Abstract
Cyclic neutropenia (CN) is a congenital hematopoietic disorder characterized by remarkably regular oscillations of blood neutrophils from near normal to extremely low levels at 21-day intervals. Recurring episodes of severe neutropenia lead to repetitive and sometimes life-threatening infections. To investigate the cellular mechanism of CN, the ultrastructure and the proliferative and survival characteristics of bone marrow–derived CD34+ early progenitors, CD33+/CD34− myeloid progenitors, and CD15+ neutrophil precursors from CN patients and healthy volunteers were studied. The ultrastructural studies showed profound apoptotic features in bone marrow progenitor cells in CN. Colony-forming assays demonstrated a 75% decrease in the number of early myeloid-committed colonies compared with controls. Long-term culture-initiating cell assays demonstrated a 6-fold increase in production of primitive progenitor cells in CN. To determine whether accelerated apoptosis might account for the underproduction of myeloid progenitors, the hematopoietic subpopulations were labeled with fluorescein isothiocyanate–annexin V and analyzed by flow cytometry. Short-term culture of CN cells resulted in apoptosis of approximately 65% of CD34+ cells, 80% of CD33+/CD34− cells, and more than 70% of CD15+ cells, as compared with 20%, 7%, and 15% apoptosis in respective control subpopulations. Evidence of accelerated apoptosis of bone marrow progenitor cells was observed in all 8 patients participating in the study, regardless of the stage in the CN cycle in which bone marrow aspirations were obtained. Granulocyte colony-stimulating factor therapy of CN patients significantly improved survival of bone marrow progenitor cells. These data indicate that ineffective production of neutrophils is due to accelerated apoptosis of bone marrow myeloid progenitor cells in CN.
Introduction
Severe chronic neutropenia includes a heterogeneous group of hematological diseases characterized by decreases in circulating neutrophils to levels associated with recurrent fevers, malaise, mouth ulcers, gingivitis, and severe infections. Two of these diseases, cyclic neutropenia (CN) and myelokathexis, are inherited as autosomal dominant disorders.1-7 In contrast, severe congenital neutropenia, also called Kostmann's syndrome or congenital agranulocytosis, is an autosomal recessive or, less frequently, an autosomal dominant disease.8 9 In all of these conditions, neutropenia occurs because of impaired formation and reduced delivery of neutrophils from the bone marrow to the peripheral circulation.
In CN, marrow aspirates and biopsies show varying degrees of “maturation arrest” depending on which day in the cycle the marrow aspirate or biopsy is performed.10-12 For example, when patients enter their neutropenic periods, neutrophils are absent from the marrow. Soon thereafter, early neutrophil precursors predominate. When examined during periods in which the peripheral neutrophil count is highest, the marrow may appear virtually normal. Marrow culture studies generally have indicated that precursor cells from most of these patients have the capacity to proliferate and mature to form neutrophils.11-13 Morphological studies have suggested that a cyclic insult to the marrow causes oscillations,14 but the cellular basis for these oscillations of marrow morphology and blood counts is not yet known.
Recent studies have implicated apoptosis, or programmed cell death, as an important regulatory mechanism in normal hematopoiesis and in the myelodysplastic syndrome.15-19 In the present study, we have examined hematopoiesis in 8 patients with CN from 4 unrelated families. In these patients, we observed an increased proliferative capacity of primitive CD34+ progenitor cells, as determined by colony-forming assays and analysis of long-term culture-initiating cells, but an impaired production of bone marrow–derived myeloid-committed progenitors. Moreover, survival of the bone marrow–derived progenitor cells was severely affected in CN. This abnormality was partially corrected by in vivo administration of G-CSF (granulocyte colony-stimulating factor).
Patients, materials, and methods
Patients and controls
After giving informed consent under a protocol approved by the Human Subjects Committee of the University of Washington, 8 patients (3 patients each from 2 families with autosomal dominant CN, and 2 patients with no family history of CN) and 10 healthy volunteers participated in these studies. The patients all had typical 21-day cycles of neutropenia, mouth ulcers, and fever. Of these patients, 6 were studied while not on G-CSF treatment for at least 3 weeks, and 4 patients were studied on G-CSF for at least 5 days. Blood samples were obtained by routine venopuncture, and heparinized marrow aspirates were obtained from the posterior iliac crest under local anesthesia by standard techniques.
Purification of bone marrow progenitor cells
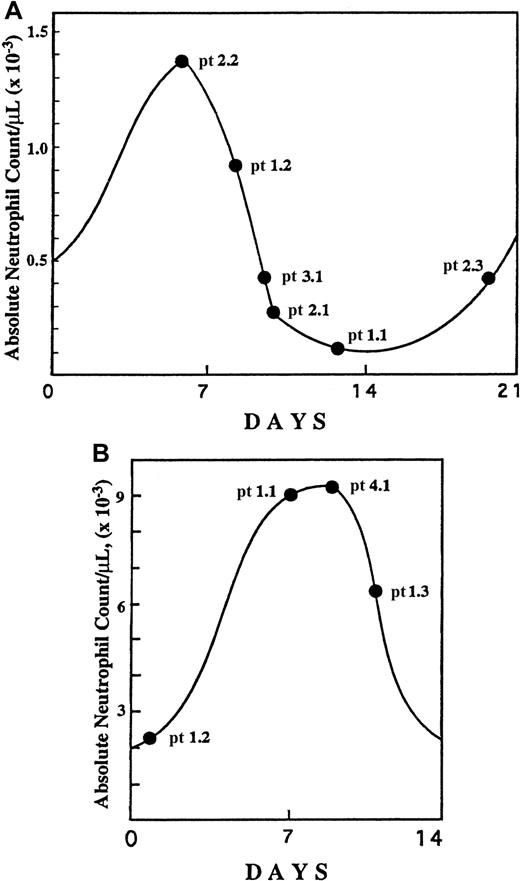
Bone marrow mononuclear cells were isolated by Ficoll-Hypaque gradient centrifugation by means of previously described methods,20 followed by manual cell counts. These cells from patients and healthy donors were then fractionated into CD34+, CD33+/CD34−, CD15+/CD33−/CD34− precursor subpopulations by means of specific monoclonal antibodies and immunomagnetic beads (Miltenyi, Auburn, CA) according to the manufacturer's recommendations. The purity of each bone marrow hematopoietic subpopulation was greater than 96% as tested by fluorescence-activated cell sorter (FACS) analysis. The specific time points of the patients' cycles at which the bone marrow aspirates were obtained from CN patients prior to and during G-CSF treatment are indicated in Figure 1.
Specific time points of the neutropenia cycle at which bone marrow aspirations were obtained from CN patients.
The aspirations were obtained prior to (A) and during (B) G-CSF treatment. The specific cycle day is based upon serial blood counts for each patient before and after the bone marrow aspiration. Numbers designate specific families and patients (eg, pt 1.2 represents family 1, patient 2).
Specific time points of the neutropenia cycle at which bone marrow aspirations were obtained from CN patients.
The aspirations were obtained prior to (A) and during (B) G-CSF treatment. The specific cycle day is based upon serial blood counts for each patient before and after the bone marrow aspiration. Numbers designate specific families and patients (eg, pt 1.2 represents family 1, patient 2).
Electron and light microscopy
Bone marrow–purified hematopoietic progenitor cells were fixed in 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer for 2 hours, washed in the same buffer, and post-fixed for 4 hours at room temperature in 2% osmium tetroxide in distilled water to which a few drops of 2% aqueous potassium-ferrocyanate were added. The cells were rinsed in distilled water, then blockstained with 0.5% aqueous uranyl acetate for 20 minutes, and again rinsed in distilled water. Cells were embedded in 2% agar in 0.1 mol/L sodium cacodylate buffer. The tip of the agar blocks containing the cell pellet was cut off, dehydrated in a graded series of ethanol, and embedded in Eponate 12 resin (Ted Pella, Inc, Redding, CA). The resultant samples were directly dehydrated and embedded in resin without agar embedding. Thin sections were cut with a diamond knife on an LKB Nova ultramicrotome (LKB, Bromma, Sweden) and collected on parlodion-coated 200-mesh copper grids (Ted Pella, Inc). The sections were stained with uranyl acetate and lead citrate and examined with a JEOL JEM 100B electron microscope (JEOL Ltd, Tokyo, Japan).
Colony-forming assays
Bone marrow–derived CD34+ cells were plated in soft agar (Difco, Detroit, MI) in Iscove's modified Dulbecco's medium (IMDM) (Gibco BRL, Grand Island, NY) containing 5 × 10−5 mol/L 2β-mercaptoethanol (Sigma, St Louis, MO) and penicillin-streptomycin, supplemented with human recombinant hematopoietic growth factor mix (20 ng/mL stem cell factor [SCF], 50 ng/mL Flt3 ligand, 10 ng/mL interleukin-3, 10 ng/mL G-CSF, and 10 ng/mL granulocyte macrophage colony-stimulating factor [GM-CSF]) in triplicate 1-mL plates at 3 × 103 cells per plate. The plates were incubated at 37°C in a humidified atmosphere containing 5% CO2. On day 15, the resultant colonies were evaluated for morphology, density, and number of cells and grouped into colony-forming units–high proliferative potential (CFU-HPPs) (high proliferative potential progenitor cells; more than 1000 cells per colony); early myeloid progenitors colony-forming units–granulocyte macrophage (CFU-GMs) (more than 100 cells per colony); and late myeloid precursor CFU-GM clusters (fewer than 50 cells per colony). The results are presented as the percentage and the number of primitive, early, and late myeloid compartments in the bone marrow, representing the mean number of morphologically distinct colonies in triplicate plates.
Long-term culture initiating cells assay
We plated 5 × 104 bone marrow–derived CD34+ cells from patients with CN and healthy volunteers per well in 1 mL IMDM in the presence of SCF (50 ng/mL), Flt-3 ligand (10 ng/mL), 2β-mercaptoethanol (10−4 mol/L), supplemented with 10% HS-5 human stromal cell–conditioned medium (kind gift of Dr Beverly Torok-Storb, Fred Hutchinson Cancer Center, Seattle, WA). Triplicate plates were maintained at 37°C in a fully humidified atmosphere of 5% CO2 in air, and cultures were fed weekly by replacing 0.5 mL of culture medium per well. At week 5, cells were harvested and plated directly into colony forming assays as described above. For each sample, the total number of secondary colonies was enumerated and used as a measure of the number of long-term culture initiating cells (LTC-ICs) present in the sample.
Apoptosis assays and flow cytometry
Apoptosis of bone marrow cells and peripheral blood neutrophils was assessed by flow cytometry with the use of annexin V binding, which allows detection of phosphatidylserine on the cell surface of apoptotic cells (Apoptosis Detection Kit, R&D Systems, Minneapolis, MN).21-23 Briefly, 5 to 20 × 104 freshly isolated cells or cells after short-term culture (16 hours) in RPMI (BioWhittaker, Walkersville, MD) in the presence of 10% autologous serum at 37°C in CO2 incubator were labeled with fluorescein isothiocyanate (FITC)/annexin V for 20 minutes at room temperature, washed twice, and analyzed by flow cytometry by means of CellQuest Analysis software (Becton Dickinson, Mountain View, CA). In some experiments bone marrow–derived CD33+/CD34− and CD15+ cells from patients or healthy volunteers were incubated overnight in the presence of 10% serum from healthy volunteers or CN patients, respectively, and analyzed by flow cytometry. A minimum of 10 000 events were counted per sample. Results are reported as a percentage of annexin V–positive cells.
Statistical analysis
For statistical analysis, the standard analysis of variance test (GraphPadPrism2.01, GraphPad Software Inc, San Diego, CA) was used. Statistical significance was defined as P < .05.
Results
Ultrastructural morphology of bone marrow progenitor cells in CN
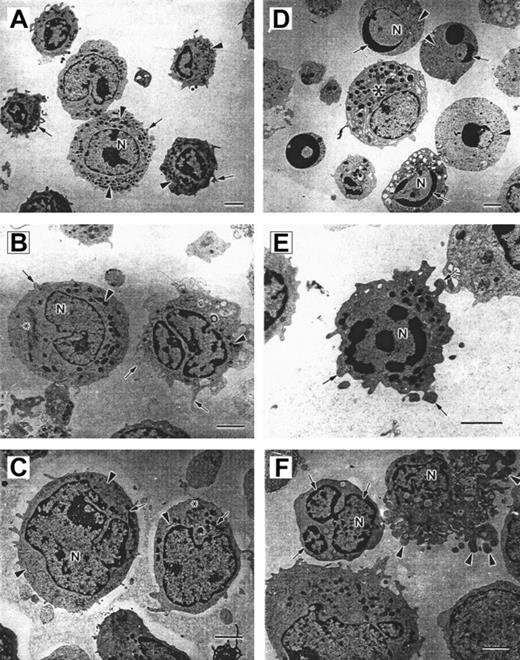
Bone marrow–derived CD34+ early progenitor, CD33+/CD34− myeloid progenitor, and CD15+ neutrophil precursor subpopulations from 3 CN patients prior to G-CSF treatment and 2 healthy volunteers were analyzed by means of electron microscopy. Representative morphology for each of these cell subpopulations is presented in Figure2. Panels A, B, and C represent bone marrow–derived CD15+, CD33+/CD34−, and CD34+ progenitor cells from healthy donors, and panels D, E, and F depict corresponding cells from patients with CN.
Micrographs of bone marrow–derived CD15+, CD33+, and CD34+ cells.
Panels A-C show cells from a healthy volunteer, and panels D-F show cells from a patient with CN. (A) Control CD15 cells. The cell surface forms few short irregular microvilli (arrows). The nucleus (N) is multilobed with irregular patches of granular heterochromatin. Electron-dense granules, mitochondria, and endoplasmic reticulum are distributed throughout the cytoplasm (arrowheads). Scale bar = 2 μm. (B) Control CD33+ cells. The cell on the right has numerous microvilli on the surface (arrows). The polymorphous nucleus (N) contains patchy granular heterochromatin. Mitochondria (arrowheads) and a Golgi complex (*) can be observed in the cytoplasm. Scale bar = 2 μm. (C) Control CD34+ cells. The cell surface forms few microvilli (*). The cells contain an irregular shaped nucleus (N) with patchy granular heterochromatin, numerous mitochondria (arrows), endoplasmic reticulum (arrowheads), and Golgi fields (*). Scale bar = 2 μm. (D) Several apoptotic cells are shown surrounding a normal cell (*). The apoptotic cells are rounded and show no microvilli on the surface. Rounded nuclei (N) contain condensed heterochromatin in distinctly circumscribed, granular masses (arrows) along the inner surface of the nuclear envelope. The cytoplasm shows few, sometimes swollen cell organelles (arrowheads) and few or no electron-dense granules. Scale bar = 2 μm. (E) CD33 cells from CN patient with irregular shape and extensive membrane blebbing on the cell surface (arrows). The nucleus (N) contains distinct patches of heterochromatin. Mitochondria and a slightly swollen endoplasmic reticulum are distributed in the cytoplasm. Scale bar = 2 μm. (F) CD34 cells from CN patients demonstrate nuclear fragmentation (arrows) and severe plasma membrane blebbing and cellular fragmentation (arrowheads). N indicates nucleus. Scale bar = 2 μm.
Micrographs of bone marrow–derived CD15+, CD33+, and CD34+ cells.
Panels A-C show cells from a healthy volunteer, and panels D-F show cells from a patient with CN. (A) Control CD15 cells. The cell surface forms few short irregular microvilli (arrows). The nucleus (N) is multilobed with irregular patches of granular heterochromatin. Electron-dense granules, mitochondria, and endoplasmic reticulum are distributed throughout the cytoplasm (arrowheads). Scale bar = 2 μm. (B) Control CD33+ cells. The cell on the right has numerous microvilli on the surface (arrows). The polymorphous nucleus (N) contains patchy granular heterochromatin. Mitochondria (arrowheads) and a Golgi complex (*) can be observed in the cytoplasm. Scale bar = 2 μm. (C) Control CD34+ cells. The cell surface forms few microvilli (*). The cells contain an irregular shaped nucleus (N) with patchy granular heterochromatin, numerous mitochondria (arrows), endoplasmic reticulum (arrowheads), and Golgi fields (*). Scale bar = 2 μm. (D) Several apoptotic cells are shown surrounding a normal cell (*). The apoptotic cells are rounded and show no microvilli on the surface. Rounded nuclei (N) contain condensed heterochromatin in distinctly circumscribed, granular masses (arrows) along the inner surface of the nuclear envelope. The cytoplasm shows few, sometimes swollen cell organelles (arrowheads) and few or no electron-dense granules. Scale bar = 2 μm. (E) CD33 cells from CN patient with irregular shape and extensive membrane blebbing on the cell surface (arrows). The nucleus (N) contains distinct patches of heterochromatin. Mitochondria and a slightly swollen endoplasmic reticulum are distributed in the cytoplasm. Scale bar = 2 μm. (F) CD34 cells from CN patients demonstrate nuclear fragmentation (arrows) and severe plasma membrane blebbing and cellular fragmentation (arrowheads). N indicates nucleus. Scale bar = 2 μm.
The hematopoietic cell subpopulations from healthy individuals exhibit normal cellular morphology. The CD15+, CD33+/CD34−, and CD34+ cells from healthy volunteers are irregularly shaped with short microvilli on the surface (Figure 2A-C). The nucleus is polymorphous with granular heterochromatin distributed throughout the nucleoplasm forming irregular patches. Mitochondria are electron-dense with well-organized cristae, and the rough endoplasmic reticulum forms typical regular narrow channels.
Bone marrow–derived CD15+, CD33+/CD34−, and CD34+ cells from patients with CN exhibit profound apoptotic features (Figure 2D-F). These cells are diminished in size and have a more rounded shape, with few short or no microvilli on the cell surface. The cell membrane forms bulbous extensions (membrane blebbing), which are characteristic of cells undergoing apoptotic cell death (Figure 2E). The nuclei are convoluted and can be fragmented into smaller rounded pieces (Figure2D,F). The heterochromatin is concentrated in very distinct, electron-dense patches that are located on the inner surface of the nuclear envelope (Figure 2D-E). In later stages of apoptosis, the cell shape becomes more irregular, with increased membrane blebbing and ultimately cell fragmentation (Figure 2F). Cell organelles are observed in different stages of deterioration. The mitochondria appear less electron-dense and slightly swollen (Figure 2F). The rough endoplasmic reticulum is abundant, and the cisternae appear expanded. These features are characteristic of early stages of apoptotic cell death.
Characteristics of bone marrow progenitor cells in CN
Recovery of bone marrow–derived CD34+ cells.
Table 1 represents the number of CD34+ cells recovered per 108 bone marrow mononuclear cells from healthy volunteers and CN patients prior to and during G-CSF treatment. The mean number of CD34+ cells from the controls (2.3 ± 1.3 × 106, n = 7) was not significantly different from that of the CN patients not on G-CSF treatment (1.9 ± 0.4 × 106, n = 7,P > .08) or CN patients on G-CSF treatment (2.2 ± 0.6 × 106, n = 4,P > .17).
Colony-forming assays.
To determine the relative distribution of bone marrow progenitor cells in CN patients, we examined the proliferative potential of bone marrow–derived CD34+ cells from 6 untreated patients from 3 families and 10 healthy volunteers. The number of high proliferative potential colony forming cells (CFU-HPPs) in CN patients not on G-CSF was significantly increased (P < .02) compared with healthy controls (Table 2). The number of early myeloid-committed progenitor cells (CFU-GM), however, was significantly decreased in CN patients prior to (P < .005) and during G-CSF treatment (P < .05) compared with normal individuals (Table 2). The numbers of more differentiated myeloid progenitors (CFU-GM late) were significantly decreased in CN patients prior to G-CSF treatment compared with healthy volunteers (P < .05). However, upon G-CSF treatment, these numbers were not significantly different from controls (P > .05).
Colony forming assays of human bone marrow–derived CD34+ cells from patients with cyclic neutropenia and healthy volunteers
| . | Controls (n = 10) . | Patients (n = 6) . | Patients on G-CSF (n = 3) . |
|---|---|---|---|
| CFU-HPP | 70 ± 10 | 94 ± 12* | 77 ± 16 |
| CFU-GM early | 44 ± 8 | 10 ± 2* | 28 ± 12* |
| CFU-GM late | 16 ± 2 | 10 ± 2* | 16 ± 6 |
| Ratio:CFU-HPP CFU-GM early | 1.6 | 9.8 | 2.8 |
| Ratio: CFU-HPP CFU-GM late | 4.4 | 9.0 | 4.8 |
| . | Controls (n = 10) . | Patients (n = 6) . | Patients on G-CSF (n = 3) . |
|---|---|---|---|
| CFU-HPP | 70 ± 10 | 94 ± 12* | 77 ± 16 |
| CFU-GM early | 44 ± 8 | 10 ± 2* | 28 ± 12* |
| CFU-GM late | 16 ± 2 | 10 ± 2* | 16 ± 6 |
| Ratio:CFU-HPP CFU-GM early | 1.6 | 9.8 | 2.8 |
| Ratio: CFU-HPP CFU-GM late | 4.4 | 9.0 | 4.8 |
Data represent mean values of triplicate experiments ± SD.
When compared with control group, the statistical difference withP value < .02 was observed.
G-CSF indicates granulocyte colony-stimulating factor; CFU-HPP, high proliferative potential colony forming cell; CFU-GM, granulocyte macrophage colony-forming unit.
LTC-IC assays.
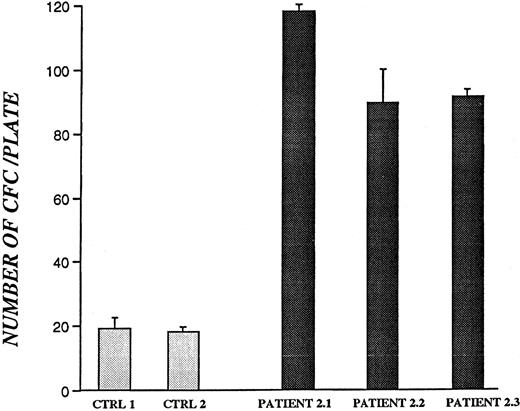
To determine whether bone marrow–derived CD34+ cells from patients with CN retain the ability to survive and produce colony-forming cells, CD34+ cells were maintained in culture for 5 weeks in the presence of HS-5–conditioned medium,24 and secondary CFU assays were performed (Figure3). Colonies were enumerated on day 15 and are presented as the number of colony-forming cells per plate. There was a 4- to 6-fold increase in the production of colony-forming cells from all 3 CN patients examined compared with healthy volunteers (P < .05).
Colony-forming assay of bone marrow–derived CD34+ cells from CN patients and healthy volunteers after 5 weeks in liquid culture.
All samples were from patients and controls not receiving G-CSF. Results for patient 2.3 are based on 30 × 104CD34+ cells plated. For all other patients and controls, 50 × 104 CD34+ cells were used. Data represent mean values of colony numbers ± SD of triplicate plates.
Colony-forming assay of bone marrow–derived CD34+ cells from CN patients and healthy volunteers after 5 weeks in liquid culture.
All samples were from patients and controls not receiving G-CSF. Results for patient 2.3 are based on 30 × 104CD34+ cells plated. For all other patients and controls, 50 × 104 CD34+ cells were used. Data represent mean values of colony numbers ± SD of triplicate plates.
Survival characteristics of peripheral blood neutrophils and bone marrow hematopoietic progenitor cells from patients with CN
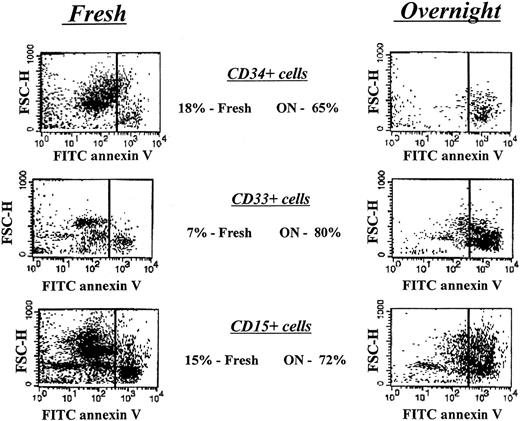
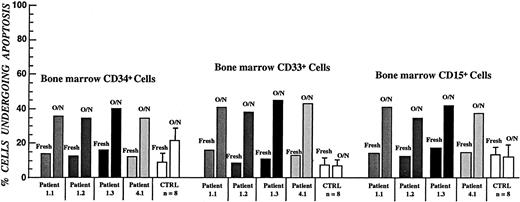
Figure 1 depicts the specific time points of the neutropenia cycle at which bone marrow aspirations from CN patients prior to and during the G-CSF treatment were obtained. The proportions of annexin V–positive cells for freshly isolated CD34+, CD33+/CD34−, and CD15+ bone marrow progenitors in CN were similar to those for the corresponding control cell subpopulations. Short-term (16 hours) culture of these cells in the presence of 10% autologous serum resulted in apoptosis in approximately 65% of CD34+ cells, 80% of CD33+/CD34− cells, and more than 70% of CD15+ cells, as compared with 20%, 7%, and 15% of apoptosis in respective control subpopulations. Representative data from the FACS analysis for one of the CN patients not receiving G-CSF (patient 1.2) are presented in Figure 4. The flow cytometry data for 6 CN patients prior to G-CSF treatment representing 3 different families are summarized in Figure5. These data indicate that the bone marrow progenitor cells undergo accelerated apoptosis in CN regardless of the stage in the neutropenia cycle examined.
Apoptosis analysis of freshly isolated and short-term–cultured bone marrow–derived hematopoietic progenitor cells from a patient with CN prior to G-CSF treatment.
Cells were labeled with FITC-conjugated annexin V and analyzed by flow cytometry as described in “Patients, materials, and methods.” Right panels represent cells undergoing apoptotic cell death.
Apoptosis analysis of freshly isolated and short-term–cultured bone marrow–derived hematopoietic progenitor cells from a patient with CN prior to G-CSF treatment.
Cells were labeled with FITC-conjugated annexin V and analyzed by flow cytometry as described in “Patients, materials, and methods.” Right panels represent cells undergoing apoptotic cell death.
Apoptotic cell death of FITC–annexin V–labeled bone marrow progenitor cells in CN as determined by flow cytometry.
All samples were from patients and controls not receiving G-CSF. Error bars represent SD of apoptosis data within each of the affected families.
Apoptotic cell death of FITC–annexin V–labeled bone marrow progenitor cells in CN as determined by flow cytometry.
All samples were from patients and controls not receiving G-CSF. Error bars represent SD of apoptosis data within each of the affected families.
To determine whether a soluble serum factor might be responsible for accelerated apoptosis, bone marrow–derived CD33+/CD34− and CD15+ cells from healthy volunteers were cultured overnight in the presence of serum from patients with CN. Patients' cells were also incubated in the presence of serum from healthy volunteers. Serum from CN patients failed to accelerate apoptosis in cells from normal volunteers, and cells from CN patients exhibited the same degree of apoptosis when incubated with autologous serum or serum from healthy volunteers (data not shown).
The viability and rate of apoptotic cell death of peripheral blood neutrophils from CN patients and healthy volunteers were also examined. No difference was observed in the survival rate of freshly isolated neutrophils from affected individuals and healthy volunteers. Examination of neutrophils following short-term culture in the presence of 10% autologous serum also revealed no significant difference (data not shown).
Effect of G-CSF on bone marrow precursor cells in CN
Colony-forming assays.
CFU assays of bone marrow–derived CD34+ cells from 3 CN patients during G-CSF treatment demonstrated a significant increase in the number of late CFU-GM clusters compared with patients not on G-CSF (Table 2, P < .05). The number of early CFU-GM colonies increased almost 3-fold compared with the pre–G-CSF state.
Apoptosis.
The rate of spontaneous apoptosis in bone marrow cell subpopulations derived from 4 patients with CN during G-CSF therapy was also examined (Figure 6). The rate of apoptotic cell death in freshly isolated cell subpopulations was not different from controls. Nevertheless, the proportion of annexin V–positive cells after short-term culture was significantly reduced (P < .05) in these patients compared with the pretreatment results for the CD34+, CD33+/CD34−, and CD15+ cell subpopulations (Figure 5), but remained significantly increased compared with the appropriate control subpopulations (Figure6).
Apoptotic cell death of bone marrow–derived hematopoietic progenitor cells from patients with CN during G-CSF treatment.
This was determined by flow cytometry.
Apoptotic cell death of bone marrow–derived hematopoietic progenitor cells from patients with CN during G-CSF treatment.
This was determined by flow cytometry.
Discussion
CN is a rare hematological disorder characterized by oscillatory production of blood cells by the bone marrow with a 21-day periodicity.1-4 In this study, we investigated the cellular defect responsible for this form of ineffective hematopoiesis through investigations of the ultrastructure, proliferative capacity, and survival characteristics of fractionated marrow progenitor cells from 8 patients. We also performed similar studies in 4 of these patients during treatment with G-CSF. Three types of evidence—morphologic, colony-forming assays, and annexin V staining—show that CD34+ cells, CD33+/CD34− cells, and CD15+ cells from CN patients undergo accelerated spontaneous apoptosis compared with normal progenitor cells. These data are consistent with the hypothesis of Mackey25 and Haurie et al26 that the pathophysiology of cyclic hematopoiesis can be attributed primarily to impaired survival of progenitor cells.
Bone marrow–derived cell subpopulations from 3 CN patients examined by electron microscopy exhibited profound degenerative changes. These features are typical of apoptotic cell death and included extended membrane blebbing, granule aggregation, cytoplasmic vacuolization, malfunctioning mitochondria characterized by low electron density, and intensive condensation of heterochromatin in the nucleus. Similar features were not observed in respective control populations. These ultrastructural data are similar to an earlier report on morphological characterization of myeloid precursors in CN.14
Proliferation assays of freshly isolated CD34+ cells revealed a defect in production of late myeloid-committed (CFU-GM), but not early (CFU-HPP) colony-forming cells in CN. Previously, we have reported a similar though less profound decrease in the proportion of myeloid-committed progenitor cells in the bone marrow of patients with myelokathexis, another congenital disorder characterized by severe neutropenia.27 Recently it was reported that bone marrow–derived CD34+ cells from neutropenic patients with Shwachman-Diamond syndrome also exhibited decreased potential to produce colony-forming cells.28
For all of the CN patients, colony-forming assays with freshly isolated CD34+ cells or with CD34+ cells maintained under long-term culture conditions demonstrated that primitive hematopoietic progenitor cells exhibit a normal or enhanced proliferative capacity compared with controls. However, these same assays demonstrated a significant reduction in production of myeloid-committed CFU-GM in CN (Table 2). The ratio of the most immature progenitors to more mature neutrophil precursors was much greater in CN patients. This finding suggests that cell loss may occur during the developmental progression from early to more differentiated progenitors in CN.
To determine which hematopoietic cell subpopulations are affected in CN, apoptosis analysis of bone marrow–derived CD34+, CD33+/CD34−, and CD15+ cell subpopulations from 8 CN patients from 4 unrelated families and 8 healthy volunteers was performed.
Accelerated spontaneous apoptotic cell death in bone marrow–derived myeloid progenitor cells from CN patients was observed in more than 60% of these cells upon short-term culture in the presence of 10% autologous serum (Figures 4 and 5). Peripheral blood neutrophils from CN patients also did not appear to undergo accelerated apoptosis, a finding that is consistent with an earlier report in which labeling of blood neutrophils for in vivo survival studies demonstrated normal survival of mature neutrophils.10 Analysis of apoptosis in control bone marrow cells in the presence of CN patients' serums did not suggest the presence of a soluble pro-apoptotic factor. However, this finding does not exclude the possibility of a pro-apoptotic factor since such a factor could be released by cells undergoing apoptosis and the experimental conditions do not completely mimic the natural state of cells in the bone marrow. Thus, it is yet to be determined whether the poor survival characteristics of patients' progenitor cells is due to an intrinsic or environmental effect in the bone marrow.
Colony-forming assays of bone marrow–derived CD34+ cells from CN patients receiving G-CSF therapy demonstrated significant increase in the production of myeloid CFU-GM in the marrow (Table 2). FACS analysis also demonstrated that G-CSF treatment improved survival characteristics of marrow progenitor cells in CN (Figure 6). Consistent with earlier reports, these data suggest that G-CSF acts as an anti-apoptotic factor leading to an improvement in survival of bone marrow progenitor cells.29-31
For the interpretation of the results, it is important to note that the total number and the range of CD34+ cells recovered per 107 bone marrow mononuclear cells in CN was not significantly different from controls (Table 1). These data indicate that the accelerated apoptosis of bone marrow progenitor cells is a specific characteristic of this disorder and does not depend on the stage of neutropenia cycle when bone marrow aspirations were obtained.
Recently, we have reported that missense, insertion or deletion mutations in neutrophil elastase are involved in pathogenesis of CN.32 Missense or deletion mutations in the gene encoding neutrophil elastase were detected in all patients with CN who participated in this study. We now hypothesize that these mutations lead to accelerated apoptosis of developing neutrophil precursors.33 Impaired survival observed in CN may be due to conformational changes in protein structure that result in aberrant subcellular localization or alterations in substrate specificity and/or binding properties of neutrophil elastase. Further investigations are in progress to characterize the molecular mechanism(s) underlying the pathogenesis of CN.
Acknowledgments
The authors thank Audrey Anna Boyard of the Severe Chronic Neutropenia International Registry, Seattle, WA, for referral of patients with CN.
Supported by National Institutes of Health grant 2RO1-DK-18951 and a grant from Amgen, Inc, Thousand Oaks, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrew A. G. Aprikyan, Department of Medicine, Box 356422, University of Washington, Seattle, WA 98195-6422; e-mail: apri@u.washington.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal