmingen) for 30 minutes at 4°C followed by specific antibodies. Excess antibody was removed by washing, and the stained cells were analyzed, using a FACSscan (Becton Dickinson, San Jose, CA). In some experiments, CD3+NK1.1+ and CD3+NK1.1− or CD3+DX5+and CD3+DX5− populations of cells were isolated by flow cytometry, using a fluorescence-activated cell sorter (FACS) Star Sorter.
51Cr release cytotoxicity assay
Target cells, Bcl1 (B-cell lymphoma H-2d), P3x63 AG8U.1 (myeloma H-2d), C6VL (leukemia H-2b), EL4 (T-cell leukemia H-2b), P815 (mastocytoma H-2d), and YAC-1 (T-cell lymphoma H-2a) were labeled with 51Cr (Dupont-NEN, Boston, MA) by incubating 1-2 × 106 cells in 300 μCi 51Cr for 2 hours at 37°C. The labeled cells were washed with phosphate-buffered saline 3 times, then distributed in 96-well plates at 2 × 104 cells/well in triplicate. Effector cells were added at the indicated ratio and incubated for 4 hours at 37°C. The cells were then pelleted by centrifugation, and aliquots of supernatant were counted in a gamma counter. The percentage of specific51Cr release was calculated according to the following formula: % specific lysis = (test release) − (spontaneous release) × 100/(maximal release) − (spontaneous release). Spontaneous release was obtained by incubating cells in media alone, and maximal release was obtained with 2% Nonidet-P40 incubation.
Cytokine production
Splenocytes (2 × 105) or expanded NKT cells at 0, 7, 14, and 21 days of culture were removed, washed, and incubated with 5 μg/mL phytohemagglutinin (PHA; Sigma Biochemicals, St. Louis, MO) or media alone in a total volume of 200 μL/well in a 96-well plate and cultured for 48 hours. The supernatant was harvested and assayed for cytokine production by enzyme-linked immunosorbent assay (ELISA) for IFN-γ, IL-4, IL-10, and tumor necrosis factor α (TNF-α; Biosource, Camarino, CA) according to manufacturer's instructions. Cytokine production was also evaluated at the same time points by RNase protection assay (Pharmingen, San Diego, CA) according to the manufacturer's instructions.
In vivo experiments
To establish malignant disease, 6- to 8-week-old Balb/c recipient mice were first injected with 103 Bcl1 leukemic cells intraperitoneally. Tumors were allowed to grow for 7 days, and then mice were irradiated with 8 cGy in 2 split fractions. On the same day, animals were rescued with 1 × 107 BM cells, and some animals received 2-4 × 107 expanded CD8+ NKT cells by intravenous injection derived from syngeneic Balb/c donor animals. Animals were observed for signs or symptoms of tumor growth (massive splenomegaly), and time from cell injection to animal death was monitored as previously described.22 The cause of death was determined by necropsy.
To explore the capacity of the CD8+ NKT cells to cause GVHD, recipient Balb/c mice were irradiated as above and transplanted with BM cells from C57BL/6 recipients with either splenocytes or expanded CD8+ NKT cells from wild-type (WT) C57BL/6 or mutant strains of mice on the B6 background at the indicated dose. Typical signs and symptoms of GVHD were observed, including ruffled fur, diarrhea, hunchback appearance, weight loss, and survival.
Statistical methods
Cytotoxicity of sorted populations of cells was evaluated, using the Student t test. Animal survival was compared, using the log-rank test.
Results
Expansion of NKT cells
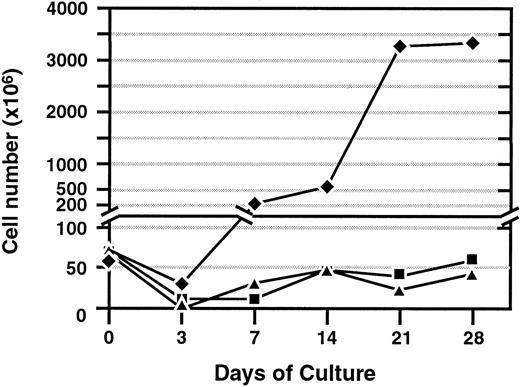
Cells were expanded from spleen, thymus, and BM from different strains of mice by incubating the cells in the presence of murine recombinant IFN-γ. One day later, the cells were transferred to a coated flask with MAb against CD3, and media were supplemented with IL-2. Expansion of cells from the spleen, thymus, and BM were assessed at regular intervals for proliferation and viability (Figure1). Under these culture conditions, cells expanded rapidly from the spleen up to 200-fold by day 21. Total cell expansion was less dramatic from the thymus and BM. These results reflect the relatively low numbers of CD8+ cells in these tissues, especially in the BM. If purified CD8+ cells are isolated from these tissues, they readily expand similar to that of splenocytes (data not shown). Cells from all tissues cultured with IL-2 alone did not expand and were not viable after 7 days in culture. Cells cultured with IL-2 and IFN-γ without CD3 MAb maintained their viability until day 14 but did not expand. Cell expansion from splenocytes was similar from C57BL/6, Balb/c, as well as the knock-out (KO) strains of mice (data not shown).
Expansion of cells from spleen, BM, and thymus.
Total cell number following ex vivo activation with interferon-γ, followed by immobilized anti-CD3 monoclonal antibody and interleukin-2 (300 U/mL). Representative results of more than 3 experiments are shown for cell expansion from each tissue source. ♦, indicates spleen; ▪, BM; and ▴, thymus.
Expansion of cells from spleen, BM, and thymus.
Total cell number following ex vivo activation with interferon-γ, followed by immobilized anti-CD3 monoclonal antibody and interleukin-2 (300 U/mL). Representative results of more than 3 experiments are shown for cell expansion from each tissue source. ♦, indicates spleen; ▪, BM; and ▴, thymus.
Phenotype of the expanded cells
FACS analysis was performed on cultures initiated from the different tissues on day 0 and every 7 days thereafter. The percentages of lymphocytes expressing CD3, CD8, CD4, αβTCR, γδTCR, CD69, CD25, CD44, B220, DX5, NK1.1, or Ly49 molecules were determined at each time point. The phenotype of the cells derived from spleen over a 21-day period is shown in Figure 2. At the initiation of culture, the phenotype of the splenocytes were typical of naive unmanipulated cells with approximately 24% CD3+, 8% CD8+, 15% CD4+, and 20% αβTCR+ (Figure 2A). These cells did not express the activation markers CD69 or CD25 but were CD44+ (Figure 2B). A small population (5%) of cells expressed the NK markers NK1.1 and DX5 (Figure 2C) with the majority of these cells being CD3− (data not shown). By day 7, there was a dramatic increase in the percentage of CD3+CD8+αβTCR+ cells. Cells were negative for both myeloid (Gr-1) and monocytic (Mac1) markers (data not shown). By day 21 of culture, virtually all of the cells were CD3+CD8+αβTCR+ with a significant percentage (20% to 50%) of the cells co-expressing the NK cell markers NK1.1 and DX5 (Figure 2A,C). A similar phenotype was observed from cells expanded from thymus and BM. Cells expressing γδTCR were relatively rare (1% to 6%) at the initiation of culture and were no longer detectable after 21 days of culture. The phenotype of the majority (> 90%) of cells after 14 to 21 days in culture from all tissue sources (BM, thymus, and spleen) was similar and found to be CD3+, CD8+, or αβTCR+ (data not shown). The expanded cells were highly activated as assessed by CD25 and CD69 expression (Figure 2B). Approximately 4% to 5% of splenocytes expressed Ly49A, C, D, or G2. However, after day 7 of culture, very few cells (< 2%) expressed these markers (data not shown). Morphologically, the expanded cells were large, granular, and vacuolated. Evaluation of TCR repertoire using a panel of Vβ MAbs showed a varied TCR usage that does not significantly change over time in culture, indicating that the expanded cells are polyclonal (data not shown). A number of functional studies were performed with these expanded cells.
Phenotypic expression of cell surface molecules at different time points.
(A) Expression of CD3, CD8, CD4, αβTCR, and γδTCR from cells initiated from splenocytes (day 0) and at days 7, 14, and 21 in culture. (B) Expression of the activation markers CD69, CD25, CD44, and B220 at day 0 and at different time points. (C) Expression of the NK markers DX-5 and NK1.1 at day 0 and at different time points. Background staining using an isotype control antibody is shown in each histogram (dotted line). Results are representative of more than 3 experiments.
Phenotypic expression of cell surface molecules at different time points.
(A) Expression of CD3, CD8, CD4, αβTCR, and γδTCR from cells initiated from splenocytes (day 0) and at days 7, 14, and 21 in culture. (B) Expression of the activation markers CD69, CD25, CD44, and B220 at day 0 and at different time points. (C) Expression of the NK markers DX-5 and NK1.1 at day 0 and at different time points. Background staining using an isotype control antibody is shown in each histogram (dotted line). Results are representative of more than 3 experiments.
Cytotoxicity against syngeneic and allogeneic tumor cell lines
Splenocytes had no cytolytic activity on day 0. However, by day 7, significant lytic activity was observed against P3x63 tumor cells with up to 65% specific lysis at an effector-to-target (E:T) ratio of 40:1. By day 14 to day 21, the CD8+ NKT cells exhibited more than 80% specific lysis against this tumor cell line at a 40:1 ratio (Figure 3A). The expanded CD8+ NKT cells maintained their cytolytic activity for up to 60 days in culture (data not shown). Cytotoxicity of the expanded CD8+ NKT cells against different tumor cell lines was assessed by a 4-hour 51Cr release assay. A variety of different tumor cell lines were used, including P815 (mastocytoma, H-2d), YAC-1 (T-cell lymphoma, H-2a), P3x63 (myeloma, H-2d), Bcl1 (mouse B-cell lymphoma, H-2d), EL4 (lymphoma, H-2b), and C6VL (thymoma, H-2b). The representative results are shown in Figure 3B, using effector cells derived from C57BL/6 mice. The expanded CD8+ NKT cells were capable of recognizing syngeneic (EL4) and allogeneic (P815, P3 × 63, Bcl1, and YAC1) targets. Interestingly, C6VL tumor cells were consistently resistant to cytolysis. A similar pattern was observed when CD8+ NKT cells were expanded from Balb/c splenocytes, where, again, all target cells except C6VL were sensitive to cytolysis. Similar cytotoxicity results were obtained, using cells derived from the thymus and BM as compared to splenocytes from B10.D2/nSnJ and FVB/J animals (data not shown), indicating that expansion of this population of cells is not strain specific. In contrast, when freshly isolated normal cells derived from BM or spleen were used as target cells, no cytolytic activity was observed (data not shown).
Cytotoxicity of ex vivo–expanded cells.
Cells derived from C57BL/6 (H-2b) were assessed at the initiation of culture and at different time points against the target cell P3 × 63 (H-2d). (B) Cytotoxicity of ex vivo–expanded CD8+ NKT cells against syngeneic and allogeneic targets. C57BL/6 (H-2b)-expanded CD8+ NKT cells at day 21 of culture were assayed for cytotoxicity against a panel of syngeneic and allogeneic target cells. Cytotoxicity was assessed after 4 hours at different target-to-effector ratios, ranging from 1:5 to 1:40. Results are representative of more than 3 experiments. ■ indicates 1:5; ▧, 1:10; ⊠, 1:20, and ▪, 1:40.
Cytotoxicity of ex vivo–expanded cells.
Cells derived from C57BL/6 (H-2b) were assessed at the initiation of culture and at different time points against the target cell P3 × 63 (H-2d). (B) Cytotoxicity of ex vivo–expanded CD8+ NKT cells against syngeneic and allogeneic targets. C57BL/6 (H-2b)-expanded CD8+ NKT cells at day 21 of culture were assayed for cytotoxicity against a panel of syngeneic and allogeneic target cells. Cytotoxicity was assessed after 4 hours at different target-to-effector ratios, ranging from 1:5 to 1:40. Results are representative of more than 3 experiments. ■ indicates 1:5; ▧, 1:10; ⊠, 1:20, and ▪, 1:40.
To better define the population of cells with cytolytic activity, the expanded CD8+ NKT cells were sorted into CD3+DX5+ and CD3+DX5−cellular subsets. The isolated cell populations were approximately 80% to 95% pure on re-analysis from different experiments (Table1). Cytotoxicity assays were performed against the P3x63 target cells. Higher cytolytic activity was consistently observed from the CD3+DX5+population of NKT cells, compared to CD3+DX5−T cells, at all E:T ratios (Table 1). The CD3+CX5− T cells also had some cytolytic activity. Similar data were obtained when the CD3+NK1.1+ population of cells was isolated by FACS (data not shown).
Cytotoxicity of purified CD3+DX5+and CD3+DX5− cells isolated by flow cytometry
| Effector-to-target ratio . | Cytolysis of sorted populations (%) (±SD) . | P value . | |
|---|---|---|---|
| CD3+DX5− . | CD3+DX5+ . | ||
| 5:1 | 31 (±1) | 52 (±1) | .0001 |
| 10:1 | 39 (±2) | 59 (±1) | .004 |
| 20:1 | 56 (±1) | 76 (±2) | .0005 |
| Effector-to-target ratio . | Cytolysis of sorted populations (%) (±SD) . | P value . | |
|---|---|---|---|
| CD3+DX5− . | CD3+DX5+ . | ||
| 5:1 | 31 (±1) | 52 (±1) | .0001 |
| 10:1 | 39 (±2) | 59 (±1) | .004 |
| 20:1 | 56 (±1) | 76 (±2) | .0005 |
Cytokine production
Cytokine production by the expanded CD8+ NKT cells was assessed for the production of IFN-γ, TNF-α, IL-4, and IL-10 by ELISA. To perform these studies, splenocytes were expanded under the culture conditions described above and harvested at weekly intervals. The harvested cells were evaluated for cytokine production with and without activation in vitro for 24 hours with PHA. Supernatants were harvested and assayed by ELISA to quantitate cytokine production. The major cytokine produced by the expanded CD8+ NKT cells was IFN-γ. Levels of IFN-γ produced by the CD8+ NKT cells increased over time in culture (Figure4A). At day 0, low levels of IFN-γ were found. However, by day 7, production of IFN-γ by the expanded cells increased dramatically and by day 21 was approximately 6000 pg/mL with or without PHA activation. TNF-α production was similarly up-regulated to approximately 1800 pg/mL by day 21. Moderate levels of IL-10 (800 pg/mL) were produced mainly early in the culture period (day 7) that declined over time. In marked contrast, little or no IL-4 (10-20 pg/mL) was detected. Cytokine production was also evaluated by RNA expression, using an RNase protection assay (Figure 4B). This assay confirmed high levels of IFN-γ messenger RNA (mRNA) as well as low levels of IL-10 and little to no IL-4 mRNA (data not shown). mRNA expression of TNF-α, TNF-β, macrophage migration inhibitory factor, and tumor growth factor β were all up-regulated over time in culture. No expression of IL-6 or IFN-β was found at initiation of cultures or at the end of expansion (Figure 4B).
Cytokine production by the expanded CD8+ NKT cells.
Splenocytes were cultured as described, and at the indicated time points cells were assayed for cytokine production with or without stimulation with PHA. (A) Production of IFN-γ, TNF-α, IL-4, and IL-10 was assayed by ELISA. ■ indicates control; ▪, PHA. (B) mRNA production for a broad array of other cytokines was assessed, using an RNase protection assay.
Cytokine production by the expanded CD8+ NKT cells.
Splenocytes were cultured as described, and at the indicated time points cells were assayed for cytokine production with or without stimulation with PHA. (A) Production of IFN-γ, TNF-α, IL-4, and IL-10 was assayed by ELISA. ■ indicates control; ▪, PHA. (B) mRNA production for a broad array of other cytokines was assessed, using an RNase protection assay.
Role of CD1d on expansion of CD8+/− NKT cells
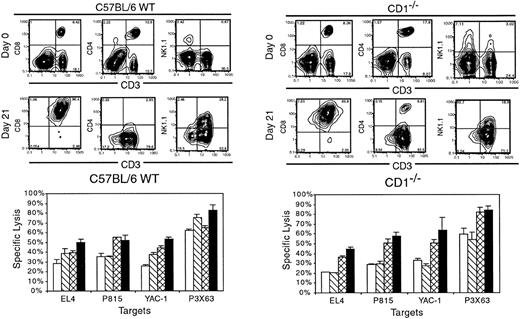
Previous investigators have determined that CD4+NK1.1+ T cells require CD1d for development.11-13 To explore the role of CD1d expression on the expansion and cytotoxicity of the NKT cells, splenocytes were isolated from both WT C57BL/6 animals and homozygous CD1−/− KO animals. At day 0 prior to expansion, the phenotype of the starting population from CD1−/−splenocytes was similar to WT with approximately 25% CD3+T cells and a slightly higher percentages of CD4+ T cells as compared to CD8+ T cells (Figure5). Both animals had low numbers of NK1.1+ cells with the majority of these cells being CD3−. Splenocytes from CD1−/− animals had a small population (approximately 3%) of CD3+NK1.1+ cells. Following ex vivo expansion under the culture conditions described above, both populations of splenocytes expanded to a similar degree (data not shown). The phenotype after 21 days in culture was also very similar with the majority of cells expressing CD3, CD8, and αβTCR. Cells derived from CD1d−/− animals had a small percentage of CD4+ cells following expansion. A significant percentage of CD3+ T cells also expressed NK1.1 (19% to 30%) that was similar in cells derived from either WT or CD1−/−splenocytes (Figure 5). Cytotoxicity was also similar with both cultures of expanded cells displaying significant cytotoxicity against a broad array of tumor cell targets. Moreover, using CD1d−/− targets that were transduced with the CD1d molecule showed essentially no difference in cytotoxicity (data not shown). These data strongly suggest that CD1d plays little to no role in the expansion or cytotoxicity of the CD8+ NKT cells.
Role of CD1d in ex vivo expansion of CD8+NKT cells.
Splenocytes were isolated from C57BL/6 WT or CD1−/−animals and cultured as described for 21 days. Phenotypic analysis was performed at days 0 and 21, as well as cytotoxicity against a panel of target cells at day 21. Data are representative of one of 3 experiments. ■ indicates 1:5; ▧, 1:10; ⊠, 1:20; and ▪, 1:40.
Role of CD1d in ex vivo expansion of CD8+NKT cells.
Splenocytes were isolated from C57BL/6 WT or CD1−/−animals and cultured as described for 21 days. Phenotypic analysis was performed at days 0 and 21, as well as cytotoxicity against a panel of target cells at day 21. Data are representative of one of 3 experiments. ■ indicates 1:5; ▧, 1:10; ⊠, 1:20; and ▪, 1:40.
In vivo antitumor effect of expanded NKT cells
The in vivo antitumor activity of the expanded CD8+NKT cells was studied in a syngeneic model system in which Balb/c mice were injected with an otherwise lethal dose of Bcl1 tumor cells. This tumor cell line results in massive splenomegaly and ultimately death of the recipient animals within approximately 2 months.22 In these studies, Balb/c mice were injected with 103 Bcl1 cells that were allowed to grow for 1 week to simulate active disease. The mice then received lethal irradiation to attempt to develop a state of minimal residual disease similar to the clinical situation. The recipient animals were then treated with an infusion of BM cells or BM plus a single injection of 2-4 × 107 expanded CD8+ NKT cells of Balb/c origin. No additional cytokines, such as IL-2, were used. A representative experiment is shown in Figure6. Animals that received radiation alone without rescue with BM cells all died within 20 days of hematopoietic failure. Animals that received both Bcl1 tumor cells and BM survived the effects of radiation, but all died with progressive tumor growth within 55 days. The animals that received the expanded CD8+NKT cells in addition to the BM and Bcl1 tumor cells had improved overall survival with approximately 50% of the animals surviving for more than 140 days (P = .004). These data demonstrate the in vivo antitumor activity of the expanded CD8+ NKT cells in a syngeneic model system. Further, these experiments demonstrate that CD8+ NKT cells do not interfere with hematopoietic engraftment.
In vivo activity of syngeneic CD8+ NKT cells after injection into tumor-bearing hosts.
Bcl1 tumor cells (H-2d; 103) were injected intraperitoneally into syngeneic Balb/c recipient animals. After 7 days, animals were lethally irradiated (400 cGy ×2) and some animals were rescued with 106 syngeneic (H-2d) BM with or without syngeneic ex vivo–expanded CD8+ NKT cells (H-2d). Length of animal survival was assessed. All animals that died after day 20 had clear evidence of Bcl1 tumor growth assessed by massive splenomegaly. ▵ indicates XRT (n = 5); ○, BM (n = 5); and ■, NK − T + BM cells (n = 15).
In vivo activity of syngeneic CD8+ NKT cells after injection into tumor-bearing hosts.
Bcl1 tumor cells (H-2d; 103) were injected intraperitoneally into syngeneic Balb/c recipient animals. After 7 days, animals were lethally irradiated (400 cGy ×2) and some animals were rescued with 106 syngeneic (H-2d) BM with or without syngeneic ex vivo–expanded CD8+ NKT cells (H-2d). Length of animal survival was assessed. All animals that died after day 20 had clear evidence of Bcl1 tumor growth assessed by massive splenomegaly. ▵ indicates XRT (n = 5); ○, BM (n = 5); and ■, NK − T + BM cells (n = 15).
CD8+/− NKT cells cause less GVHD than splenocytes
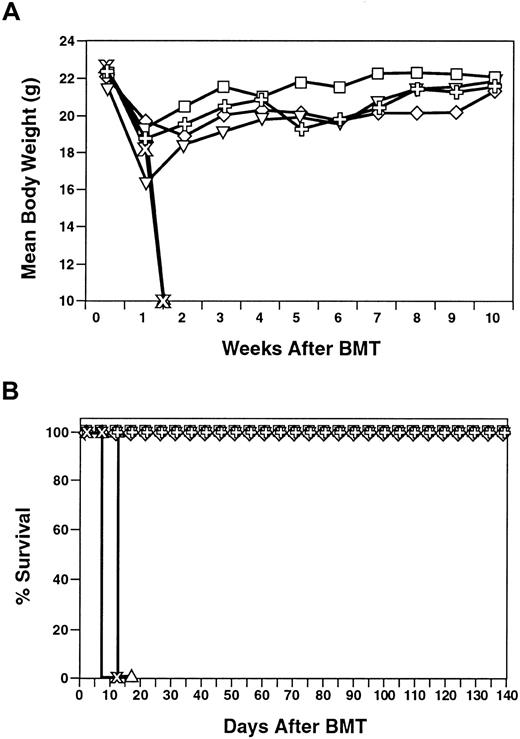
CD8+ NKT cells are readily expandable, have in vitro cytotoxic activity, and protect syngeneic animals from an otherwise lethal dose of Bcl1 tumor cells. To explore the potential of this population of cells in the allogeneic transplant setting, experiments were performed to evaluate the role CD8+ NKT cells in the induction of GVHD when transplanted across major histocompatibility barriers (C57BL/6 → Balb/c). In this system, BM cells alone do not cause GVHD, and GVHD is induced with naive splenocytes. Injection of 2.5 × 106 unmanipulated splenocytes resulted in acute GVHD and death within 20 days (Figure 7). Interestingly, CD8+ NKT cells had a much lower propensity for GVHD induction with little to no GVHD following injection of 5 × 106, 10 × 106, and even 20 × 106 cells (Figure 7). Although these animals did experience mild weight loss (Figure 7A) as compared to animals that were transplanted with BM alone, all of the animals survived (Figure7B). The CD8+ NKT cells were not capable of preventing GVHD by splenocytes (data not shown).
Induction of GVHD by CD8+ NKT cells across histocompatibility barriers.
Donor (C57BL/6) (H-2b) splenocytes or expanded CD8+ NKT cells at the indicated doses were injected into lethally irradiated Balb/c (H-2d) recipients. Mean body weight (top panel) and survival (bottom panel) are presented. ▵ indicates XRT;  , splenocytes; ■, BM; ✙, NK − T cells 20 × 106; ⋄, NK − T cells 10 × 106; and ▿, NK − T cells 5 × 106.
, splenocytes; ■, BM; ✙, NK − T cells 20 × 106; ⋄, NK − T cells 10 × 106; and ▿, NK − T cells 5 × 106.
Induction of GVHD by CD8+ NKT cells across histocompatibility barriers.
Donor (C57BL/6) (H-2b) splenocytes or expanded CD8+ NKT cells at the indicated doses were injected into lethally irradiated Balb/c (H-2d) recipients. Mean body weight (top panel) and survival (bottom panel) are presented. ▵ indicates XRT;  , splenocytes; ■, BM; ✙, NK − T cells 20 × 106; ⋄, NK − T cells 10 × 106; and ▿, NK − T cells 5 × 106.
, splenocytes; ■, BM; ✙, NK − T cells 20 × 106; ⋄, NK − T cells 10 × 106; and ▿, NK − T cells 5 × 106.
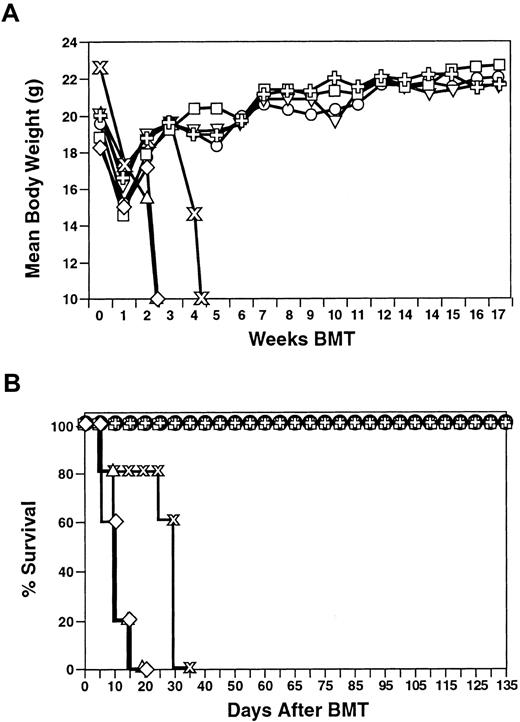
To explore the mechanism of action of the CD8+ NKT cells, a series of animals deficient in key effector molecules were used, including fas deficient, fas ligand deficient, as well as KO animals lacking IL-2, IFN-γ, and perforin. CD8+ NKT cells were readily expandable from these different strains of animals and displayed a similar cell surface phenotype (data not shown). Cytokine production was also similar except for cells derived from the IFN-γ−/− animals that, as expected, did not produce IFN-γ. Perforin was critically important for cytotoxicity both in vitro and in vivo since CD8+ NKT cells expanded from perforin animals had no cytotoxic activity in vitro and were ineffective in protecting animals from Bcl1 tumor challenge (M Verneris, submitted for publication). Fas and fas ligand appeared to play little role in antitumor responses. In a representative transplantation experiment, expanded CD8+NKT cells derived from IL-2, fas ligand deficient, fas deficient, and perforin animals were similar to WT in that they caused little to no GVHD with all animals surviving more than 180 days (Figure8). In marked contrast, CD8+NKT cells from IFN-γ KO animals resulted in the induction of acute lethal GVHD with rapid weight loss and animal death within 20 days of injection (Figure 8). IFN-γ production by the donor CD8+NKT cells and not recipient cells was required since IFN-γ−/− recipients did not develop GVHD when injected with WT CD8+ NKT cells (data not shown). Induction of GVHD was rapid, similar to that observed with splenocytes, although the number of CD8+ NKT cells was 10 times higher than splenocytes in all experiments. In some experiments, a small percentage of animals transplanted with CD8+ NKT cells derived from IFN-γ−/− splenocytes eventually recovered (approximately 20%). These data indicated that IFN-γ production by the donor CD8+ NKT cells is critical to the lack of GVHD observed across allogeneic barriers in this model system.
Role of interferon-γ production in GVHD induction.
CD8+ NKT cells were expanded from different deficient and KO strains of animals on the C57BL/6 background as indicated. Cells (2 × 107) were adoptively transferred into lethally irradiated (400 cGy ×2) Balb/c (H-2d) mice rescued with 107 BM derived from C57BL/6 (H-2b) animals and monitored for signs and symptoms of GVHD (weight loss) and survival. Mean body weight (top panel) and overall survival (bottom panel) are presented. Results of IFN-γ KO animals are representative of 5 experiments. ▵ indicates XRT; , splenocytes; ⋄, IFN-γ; ■, IL-2; ○, gld; ▿, Ipr; and ✙, pfp.
, splenocytes; ⋄, IFN-γ; ■, IL-2; ○, gld; ▿, Ipr; and ✙, pfp.
Role of interferon-γ production in GVHD induction.
CD8+ NKT cells were expanded from different deficient and KO strains of animals on the C57BL/6 background as indicated. Cells (2 × 107) were adoptively transferred into lethally irradiated (400 cGy ×2) Balb/c (H-2d) mice rescued with 107 BM derived from C57BL/6 (H-2b) animals and monitored for signs and symptoms of GVHD (weight loss) and survival. Mean body weight (top panel) and overall survival (bottom panel) are presented. Results of IFN-γ KO animals are representative of 5 experiments. ▵ indicates XRT; , splenocytes; ⋄, IFN-γ; ■, IL-2; ○, gld; ▿, Ipr; and ✙, pfp.
, splenocytes; ⋄, IFN-γ; ■, IL-2; ○, gld; ▿, Ipr; and ✙, pfp.
Discussion
T cells with NK cell activity have been described in both murine and human tissues.1-4 NKT cells have generated considerable interest due to the ability of this population of cells to produce cytokines such as IL-4 and to possibly play a role in immune regulation and surveillance.10,11 To date, 2 populations of NKT cells have been described. One population of NKT cells is primarily CD4+ or lacks expression of either CD4 or CD8, co-expresses the NK marker NK1.1, and has a restricted TCR repertoire expression.5,6,23 This population of NKT cells is found primarily in the liver and thymus, recognizes CD1d and, on stimulation produces large quantities of IL-4.8,10CD4+NK1.1+ cells have also been found to suppress GVHD.24 More recently, a second population of NKT cells has been described that is primarily CD8+, also co-expresses NK1.1, and has a more diverse and variable TCR repertoire.14 This population of NKT cells does not depend on CD1d and has been found primarily in the spleen and BM. Little is known about the biological function of these CD8+ NKT cells that are found at low frequency (approximately 1% to 3%) in the spleen and BM.
A major advantage of T-cell populations is that they are readily expandable on activation. In this report we have used similar cytokine conditions that result in the expansion of both murine and human T cells from peripheral blood.17,18,20 These conditions include the cytokines IFN-γ and IL-2 along with a T-cell mitogenic stimulus (anti-CD3). In murine systems, immobilization of the CD3 MAb is required for optimal T-cell expansion. IL-2 is also required since cell expansion is greatly limited in the absence of this cytokine, and the cells do not survive past approximately 7 days in culture. At the beginning of the culture, there is a mixed population of cells in the spleen, including both CD4+ and CD8+ T cells. There is also a population of CD3−NK1.1+ NK cells and a small population (approximately 1%) of CD3+NK1.1+ T cells. Following 14 to 21 days in culture, dramatic expansion of CD8+ T cells occurs with 20% to 50% co-expressing the NK markers NK1.1 and DX5. In addition, virtually all of the cells in these cultures are αβTCR+. Ly49 molecules are expressed on only a minority of cells in contrast to NK cells. The expanded CD8+ NKT cells produce cytokines of the TH1 type, including large amounts of IFN-γ and TNF-α. Small amounts of IL-10 are produced with virtually no IL-4 production in marked contrast to the previously described CD4+ NKT cells. These expanded cells express phenotypic properties of NK cells (ie, NK1.1 and DX5) and display NK functional properties demonstrated by lysis of a broad array of both syngeneic and allogeneic tumor targets without prior exposure. Interestingly, some targets are not effectively lysed (ie, C6VL). Lysis does not correlate with class I MHC expression since both sensitive and resistant targets express class I. The CD8+ NKT cells did not lyse normal BM or spleen cells in vitro, and administration of the CD8+ NKT cells did not inhibit reconstitution of BM following an otherwise lethal dose of irradiation. Direct experiments combining CD8+ NKT cells with purified murine hematopoietic stem cells of the phenotype Sca-1+ThyloLin− have also demonstrated that the CD8+ NKT cells do not interfere with engraftment (MR Verneris, submitted for publication). The potential for CD8+ NKT cells to facilitate hematopoietic stem cell engraftment is under investigation. The majority of the cytotoxic activity is found in the CD3+DX5+ (or CD3+NK1.1+) population of cells, although the CD3+DX5− population retains some, albeit less, biological activity. Cytotoxic activity increases over time in culture with concomitant expression of cytotoxic molecules such as perforin and granzymes. Moreover, cytotoxicity is dependent on perforin since expanded cells derived from perforin-deficient mice have no cytotoxic activity either in vitro or in vivo (MR Verneris, submitted for publication) similar to other populations of cytotoxic lymphocytes.25 Fas ligand appears to play a limited role in contrast to freshly isolated CD8+ T cells.26 The lack of significant expression of Ly49 molecules suggests that these molecules play a limited role in the observed cytotoxicity. Additional studies are required to phenotypically define and elucidate the cytotoxic mechanisms involved in target cell recognition.
With the use of these culture conditions that favor T-cell expansion, large numbers of CD8+ NKT cells can be readily expanded that can be used for both in vitro and in vivo studies. CD1d is not involved in expansion and cytotoxicity of these CD8+ NKT cells, in contrast to the critical role of CD1d in the development and function of CD4+ NKT cells.11,13 The lack of CD1d involvement was demonstrated, using KO mice deficient in this molecule. Cell expansion and phenotype of the cells were identical when splenocytes from CD1d−/− mice were compared to WT animals. These data are consistent with the observations of Eberl et al14 in which the CD8+ NKT cells observed in BM and spleen were also found in CD1d−/− animals; however, the CD4+ and double-negative NKT cells were markedly reduced in these KO animals.
To explore the potential role of CD8+ NKT cells in vivo, experiments were performed in both the syngeneic and allogeneic setting. Expanded CD8+ NKT cells were capable of protecting a significant proportion of mice from an otherwise lethal challenge of Bcl1 lymphoma cells. In vivo activity was not dependent on IL-2 administration, and no toxicity was observed following the intravenous injection of up to 50 × 106 CD8+ NKT cells. Relatively large numbers of CD8+ NKT cells were used in the in vivo experiments; however, since the cells are readily expandable, this was easily achieved. In other studies, following stimulation with anti-CD3 and IL-2 and short-term culture in vivo, biological activity was also observed; however, IL-2 was required.16 27-29Additional studies are under way to determine whether exogenous administration of cytokines such as IL-2 or IL-12 increases the in vivo activity of this cell population.
To further explore the role of CD8+ NKT cells in the allogeneic transplantation setting, a number of studies were performed to determine whether this population of cells is capable of the induction of GVHD. Doses of between 5 and 20 × 106CD8+ NKT cells were used that did not result in clinically significant GVHD. Animals that received CD8+ NKT cells had much improved survival in contrast to animals that received unmanipulated splenocytes, all of whom died within 20 days even at 10 times lower cell numbers. A different strain combination of B10.BR (H-2a) donor-activated T cells into Balb/c (H-2d) recipients also produced markedly reduced GVHD as compared to freshly isolated splenocytes.21 In other studies, NK cells have also been found to be capable of crossing histocompatibility barriers without causing GVHD.30
A number of hypotheses can be proposed to explain why CD8+NKT cells cause much less GVHD than unmanipulated splenocytes. One possibility, as discussed above, is that GVHD in this model system is primarily caused by CD4+ T cells. However, this does not explain the observed results since freshly isolated CD8+ T cells (> 1 × 106) are capable of causing GVHD in this strain combination.31 In our experiments, up to 20 × 106 CD8+ NKT cells were injected with minimal GVHD. A second possibility is that activation of the T cells results in a change in the cells such that GVHD is attenuated. This could include the production of protective cytokines. In addition, activation of CD4+ T cells also results in attenuation of GVHD21 (and our unpublished observations). A third possibility is that the activated CD8+ NKT cells do not home properly. In fact, cultured T cells have been shown to home preferentially to the lungs and liver.21,32,33 Although homing was not directly assessed in this study, the CD8+NKT cells were capable of protecting syngeneic and allogeneic recipients from a lethal dose of Bcl1 (this study and MR Verneris, submitted for publication). In fact, donor-derived CD8+ NKT cells could be found in the peripheral blood of recipient mice for at least 3 weeks following injection. Therefore, it seems unlikely that homing differences alone explain the observed results. A fourth possibility is that following allorecognition, previously activated T cells may undergo activation-induced cell death (AICD) that has been described for activation through the CD3/TCR complex.34,35AICD is thought to proceed through fas ligand–fas interactions, although other molecules may also be important.36 37
To explore the mechanisms underlying the biological activity of the CD8+ NKT cells, a number of different mouse strains lacking key effector molecules were used. We studied CD8+ NKT cells derived from fas ligand–defective, fas defective, and perforin, IL-2 and IFN-γ KO animals since these molecules were thought to potentially play important roles in effector cell function. CD8+ NKT cells were readily expandable from all of these different strains of animals. Cells derived from perforin animals lacked cytotoxic activity both in vitro and in vivo (MR Verneris, submitted for publication, 2001). CD8+ NKT cells expanded from animals deficient of IL-2, fas, fas ligand, and perforin or from defective strains of mice were similar to WT cells in that they caused little to no GVHD across major histocompatibility barriers. This observation makes it unlikely that AICD mechanisms alone explain the lack of GVHD. In marked contrast, cells expanded from IFN-γ–deficient animals resulted in acute lethal GVHD with similar kinetics to unmanipulated splenocytes. Splenocytes from IFN-γ–deficient animals expanded in a similar fashion and were phenotypically and functionally identical to WT cells other than the lack of IFN-γ production.
The role of IFN-γ production in GVHD induction is complex and depends on the timing of administration. When administered late, IFN-γ exacerbates GVHD. In contrast, early administration has a protective effect.38 Yang et al39 have demonstrated that IL-12 injected at the same time as the BM transplant protected animals from GVHD and that this protection is dependent on the ability of the donor cells to produce IFN-γ. Similarly, in our studies the high levels of IFN-γ produced by the expanded CD8+ NKT cells appears to protect animals from otherwise lethal GVHD. IFN-γ production is required by the donor and not recipient animals since WT CD8+ NKT cells injected into IFN-γ KO animals did not cause GVHD (data not shown). The mechanism by which IFN-γ production dampens the allogeneic response requires further study and may affect allorecognition or clonal expansion following recognition. In addition, IFN-γ may affect the recruitment of other populations of cells responsible for GVHD progression.
The relationship of the expanded CD8+ NKT cells to that of freshly isolated CD8+NK1.1+ cells requires further evaluation. However, these studies are difficult to perform due to the paucity of these cells in fresh tissues. NK1.1 expression may represent an activation marker on T cells. This has recently been demonstrated on virus-specific CD8+ and CD4+ T cells40 and on γ/δT cells after Salmonellainfection.41
We have performed similar studies with human peripheral blood lymphocytes. Following 14 to 21 days in culture, the majority (> 90%) of cells are CD3+ with a significant percentage of the cells co-expressing the human NK marker CD56.20,42Ex vivo–expanded human CD3+CD56+ T cells produce cytokines of the TH1 type and have broad non–MHC-restricted cytotoxicity against a variety of tumor cell lines as well as autologous and allogeneic fresh tumor isolates.43,44 Stimulation with IFN-γ results in IL-12 production from monocytes as well as up-regulation of CD2 that interacts with leukocyte function-associated antigen-3 for optimal in vitro expansion of the human CD3+CD56+ effector cells.45 We have used murine models to evaluate the biological activity of these human ex vivo–expanded effector cells and have shown that they are superior to IL-2–activated NK cells against human lymphoma cells engrafted into severe combined immunodeficient mice and do not require exogenous IL-2.20 These cells can also be expanded from patients with hematologic malignancies such as chronic myelogenous leukemia in which they are not part of the malignant clone and have antileukemic potential.44 We have also used a novel bioluminescent tumor model to further document the biological activity of these effector cells.46 On the basis of these experimental observations, the autologous ex vivo–expanded NKT cells are currently being evaluated in phase I/II clinical trials in patients with advanced-stage lymphomas. The potential benefit of these populations of NKT cells in the allogeneic setting is supported by these murine models in which induction of GVHD is significantly attenuated.
In summary, in this report we have used culture conditions that result in the dramatic expansion of CD8+ NKT cells that share functional and phenotypic properties of both cytotoxic T and NK cells. These expanded CD8+ NKT cells are not dependent on CD1d for growth or cytotoxicity and produce cytokines of the TH1 type. The expanded CD8+ NKT cells have potent in vitro cytotoxicity and protect animals from an otherwise lethal tumor challenge. In addition, the CD8+ NKT cells do not cause significant GVHD even across major histocompatibility barriers at least in part due to the endogenous production of IFN-γ. These data delineate the biological activity of expanded CD8+ NKT cells that could have important clinical utility.
The helpful discussions with Drs Samuel Strober and Irving Weissman are gratefully acknowledged. Dr Strober also provided the CD1d−/− mice and CD1d-transduced cell lines. The statistical input of Ruby Wong, PhD, is greatly appreciated. The expert secretarial assistance of Sara Clark is appreciated.
Supported by grants 2PO1CA49605, HL-57443, and RO1CA80006 from the National Institutes of Health. M.R.V. is supported by a scholarship from the American Society of Hematology. J.A.S. is supported in part by a Burroughs-Wellcome Fund Career Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert S. Negrin, Rm H1353, Stanford University Hospital, Stanford, CA 94305; e-mail: negrs@leland.stanford.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal