Abstract
Chemokines and adhesion molecules such as integrins play a major part in the trafficking, extravasation, and recruitment of leukocytes to inflammatory sites. This study investigated the effects of β2 integrin engagement on chemokine production by freshly isolated human monocytes. We found that ligation of CD11b or CD11c but not CD11a α chains of β2 integrins by antibodies or soluble CD23 (sCD23) fusion proteins rapidly induced transcription and secretion of interleukin 8, macrophage inflammatory protein (MIP) 1α, and MIP-1β. Because the promoters of these chemokine genes contain κB binding sites, we assessed the possible role of nuclear factor–κB (NF-κB) in controlling induction of the genes through β2 integrin engagement. Electrophoretic mobility shift assays showed that sCD23 or antibodies to CD11b or to CD11c up-regulated DNA-binding activity of NF-κB. Activation of NF-κB was accompanied by degradation of its cytosolic inhibitor IκB-α. Blockade of depletion of IκB-α by proteasome inhibitors (proteasome inhibitor I or acetyl-leucinyl-leucinyl-norleucinal) led to concomitant inhibition of NF-κB DNA-binding activity and expression of MIP-1α and MIP-1β messenger RNA induced by β2 integrin ligation. These results suggest that triggering of CD11b or CD11c β2 integrin on primary human monocytes provides activation signals leading to nuclear translocation of NF-κB and subsequent secretion of MIP-1α and MIP-1β that may have an important role in recruitment of other inflammatory cells during initiation of an inflammatory response.
Introduction
Recruitment of circulating leukocytes to an inflammatory site in response to stimuli such as infectious agents (viruses, bacteria, and protozoans) or noninfectious processes (trauma, autoimmune disorders, and ischemia-reperfusion injury) is a crucial step in the development of both acute and chronic inflammatory responses. In all these conditions, extravasation of circulating leukocytes requires communication with vascular endothelial cells that in turn depends on an interrelated network of events involving the finely regulated action of inflammatory cytokines (ie, interleukin [IL] 1 and tumor necrosis factor [TNF]-α), adhesion molecules (notably integrins), and chemoattractant cytokines called chemokines.
Chemokines are highly conserved, small, secreted or membrane-bound cytokines with molecular masses of 6 to 14 kd and a characteristic 4-cysteine motif in their amino acid sequence.1-3 Two main subfamilies are defined according to the position of the first 2 cysteine residues. In the CXC (or α-chemokine) subfamily, these residues are separated by one amino acid, whereas in the CC (or β-chemokine) subfamily, they are adjacent. The β-chemokines, which include monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein (MIP) 1α, MIP-1β, and regulated on activation, normal T-cell expressed and secreted (RANTES) molecules, are potent chemotactic agents for monocytes.4 5 The migratory behavior of monocytes in response to a concentration gradient of these soluble chemotactic factors also depends on adhesion molecules, particularly the leukocyte-specific β2 integrins.
Integrins are part of a family of heterodimeric transmembrane glycoproteins that recognize a variety of ligands or counterreceptors, including extracellular matrix and cell-surface and plasma proteins, and that consequently control numerous physiologic functions, such as adhesion, locomotion, chemotaxis, and phagocytosis.6Expression of β2 integrins is restricted to leukocytes, with distribution varying among leukocyte subtypes. The β2 integrins share a common β chain (CD18) and have at least 4 distinct α chains noncovalently associated with CD18: lymphocyte functional antigen 1 (αLβ2, CD11a/CD18), macrophage antigen 1 (Mac-1; αMβ2, CR3, CD11b/CD18), p150,95 (αXβ2, CR4, CD11c/CD18), and a newly characterized member, αDβ2(CD11d/CD18).7-9 Interestingly, some chemokines (MCP-1, MIP-1α, MIP-1β, and RANTES) increase surface expression of CD11b/CD18 and CD11c/CD18 on human monocytes.10-12
The best characterized ligands of CD11a/CD18 are intracellular adhesion molecule (ICAM) 1 (CD54), ICAM-2 (CD102), and ICAM-3 (CD50), all of which are members of the immunoglobulin superfamily.13-17CD11b/CD18 also binds to ICAM-1 and other soluble ligands, including fibrinogen, complement fragment iC3b, coagulation factor X, arginine-glycine-aspartic acid sequences, heparin-like glycosaminoglycans, certain forms of collagen, and bacterial lipopolysaccharide (LPS).18-25 The functional role and ligands of CD11c/CD18 have not been well defined but appear to be similar to those of Mac-1, ie, implicated in adhesion of monocytes to endothelium26 and binding to iC3b, fibrinogen, LPS, and type I collagen.27-30
Human CD23, the low-affinity receptor for IgE (FcεRII), is a 45-kd type II membrane glycoprotein expressed on many hematopoietic cell types, including B lymphocytes and monocytes. CD23 is cleaved into biologically active soluble fragments (sCD23), and this results in generation of a relatively stable 25-kd fragment.31 CD23 may be involved in a variety of IgE- or CD21-dependent biologic activities, such as cell-cell adhesion, B-cell survival in germinal centers, histamine release from basophils, and regulation of IgE production.32,33 It was also reported that CD23 is a functional ligand for CD11b and CD11c on human and murine monocytes.34-37
Although β2 integrins promote cellular adhesion in various immune-inflammatory processes, they also function like most transmembrane receptors that are capable of transmitting outside-in signals elicited by ligand binding and resulting in cellular effector responses. In monocytes, triggering of β2 integrins by monoclonal antibodies (mAbs) or sCD23 leads to production of nitric oxide and inflammatory cytokines (TNF-α, IL-1β, and IL-6), induction of procoagulant activity, and up-regulation of cell-surface molecule expression.34,36-40 Such interactions could play a role in chronic inflammatory diseases, including systemic lupus erythematosus, inflammatory bowel disease, Sjögren syndrome, glomerulonephritis, and rheumatoid arthritis, in which increased levels of CD23 have been observed.35 However, little is known about the intracellular events regulating these cell functions. Therefore, we previously analyzed the signaling pathways leading to IL-1β production with β2 integrin engagement on human monocytes.41
Materials and methods
Reagents
RPMI 1640 medium, phosphate-buffered saline (PBS), penicillin, streptomycin, and l-glutamine were supplied by Life Technologies (Paisley, United Kingdom). Low-endotoxin fetal-calf serum (FCS) was from Seromed (Biochrom, Berlin, Germany). The Ficoll-Paque device was from Pharmacia (Uppsala, Sweden). Polymyxin B sulfate, neuraminidase, N-acetyl-leucinyl-leucinyl-norleucinal (ALLN), and all other chemicals were obtained from Sigma (St Louis, MO). The α-phosphorus 32 (32P) uridine triphosphate (1.115 GBq [3000 Ci] /mmol) was from Hartmann Analytic (Braunschweig, Germany). Proteasome inhibitor I (PSI) was obtained from Calbiochem (La Jolla, CA), human recombinant TNF-α from Biogen (Cambridge, MA), and soluble TNF receptor (sTNFR)-p55 from Amgen (Thousand Oaks, CA). Culture media contained less than 0.15 U/mL endotoxin on chromogenic Limulus amebocyte lysate assay.
mAbs and recombinant chimeric proteins
Anti-CD11a were from the Binding Site (Birmingham, United Kingdom; IgG1, clone BU17) and Pharmingen (San Diego, CA; IgG2b, clone G43-25B). Anti-CD11b were from R&D Systems (Minneapolis, MN; IgG1, clone 44) and Serotec (Oxford, United Kingdom; IgG1, clone ICRF44). Anti-CD11c were from the Binding Site (IgG1; clone BU15) and R&D Systems (IgG1, clone 3.9). The isotype mAb controls were from Pharmingen.
Human recombinant fusion proteins for sCD23 were the kind gift of Dr M. Bird (Glaxo Wellcome, Stevenage, United Kingdom). The human fusion protein ZZ-CD23 consists of the lectin domain of human CD23 linked to the protein A IgG binding domain (ZZ) and is produced in insect cells as described previously.44 ZZ-CD23 can form oligomers in solution. For these studies, we used polymeric ZZ-CD23 purified by gel filtration as described previously for mouse ZZ-CD23.36 ZZ–P selectin and ZZ–E selectin fusion proteins were used as negative controls in all experiments. MBP-CD23 chimeric protein consists of maltose binding protein fused to the C-terminal 25-kd form of human CD23. It was expressed in soluble form in Escherichia coli, purified by affinity chromatography on amylose resin, and processed to remove endotoxin by repeated passage through Detoxi-gel (Pierce, Rockford, IL).
Isolation of human monocytes
Monocytes from fresh peripheral blood of healthy volunteers were prepared as described previously.45 Briefly, peripheral blood mononuclear cells (PBMC) isolated by using a Ficoll density gradient were incubated at a concentration of 50 × 106cells/mL in RPMI 1640 medium containing 10% heat-inactivated FCS for 40 minutes at 4°C, with rotation leading to monocyte aggregation. This was followed by 10 minutes of incubation on ice. Pellets of aggregated enriched monocytes were separated from nonaggregated PBMC by a gradient using FCS. Enriched monocyte preparations were further depleted of T cells and natural killer cells by rosetting with neuraminidase-treated sheep red blood cells. Final monocyte preparations routinely contained more than 90% CD14+cells, less than 1% CD3+ cells, and less than 1% CD19+ cells. Cellular viability was greater than 90% on trypan blue exclusion. Polymyxin B (1 μg/mL) was present throughout the isolation procedure and during activation experiments to rule out contamination by low levels of endotoxin.46 Furthermore, to prevent activation on adhesion, monocytes were cultured and stimulated in polypropylene tubes unless indicated otherwise.
Monocyte activation and measurement of MIP-1α and MIP-1β
Freshly isolated monocytes were cultured in flat-bottomed, 96-well tissue-culture trays (Corning Costar, Kennebunk, ME) at a concentration of 50 × 103 cells/well in complete RPMI medium in the presence of polymyxin B. Monocytes were cultured for various times in the presence of anti–β2 integrin mAbs or recombinant sCD23 fusion proteins. Culture supernatants were then tested for production of MIP-1α and MIP-1β by enzyme-linked immunosorbent assays (ELISA; R&D Systems, Abingdon, United Kingdom). The limits of detection were 10 pg/mL and 4 pg/mL, respectively.
RNA extraction and RNase protection assays
Human monocytes (5-10 × 106 cells) were starved for 14 hours in RPMI 1640 medium supplemented with 1% FCS in polypropylene tubes (Falcon; Becton Dickinson, Heidelberg, Germany). Cells were harvested, resuspended in 500 μL RPMI and HEPES containing 1% FCS, and incubated in 2-mL tubes (Eppendorf, Germany) at 37°C with or without effectors. Total RNA was isolated by lysing the cells with Trizol reagent (Life Technologies) according to the manufacturer's instructions and analyzed for the level of expression of MIP-1α, MIP-1β, IL-8, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) messenger RNA (mRNAs) by RiboQuant RNase protection assay (RPA) using the hck-5 multiprobe template set from Pharmingen. Briefly, riboprobes were labeled with 32P and hybridized overnight in solution with 1 to 2 μg RNA. The hybridized RNA was digested with RNase, and the remaining “RNase-protected” probes were purified, resolved on denaturating polyacrylamide gels, and imaged autoradiographically according to the RiboQuant protocol.
Western blot analysis
Nonadherent monocytes were starved for 14 hours in RPMI 1640 medium supplemented with 1% FCS in polypropylene tubes, and 7 × 106 cells were stimulated for various times in 2-mL polypropylene tubes at 37°C with or without effectors. After incubation, monocytes were washed twice with 1 mL ice-cold PBS and lysed in buffer A, which consisted of 50 mM HEPES (pH 7.5), 150 mM sodium chloride (NaCl), 0.8 mM magnesium chloride, 5 mM ethylene glycol tetraacetic acid (EGTA), 1% Nonidet P-40 (NP-40), 1 mM phenylmethyl sulfonyl fluoride (PMSF), 15 μg/mL leupeptin, 1 μM pepstatin, 1 mM sodium fluorescein (NaF), and 1 mM sodium orthovanadate (Na3VO4). The crude lysates were centrifuged at 15 000g for 20 minutes at 4°C, and protein concentrations in the supernatants were determined by using Bradford reagent (Bio-Rad, Hercules, CA). For Western blot analysis, total cell lysates (50 μg proteins) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Hybond electrogenerated chemiluminescence (ECL) membrane (Amersham, United Kingdom). The blots were probed with anti–IκB-α polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Secondary horseradish peroxidase–conjugated goat antirabbit antibody was supplied by Dako (Copenhagen, Denmark). Antibody-bound proteins were detected with the Amersham ECL system. Autoradiographic results were quantified by densitometric scanning on a laser densitometer equipped with ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Preparation of nuclear extracts and electrophoretic mobility shift assays
Nuclear extracts were prepared as described by Schreiber et al.47 Briefly, monocytes (10-15 × 106cells) were lysed in 200 μL buffer A (10 mM HEPES [pH 7.9], 10 mM potassium chloride [KCl], 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], 1 mM PMSF, 15 μg/mL leupeptin, 1 μM pepstatin, 1 mM NaF, and 1 mM Na3VO4) containing 0.6% NP-40. The lysates were centrifuged and nuclear proteins were extracted from pellets in buffer B (20 mM HEPES [pH 7.9], 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, and 1 mM DTT). The specific κB DNA probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was from Santa Cruz Biotechnology. Binding reactions were done with 5 μg nuclear proteins incubated for 25 minutes at 25°C with the radiolabeled κB probe (25 000 cpm) in 20 μL binding buffer (10 mM Tris [pH 7.5], 60 mM KCl, 1 mM EDTA, 10% glycerol, 1 mM DTT, and 1 mg/mL bovine serum albumin) in the presence of 100 ng poly dI-dC and 1 μg sonicated salmon-sperm DNA. DNA-protein complexes were separated on a 6% nondenaturing polyacrylamide gel in 0.5 × Tris-borate EDTA. When indicated, an excess of cold competitor oligonucleotides (κB or the unspecific nuclear factor [NF] 1 consensus binding site 5′-TTTTGGATTGAAGCCAATATGATAA-3′) was preincubated for 15 minutes with nuclear extracts.
Results
Anti-CD11b and anti-CD11c mAbs and sCD23 fusion proteins are potent inducers of chemokine mRNA expression in human monocytes
We and others34,36,41,48 previously established that ligation of β2 integrins at the surface of human monocytes mediates outside-in signaling leading to synthesis and secretion of inflammatory cytokines, notably IL-1β. Because monocytes are also an important source of chemokine production,49 we here investigated the effects of β2 integrin engagement on modulation of chemokine expression.
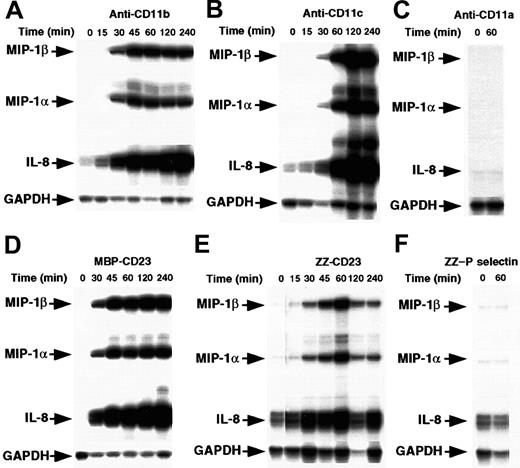
Enriched human monocytes (85%-90% CD14+ cells) were starved for 14 hours in medium supplemented with 1% FCS and then cultured under nonadherent conditions in polypropylene tubes for 15 minutes to 4 hours in the presence of either mAbs raised against CD11a, CD11b, or CD11c β2 integrin α chains or sCD23 chimeric proteins. The concentrations used were those previously found to induce IL-1β production.41 Cells were then analyzed for chemokine mRNA expression by RPA (Figure1). We found that incubation of monocytes with anti-CD11b or anti-CD11c mAbs led rapidly to a marked induction of MIP-1α, MIP-1β, and IL-8 mRNAs. These were detected after 30 minutes and maintained after 4 hours of activation. Similarly, stimulation of monocytes by 2 distinct sCD23 fusion proteins, MBP-CD23 and ZZ-CD23, through CD11b and CD11c counterreceptors, resulted in a potent increase in steady-state levels of MIP-1α, MIP-1β, and IL-8 mRNAs. Interestingly, we did not observe induction of mRNAs of MCP-1 or interferon-γ–inducible protein 10 (IP-10), 2 chemokines produced by monocytes under different activation conditions (data not shown). Furthermore, anti-CD11a mAbs and ZZ–P selectin chimeric protein had no effect on chemokine mRNA levels (Figure 1C and 1F), as was already reported for IL-1β synthesis.41
Time course of MIP-1α and MIP-1β mRNA induction by β2 integrin engagement.
Nonadherent human monocytes (7 × 106 cells) were left untreated or stimulated for various times with the following effectors: anti-CD11b (ICRF44, 2 μg/mL; A) anti-CD11c (BU15, 2 μg/mL; B), anti-CD11a (BU17, 2 μg/mL; C) MBP-CD23 (1 μg/mL; D) ZZ-CD23 (1 μg/mL; E), and ZZ–P selectin (5 μg/mL; F). Cells were then harvested and total RNA was isolated and analyzed by RPA for expression of MIP-1α, MIP-1β, IL-8, and GAPDH mRNAs. Results are representative of 2 distinct experiments.
Time course of MIP-1α and MIP-1β mRNA induction by β2 integrin engagement.
Nonadherent human monocytes (7 × 106 cells) were left untreated or stimulated for various times with the following effectors: anti-CD11b (ICRF44, 2 μg/mL; A) anti-CD11c (BU15, 2 μg/mL; B), anti-CD11a (BU17, 2 μg/mL; C) MBP-CD23 (1 μg/mL; D) ZZ-CD23 (1 μg/mL; E), and ZZ–P selectin (5 μg/mL; F). Cells were then harvested and total RNA was isolated and analyzed by RPA for expression of MIP-1α, MIP-1β, IL-8, and GAPDH mRNAs. Results are representative of 2 distinct experiments.
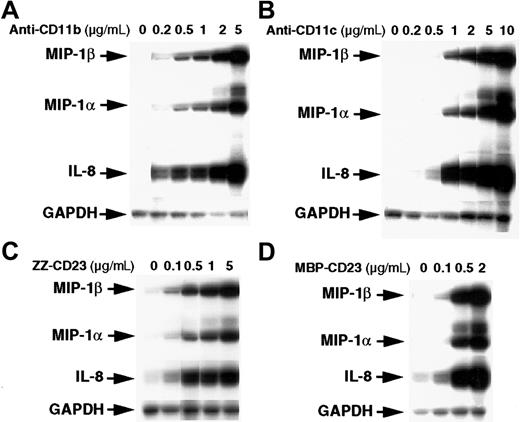
The stimulatory effect of β2 integrin triggering on chemokine gene expression was dose dependent (Figure2). Activation of monocytes by anti-CD11b or anti-CD11c mAbs stimulated MIP-1α, MIP-1β, and IL-8 mRNAs at concentrations as low as 0.2 to 0.5 μg/mL and reached maximal levels at 5 to 10 μg/mL mAbs. Similarly, chemokine mRNAs were induced at a concentration of 0.1 μg/mL ZZ-CD23 or MBP-CD23 fusion proteins and maximal activation was reached with 2 to 5 μg/mL. These results constitute the first evidence that engagement of CD11b and CD11c is a potent and selective inducer of MIP-1α, MIP-1β, and IL-8 mRNA expression in human monocytes.
Dose-dependent stimulatory effect of anti-CD11b and anti-CD11c mAbs and sCD23 chimeric proteins on the steady-state level of MIP-1α and MIP-1β mRNAs.
Monocytes (7 × 106 cells) were left untreated or incubated for 1 hour with various concentrations of the following effectors: anti-CD11b (ICRF44; A), anti-CD11c (BU15; B), ZZ-CD23 (C), and MBP-CD23 (D). RNA was isolated and analyzed for expression of MIP-1α, MIP-1β, IL-8, and GAPDH mRNAs. Results are representative of 2 distinct experiments.
Dose-dependent stimulatory effect of anti-CD11b and anti-CD11c mAbs and sCD23 chimeric proteins on the steady-state level of MIP-1α and MIP-1β mRNAs.
Monocytes (7 × 106 cells) were left untreated or incubated for 1 hour with various concentrations of the following effectors: anti-CD11b (ICRF44; A), anti-CD11c (BU15; B), ZZ-CD23 (C), and MBP-CD23 (D). RNA was isolated and analyzed for expression of MIP-1α, MIP-1β, IL-8, and GAPDH mRNAs. Results are representative of 2 distinct experiments.
Ligation of CD11b and CD11c β2 integrins triggers MIP-1α and MIP-1β release on human monocytes
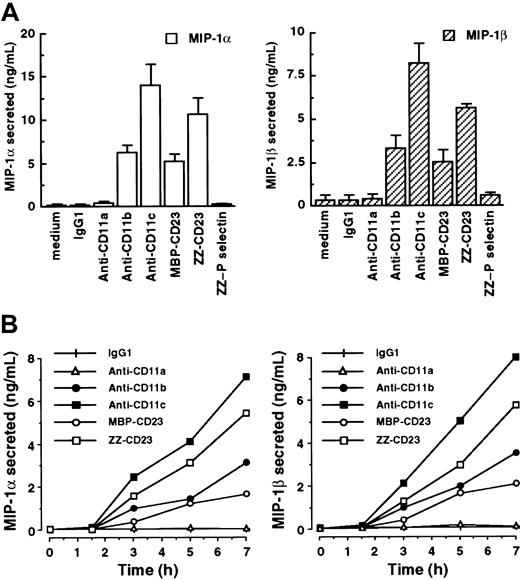
In light of evidence that adhesion-dependent signaling can, under some circumstances, induce mRNAs without synthesis of the corresponding proteins by monocytes,50 we investigated the effects of β2 integrin triggering on MIP-1α and MIP-1β secretion in our system (Figure 3). Freshly isolated human monocytes constitutively produced low levels of or no MIP-1α and MIP-1β. However, after stimulation of monocytes by anti-CD11b mAbs, anti-CD11c mAbs, MBP-CD23, or ZZ-CD23 for 7 hours, release of high amounts of MIP-1α in culture supernatants was observed (6.27 ± 0.76 ng/mL, 14.05 ± 2.31 ng/mL, 5.15 ± 0.82 ng/mL, and 10.66 ± 1.85 ng/mL, respectively; Figure 3A). Under these conditions, MIP-1β secretion was also markedly stimulated (3.32 ± 0.67 ng/mL, 8.22 ± 1.14 ng/mL, 2.55 ± 0.64 ng/mL, and 5.64 ± 0.2 ng/mL, respectively). Conversely, incubation with either IgG1 isotype control mAb, anti-CD11a mAbs, or ZZ–P selectin fusion protein had no effect on MIP-1α and MIP-1β secretion, thereby confirming that the induction was not due to nonspecific activation through monocyte Fc receptors or mediated by the ZZ motif of CD23 fusion proteins. Evaluation of the time course of MIP-1α and MIP-1β secretion with β2 integrin engagement showed that both chemokines were detected as early as 3 hours after stimulation and persisted for more than 24 hours (Figure 3B and data not shown). Furthermore, these data were consistent with the kinetics of induction of MIP-1α and MIP-1β mRNAs (Figure 1).
Engagement of CD11b and CD11c by mAbs or sCD23 fusion proteins rapidly stimulates secretion of MIP-1α and MIP-1β on freshly isolated human monocytes.
(A) Human monocytes (50 × 103 cells/well) were incubated for 7 hours in medium alone or in the presence of the following antibodies or recombinant fusion proteins: isotype control IgG1 (5 μg/mL), anti-CD11a (clone BU17, 5 μg/mL), anti-CD11b (clone ICRF44, 20 μg/mL), anti-CD11c (clone BU15, 5 μg/mL), ZZ-CD23 and MBP-CD23 (0.5 μg/mL), and ZZ–P selectin (5 μg/mL). Secreted MIP-1α and MIP-1β in the culture supernatants were detected by ELISA. Data are mean ± SE values (n = 3). Similar results were obtained with anti-CD11a (clone G43-25B), anti-CD11b (clone 44), and anti-CD11c (clone 3.9) mAbs (data not shown). (B) Monocytes were cultured for various times under the same conditions as the experiment depicted in panel A. Then, MIP-1α and MIP-1β were detected in the culture supernatants by ELISA. Data are mean ± SD values from triplicate performance of one experiment representative of 2 others (for each condition, SD was < 5%).
Engagement of CD11b and CD11c by mAbs or sCD23 fusion proteins rapidly stimulates secretion of MIP-1α and MIP-1β on freshly isolated human monocytes.
(A) Human monocytes (50 × 103 cells/well) were incubated for 7 hours in medium alone or in the presence of the following antibodies or recombinant fusion proteins: isotype control IgG1 (5 μg/mL), anti-CD11a (clone BU17, 5 μg/mL), anti-CD11b (clone ICRF44, 20 μg/mL), anti-CD11c (clone BU15, 5 μg/mL), ZZ-CD23 and MBP-CD23 (0.5 μg/mL), and ZZ–P selectin (5 μg/mL). Secreted MIP-1α and MIP-1β in the culture supernatants were detected by ELISA. Data are mean ± SE values (n = 3). Similar results were obtained with anti-CD11a (clone G43-25B), anti-CD11b (clone 44), and anti-CD11c (clone 3.9) mAbs (data not shown). (B) Monocytes were cultured for various times under the same conditions as the experiment depicted in panel A. Then, MIP-1α and MIP-1β were detected in the culture supernatants by ELISA. Data are mean ± SD values from triplicate performance of one experiment representative of 2 others (for each condition, SD was < 5%).
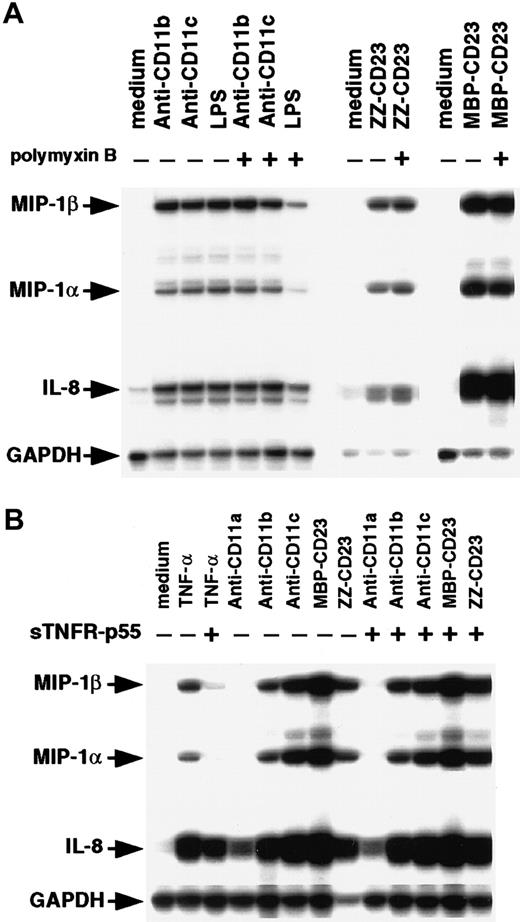
Because monocytes are highly sensitive to endotoxin, we compared the effects of β2 integrin engagement by mAbs or sCD23 fusion proteins on chemokine expression in the presence and absence of polymyxin B (Figure 4A). Polymyxin B (10 μg/mL) had no effect on MIP-1α, MIP-1β, and IL-8 mRNAs induced by anti-CD11b, anti-CD11c, or sCD23 chimeras, but it strongly blocked (70%-80% inhibition) expression of the chemokine transcripts induced by a large amount of LPS (200 ng/mL). Engagement of CD11b and CD11c on monocytes by mAbs or sCD23 was previously found to trigger release of TNF-α.34,37,48 Moreover, TNF-α is known to stimulate MIP-1α synthesis.51 52 To rule out the possibility that chemokine production was induced by β2 integrin triggering resulting from autocrine TNF-α production, we did experiments in the presence and absence of the TNF-α–blocking agent, sTNFR-p55 (Figure 4B). As expected, sTNFR-p55 totally blocked MIP-1α and MIP-1β mRNA expression induced by soluble TNF-α, but it did not affect expression induced by anti-CD11 mAbs or sCD23 fusion proteins. Taken together, these results indicate that CD11b and CD11c β2 integrin ligation is a potent way to activate MIP-1α and MIP-1β expression on human monocytes at both the mRNA and protein level. The findings also show that this chemokine production is not due to either endotoxin contamination or autocrine TNF-α production.
Effect of polymyxin B and sTNFR-p55 on induction of MIP-1α and MIP-1β mRNAs.
(A) Nonadherent human monocytes (7 × 106 cells) were left untreated or stimulated for 1 hour at 37°C with either anti-CD11b (5 μg/mL), anti-CD11c (5 μg/mL), LPS (200 ng/mL), ZZ-CD23 (1 μg/mL), or MBP-CD23 (1 μg/mL) in the presence or absence of polymyxin B (10 μg/mL). (B) Monocytes (7 × 106cells) were left untreated or cultured for 1 hour at 37°C with anti-CD11a (5 μg/mL), anti-CD11b (5 μg/mL), anti-CD11c (5 μg/mL), MBP-CD23 (1 μg/mL), ZZ-CD23 (1 μg/mL), or soluble TNF-α (10 ng/mL) in the presence or absence of sTNFR-p55 (10−8 M). RNA was isolated and analyzed by RPA for expression of MIP-1α, MIP-1β, IL-8, and GAPDH mRNAs. Results are representative of 2 distinct experiments.
Effect of polymyxin B and sTNFR-p55 on induction of MIP-1α and MIP-1β mRNAs.
(A) Nonadherent human monocytes (7 × 106 cells) were left untreated or stimulated for 1 hour at 37°C with either anti-CD11b (5 μg/mL), anti-CD11c (5 μg/mL), LPS (200 ng/mL), ZZ-CD23 (1 μg/mL), or MBP-CD23 (1 μg/mL) in the presence or absence of polymyxin B (10 μg/mL). (B) Monocytes (7 × 106cells) were left untreated or cultured for 1 hour at 37°C with anti-CD11a (5 μg/mL), anti-CD11b (5 μg/mL), anti-CD11c (5 μg/mL), MBP-CD23 (1 μg/mL), ZZ-CD23 (1 μg/mL), or soluble TNF-α (10 ng/mL) in the presence or absence of sTNFR-p55 (10−8 M). RNA was isolated and analyzed by RPA for expression of MIP-1α, MIP-1β, IL-8, and GAPDH mRNAs. Results are representative of 2 distinct experiments.
Activation of NF-κB DNA-binding activity and degradation of IκB-α in monocytes triggered by anti-CD11b and anti-CD11c mAbs or sCD23 chimeras
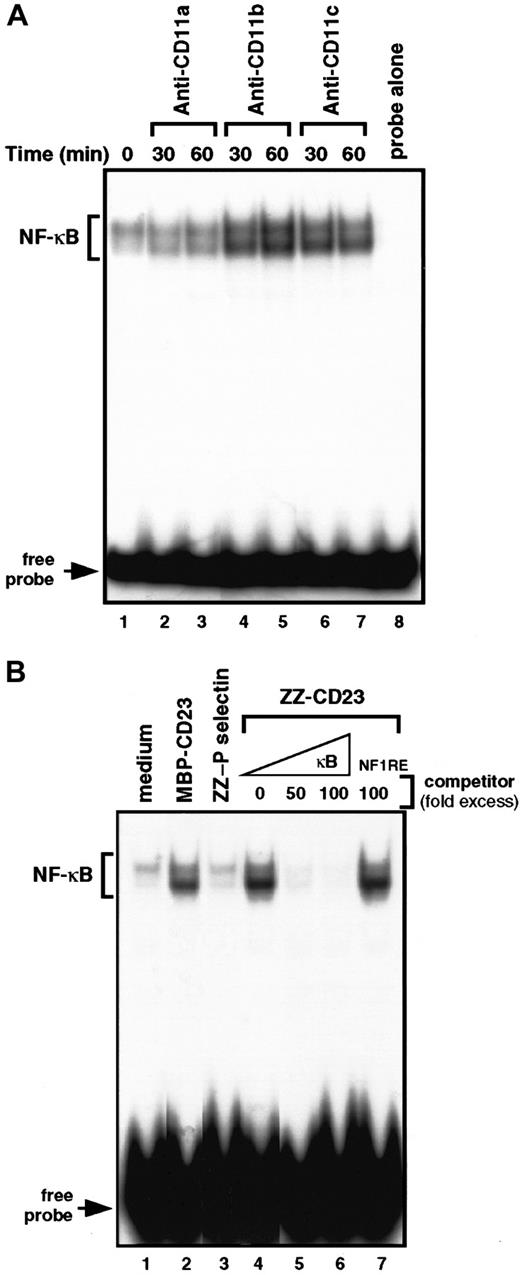
To gain insight into the molecular mechanisms by which β2 integrin engagement elicits induction of chemokines in monocytes, we investigated the NFs that could control MIP-1α and MIP-1β mRNA transcription. MIP-1α and MIP-1β promoters were cloned, characterized, and shown to contain κB response elements.53 Therefore, we concentrated on the DNA-binding activity of NF-κB with β2 integrin triggering on human monocytes (Figure 5). We performed electrophoretic mobility shift assays on nuclear extracts prepared from cells treated with anti-CD11b mAbs, anti-CD11c mAbs, or sCD23 chimeric proteins. Gel-shift studies using a consensus target sequence for NF-κB revealed a constitutive low level of DNA-binding activity in untreated nonadherent monocytes that was not altered during incubation with anti-CD11a mAbs or ZZ–P selectin. Interestingly, triggering of CD11b or CD11c by mAbs or sCD23 fusion proteins resulted in a significant increase in NF-κB DNA-binding activity after 30 to 60 minutes of activation (Figure 5A, lanes 4-7, and Figure 5B, lanes 2 and 4). The specificity of the protein-DNA complexes induced by β2 integrin engagement was confirmed by the finding that incubation with an excess (50-100 fold) of unlabeled κB oligonucleotide prevented ZZ-CD23–induced binding to the32P-labeled κB probe (Figure 5B, lanes 5-6), whereas incubation with an NF-1 oligonucleotide did not (lane 7).
Engagement of β2 integrins at the monocyte cell surface stimulates NF-κB DNA-binding activity.
(A) Nonadherent monocytes were left untreated (lane 1) or stimulated for 30 and 60 minutes with anti-CD11a (BU17, 5 μg/mL; lanes 2 and 3), anti-CD11b (ICRF44, 5 μg/mL; lanes 4 and 5), or anti-CD11c (BU15, 5 μg/mL; lanes 6, 7) mAbs. Nuclear extracts were prepared and NF-κB DNA-binding activity was assessed in bandshift experiments. Lane 8 shows the migration of the radiolabeled κB probe in the absence of nuclear extracts. (B) Nonadherent monocytes were incubated for 30 minutes with medium alone (lane 1), MBP-CD23 (2 μg/mL; lane 2), ZZ–P selectin (5 μg/mL; lane 3), or ZZ-CD23 (5 μg/mL; lane 4). NF-κB DNA-binding activity was measured in 5 μg nuclear extracts in bandshift experiments. The specificity of the DNA-binding complex was analyzed by incubation with a 50- and 100-fold excess of unlabeled κB probe (lanes 5 and 6) or a 100-fold excess of NF-1 oligonucleotide (lane 7) under the conditions depicted in lane 4. Results are the most representative of 4 distinct experiments.
Engagement of β2 integrins at the monocyte cell surface stimulates NF-κB DNA-binding activity.
(A) Nonadherent monocytes were left untreated (lane 1) or stimulated for 30 and 60 minutes with anti-CD11a (BU17, 5 μg/mL; lanes 2 and 3), anti-CD11b (ICRF44, 5 μg/mL; lanes 4 and 5), or anti-CD11c (BU15, 5 μg/mL; lanes 6, 7) mAbs. Nuclear extracts were prepared and NF-κB DNA-binding activity was assessed in bandshift experiments. Lane 8 shows the migration of the radiolabeled κB probe in the absence of nuclear extracts. (B) Nonadherent monocytes were incubated for 30 minutes with medium alone (lane 1), MBP-CD23 (2 μg/mL; lane 2), ZZ–P selectin (5 μg/mL; lane 3), or ZZ-CD23 (5 μg/mL; lane 4). NF-κB DNA-binding activity was measured in 5 μg nuclear extracts in bandshift experiments. The specificity of the DNA-binding complex was analyzed by incubation with a 50- and 100-fold excess of unlabeled κB probe (lanes 5 and 6) or a 100-fold excess of NF-1 oligonucleotide (lane 7) under the conditions depicted in lane 4. Results are the most representative of 4 distinct experiments.
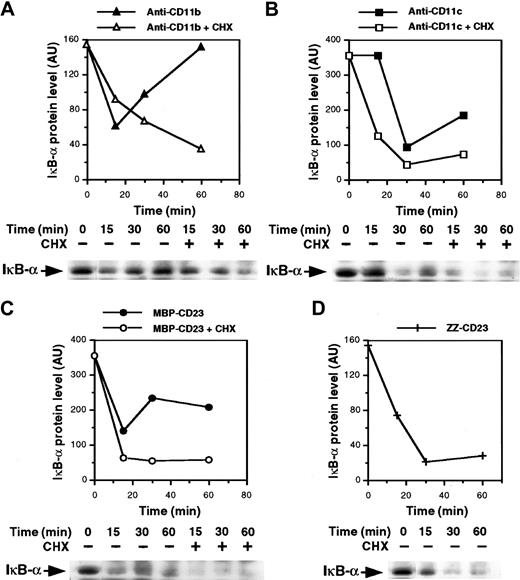
The mechanism underlying NF-κB activation is known. It involves stimulus-induced serine phosphorylation, ubiquitination, and subsequent proteolysis of NF-κB inhibitory proteins (IκB) by the proteasome complex, leading to activation and nuclear translocation of the transcription factor.54-57 Thus, we analyzed expression of IκB-α protein in the cytosol of monocytes after β2integrin ligation. As shown in Figure 6A and 6B, the level of IκB-α decreased rapidly after 15 and 30 minutes of activation with anti-CD11b and anti-CD11c mAbs, respectively, and then increased again, probably because of IκB-α neosynthesis. Similarly, the MBP-CD23 chimera induced a rapid and transient depletion of IκB-α (Figure 6C). Moreover, the IκB-α down-regulation appeared more sustained in cells treated with ZZ-CD23 fusion protein, probably because the concentration used was higher than that of MBP-CD23 (5 μg/mL versus 1 μg/mL). In contrast, IκB-α remained stable after 30 minutes of stimulation with 10 μg/mL of either anti-CD11a mAbs or ZZ–P selectin fusion protein (data not shown). Because NF-κB up-regulates transcription of its own inhibitor,58 we next preincubated monocytes with cycloheximide to prevent new IκB-α synthesis. Under these conditions, depletion of IκB-α induced by anti-CD11b and anti-CD11c mAbs or sCD23 fusion proteins was more pronounced and prolonged.
Ligation of β2 integrins induces IκB-α degradation.
Nonadherent monocytes were preincubated for 30 minutes with or without 10 μg/mL cycloheximide and then stimulated for various times with anti-CD11b mAb (10 μg/mL; A), anti-CD11c mAb (10 μg/mL; B), MBP-CD23 (1 μg/mL; C) or ZZ-CD23 (5 μg/mL; D). Whole-cell extracts were prepared and 50 μg protein was separated on 10% SDS-PAGE and transferred to nitrocellulose membranes. IκB-α proteins were revealed with anti–IκB-α polyclonal serum followed by an ECL reaction. Diagrams represent the densitometric scanning of IκB-α protein levels in each Western blot. Results are representative of 2 experiments.
Ligation of β2 integrins induces IκB-α degradation.
Nonadherent monocytes were preincubated for 30 minutes with or without 10 μg/mL cycloheximide and then stimulated for various times with anti-CD11b mAb (10 μg/mL; A), anti-CD11c mAb (10 μg/mL; B), MBP-CD23 (1 μg/mL; C) or ZZ-CD23 (5 μg/mL; D). Whole-cell extracts were prepared and 50 μg protein was separated on 10% SDS-PAGE and transferred to nitrocellulose membranes. IκB-α proteins were revealed with anti–IκB-α polyclonal serum followed by an ECL reaction. Diagrams represent the densitometric scanning of IκB-α protein levels in each Western blot. Results are representative of 2 experiments.
Proteasome inhibitors prevent NF-κB activation mediated by β2 integrin ligation
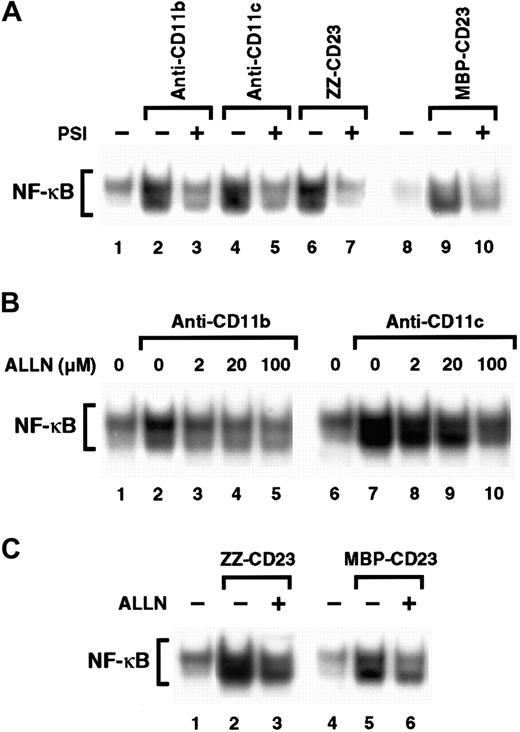
Inhibitors of the proteasome complex block IκB degradation and are therefore potent NF-κB inhibitors.59,60 However, proteolysis-independent pathways for NF-κB activation have also been observed.61 Therefore, we investigated whether 2 proteasome inhibitors, PSI and ALLN, would affect NF-κB activation induced by β2 integrin engagement. We found that preincubation of monocytes with PSI, a specific inhibitor of 20S proteasome chymotrypsin-like activity, abolished NF-κB DNA-binding activity induced by either anti-CD11b and anti-CD11c mAbs or sCD23 fusion proteins (Figure 7A). Similarly, ALLN significantly inhibited NF-κB activation by anti-CD11b and anti-CD11c mAbs in a dose-dependent manner, (Figure 7B), and NF-κB mobilization mediated by sCD23 chimeras was markedly suppressed by the maximal dose of ALLN (100 μM; Figure 7C).
Effect of proteasome inhibitors on NF-κB activation induced by β2 integrin triggering.
(A) Nonadherent monocytes (10-15 × 106 cells) were not preincubated (lanes 1, 2, 4, 6, 8, 9) or were preincubated with 100 μM PSI for 1 hour (lanes 3, 5, 7, and 10) and then stimulated with medium alone (lanes 1 and 8), anti-CD11b mAb (10 μg/mL; lanes 2 and 3), anti-CD11c mAb (10 μg/mL; lanes 4 and 5), ZZ-CD23 (5 μg/mL; lanes 6 and 7), or MBP-CD23 (2 μg/mL; lanes 9 and 10) for 30 minutes. (B) Nonadherent monocytes were pretreated with increasing amounts of ALLN (2, 20, and 100 μM) for 1 hour (lanes 3-5 and 8-10) and then stimulated with mAbs to CD11b (10 μg/mL) or CD11c (10 μg/mL) for 30 minutes. (C) Nonadherent monocytes were incubated for 1 hour without ALLN (lanes 1, 2, 4, and 5) or with 100 μM ALLN (lanes 3 and 6) before stimulation for 30 minutes with ZZ-CD23 (5 μg/mL; lanes 2 and 3), MBP-CD23 (2 μg/mL; lanes 5 and 6), or medium alone (lanes 1 and 4). Nuclear extracts were prepared and analyzed for NF-κB DNA-binding activity in bandshift experiments. Results are representative of 3 similar experiments.
Effect of proteasome inhibitors on NF-κB activation induced by β2 integrin triggering.
(A) Nonadherent monocytes (10-15 × 106 cells) were not preincubated (lanes 1, 2, 4, 6, 8, 9) or were preincubated with 100 μM PSI for 1 hour (lanes 3, 5, 7, and 10) and then stimulated with medium alone (lanes 1 and 8), anti-CD11b mAb (10 μg/mL; lanes 2 and 3), anti-CD11c mAb (10 μg/mL; lanes 4 and 5), ZZ-CD23 (5 μg/mL; lanes 6 and 7), or MBP-CD23 (2 μg/mL; lanes 9 and 10) for 30 minutes. (B) Nonadherent monocytes were pretreated with increasing amounts of ALLN (2, 20, and 100 μM) for 1 hour (lanes 3-5 and 8-10) and then stimulated with mAbs to CD11b (10 μg/mL) or CD11c (10 μg/mL) for 30 minutes. (C) Nonadherent monocytes were incubated for 1 hour without ALLN (lanes 1, 2, 4, and 5) or with 100 μM ALLN (lanes 3 and 6) before stimulation for 30 minutes with ZZ-CD23 (5 μg/mL; lanes 2 and 3), MBP-CD23 (2 μg/mL; lanes 5 and 6), or medium alone (lanes 1 and 4). Nuclear extracts were prepared and analyzed for NF-κB DNA-binding activity in bandshift experiments. Results are representative of 3 similar experiments.
NF-κB activation is a prerequisite for CD11b and CD11c signaling of MIP-1α and MIP-1β expression in monocytes
To determine the role of the NF-κB pathway in controlling MIP-1α and MIP-1β up-regulation induced by CD11b or CD11c triggering, we assessed the effects of proteasome inhibitors on expression of chemokine transcripts (Figure8). Preincubation of monocytes with 40 μM PSI abrogated or strongly decreased expression of MIP-1α and MIP-1β induced by anti-CD11b and anti-CD11c mAbs or sCD23 fusion proteins (Figure 8A, 8C, and 8D). At the highest concentration used (100 μM), ALLN markedly inhibited MIP-1α and MIP-1β gene expression on β2 integrin engagement (Figure 8B, 8C, and8D). Both PSI and ALLN appeared to be more effective in blocking induction of steady-state mRNA levels of MIP-1β than those of MIP-1α. The constitutive level of GAPDH mRNA was not altered under these conditions. Interestingly, IL-8 mRNA was not inhibited by PSI or ALLN; instead, it tended to be potentiated by them. Taken together, these results indicate that activation of NF-κB is mandatory for up-regulation of MIP-1β gene expression and, to a lesser extent, MIP-1α gene expression with CD11b and CD11c β2integrin engagement.
Effect of proteasome inhibitors on steady-state levels of MIP-1α, MIP-1β, and IL-8 mRNAs induced by anti-CD11b/c mAbs or sCD23 fusion proteins.
(A) Nonadherent monocytes (7 × 106 cells) were not preincubated or were preincubated with 40 μM PSI for 1 hour and then stimulated for 30 minutes with medium alone, anti-CD11a (5 μg/mL), anti-CD11b (5 μg/mL), or anti-CD11c (5 μg/mL) mAbs. (B) Cells were pretreated with increasing amounts of ALLN (2, 20, and 100 μM) for 1 hour and then stimulated with anti-CD11b (5 μg/mL) or anti-CD11c (5 μg/mL) mAbs for 30 minutes. (C,D) Monocytes were not preincubated or were preincubated for 1 hour with 40 μM PSI or 100 μM ALLN before stimulation for 30 minutes with ZZ-CD23 (2 μg/mL), MBP-CD23 (1 μg/mL), or medium alone. Total RNAs were isolated and analyzed by RPA. Results are representative of 3 similar experiments.
Effect of proteasome inhibitors on steady-state levels of MIP-1α, MIP-1β, and IL-8 mRNAs induced by anti-CD11b/c mAbs or sCD23 fusion proteins.
(A) Nonadherent monocytes (7 × 106 cells) were not preincubated or were preincubated with 40 μM PSI for 1 hour and then stimulated for 30 minutes with medium alone, anti-CD11a (5 μg/mL), anti-CD11b (5 μg/mL), or anti-CD11c (5 μg/mL) mAbs. (B) Cells were pretreated with increasing amounts of ALLN (2, 20, and 100 μM) for 1 hour and then stimulated with anti-CD11b (5 μg/mL) or anti-CD11c (5 μg/mL) mAbs for 30 minutes. (C,D) Monocytes were not preincubated or were preincubated for 1 hour with 40 μM PSI or 100 μM ALLN before stimulation for 30 minutes with ZZ-CD23 (2 μg/mL), MBP-CD23 (1 μg/mL), or medium alone. Total RNAs were isolated and analyzed by RPA. Results are representative of 3 similar experiments.
Discussion
Numerous proinflammatory and anti-inflammatory genes, as well as genes not known to be related to immunity, are likely to be regulated in response to engagement of integrins. Growing evidence supports the idea that in addition to their crucial function in cell-adhesion reactions during immune-inflammatory mechanisms, β2 integrins also generate outside-in cellular signaling that leads to cell activation. We demonstrated previously that triggering of CD11b or CD11c α chains of β2 integrins by mAbs or chimeric sCD23 on freshly isolated human monocytes rapidly activates signaling pathways of extracellular signal–regulated kinases 1 and 2 and p38–stress-activated protein kinase 2 that cooperate in controlling IL-1β production.41 Our current results provide new evidence that CD11b and CD11c engagement can also have a profound effect on chemokine release and consequently on recruitment of inflammatory cells during initiation of an inflammatory response.
Many studies have established that chemokines not only induce leukocyte migration but that they also facilitate leukocyte adhesion, particularly through their stimulatory effect on cell-surface expression and avidity of both CD11b and CD11c but not CD11a.10-12,62,63 Conversely, interaction of monocytes with endothelial cells, platelets, or fibroblasts was reported to increase production of chemokines, particularly MIP-1α.43 64-67 However, the identity of the adhesion molecules expressed on monocytes and responsible for contact-induced chemokine secretion has not been determined.
In the current study, we found that direct triggering of CD11b and CD11c but not CD11a α chains of β2 integrins on primary human monocytes stimulated synthesis of IL-8, MIP-1α, and MIP-1β. IL-8 production was previously reported to be enhanced through β2 integrin aggregation on human polymorphonuclear neutrophils.68 The current study is the first to show that ligation of CD11b or CD11c by mAbs up-regulates MIP-1α and MIP-1β expression and secretion on primary human monocytes. Our findings also indicate that the stimulation of MIP-1α and MIP-1β production by anti-CD11b and anti-CD11c mAbs is physiologically relevant, since such stimulation can also be produced by the natural ligand of these integrins, sCD23. The induction probably takes place at the transcriptional level according to the rapid time course of induction of MIP-1α and MIP-1β mRNA. Interestingly, the chemokine production induced by CD11b or CD11c ligation was selective for MIP-1α, MIP-1β, and IL-8, since it did not take place on several other CC or CXC chemokines, including MCP-1, RANTES, and IP-10. As in our study of IL-1β production,41 we can here rule out the possibility that endotoxin contamination contributed to activation of expression of the MIPs, since addition of polymyxin B sulfate, which binds to and neutralizes LPS,46 did not reduce the stimulatory activities of anti-CD11b and anti-CD11c mAbs or sCD23 fusion proteins but did markedly block the effect of 200 ng/mL LPS.
Proinflammatory cytokines, particularly IL-1β and TNF-α, are potent inducers of in vitro and in vivo MIP-1α production on several cell types, including peripheral blood monocytes, alveolar macrophages, inflammatory fibroblasts, and osteosarcoma cells.51,52,64,69 Furthermore, we and others34,38,39,41 70-72 found that engagement of β2 integrins by different methods, including binding to specific mAbs, adherence to surface coated with fibrinogen, incubation with sCD23, and direct contact with T cells, plays an important role in the production of IL-1β and TNF-α on monocytes. However, using sTNFR or IL-1Ra (data not shown), we showed that, in our system, expression of MIP-1α, MIP-1β, and IL-8 induced by engagement of CD11b or CD11c was not mediated by prior secretion of TNF-α or IL-1β.
In monocytes, induction of genes expressing inflammatory mediators such as adhesion molecules, cytokines, and chemokines is regulated partly by members of the NF-κB–Rel family of transcription factors. Furthermore, NF-κB was reported to control transcription of MIP-1α and IL-8.53,73 Thus, the aim of our study was to assess the role of the transcription factor NF-κB in the control of MIP-1α, MIP-1β, and IL-8 expression in response to ligation of β2 integrins on primary human monocytes. We demonstrated for the first time that specific engagement by mAbs of CD11b or CD11c but not CD11a on nonadherent human monocytes induced nuclear translocation of NF-κB. These results are in agreement with previous studies showing that fibrinogen activates NF-κB transcription by signaling through CD11b-CD18 in U937 monocytic leukemia cells.74 To our knowledge, our study is the first to show that stimulation of human monocytes by sCD23 leads to activation of NF-κB.
In addition, using the proteasome-specific inhibitors PSI and ALLN to block NF-κB activation, we found new evidence of the importance of this transcription factor in the control of MIP-1β expression and, to a lesser extent, MIP-1α expression induced by ligation of CD11b or CD11c. Interestingly, these NF-κB–blocking agents slightly potentiated IL-8 steady-state mRNA induced by β2 integrin engagement. This finding is in agreement with a previous investigation showing that PSI inhibits LPS-induced IL-8 gene transcription while increasing mRNA stability, thereby producing a slight increase in IL-8 production.75
Chemokines have been found in tissues in several pathologic conditions characterized by distinct leukocytic infiltrates, including rheumatoid arthritis, sepsis, atherosclerosis, asthma, psoriasis, ischemia-reperfusion injury, and many pulmonary disorders.1,49 MIP-1α and MIP-1β are particularly elevated in synovial fluids of patients with rheumatoid arthritis and osteoarthritis, respectively, and may consequently play a role in recruiting monocytes to inflamed joints.76,77 Furthermore, MIP-1α is produced by osteoblasts and stimulates the motility of osteoclasts, which could in turn modulate bone resorption in inflammatory disorders.52,78 On the other hand, high levels of sCD23 have been detected in serum and synovial fluids of patients with rheumatoid arthritis and associated with worsening of the disease.79-81 Synovial macrophages also express high levels of CD23 and its counter-ligands CD11b and CD11c.80 82 These data suggest that an interaction of sCD23 or CD23 expressed on macrophages with CD11b or CD11c could play a major part in production of MIP-1α and MIP-1β in the joint and may therefore constitute an important mechanism sustaining continuous recruitment of inflammatory cells and maintaining chronic inflammation. Thus, further understanding of the molecular mechanisms that control production of chemokines induced by β2 integrin engagement may lay the foundation for new anti-inflammatory treatments.
Two major strategies for inhibiting chemokine activity to limit leukocyte migration in chronic inflammatory diseases have been proposed—one involving the use of antiligand and the other the use of antireceptor.83 An alternative approach, blockage of leukocyte-adhesion mechanisms, is also being tested in humans.84,85 Chemokine activities could also be modulated by interfering in signal-transduction pathways, eg, by blocking NF-κB by adenoviral infection with IκB-α.86-88
In conclusion, this study identified a novel link between CD11b and CD11c β2 integrins and expression of MIP-1α and MIP-1β chemokines in monocytes. The results extend previous findings on the important role of these adhesion molecules in regulating host immune defense mechanisms and inflammatory processes.
We thank Dr M. Bird for kindly providing ZZ-CD23, MBP-CD23, and ZZ-selectin fusion proteins, M.T. Kauffmann for technical assistance, and R. Rehm for critical reading of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Roger Rezzonico, INSERM U364, Faculté de Médecine, Avenue de Valombrose, 06107 Nice Cedex 02, France; e-mail: rezzonic@unice.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal