Abstract
With the recent cloning and characterization of thrombopoietin, appreciation of the molecular events surrounding megakaryocyte (MK) development is growing. However, the final stages of platelet formation are less well understood. Platelet production occurs after the formation of MK proplatelet processes. In a study to explore the molecular mechanisms underlying this process, mature MKs isolated from suspension murine bone marrow cell cultures were induced to form proplatelets by exposure to plasma, and the role of various cell-signaling pathways was assessed. The results showed that (1) bis-indolylmaleimide I, which blocks protein kinase C (PKC) activation; (2) down-modulation of conventional or novel classes of PKC by phorbol myristate acetate; and (3) ribozymes specific for PKCα each inhibited proplatelet formation. Inhibition of several MAP kinases, PI3 kinase, or protein kinase A failed to affect MK proplatelet formation. To gain further insights into the function of PKCα in proplatelet formation, its subcellular localization was investigated. In cultures containing active proplatelet formation, cytoplasmic polymerized actin was highly aggregated, its subcellular distribution was reorganized, and PKCα colocalized with the cellular actin aggregates. A number of MK manipulations, including blockade of integrin signaling with a disintegrin or inhibition of actin polymerization with cytochalasin D, interrupted actin reorganization, PKC relocalization, and proplatelet formation. These findings suggest an important role for PKCα in proplatelet development and suggest that it acts by altering actin dynamics in proplatelet-forming MKs. Identification of the upstream and downstream pathways involved in proplatelet formation should provide greater insights into thrombopoiesis, potentially allowing pharmacologic manipulation of the process.
Introduction
Platelet formation represents the terminal stage of megakaryocyte (MK) development. This process, during which a large polyploid cell fragments into thousands of anucleate progeny, is virtually unique in mammalian cell biology. In addition to its intrinsic interest to cell biologists, the generation of platelets holds much medical interest. Several clinical states characterized by inadequate platelet production, such as myelodysplasia, may be due, at least in part, to failure of platelet formation from seemingly normal numbers of MKs. Moreover, although thrombopoietin (TPO), the major regulator of MK development, has been cloned and is undergoing clinical testing, the therapeutic response is slow because of the long time required for MK progenitor cells to mature into platelets. Theoretically, direct stimulation of MK platelet formation would be more rapid. It is clear that a better understanding of the mechanisms of platelet formation may lead to improved therapies for thrombocytopenia or pharmacologic inhibition of this process in the treatment of thrombocytosis.
It has long been observed that fully mature MKs form proplatelets in culture. Proplatelets are long cytoplasmic processes protruding from the MK surface composed of platelet-sized nodes separated by constrictions. The morphology and ultrastructure of in vitro proplatelet formation have been well described.1,2Recently, an excellent real-time morphologic demonstration of this process was captured by videomicroscopy by Italiano et al.3 Fragmentation of proplatelets can give rise to functional platelets in culture.4 Moreover, proplatelets have also been identified and characterized by electron microscopy in bone marrow sections, providing strong evidence that they form in vivo.5-7 In addition, the ability to form proplatelets in culture is correlated with in vivo platelet production in the NF-E2 knockout mouse.8 It is becoming clear that proplatelet formation is a major mechanism of normal platelet formation.
The molecular mechanisms controlling proplatelet formation are unclear. Because of the dramatic morphologic changes that characterize this process, cytoskeletal reorganization has been a focus of much study. Microtubules have been demonstrated in proplatelet processes by both electron and fluorescent microscopy.2,3,9,10 Moreover, pharmacologic disruption of microtubule polymerization markedly inhibits proplatelet formation,11-13 and their stabilization results in abnormal proplatelet processes,13suggesting that microtubule reorganization is also critical for this event. However, although tubulin is critical for the process,3,14 the precise role of actin in proplatelet formation is less clear. In various studies, inhibition of actin polymerization has been shown to enhance,13inhibit,15 or cause abnormal proplatelets3 in different model systems. This discrepancy may be due to species differences or differing concentrations of the actin polymerization inhibitors used in the studies, or actin might perform different functions at different stages of proplatelet formation.
Several studies suggest that proplatelet formation is regulated by extracellular signals. In serum-containing human whole bone marrow cultures, only small numbers of proplatelets are observed. However, if purified MKs are cultured in the absence of serum, many proplatelets are detectable.16 These data suggest that certain cells or serum may exert an inhibitory effect on human proplatelet formation. Thrombin has been shown to be a component in serum that inhibits proplatelet formation in vitro.17,18 In contrast, coating the culture vessels with various extracellular matrices10or adding plasma4,9 stimulates proplatelet formation. The extracellular matrix components that promote proplatelet formation have been identified as vitronectin19,20 and glycosaminoglycan-serglycin21 in different models. A role for these proplatelet promoting factors is particularly attractive because proplatelet formation occurs in the perivascular areas of the marrow, precisely where these substances reside.7 22Despite these morphologic and cell culture clues, however, the specific signal transduction pathways governing proplatelet formation are still poorly defined. In the present series of experiments, we used a murine model of MK proplatelet formation and began to explore the molecular mechanisms of this process.
Materials and methods
Reagents
EMF-10 was a gift of Dr Stefan Niewiarowski (Temple University, Philadelphia, PA). Signaling molecule inhibitors studied included bis-indolylmaleimide I HCl (BIM), phorbol myristate acetate (PMA), PD 98059, Ly 294002, KT 5720, and SB 203580, all purchased from Calbiochem (La Jolla, CA). Matrigel was purchased from Collaborative Biomedical Products (Bedford, MA). All other reagents were obtained from Sigma (St Louis, MO) unless otherwise specified.
Proplatelet assay
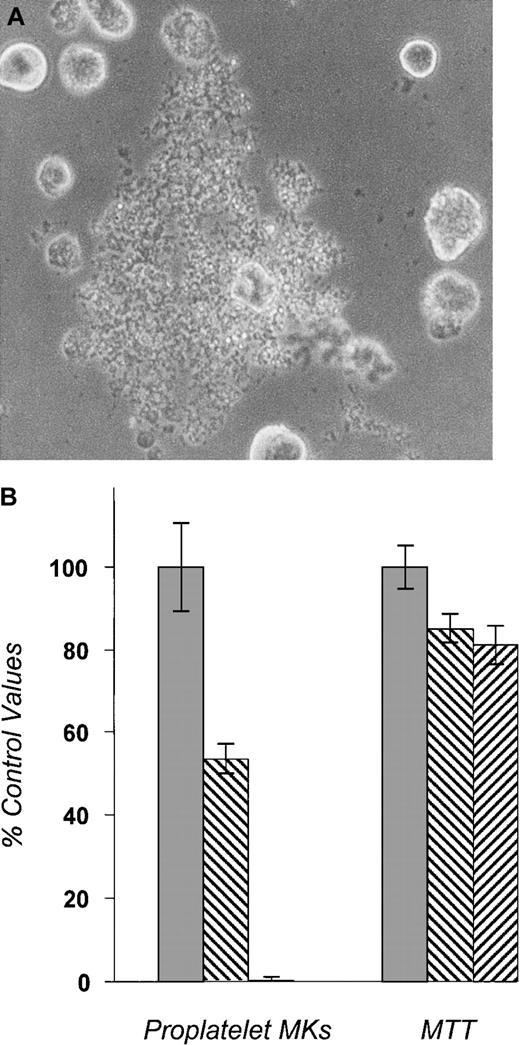
BDF-1 mice (Jackson Laboratories, Bar Harbor, ME) were injected subcutaneously with pure recombinant human TPO for 5 days (2 μg/d; ZymoGenetics Inc, Seattle, WA) to expand MK progenitors and were then anesthetized and killed. Bone marrow cells were flushed from femurs and tibiae and cultured in Iscove's modified Dulbecco's medium (IMDM) supplemented with 1% Nutridoma (Boehringer Mannheim, Indianapolis, IN) with penicillin, streptomycin, and L-glutamine. Cells were incubated (37°C, 5% CO2) with 37.5 ng/mL recombinant murine TPO (ZymoGenetics) for 72 hours. Mature MKs were purified over a discontinuous bovine serum albumin (BSA) density gradient (0%/2%/4%) as described previously.23 Purified MKs were then cultured in IMDM/1% Nutridoma in the presence of 10% human plasma and 50 ng/mL recombinant human TPO with or without pharmacologic inhibitors at indicated concentrations. About 3000 to 5000 cells in 100 μL of medium were plated per well in a 96-well plate. Four to 5 wells per condition were assessed in each experiment. At 48 hours, the time of maximal proplatelet formation, the number of proplatelet-bearing MKs was counted by inverted microscopy (Figure1A). To measure MK cell viability, we added 3,4,5-dimethylthiazole-2-yl-2,5-diphenyl tetrazolium bromide (MTT; Sigma) to each well at 1 mg/mL final concentration and incubated them for 4 to 6 hours. Cells were then lysed overnight, and absorbance at 570 to 630 nm was measured by an enzyme-linked immunosorbent assay plate reader. The MTT reading reflects the total viable MK mass per well and correlated well with trypan blue exclusion as a measure of cell membrane integrity (data not shown). All proplatelet experiments were performed at least 3 times.
Proplatelet assay.
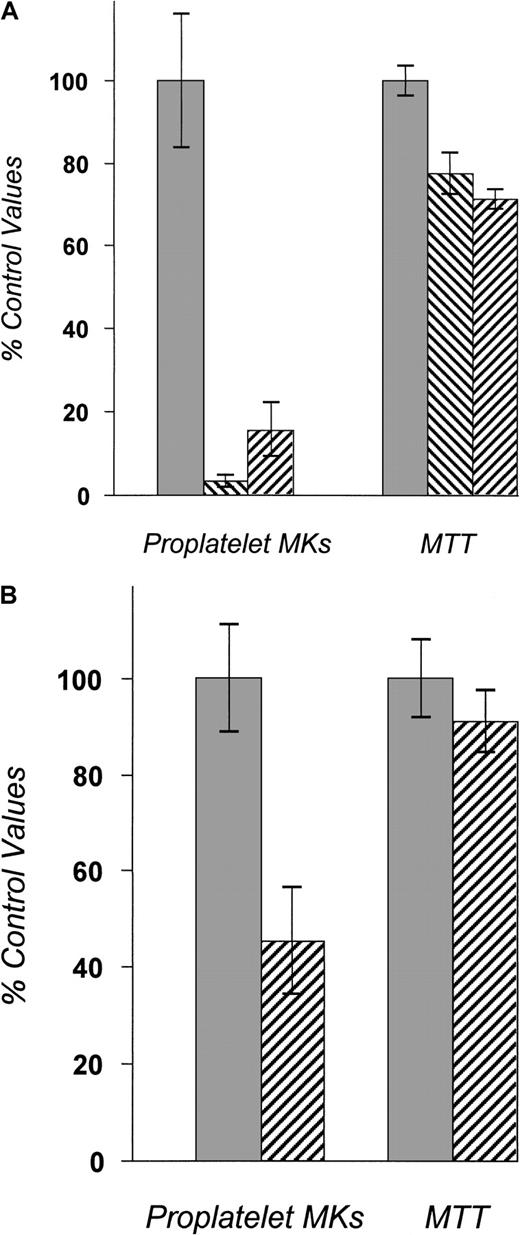
Bone marrow cells from BDF1 mice were cultured in TPO-containing media for 72 hours. Mature megakaryocytes (MKs) were purified by unit gravity sedimentation and then cultured in the presence of 10% human plasma for 48 additional hours with or without pharmacologic inhibitors. All experimental conditions contained 50 ng/mL recombinant human TPO. The number of proplatelet-bearing MKs was then enumerated by visual inspection of undisturbed cells. MTT was then added to the culture to reflect the total number of living cells. The data shown are representative results from 3 separate experiments. In each experiment, proplatelet-bearing MK counts were performed in 4 to 5 separate wells per condition tested. (A) A proplatelet-bearing MK. (B) The effects of PKC inhibitors on proplatelet formation. Bis-indolylmaleimide I (500 nmol/L) and prolonged incubation of PMA (100 ng/mL) decreased the number of proplatelet-forming MKs. The numbers of viable MKs measured by MTT assay (shown in the right set of bars) are only modestly affected. ░, DMSO; ▧, BIM 0.5 μM; ▨, PMA 100 ng/mL.
Proplatelet assay.
Bone marrow cells from BDF1 mice were cultured in TPO-containing media for 72 hours. Mature megakaryocytes (MKs) were purified by unit gravity sedimentation and then cultured in the presence of 10% human plasma for 48 additional hours with or without pharmacologic inhibitors. All experimental conditions contained 50 ng/mL recombinant human TPO. The number of proplatelet-bearing MKs was then enumerated by visual inspection of undisturbed cells. MTT was then added to the culture to reflect the total number of living cells. The data shown are representative results from 3 separate experiments. In each experiment, proplatelet-bearing MK counts were performed in 4 to 5 separate wells per condition tested. (A) A proplatelet-bearing MK. (B) The effects of PKC inhibitors on proplatelet formation. Bis-indolylmaleimide I (500 nmol/L) and prolonged incubation of PMA (100 ng/mL) decreased the number of proplatelet-forming MKs. The numbers of viable MKs measured by MTT assay (shown in the right set of bars) are only modestly affected. ░, DMSO; ▧, BIM 0.5 μM; ▨, PMA 100 ng/mL.
Western blot analysis
To determine the protein kinase C (PKC) isoform expression profile, we harvested primary MKs from a BSA gradient as described earlier. Cells were then lysed and protein concentrations were measured using the protein/DC assay (BioRad, Hercules, CA). The lysates from BaF3 or UT7 cells were used as positive controls. Fifty micrograms of protein per lane was denatured by boiling for 5 minutes in Laemmli's buffer and separated by 7.5% polyacrylamide gel electrophoresis. Proteins were then transferred to nitrocellulose membranes (Schleicher & Schuell, Keane, NH) and blocked for 1 hour with 3% BSA/TBST (10 mM Tris, 150 mM NaCl, 0.05% Tween 20, pH 8.0). Isoform-specific PKC antibodies were purchased from Transduction Labs (San Diego, CA). The membranes were incubated overnight at 4°C with primary antibodies in 3% BSA/TBST at the dilution recommended by the supplier. After 4 5-minute washes in TBST, the blots were gently rocked in 1:3000 goat–antimouse antibody coupled to horseradish peroxidase (BioRad) in 3% BSA/TBST for 1 hour at room temperature. Membranes were then washed 4 times, incubated with enhanced chemiluminescent reagents (NEN Life Science Products, Inc, Boston, MA), and exposed to film.
PKCα ribozyme studies
All PKCα ribozymes (R1, R2, fluorescein isothiocyanate [FITC]-conjugated ribozyme, and a control molecule called RC) were gifts generously provided by Dr Mouldy Sioud (Norwegian Radium Hospital, Oslo, Norway). The binding site for the R1 ribozyme is 5′GGGGGACCAUGGCUGACG3′, and the site for the R2 is 5′GGGGGACCAUGGCUGACGUUU3′. The control ribozyme binding sequence is identical to R1 but in the reverse orientation. The method of liposomal transfection was performed as described.24 The concentrations of ribozyme and DOTAP liposomal transfection reagent (Boehringer Mannheim) were determined from 2 pilot experiments using FITC-conjugated ribozyme. The transfection mixtures were prepared by mixing DOTAP in OptiMEM I (Life Technologies, Rockville, MD) and Ribozyme in OptiMEM I and incubating at room temperature for 30 minutes before adding to cells. The volume of the added ribozyme/DOTAP mixtures was 10 μL per 100 μL of cells in each well. The final concentration of DOTAP was 10 μg/mL and the final concentration of each ribozyme was 2 μmol/L. Under these conditions, about 50% of the cells were FITC positive as observed by fluorescent microscopy at 24 hours after transfection.
Immunofluorescent microscopy
MKs were cultured under the conditions described earlier. After 48 hours in human plasma, cytospins were prepared on a Superfrost/plus microscope slide (Fisher Scientific, Pittsburgh, PA). Cells were fixed for 10 minutes with 10% neutral-buffered formalin (Sigma) and permeabilized for 5 minutes with 0.1% Triton X-100 in phosphate-buffered saline (PBS). The slides were then blocked for at least 1 hour at room temperature by 10% fetal bovine serum (Hyclone, Logan, UT) in PBS. For polymerized actin staining, 1:100 rhodamine-conjugated phalloidin (Molecular Probes, Eugene, OR) in 3% BSA/TBS (0.1 mol/L Tris, pH 7.4, 150 mmol/L NaCl, 0.1% Triton X-100) was added for 1 hour at room temperature. For PKC staining, 1:100 anti-PKC antibody (Transduction Labs) in 3% BSA/TBS was added and incubated at 4°C overnight. Slides were washed with PBS for 10 minutes, 3 times each. FITC-conjugated goat–antimouse antibody (American Qualex, La Mirada, CA) 1:100 in 1% BSA/TBS was then added and incubated at room temperature for 1 hour. Slides were washed 3 times with PBS, mounted with Vectashield mounting medium with DAPI (4′,6-diamidino-2-phenylindole) or Vectashield mounting medium with propidium iodide to stain nuclear DNA (Vector Laboratories, Burlingame, CA), and examined by fluorescent microscopy. For PKC/actin double staining, 1:100 rhodamine-conjugated phalloidin was added concomitantly with the secondary antibody. In some experiments, MKs were fixed in culture with 2% paraformaldehyde for 10 minutes to preserve proplatelet processes. Rhodamine-phalloidin/DAPI staining was then performed on cytospin preparations.
Results
Inhibition of PKC reduces proplatelet formation
To study the molecular basis of the final stages of platelet formation, we developed a murine model of proplatelet formation. In contrast to other animal models that use various extracellular matrix materials as a substratum for proplatelet formation,10,18-20 very few proplatelet-bearing murine MKs were found in tissue culture dishes coated with vitronectin, collagen, or Matrigel. MKs grown in a serum-free medium (IMDM containing 1% Nutridoma) do not produce proplatelets, as opposed to that reported for human MKs.15 However, a number of proplatelet-bearing MKs were seen in cultures containing either serum or plasma. Culture medium with 10% human plasma was found to maximally and reproducibly stimulate proplatelet formation. At 48 hours of culture, about 10% of BSA-gradient–purified mature MKs were actively forming numerous proplatelet processes (Figure 1A), a figure that likely underestimates the true number of MKs that form proplatelets in such cultures because of the rapid elimination of MKs that have completed platelet formation.
To investigate the intracellular molecular pathways responsible for proplatelet formation, we tested various signaling inhibitors. The extracellular signal-regulated protein kinase (ERK)–mitogen-activated protein kinase (MAPK) pathway inhibitor PD 98059 (at 50 μmol/L), the protein kinase A (PKA) inhibitor KT 5720 (at 200 nmol/L), or the p38 MAPK pathway inhibitor SB 203580 (at 20 μmol/L) did not inhibit proplatelet formation or morphology at concentrations we have previously found to affect other aspects of MK development25 (data not shown). At higher doses of these inhibitors, the number of proplatelet-forming MKs was decreased, but the viable cell numbers determined by MTT assay were also markedly affected, suggesting nonspecific toxicity. The phosphoinositide-3-kinase (PI3K) inhibitor Ly 294002 (at 8 μmol/L) also decreased proplatelet-bearing MK numbers and total MK numbers proportionately, reflecting the importance of this pathway on cell survival26 but making it difficult to determine whether this signaling mediator also plays an independent role in proplatelet development. Only PKC inhibitors at the indicated doses could markedly inhibit proplatelet formation without significantly affecting cell survival (Figure 1B). Addition of BIM and incubation of cells with PMA for 48 hours both inhibited PKC activity and expression and decreased the number of MKs bearing proplatelets. PKCα blockade did not simply alter the kinetics of proplatelet formation; serial examination of our cultures failed to reveal any evidence of a delay in proplatelet formation. At doses of greater than or equal to 3 μmol/L, BIM almost completely inhibited proplatelet formation, but also significantly decreased cell survival (data not shown). Although short-term (minutes) incubation with PMA activates some PKC isoforms, after 48 hours several PKC isoforms are down-modulated. We also found that MK PKCα was down-modulated by PMA in our culture conditions (Figure2B). Because of its inhibitory effects without affecting cell viability, PMA was used as a PKC inhibitor in further studies of proplatelet formation.
PKC isoform expression in MKs.
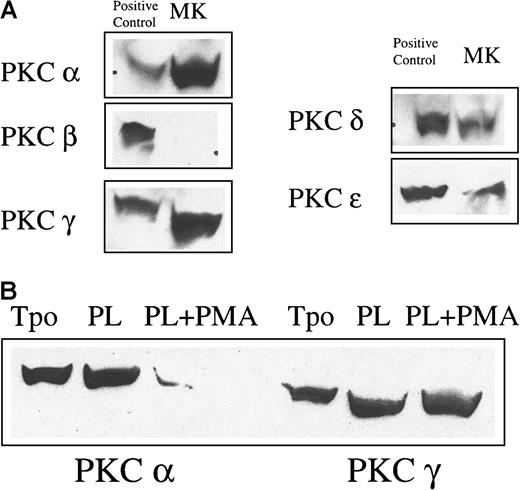
(A) The expression of conventional and novel isoforms of PKC in MKs was tested by Western blot analysis. MKs were purified from cultures by unit gravity sedimentation and lysed. Fifty micrograms of protein was loaded per lane. The positive controls for PKCα and β were UT7 cells and those for PKCγ, δ, and ε were BaF3 cells. (B) PKC isoform downmodulation by PMA in megakaryocytes. Purified MKs were cultured in TPO alone, TPO and plasma, or TPO and plasma and 100 ng/mL PMA for 48 hours. Cells were then lysed and examined for PKC isoform expression by Western blot analysis. Chronic PMA stimulation markedly down-modulated PKCα, but not PKCγ expression.
PKC isoform expression in MKs.
(A) The expression of conventional and novel isoforms of PKC in MKs was tested by Western blot analysis. MKs were purified from cultures by unit gravity sedimentation and lysed. Fifty micrograms of protein was loaded per lane. The positive controls for PKCα and β were UT7 cells and those for PKCγ, δ, and ε were BaF3 cells. (B) PKC isoform downmodulation by PMA in megakaryocytes. Purified MKs were cultured in TPO alone, TPO and plasma, or TPO and plasma and 100 ng/mL PMA for 48 hours. Cells were then lysed and examined for PKC isoform expression by Western blot analysis. Chronic PMA stimulation markedly down-modulated PKCα, but not PKCγ expression.
PKCα is necessary for proplatelet formation
Three classes of PKC isoforms have been described based on their responsiveness to physiologic stimuli. The conventional class, isoforms α, β, and γ, responds to stimulation with both calcium and diacylglycerol (DAG). The novel class, isoforms δ, ε, θ, and η, responds to DAG, but not calcium. Finally, the atypical class, isoforms ζ, λ, and ι, does not respond to either stimulus. Chronic stimulation by PMA, a DAG analog, down-modulates expression of the DAG-responsive conventional and novel isoforms of PKC. Because proplatelet formation is markedly inhibited by this agent, one or more members of the first 2 classes of PKC are likely to play an important role in proplatelet formation. The expression of conventional and novel isoforms of PKC was thus investigated in primary MKs by Western blot analysis. We found that PKC isoforms α, γ, δ, and ε are expressed in murine MKs, but that the β isoform is not (Figure 2A).
To confirm that PMA actually down-modulates PKC isoforms in MKs, we tested the expression of PKCα and PKCγ by Western blot analysis (Figure 2B). MK PKCα expression was markedly decreased by chronic stimulation with PMA. In contrast, the PKCγ isoform was unchanged, suggesting that PKCα may play an important role in proplatelet formation. However, the studies thus far presented all depended on chemical inhibitors of activation or expression. To confirm this hypothesis, we attempted to specifically inhibit PKCα expression with targeted ribozymes. Such reagents have been used successfully both in cell lines and primary CD34+ cells in vitro24and in vivo.27
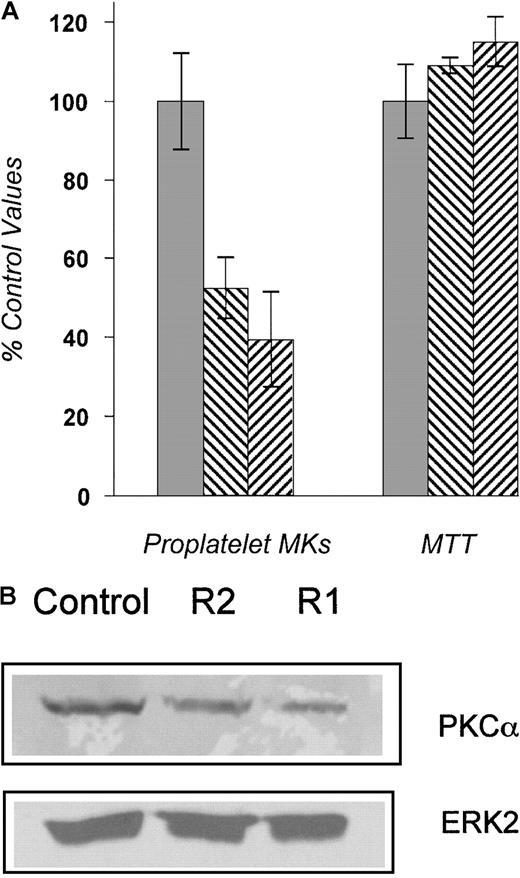
To establish appropriate transfection methods and to quantitate transduction efficiencies, we transfected an FITC-conjugated ribozyme into primary MKs using a liposomal method. The conditions of culture were similar to the proplatelet assay. Twenty-four hours after incubation, cells were examined under fluorescent microscopy to evaluate ribozyme uptake. About 50% of cells were FITC positive. Next, 2 PKCα-specific ribozymes and a control ribozyme were tested; the R1 ribozyme reduced PKCα expression to 51% of control values, and the R2 ribozyme reduced it to 25% of control in a Western blotting experiment (Figure 3B). When applied to cell cultures of purified MKs, R1 and R2 decreased the number of proplatelet-bearing MKs to 38% to 50% of that seen in control ribozyme-containing cultures (Figure 3A). Given that proplatelet formation was lost in about the same proportion of MKs that were successfully transfected by ribozyme, the level to which PKCα was reduced, and the findings of the chemical inhibitor studies, these results suggest that PKCα plays an important role in MK proplatelet formation.
PKCα-specific ribozymes substantially inhibit proplatelet formation.
(A) The proplatelet assay was performed in the presence of ribozymes R1, R2, or the control ribozyme, which is identical to R1 except that the binding site is present in reverse orientation. The numbers of proplatelet-bearing MKs, expressed as a percentage of the control, were significantly decreased in the presence of R1 and R2. No effect on total cell number was found. The experiments were performed 3 times with similar results. ░, control ribozyme; ▧, R1 ribozyme; ▨, R2 ribozyme. (B) Equal numbers of MKs were transfected with ribozymes. PKCα protein expression was then assessed by Western blot analysis 48 hours after transfection and quantified by phosphoimager. The amount of PKCα expression in R1- and R2-transfected MKs was 25% and 51% of the control, respectively (upper panel). The blot was stripped and reprobed with the ERK2 MAPK antibody to ensure an equal number of cells per lane (lower panel).
PKCα-specific ribozymes substantially inhibit proplatelet formation.
(A) The proplatelet assay was performed in the presence of ribozymes R1, R2, or the control ribozyme, which is identical to R1 except that the binding site is present in reverse orientation. The numbers of proplatelet-bearing MKs, expressed as a percentage of the control, were significantly decreased in the presence of R1 and R2. No effect on total cell number was found. The experiments were performed 3 times with similar results. ░, control ribozyme; ▧, R1 ribozyme; ▨, R2 ribozyme. (B) Equal numbers of MKs were transfected with ribozymes. PKCα protein expression was then assessed by Western blot analysis 48 hours after transfection and quantified by phosphoimager. The amount of PKCα expression in R1- and R2-transfected MKs was 25% and 51% of the control, respectively (upper panel). The blot was stripped and reprobed with the ERK2 MAPK antibody to ensure an equal number of cells per lane (lower panel).
Correlation between actin reorganization and proplatelet formation
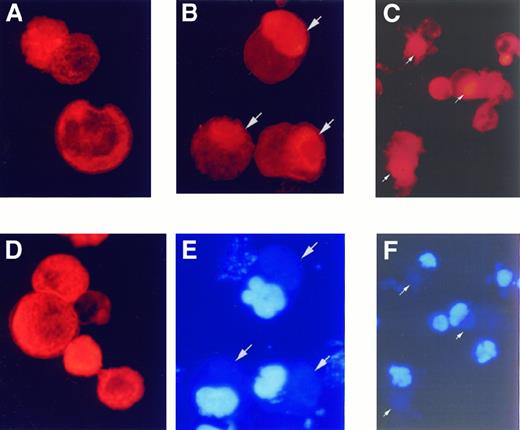
The tremendous morphologic change seen during MK proplatelet formation suggests that a massive cytoskeletal reorganization occurs as part of the process. Actin dynamics during MK proplatelet formation were investigated using rhodamine-conjugated phalloidin to detect alterations in the level of polymerized actin and its subcellular distribution. In plasma-free cultures of murine MKs, low levels of polymerized actin are evenly distributed throughout the cytoplasm (Figure 4A) and there is no proplatelet formation. In contrast, in plasma-containing cultures that promote proplatelet formation, polymerized actin aggregates are found in most cells concentrated in a mass (Figure 4B). The DNA of the same cells was stained with DAPI in Figure 4E, indicating that these actin aggregates are not associated with the nucleus. Down-modulation of PKCs by incubation with PMA for the duration of the culture prevented actin reorganization in the plasma-containing MK cultures (Figure 4D). The result was the same for another PKC inhibitor, BIM, at 3 μmol/L (data not shown). Proplatelet processes could be observed emanating from MKs in another experiment in which the cells were preserved with 2% paraformaldehyde in culture before cytospin preparation. By rhodamine-phalloidin staining, approximately 50% to 60% of cells display actin aggregation and 10% of cells form proplatelets. Polymerized actin aggregation can be seen in the proplatelet-forming MKs (Figure 4C,F). In these cells, the actin aggregates can be seen adjacent to the base of proplatelet formation.
Actin reorganization during proplatelet formation.
The distribution of polymerized MK actin was investigated after cytocentrifugation by staining with rhodamine-conjugated phalloidin (red) and examining by fluorescent microscopy. (A) In the absence of plasma, polymerized actin is evenly distributed throughout the cells, and there is no proplatelet formation as determined from visual inspection of undisturbed cultures before cytocentrifugation. (B) In plasma-containing cultures that promote proplatelet formation, there are F-actin aggregates in the cells (arrows). These aggregates are not nuclei, as demonstrated by DAPI staining of cells in the same field to show nuclear DNA in blue (E). (C) F-actin staining in proplatelet-forming MKs. MKs grown in 10% plasma were fixed in culture with 2% paraformaldehyde to preserve proplatelet processes before cytospin preparation. At 48 hours, F-actin aggregations can be found in proplatelet-forming MKs (arrows). (D) PKC down-modulation by prolonged incubation of PMA prevented actin reorganization in plasma-containing culture, but did not reduce the amount of F-actin. (F) Nuclear DNA staining of cells in panel C was revealed by DAPI staining.
Actin reorganization during proplatelet formation.
The distribution of polymerized MK actin was investigated after cytocentrifugation by staining with rhodamine-conjugated phalloidin (red) and examining by fluorescent microscopy. (A) In the absence of plasma, polymerized actin is evenly distributed throughout the cells, and there is no proplatelet formation as determined from visual inspection of undisturbed cultures before cytocentrifugation. (B) In plasma-containing cultures that promote proplatelet formation, there are F-actin aggregates in the cells (arrows). These aggregates are not nuclei, as demonstrated by DAPI staining of cells in the same field to show nuclear DNA in blue (E). (C) F-actin staining in proplatelet-forming MKs. MKs grown in 10% plasma were fixed in culture with 2% paraformaldehyde to preserve proplatelet processes before cytospin preparation. At 48 hours, F-actin aggregations can be found in proplatelet-forming MKs (arrows). (D) PKC down-modulation by prolonged incubation of PMA prevented actin reorganization in plasma-containing culture, but did not reduce the amount of F-actin. (F) Nuclear DNA staining of cells in panel C was revealed by DAPI staining.
Thrombin has been reported to cause actin aggregates in primary MKs.28 Because there may be trace amounts of thrombin in plasma, we used a thrombin inhibitor in additional studies; actin polymerization, actin aggregation, and proplatelet formation were not inhibited by the specific thrombin inhibitor hirudin (data not shown). Therefore, thrombin is not responsible for this cytoskeletal change.
To begin to determine whether plasma-induced MK actin reorganization plays an important role in proplatelet formation, and whether this represents an important target for PKC inhibition, we interfered with actin dynamics by 2 other experimental manipulations: MK integrin blockade and inhibition of actin polymerization. Using 2 disintegrins, including kistrin, a snake venom–derived, broad-spectrum disintegrin, and the α5β1 integrin specific inhibitor EMF-10, we found that interruption of integrin function in mature MKs reduced the numbers of proplatelet-bearing MKs (Figure5A). As shown, both kistrin and EMF-10 markedly inhibited proplatelet formation. Because the viable MK mass measured by an MTT reduction assay was only modestly decreased during the culture period, it is unlikely that the effects on proplatelet formation were secondary to nonspecific toxicity. Consistent with our hypothesis, kistrin, which markedly inhibited proplatelet formation, also inhibited actin polymerization induced by plasma (data not shown). We also blocked actin polymerization with cytochalasin D; this acted to substantially reduce total numbers of proplatelet-bearing MKs in culture (Figure 5B) and to reduce F-actin aggregation. Cytochalasin D has also been reported to morphologically alter proplatelet formation, inducing nonbranching forms3; we observed the same phenomenon. Thus, because several experimental manipulations that reduce MK proplatelet formation (disintegrins, actin polymerization inhibition, PKC blockade) also interfere with F-actin aggregation, these results suggest that one potentially important target of PKCα is F-actin.
Effect of MK integrin blockade and inhibition of actin polymerization.
(A) The effects of disintegrin on proplatelet formation. Both kistrin (10 ng/mL) and EMF-10 (50 ng/mL) markedly decreased the numbers of proplatelet-bearing MKs (shown in the left-hand set of bars), expressed as a percentage of control diluent (dimethylsulfoxide; DMSO). The numbers of viable MKs, shown in the right set of bars, are only modestly affected. ░, DMSO; ▧, kistrin; ▨, EMF-10. (B) We tested whether actin reorganization is essential for MK proplatelet formation using cytochalasin D (5 μmol/L). Cytochalasin D significantly decreased the number of proplatelet-bearing MKs in culture. However, at this dose of cytochalasin, some actin aggregates could still be observed. ░, DMSO; ▨, cyto D 0.5 μM.
Effect of MK integrin blockade and inhibition of actin polymerization.
(A) The effects of disintegrin on proplatelet formation. Both kistrin (10 ng/mL) and EMF-10 (50 ng/mL) markedly decreased the numbers of proplatelet-bearing MKs (shown in the left-hand set of bars), expressed as a percentage of control diluent (dimethylsulfoxide; DMSO). The numbers of viable MKs, shown in the right set of bars, are only modestly affected. ░, DMSO; ▧, kistrin; ▨, EMF-10. (B) We tested whether actin reorganization is essential for MK proplatelet formation using cytochalasin D (5 μmol/L). Cytochalasin D significantly decreased the number of proplatelet-bearing MKs in culture. However, at this dose of cytochalasin, some actin aggregates could still be observed. ░, DMSO; ▨, cyto D 0.5 μM.
PKCα is translocated to F-actin aggregates during proplatelet formation
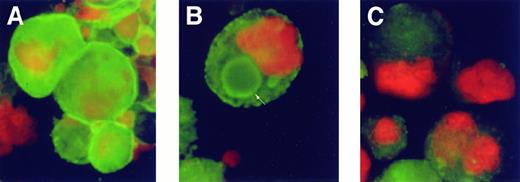
The subcellular localization of PKC isoforms in proplatelet-forming MKs was studied using immunofluorescence. As shown in Figure 6A, in the absence of plasma, PKCα distributed throughout the cells, possibly primarily localized to the cell membrane. In the presence of plasma, when MKs were forming proplatelets, PKCα was localized in a ring- or ball-like pattern in the cytoplasm of the cells (Figure 6B). This distribution was not found when cells were stained with the isotype-matched primary antibody control. After exposure to PMA for 48 hours, PKCα expression was markedly decreased (Figure 6C), consistent with the Western blot result (Figure 2B). No specific change in the pattern of staining of PKCδ and ε was detectable by immunostaining in the presence or absence of plasma or PMA (data not shown).
PKC isoform translocation in response to plasma.
Purified MKs were cultured in TPO either without plasma, with plasma, or with plasma and 100 ng/mL PMA for 48 hours; stained; and examined by immunofluorescent microscopy. PKC isoform α was labeled with FITC-conjugated specific antibodies (green), and nuclear DNA was labeled by propidium iodide (orange). (A) In the absence of plasma, PKCα was distributed throughout the cells with bright-green staining of the cell periphery. (B) In the presence of plasma, when MKs were forming proplatelets, PKCs were localized as a ring-like pattern (arrow) and peripheral staining was reduced. (C) Prolonged incubation with PMA down-modulated PKCα expression and prevented formation of this pattern of PKC distribution.
PKC isoform translocation in response to plasma.
Purified MKs were cultured in TPO either without plasma, with plasma, or with plasma and 100 ng/mL PMA for 48 hours; stained; and examined by immunofluorescent microscopy. PKC isoform α was labeled with FITC-conjugated specific antibodies (green), and nuclear DNA was labeled by propidium iodide (orange). (A) In the absence of plasma, PKCα was distributed throughout the cells with bright-green staining of the cell periphery. (B) In the presence of plasma, when MKs were forming proplatelets, PKCs were localized as a ring-like pattern (arrow) and peripheral staining was reduced. (C) Prolonged incubation with PMA down-modulated PKCα expression and prevented formation of this pattern of PKC distribution.
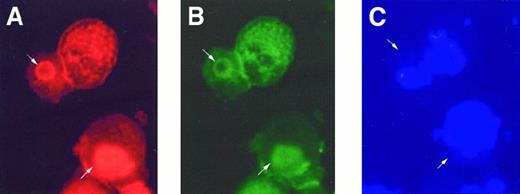
To determine whether PKC isoforms localize to actin during proplatelet formation, we triply stained MKs for F-actin, PKC isoforms, and DNA. As shown in Figure 7, polymerized actin and PKCα colocalize, and are distinct from the nuclei of cells, under conditions in which proplatelets are induced to form. These data suggest that PKCα may play a role in F-actin aggregation during proplatelet formation.
PKCα colocalizes with actin aggregate.
After incubation with plasma for 48 hours, cells were triply stained with (A) rhodamine-conjugated phalloidin for F-actin (red), (B) anti-PKCα (green), and (C) DAPI (blue) for nuclear DNA. PKCα was found to be colocalized with F actin aggregations (arrow), but not the nuclei.
PKCα colocalizes with actin aggregate.
After incubation with plasma for 48 hours, cells were triply stained with (A) rhodamine-conjugated phalloidin for F-actin (red), (B) anti-PKCα (green), and (C) DAPI (blue) for nuclear DNA. PKCα was found to be colocalized with F actin aggregations (arrow), but not the nuclei.
Discussion
Although TPO is the major regulator of MK development, by itself the hormone is not sufficient to promote maximal proplatelet formation in culture.28-30 Identification of (an)other extracellular signal(s) affecting this process is likely to be very useful in controlling platelet production clinically. To identify the intracellular signaling pathways responsible for proplatelet formation, we tested inhibitors of the PI3K, ERK, p38 MAPK, PKA, and PKC pathways in mature murine MK cultures. The major findings of our study are that blockade of PKC function seriously impairs MK proplatelet formation and that the kinase is redistributed to a large aggregate of F-actin that forms only under conditions associated with proplatelet development. PKC inhibition, secondary to exposure to PMA or incubation with BIM, substantially blocked proplatelet formation at inhibitor levels that avoided general cellular toxicity. Inhibition of MAPKs, PKA, and PI3K failed to affect proplatelet formation at inhibitor concentrations that avoid cell survival or proliferation effects.
More than 10 isoforms of PKC have been described that differ in their tissue-specific expression, mechanisms of control, substrates, and roles in cellular physiology. Isoform-specific inhibition of PKC could provide a rational target for pharmacologic therapy of disorders linked to specific PKC pathways. Clinical trials of PKC isoform inhibition are underway in patients with cancer and other diseases. Therefore, we sought to identify the specific isoforms of PKC involved in proplatelet process formation. Specific inhibition of PKCα, using a ribozyme strategy, profoundly affected proplatelet formation. Therefore, PKCα may play a crucial role in MK proplatelet formation. Some of our studies used the phorbol ester PMA to block PKC expression. Certainly, PMA may have additional effects on MK physiology. However, our results are unlikely to be experimental artifacts secondary to nonspecific effects of the inhibitors because 3 different methods of PKC inhibition with 3 different mechanisms of action yielded similar results. Furthermore, cell viability was not significantly affected in any of the experiments. Notably, the usual concentrations of BIM in culture reported in the literature were in the micromolar range. Inhibition of proplatelet formation by a lower dose, 500 nmol/L, of BIM supports the crucial role of PKCα because BIM has the lowest Ki for the PKCα isoform. At higher doses, BIM can inhibit several isoforms of PKC, including the atypical isoform, PKCζ. It is possible that other isoforms may be necessary for MK survival because the MTT result was significantly affected by BIM at greater than or equal to 3 μmol/L. The signal and signaling pathway leading to PKCs are still unknown. Integrins, which are important in this process, are obvious candidates to initiate PKCα signaling. However, the pathway from integrin to PKC has not yet been described. PKCα responds to both Ca++ and DAG. Therefore, it is possible that the signal derives from a serpentine receptor/heterotrimeric G-protein/phospholipase C (PLC) pathway or from a tyrosine kinase–cytokine receptor/PLCγ pathway. TPO has been reported to activate the PKC pathway in one cell line,31 but there is no evidence that this occurs in primary MKs. Therefore, the pathways upstream of PKC in MKs need to be further defined.
The pathway downstream of PKCα regulating actin cytoskeletal reorganization in this system also remains to be elucidated. Various proteins have been identified recently to function in actin rearrangement in association with PKC. These proteins are the MARCKS (myristoylated alanine-rich C-kinase substrate) family of proteins,32-34 pleckstrin,35 and the Wiskott-Aldrich syndrome proteins WASP36,37 and N-WASP.38,39 The data on the effects of WASP mutations on proplatelet formation are conflicting. One report suggested that WASP is important for this process,40 but the other could not reproduce this result.41 By immunostaining, WASP was not colocalized with the actin cytoskeleton in our system (data not shown).
Recently, a PKCα antisense oligonucleotide has been used in phase I studies to treat cancer because PKCα is important for the growth of various types of tumors.42 43 Notably, the most common hematologic side effect of PKCα inhibition in vivo is thrombocytopenia. It is possible that inhibition of proplatelet formation is the mechanism of this toxicity. Specific modulation of this PKC isoform is a potential target for drug development in treating disorders with abnormal platelet production.
In this study, we have shown that proplatelet formation was markedly inhibited by kistrin, a broad-spectrum disintegrin, which suggests that integrin activation may play an important role in this process. This result is consistent with previous studies showing that some components of the extracellular matrix induce proplatelet formation in bovine and guinea pig MKs in serum-containing cultures.10,19 Notably, EMF-10, a disintegrin relatively specific for integrin α5β1, could only partially inhibit proplatelet formation compared with the broad-spectrum disintegrin kistrin, again suggesting that multiple integrins may be required for full proplatelet formation. Consistent with these data, neither we nor others could significantly inhibit proplatelet formation using blocking antibodies against the single integrins α4, α5, α1, and α3 (Leven20 and data not shown).
A notable feature of the murine model of proplatelet formation described in this work is the changes induced in cellular actin polymerization. Aggregation of polymerized actin occurred in most of the cells under proplatelet-promoting conditions, but was markedly decreased in the absence of plasma, in the presence of a disintegrin, when actin polymerization was inhibited, or when cultured with PKC inhibitors, all of which blocked proplatelet formation. Therefore, actin reorganization correlates well with proplatelet formation, suggesting (but not yet proving) a cause-effect relationship. Supporting this hypothesis, actin aggregation is detectable in proplatelet-forming MKs (Figure 4D). We also found that PKCα is translocated to these large cellular actin aggregates during proplatelet formation. This result suggests that PKCα may regulate this cytoskeletal reorganization, supporting our findings that actin polymerization was inhibited by PKC inhibition or down-modulation. PKCγ is also translocated to the actin cytoskeleton in plasma-containing culture (data not shown), but, in contrast to PKCα, it was not down-modulated by PMA, a strong proplatelet inhibitor of primary MKs, suggesting that this isoform is not sufficient for proplatelet formation.
In summary, an experimental model of proplatelet formation in murine MKs has been developed. Proplatelet formation is inhibited by disintegrins and PKC inhibitors, suggesting that integrin signaling and PKC play important roles in this process. Actin aggregation is induced in MKs only under conditions that promote proplatelet formation. Additionally, prevention of actin polymerization by cytochalasin inhibits proplatelet formation. These findings suggest that actin polymerization may also be important for this process. Because PKCα was the isoform found to be critical for proplatelet formation, its pharmacologic modulation may have clinical relevance in disorders of platelet formation.
Acknowledgments
We thank Drs Stefan Niewiarowski, Mouldy Sioud, and Donald Foster for kindly providing the vital reagents.
Supported by Chulalongkorn University, Bangkok, Thailand (P.R.) and National Institutes of Health grants R01 CA31615 and R01 DK49855 (K.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth Kaushansky, Division of Hematology, Box 357710, University of Washington, Seattle, WA 98195; e-mail:kkaushan@u.washington.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal