Abstract
Using 5-color fluorescence-activated cell sorting, we isolated a subset of murine pluripotent hematopoietic stem cells (PHSC) with the phenotype Lin− Sca+ kit+CD38+ CD34− that appears to fulfill the criteria for most primitive PHSC. In the presence of whole bone marrow (BM) competitor cells, these cells produced reconstitution in lethally irradiated primary, secondary, and tertiary murine transplant recipients over the long term. However, these cells alone could not produce reconstitution in lethally irradiated recipients. Rapid proliferation of these cells after BM transplantation required the assistance of another BM cell subset, which has the phenotype Lin− Sca+ kit+ CD38−CD34+.
Introduction
Pluripotent hematopoietic stem cells (PHSC) are a population of cells that reside in the bone marrow (BM) and maintain all cells of lymphoid and myeloid lineage over the long term. In mice, the frequency of long-term reconstituting (LTR) PHSC is about 1 in 100 000 whole BM cells.1,2 In accordance with their characteristics after BM transplantation (BMT), cells in murine BM with multilineage repopulating ability can be divided into 2 groups: the LTR cells, which can support hematopoiesis for more than 6 months in irradiated recipients, and the short-term reconstituting (STR) cells, which can repopulate blood elements for several weeks.3-5Using counterflow centrifugal elutriation, Jones et al6separated murine BM and found that cells with the STR property provided unsustained early engraftment in BM, whereas cells with the LTR property provided sustained but delayed engraftment in BM, spleen, and thymus. However, the cells that produced sustained repopulation could not protect animals from lethal irradiation. On the basis of these types of observations, it has been hypothesized that LTR cells proliferate slowly and can produce only delayed but sustained engraftment. Thus, to detect the subpopulation, or subset, of PHSC that can produce long-term engraftment, it appeared necessary to cotransplant early-engraftment cells along with the putative LTR cells into lethally irradiated recipients so that the host would survive the initial aplasia. The LTR cells are assumed to be the “true” stem cell.
PHSC can be isolated and characterized according to their immunophenotype by using fluorescence-activated cell sorting (FACS) and antibodies to the cell-surface markers. Spangrude et al7defined a BM cell subset with the surface markers Lin−/loSca-1+ Thy1.1lo. These cells were found to be 1000 to 2000 times enriched for the subset of PHSC with LTR ability. Further characterization of these cells indicated that they represent a heterogeneous population that includes both LTR cells and cells able to provide only STR ability.8,9 Using 4-color FACS, Osawa et al10 demonstrated that the most active murine LTR PHSC are CD34 negative (CD34−). A similar proposal was made regarding human stem cells. Three groups have reported data showing that human CD34− cells have LTR activity and may be more primitive than CD34+ cells.11-13 In addition, Randall et al14 studied another surface marker, CD38, and reported that murine LTR PHSC are CD38+.
To characterize murine PHSC in more detail and to study their proliferation after BMT, we developed and used 5-color FACS analysis and cell fractionation. The subsets of PHSC that were isolated were studied with long-term competitive repopulation and short-term repopulation assays. Using these approaches, we determined that the most active LTR cells in mouse BM are lineage-negative cells with Sca+ kit+ CD38+ CD34−(hereafter abbreviated 38+34−) surface markers and that another lineage-negative subset, with the phenotype Sca+ kit+ CD38− CD34+(38−34+), plays an important role in supporting the rapid proliferation of 38+34−cells after BMT.
Materials and methods
Mice
C57BL/6J (Ly5.2) male mice were obtained from B & K Universal Inc (Fremont, CA) and used as the irradiated recipients. Congenic C57BL/6JL-Ly5.1-Pep3b (Ly5.1) mice and the first-filial-generation (F1) hybrid of Ly5.2 and Ly5.1 mice were bred at the Jackson Laboratory (Bar Harbor, ME) and the animal facility at the University of Southern California (USC; Los Angeles, CA) and used as sources of the hematopoietic progenitor population. All animals were housed under specific pathogen-free conditions and given acidified drinking water and autoclaved chow ad libitum. Mice used in the experiments were 8 to 12 weeks of age. The study protocol was approved by the USC Animal Care and Use Committee.
Antibodies
The antibodies used in the lineage cocktail were anti-Mac-1 (M1/70), anti-Gr-1 (RB6-8C5), anti-B220 (RA3-6B2), anti-CD3e (145-2C11), anti-CD4 (RM4-5), anti-CD5 (53-7.3), anti-CD8a (53-6.7), and anti-erythroid (Ter119); all were biotinylated when used. Other antibodies were anti-Ly5.2 (104), anti-Ly5.1 (A20), anti-c-kit (2B8) labeled with allophycocyanin (APC), anti-Sca-1 (E13-161.7) labeled with phycoerythrin (PE), and anti-CD34 (RAM34) labeled with fluorescein isothiocyanate (FITC). All antibodies listed above were from Pharmigen (San Diego, CA). Anti-CD38 (NIM-R5) labeled with red 613 (R613) was conjugated under contract by Southern Biotech (Birmingham, AL). Streptavidin-conjugated peridinin chlorophyll protein (perCP) was from Becton Dickinson (San Jose, CA). The goat antirat or streptavidin-conjugated magnetic beads were from Miltenyi Biotech Inc (Auburn, CA).
Preparation and isolation of hematopoietic progenitors
BM cells were harvested from the femurs and tibias of Ly5.1 or F1 mice. After lysis of red blood cells with ammonium chloride lysis buffer (Ortho-mune Lysing Reagent; Ortho, Raritan NJ), cells were stained with biotinylated antibodies to lineage markers. Lin+ cells were depleted with streptavidin-conjugated magnetic beads by using a CS column (Miltenyi Biotech). The lineage-depleted cells were collected and incubated with perCP and streptavidin, anti-Sca-1 (PE), anti-c-kit (APC), anti-CD34 (FITC), and anti-CD38 (R613). Stained cells were sorted with a customized Elite machine (Coulter, Miami, FL) equipped with a 15 mW argon laser tuned at 488 nm (for FITC, PE, R613 and perCP excitations) and a 10 mW helium-neon laser tuned at 610 nm for APC excitation. The fourth and fifth photomultiplier tubes (PMTs) were replaced with customized PMTs (4526A photomultiplier; Burle Industries, Lancaster, PA) with increased sensitivity in the higher wavelengths. Forward light scatter was detected with a 488 bp10 and an ND1.0 filter. For FITC, PE, R613 and perCP, 520 to 530, 555 to 595, 605 to 615, and 670 to 680 filters, respectively, were used; for APC, a 670 to 680 filter was used. Because both perCP and APC were detected with the fifth PMT, a time delay of 40 milliseconds was established for the perCP signal. Compensation was adjusted to achieve optimal signals from each fluorochrome when used simultaneously. Restricted sorting variables were chosen with the purity-1 mode, 1-drop-sort envelope, and coincidence-abort system on. Residual erythrocytes, debris, and doublets were excluded by forward- and side-scatter gating. The carryover lineage-positive cells were excluded by gating out the perCP-positive cells. Different subsets were sorted according to the gating variables.
Short- and long-term repopulation assays
The sorted cells were mixed with different numbers of competitor cells and transplanted through a tail vein into lethally irradiated mice that received a single (lethal) dose of 9.5 Gy from dual, opposed sources of cesium 131. For analysis of reconstitution in the mice, either BM or peripheral blood (PB) cells (from the tail) were collected in phosphate-buffered saline (PBS) and assayed for the presence of Ly5.1 (donor) cells of each lineage. Red blood cells were lysed with ammonium chloride lysis buffer and washed with PBS and 1% bovine serum albumin. The remaining nucleated cells were stained for lineage markers and Ly5.1 antibody. Anti-CD3, anti-CD4, and anti-CD8a were used to identify T cells, B220 was used for B cells, anti-Mac-1 for macrophages, and anti-Gr1 for granulocytes.
Assay of colony-forming units–spleen (CFU-S)
CFU-S assays were performed as described previously.15 Sorted cells from Ly5.1 mice were injected through the tail vein into lethally irradiated animals. Twelve days after the injection, the mice were killed and the spleens were removed and fixed. Macroscopic colonies were counted by inspection.
Results
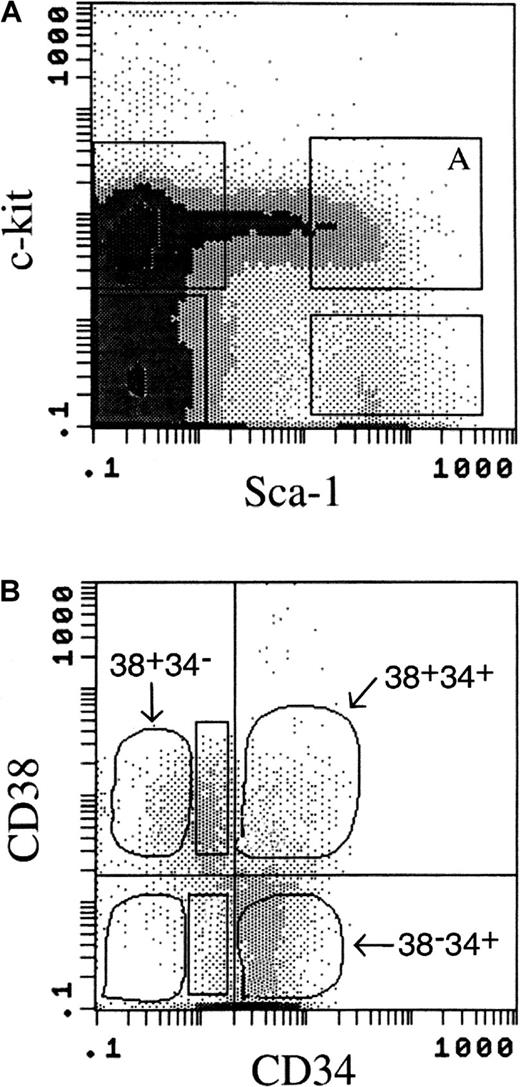
38+34− cells are the subset of PHSC with the greatest LTR ability. Previous reports indicated that the LTR activity is in the Lin− Sca+ kit+fraction of PHSC.7-9 Our data confirmed this observation (data not shown). Lineage-negative BM cells that were positive for Sca-1 and c-kit expression (Figure 1A) were further fractionated by using the expression of both CD38 and CD34 (Figure 1B). In lineage-negative Sca+ kit+ BM cells, there are 4 possible CD38/CD34 expression profiles: Sca+ kit+ CD38+ CD34−(38+34−), Sca+ kit+CD38+ CD34+ (38+34+), Sca+ kit+ CD38− CD34+(38−34+), and Sca+kit+ CD38− CD34−(38−34−) cells. The frequency of each of these subsets in whole BM with our gating variables is shown in Table1. Post-sorting analysis of the sorted cells indicated that the purity of the 38+34−subset was 97% to 99%, with 1% to 2% contamination from 38+34+ cells; the other subsets had similar purity.
Flow cytometric analysis of the surface-marker–expression profile of murine bone marrow (BM) cells.
Lineage-positive cells were removed by CS column before flow cytometry. (A) Expression of Sca-1 and c-kit on the cell surface was gated as shown; Lin− Sca+ kit+cells were gated as shown in box A. (B) Expression of CD38 and CD34 on the cell surface of the Lin− Sca+kit+ cells was used to separate the cell population shown in box A in Figure 1A into the following 4 subsets: Sca+kit+ CD38+ CD34−(38+34−), Sca+ kit+CD38+ CD34+ (38+34+), Sca+ kit+ CD38−CD34+(38−34+), and Sca+kit+ CD38− CD34−(38−34−). Two other subpopulations examined (Figure 3) were Sca+ kit+ CD38+CD34lo (38+34lo) and Sca+ kit+ CD38− CD34lo(38−34lo) (rectangular gates). Cells in each population were sorted and collected for analysis in a competitive repopulation assay.
Flow cytometric analysis of the surface-marker–expression profile of murine bone marrow (BM) cells.
Lineage-positive cells were removed by CS column before flow cytometry. (A) Expression of Sca-1 and c-kit on the cell surface was gated as shown; Lin− Sca+ kit+cells were gated as shown in box A. (B) Expression of CD38 and CD34 on the cell surface of the Lin− Sca+kit+ cells was used to separate the cell population shown in box A in Figure 1A into the following 4 subsets: Sca+kit+ CD38+ CD34−(38+34−), Sca+ kit+CD38+ CD34+ (38+34+), Sca+ kit+ CD38−CD34+(38−34+), and Sca+kit+ CD38− CD34−(38−34−). Two other subpopulations examined (Figure 3) were Sca+ kit+ CD38+CD34lo (38+34lo) and Sca+ kit+ CD38− CD34lo(38−34lo) (rectangular gates). Cells in each population were sorted and collected for analysis in a competitive repopulation assay.
Summary of long-term competitive repopulating ability of subsets of pluripotent hematopoietic stem cells (PHSC)
| PHSC subset . | Competitor cells: 2 × 105 WBM cells . | Competitor cells: 4 × 105 WBM cells . | ||
|---|---|---|---|---|
| 45 donor cells . | 100 donor cells . | 10 donor cells . | 100 donor cells . | |
| 38+34− | 22.5 ± 6.4 | 53.6 ± 10 | 4.3 ± 0.9 | 19.1 ± 3.3 |
| RU = 1.3 (12; 100%) | RU = 2.3 (9; 100%) | RU = 1.8 (29; 79%) | RU = 0.9 (30; 100%) | |
| 38+34+ | 0.7 | 3.0 ± 0.6 | 0.8 ± 0.09 | 5.8 ± 1.8 |
| RU = 0.03 (5; 20%) | RU = 0.06 (8; 100%) | RU = 0.3 (22; 13%) | RU = 0.25 (22; 77%) | |
| 38−34+ | Background | 3.9 ± 1 | 0.9 ± 0.2 | 0.7 ± 0.06 |
| RU = 0 (5; 0%) | RU = 0.08 (10; 90%) | RU = 0.39 (18; 11%) | RU = 0.03 (29; 30%) | |
| 38−34− | Not determined | Not determined | 0.7 ± 0.2 | Not determined |
| RU = 0.28 (18; 11%) | ||||
| PHSC subset . | Competitor cells: 2 × 105 WBM cells . | Competitor cells: 4 × 105 WBM cells . | ||
|---|---|---|---|---|
| 45 donor cells . | 100 donor cells . | 10 donor cells . | 100 donor cells . | |
| 38+34− | 22.5 ± 6.4 | 53.6 ± 10 | 4.3 ± 0.9 | 19.1 ± 3.3 |
| RU = 1.3 (12; 100%) | RU = 2.3 (9; 100%) | RU = 1.8 (29; 79%) | RU = 0.9 (30; 100%) | |
| 38+34+ | 0.7 | 3.0 ± 0.6 | 0.8 ± 0.09 | 5.8 ± 1.8 |
| RU = 0.03 (5; 20%) | RU = 0.06 (8; 100%) | RU = 0.3 (22; 13%) | RU = 0.25 (22; 77%) | |
| 38−34+ | Background | 3.9 ± 1 | 0.9 ± 0.2 | 0.7 ± 0.06 |
| RU = 0 (5; 0%) | RU = 0.08 (10; 90%) | RU = 0.39 (18; 11%) | RU = 0.03 (29; 30%) | |
| 38−34− | Not determined | Not determined | 0.7 ± 0.2 | Not determined |
| RU = 0.28 (18; 11%) | ||||
Individual subsets were isolated by 5-color fluorescence-activated cell sorting (FACS). Then, 10, 45, or 100 Ly5.1 donor cells and the indicated number of Ly5.2 whole bone marrow (WBM) competitor cells were injected into lethally irradiated Ly5.2 mice. Peripheral blood (PB) was collected at various times during the next 12 months and examined for engraftment of Ly5.1 donor cells. Only the data from 12 months after bone marrow transplantation (BMT) are shown. The frequency of the subsets of PHSC in the WBM was 2.2 cells/100 000 cells for 38+34−, 5.1 cells/100 000 cells for 38+34+, 15.0 cells/100 000 cells for 38−34+, and 0.83 cells/100 000 cells for 38−34−. Plus-minus values are the mean ± SE percentages of donor cells in total PB cells (ie, the donor-cell contribution). RU indicates repopulating units as defined by Jordan et al.22 An RU is the repopulating-ability equivalent to 100 000 untreated BM cells from adults and is calculated, for 100 cells, according to the following equation: donor RU = (% donor cells × competitor RU)/(100 − % donor cells). The RU of competitor cells here is 2 or 4 (there is 1 RU/100 000 WBM cells). The mean values for donor RU for the 3 subsets of PHSC studied were 1.6 for 38+34−, 0.16 for 38+34+, and 0.12 for 38−34+. The values in parentheses are the number of mice in each experiment and the percentage of engraftment. The data are from 8 independent experiments.
To determine which of the tested subsets have the properties of self-renewal and multilineage repopulation, extensive competitive repopulation assays were performed with 38+34−, 38+34+, and 38−34+ cells. Restricted gating variables and cell-sorting strategies were used to avoid contamination from cells in adjacent regions (Figure 1B). After transplantation to lethally irradiated mice, long-term engraftment of the transplanted donor cells was monitored by periodic analysis of the PB of recipient animals to detect donor-derived lineage cells. The LTR ability (12 months after BMT) from each fractionated subset is shown in Table 1. The 38+34− cells consistently yielded significantly greater long-term, multilineage engraftment than the other 3 subsets. The 38+34+ and 38−34+ subsets demonstrated some LTR activity (Table 1). In 2 experiments, 38−34− cells had an average of 0.7% donor-cell contribution from 2 mice with positive results (of a total of 15 mice) when 10 donor cells were transplanted. The mean donor repopulating unit (RU) value (Table 1) for the 38+34− cells (1.6) was 10 times the RU value for 38+34+ cells (0.16), and 13 times the value for 38−34+ cells (0.12).
We investigated the kinetics of multilineage repopulation by analyzing the lineage-cell reconstitution in PB at 5, 16, 34, and 56 weeks after BMT (Figure 2). We observed greater donor-cell reconstitution from the 38+34−subset than from either the 38+34+ or the 38−34+ subsets. The data indicated that cells from all lineages (lymphoid: T and B cells; and myeloid: macrophages and granulocytes) were already present by 5 weeks after BMT. Myeloid-cell values reached their peak level rapidly (values were already at a plateau 5 weeks after BMT), whereas lymphoid cells were delayed (16 weeks after BMT; Figure 2).
Repopulation kinetics of subsets of pluripotent hematopoietic stem cells (PHSC).
Bone marrow transplantation (BMT) was performed as follows: 100 Ly5.1 donor cells (from 1 of the 3 subsets, 38+34−[●], 38+34+ [■], or 38−34+ [▴]) and 2 × 105Ly5.2 whole BM competitor cells were transplanted into Ly5.2 lethally irradiated mice. Blood samples were collected at the indicated times after BMT and examined for the presence of Ly5.1 multilineage reconstitution (marked on y-axis). The data suggest that all 3 subsets have short-term multilineage reconstituting ability but that only the 38+34− subset of PHSC has marked long-term reconstituting ability. T indicates T lymphocytes; B, B lymphocytes; M, macrophages; and G, granulocytes. Data are from 2 independent experiments using 3 to 6 mice per group in each experiment; the values presented are means ± SE.
Repopulation kinetics of subsets of pluripotent hematopoietic stem cells (PHSC).
Bone marrow transplantation (BMT) was performed as follows: 100 Ly5.1 donor cells (from 1 of the 3 subsets, 38+34−[●], 38+34+ [■], or 38−34+ [▴]) and 2 × 105Ly5.2 whole BM competitor cells were transplanted into Ly5.2 lethally irradiated mice. Blood samples were collected at the indicated times after BMT and examined for the presence of Ly5.1 multilineage reconstitution (marked on y-axis). The data suggest that all 3 subsets have short-term multilineage reconstituting ability but that only the 38+34− subset of PHSC has marked long-term reconstituting ability. T indicates T lymphocytes; B, B lymphocytes; M, macrophages; and G, granulocytes. Data are from 2 independent experiments using 3 to 6 mice per group in each experiment; the values presented are means ± SE.
Secondary BMT was performed 1 year after primary BMT. Whole BM or sorted Ly5.1 cells from the primary recipients (which originally had reconstitution with 100 Ly5.1 38+34−, 38+34+, or 38−34+cells) were transplanted into secondary lethally irradiated Ly5.2 recipients. Seven months after the secondary BMT, all the mice that received BM cells containing cells derived from 38+34− showed engraftment in the PB from donor (Ly5.1)-originated cells (Table 2). However, there was no detectable Ly5.1 engraftment in the secondary recipient if the primary donor cells were 38+34+ or 38−34+ (data not shown). Similarly, tertiary recipients had positive results (45% ± 5% of donor-derived cells in PB) 6 months after receiving whole BM cells obtained from secondary recipients 12 months after BMT.
Reconstitution in secondary recipients from primary 38+34− donor cells
| Donor cells . | Months after BMT . | No. of mice . | % Ly5.1+ . | T and B lymphocytes . | Macrophages (M) and granulocytes (G) . |
|---|---|---|---|---|---|
| WBM from 1st recipients (1 × 106) | 12 | 7 | 31 ± 5 | T: 33.5 ± 2.9 | M: 39 ± 3 |
| B: 44.8 ± 4.5 | G: 35.2 ± 2.9 | ||||
| Sorted Ly5.1 cells from 1st recipients (1.4 × 106) | 7 | 7 | 79 ± 5 | T: 70.3 ± 8.2 | M: 63.3 ± 6 |
| B: 91.3 ± 4.3 | G: 55.1 ± 6 | ||||
| Sorted Ly5.1 cells from 1st recipients (1 × 106) | 8 | 6 | 40 ± 8 | 47.5 ± 8.2 | 49.7 ± 36.6 |
| Donor cells . | Months after BMT . | No. of mice . | % Ly5.1+ . | T and B lymphocytes . | Macrophages (M) and granulocytes (G) . |
|---|---|---|---|---|---|
| WBM from 1st recipients (1 × 106) | 12 | 7 | 31 ± 5 | T: 33.5 ± 2.9 | M: 39 ± 3 |
| B: 44.8 ± 4.5 | G: 35.2 ± 2.9 | ||||
| Sorted Ly5.1 cells from 1st recipients (1.4 × 106) | 7 | 7 | 79 ± 5 | T: 70.3 ± 8.2 | M: 63.3 ± 6 |
| B: 91.3 ± 4.3 | G: 55.1 ± 6 | ||||
| Sorted Ly5.1 cells from 1st recipients (1 × 106) | 8 | 6 | 40 ± 8 | 47.5 ± 8.2 | 49.7 ± 36.6 |
Twelve months after primary transplantation of 100 Ly5.1 38+34− donor cells, WBM or Ly5.1-marker-sorted BM cells from the Ly5.2 primary recipient mice were infused into lethally irradiated Ly5.2 secondary recipients. PB was collected at the times indicated, and the total percentage of Ly5.1 donor-cell engraftment, as well as the percentage of Ly5.1 donor-cell engraftment in the different lineages, was determined by FACS analysis. The level of Ly5.1-positive cells in the WBM from the first recipients (first row of table) was 51.6%. Plus-minus values are mean ± SE percentages.
The CFU-S12 assay15,16 detects a subset of PHSC at a specific early stage of hematopoietic maturation. Several investigators have separated CFU-S12 from LTR activity and suggested that CFU-S12 represent STR cells but not LTR cells.6 The frequency of formation of CFU-S12(one colony from 675 38+34− cells, or 214 38+34+ cells, or 38 38−34+ cells) indicates that, among the 3 subsets we tested, the highest CFU-S12 activity is in the 38−34+ cells.
CD38 expression is an important phenotype of LTR cells
Because the Lin− Sca+ kit+CD34lo population defined by Osawa et al10showed LTR properties, we investigated the importance of CD38 expression in this cell population, which includes both CD38+ and CD38− cells. BMT was performed with 100 Ly5.1 cells from either the 38+34lo(Sca+ kit+ CD38+CD34lo) or the 38−34lo(Sca+ kit+ CD38−CD34lo) subsets (Figure 1B, rectangles) and 4 × 105 Ly5.2 competitor cells. Blood was collected periodically for assays of multiple-lineage reconstitution by the Ly5.1 donor cells. Equivalent reconstitution from both subsets was observed as early as 3 weeks after BMT in both lymphoid and myeloid lineages. However, engraftment from the 38−34lo subset then decreased, whereas that from the 38+34losubset increased over time (Figure 3). These data indicate that the cells with the CD38+ phenotype were more primitive and had better self-renewal ability than the CD38− cells, although both cell populations had the same level of CD34 expression.
Repopulation kinetics of 38+34loand 38−34lo cells.
BMT was performed as follows: 100 Ly5.1 donor cells (either 38+34lo [●]or 38−34lo [■] cells) and 4 × 105 Ly5.2 whole BM competitor cells were transplanted into Ly5.2 lethally irradiated recipient mice. Peripheral blood was examined periodically. The data for CD34lo cells, together with the data for CD34− cells, suggest that only CD38+ cells can provide long-term reconstitution efficiently. T indicates T lymphocytes; B, B lymphocytes; M, macrophages; and G, granulocytes. Data are from 2 independent experiments using 6 to 8 mice per group in each experiment; the values presented are means ± SE.
Repopulation kinetics of 38+34loand 38−34lo cells.
BMT was performed as follows: 100 Ly5.1 donor cells (either 38+34lo [●]or 38−34lo [■] cells) and 4 × 105 Ly5.2 whole BM competitor cells were transplanted into Ly5.2 lethally irradiated recipient mice. Peripheral blood was examined periodically. The data for CD34lo cells, together with the data for CD34− cells, suggest that only CD38+ cells can provide long-term reconstitution efficiently. T indicates T lymphocytes; B, B lymphocytes; M, macrophages; and G, granulocytes. Data are from 2 independent experiments using 6 to 8 mice per group in each experiment; the values presented are means ± SE.
All 3 subsets of PHSC (38+34−, 38+34+, and 38−34+) start proliferating in BM efficiently after transplantation
For successful long-term engraftment, injected stem cells must reach the proper microenvironment in the BM for proliferation, a process called homing. To compare the long-term engraftment ability of the cell subsets, it is important to determine whether the compared cells can home to and repopulate the BM with equal efficiency. We examined the reconstitution of infused cells in BM early after BMT (8 days) to provide an indicator of whether the cells homed to and repopulated the BM. With 2 × 105 Ly5.2 competitor cells, 200 Ly5.1 cells of each subset were injected separately into Ly5.2 lethally irradiated mice. For all 3 subsets of PHSC, donor-derived cells were detected 8 days after BMT in BM and 14 days after BMT in PB (Table 3). These data indicate that there does not appear to be any preference in regard to homing or proliferation of one cell population over the others in this experimental setting.
Engraftment of subsets of PHSC after BMT
| PHSC subset . | BM . | PB . | |
|---|---|---|---|
| 8 days after BMT . | 14 days after BMT . | 14 days after BMT . | |
| 38+34− | 11.4 ± 3.2 (10) | 19.0 ± 5.7 (11) | 5.9 ± 1.3 (11) |
| 38+34+ | 14.5 ± 3.7 (9) | 13.1 ± 3.8 (7) | 6.6 ± 1.6 (6) |
| 38−34+ | 14.5 ± 4.2 (10) | 24.2 ± 8.0 (9) | 7.7 ± 2.8 (8) |
| PHSC subset . | BM . | PB . | |
|---|---|---|---|
| 8 days after BMT . | 14 days after BMT . | 14 days after BMT . | |
| 38+34− | 11.4 ± 3.2 (10) | 19.0 ± 5.7 (11) | 5.9 ± 1.3 (11) |
| 38+34+ | 14.5 ± 3.7 (9) | 13.1 ± 3.8 (7) | 6.6 ± 1.6 (6) |
| 38−34+ | 14.5 ± 4.2 (10) | 24.2 ± 8.0 (9) | 7.7 ± 2.8 (8) |
Two hundred Ly5.1 cells sorted from each subset of PHSC and 2 × 105 Ly5.2 competitor cells were transplanted into lethally irradiated Ly5.2 recipient mice. BM and PB were analyzed for donor-cell engraftment (Ly5.1-positive cells) at the indicated times after BMT. The data represent a summary of 4 experiments. Plus-minus values are mean ± SE percentages; values in parentheses are the total number of mice in the experiment.
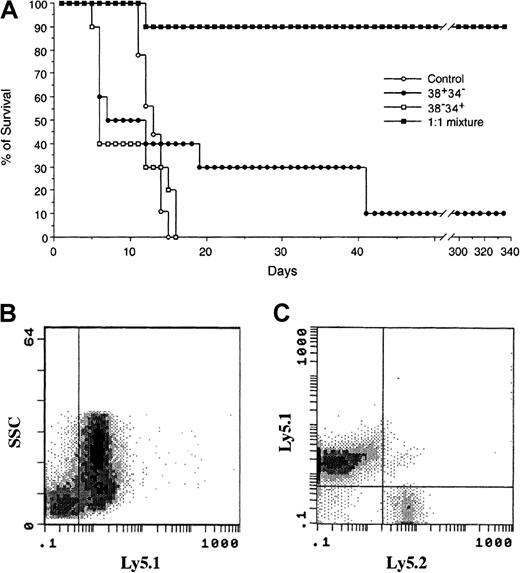
Efficient radioprotection requires both 38+34− and 38−34+cells
Because we observed both early engraftment (8 days after BMT in BM and 14 days after BMT in PB) and sustained engraftment (12 months after BMT) with 38+34− cells, we investigated whether 38+34− cells can function as both radioprotective (STR) cells and LTR cells. With a fixed total cell number, either 300 cells (Figure 4A) or 1000 (data not shown), neither 38+34− nor 38−34+ cells alone could efficiently protect the mice from lethal irradiation. However, when the 2 subsets of PHSC were mixed in a 1:1 ratio (maintaining a total cell number of 300 or 1000 cells), 90% of the mice survived (in both experimental settings; Figure 4A). When blood samples from mixed-group animals were examined 32 weeks after BMT, more than 80% of the reconstituting cells were found to have the donor marker Ly5.1 (Figure 4B). Among these Ly5.1 cells, 98% were from the 38+34− subset of PHSC (Figure 4C). These data are consistent with the findings of Osawa et al,10 who concluded that although Lin−Sca+ kit+ CD34− cells are LTR cells, they could not rescue animals from lethal irradiation.
Survival curves for lethally irradiated mice as a measure of the radioprotective capacity of subsets of PHSC.
(A) Lethally irradiated Ly5.2 mice were given transplants of either 300 38+34− cells (carrying the Ly5.1 surface marker), 300 38−34+ cells (carrying both the Ly5.1 and the Ly5.2 surface markers and obtained by using cells from a first-filial-generation hybrid of Ly5.1 and Ly5.2), or 300 cells as a 1:1 mixture of the 2 subsets (150 cells of each). Only the 1:1 mixture provided both short- and long-term reconstitution. (B) In peripheral blood collected 8 months after BMT of the 1:1 mixture, more than 80% of the blood cells were derived from the donor (ie, Ly5.1-positive cells), and (C) 98% were derived from 38+34−cells (ie, only Ly5.1-positive cells). The data suggest that reconstitution came primarily from the 38+34−subset of PHSC, not the 38−34+ subset, although the 38−34+ subset is required for efficient radioprotection.
Survival curves for lethally irradiated mice as a measure of the radioprotective capacity of subsets of PHSC.
(A) Lethally irradiated Ly5.2 mice were given transplants of either 300 38+34− cells (carrying the Ly5.1 surface marker), 300 38−34+ cells (carrying both the Ly5.1 and the Ly5.2 surface markers and obtained by using cells from a first-filial-generation hybrid of Ly5.1 and Ly5.2), or 300 cells as a 1:1 mixture of the 2 subsets (150 cells of each). Only the 1:1 mixture provided both short- and long-term reconstitution. (B) In peripheral blood collected 8 months after BMT of the 1:1 mixture, more than 80% of the blood cells were derived from the donor (ie, Ly5.1-positive cells), and (C) 98% were derived from 38+34−cells (ie, only Ly5.1-positive cells). The data suggest that reconstitution came primarily from the 38+34−subset of PHSC, not the 38−34+ subset, although the 38−34+ subset is required for efficient radioprotection.
Early proliferation of 38+34− cells after transplantation requires the presence of another subset of PHSC
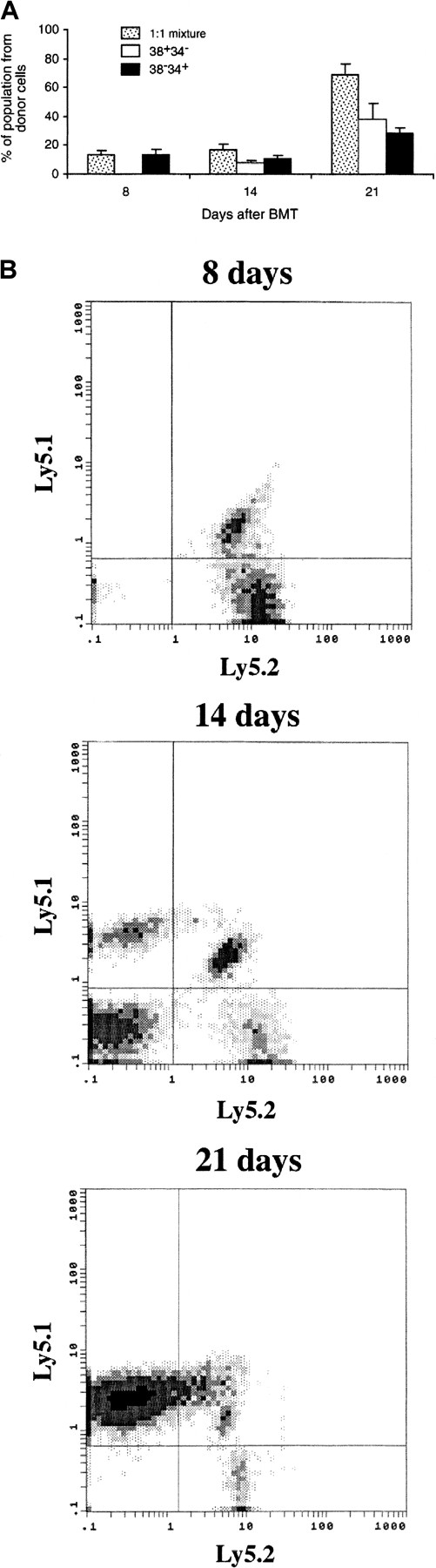
The radioprotection study raised the question of why 38+34− cells, which engrafted by 8 days after BMT in the competitive repopulation assay, could not protect lethally irradiated animals. Because 2 × 105 whole BM cells were supplied in the competitive repopulation assay but not in the radioprotection study, we wondered whether some other type of cell in the BM might be necessary to support early proliferation of the 38+34− cells. Therefore, we examined the efficiency of engraftment of 38+34− and 38−34+ cells in the setting of the radioprotection study, ie, with no competitor cells present. A total of 400 cells from either 1 subset or a mixture of 2 subsets identifiable by different genetic markers were used. Of 19 mice (9 individual experiments) that received 400 38+34− cells, 2 had a low level (3%) of donor-cell reconstitution in the BM 8 days after BMT, whereas all the mice that received 38−34+ cells or a mixture of cells had donor-cell engraftment (18.0 ± 6.2% and 13.0 ± 3.8%, respectively).
We then determined the contribution of each subset in the mixed-cell group. As shown in Figure5, all the reconstitution in BM 8 days after BMT was from the 38−34+ cells. However, reconstitution from 38+34− cells in BM was comparable with 38−34+ cells 14 days after BMT and increased additionally 21 days after BMT. Twenty-eight days after BMT, more than 95% of donor-cell–derived engraftment in PB was from 38+34− cells (data not shown). These data suggested the hypothesis that the 38−34+ cells prepared an environment for rapid proliferation of the 38+34− cells when the 2 subsets were cotransplanted. To test this hypothesis, we injected 38−34+ cells 7 days before the injection of 38+34− cells (Figure6A). Under these conditions, the engraftment of 38+34− cells was immediate, ie, it had occurred by 7 days after the second transplantation (Figure 6B).
Early time course of BM reconstitution.
Lethally irradiated Ly5.2 mice were given transplants of either 400 38+34− cells (carrying only the Ly5.1 surface marker), 400 38−34+ cells (carrying both the Ly5.1 and the Ly5.2 surface markers) or 400 cells in a 1:1 mixture of the 2 subsets (200 cells of each). At each time point, 2 to 4 animals were killed, their BM cells extracted, and the percentages of Ly5.1 and Ly5.1-Ly5.2 cells determined. (A) Summary of the results. (B) Representative fluorescence-activated cell-sorting analysis of donor-cell BM engraftment from the 1:1 mixture at 3 time points. The data suggest that the 38+34− subset of PHSC cannot engraft rapidly (ie, by 8 days after BMT) but can engraft by 14 days after BMT. Data are from 4 independent experiments using 2 to 4 mice per group in each experiment; the values presented are means ± SE.
Early time course of BM reconstitution.
Lethally irradiated Ly5.2 mice were given transplants of either 400 38+34− cells (carrying only the Ly5.1 surface marker), 400 38−34+ cells (carrying both the Ly5.1 and the Ly5.2 surface markers) or 400 cells in a 1:1 mixture of the 2 subsets (200 cells of each). At each time point, 2 to 4 animals were killed, their BM cells extracted, and the percentages of Ly5.1 and Ly5.1-Ly5.2 cells determined. (A) Summary of the results. (B) Representative fluorescence-activated cell-sorting analysis of donor-cell BM engraftment from the 1:1 mixture at 3 time points. The data suggest that the 38+34− subset of PHSC cannot engraft rapidly (ie, by 8 days after BMT) but can engraft by 14 days after BMT. Data are from 4 independent experiments using 2 to 4 mice per group in each experiment; the values presented are means ± SE.
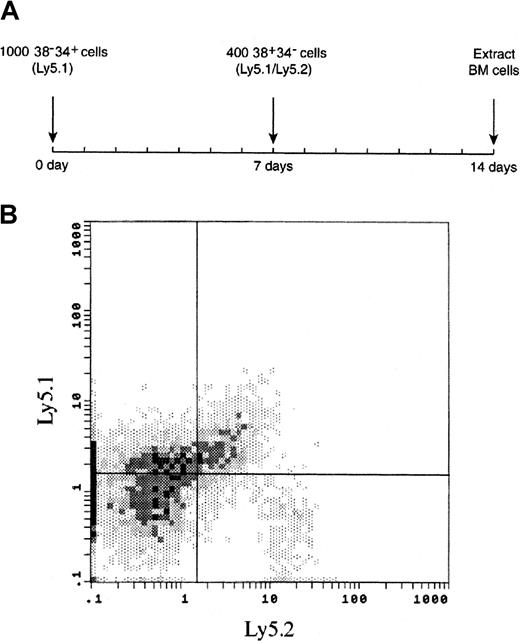
Early time course of BM reconstitution from the 38+34− subset of PHSC after previous engraftment of 38−34+ cells.
(A) Lethally irradiated Ly5.2 mice were given transplants of 1000 38−34+ cells (carrying the Ly5.1 surface marker). Seven days later, each animal received an injection of 400 38+34− cells (carrying both the Ly5.1 and the Ly5.2 surface markers). Engraftment of 38+34−and 38−34+ cells in the BM was examined 7 days later. (B) A representative FACS analysis of BM from a recipient mouse 7 days after the injection of 38+34− cells; mean ± SE engraftment was 27.9% ± 6.9% for 38−34+ cells and 7.8% ± 1.5% for 38+34− cells. Two independent experiments were performed using 5 to 8 mice per group in each experiment. The data suggest that the 38−34+ subset of PHSC supports early engraftment of 38+34− cells because, as shown in Figure 5, engraftment of 38+34− cells was undetectable 8 days after transplantation.
Early time course of BM reconstitution from the 38+34− subset of PHSC after previous engraftment of 38−34+ cells.
(A) Lethally irradiated Ly5.2 mice were given transplants of 1000 38−34+ cells (carrying the Ly5.1 surface marker). Seven days later, each animal received an injection of 400 38+34− cells (carrying both the Ly5.1 and the Ly5.2 surface markers). Engraftment of 38+34−and 38−34+ cells in the BM was examined 7 days later. (B) A representative FACS analysis of BM from a recipient mouse 7 days after the injection of 38+34− cells; mean ± SE engraftment was 27.9% ± 6.9% for 38−34+ cells and 7.8% ± 1.5% for 38+34− cells. Two independent experiments were performed using 5 to 8 mice per group in each experiment. The data suggest that the 38−34+ subset of PHSC supports early engraftment of 38+34− cells because, as shown in Figure 5, engraftment of 38+34− cells was undetectable 8 days after transplantation.
Discussion
Stem-cell transplantation and stem-cell gene therapy have potential for broad clinical applications. However, there is still insufficient understanding of the regulatory mechanisms and kinetics of stem-cell proliferation after BMT. Although BMT is widely used clinically, it is a cumbersome procedure. In addition, efficient gene transfer into LTR human stem cells has been only partly successful, thereby inhibiting development of stem-cell gene therapy. We used a murine model system in studies to increase understanding of hematopoietic stem cells and the proliferation of stem cells after BMT.
The lineage-negative subset of PHSC that is positive for Sca-1, c-kit, and CD38 but negative for CD34 (the 38+34−subset) appears to be the pivotal cell in the mouse stem-cell hierarchy. We found that 38+34− cells have both long-term and short-term repopulating ability: they can engraft rapidly after BMT (8 days), they can produce reconstitution in animals over the long term (Table 1), they can effect reconstitution in animals receiving secondary and tertiary transplants (Table 2 and data not shown), they form few CFU-S12, and they represent 0.0022% of the nucleated cells in the BM (ie, approximately 2 cells/100 000).
For the other 3 subsets (the 38+34+, 38−34+, and 38−34−cells), we observed some long-term repopulating ability (Table 1) but no ability to reconstitute a secondary transplant. Although it is possible that the LTR activity we observed was due to contamination from 38+34− cells during cell sorting, we used stringent sorting conditions; thus, contamination seems less likely an explanation for this finding than that a low level of LTR activity is an intrinsic property of 38+34−, 38−34+, and 38−34−cells.
The combined expression profile of CD38 and CD34 from the FACS analysis (Figure 1B) indicated that most Lin− Sca+kit+ CD34− cells are CD38+ and that most of the CD34+ cells in the Lin−Sca+ kit+ subset are CD38−. Therefore, our data support the conclusions of both Osawa et al10 and Randall et al14 that the LTR cells in mice are cells with the immunophenotype Lin−Sca+ kit+ CD34− as well as Lin− Sca+ kit+ CD38+.
Zijlmans et al23 suggested that the early phase of engraftment after murine blood transplantation is mediated by hematopoietic stem cells, which are defined by the phenotype Lin− WGA+ Rh−. Using modified rhodamine staining, Zijlmans et al subdivided this population into Rho−/Rho (VP)+, which showed short-term repopulating activity, and Rho−/Rho (VP)−, which showed long-term repopulating activity. We speculate that the cells with the phenotype Rho−/Rho (VP)+ might be the same cells as the 38−34+ cells in our study and that the Rho−/Rho (VP)− cells might be the same as our 38+34− cells. However, because the variables assessed in the study by Zijlmans et al were animal survival (> 4 weeks) and blood cell counts, it is difficult to identify the contribution from each subset when evaluating the early-engraftment kinetics. Our experimental design overcame this problem. Using Ly5.1 and F1 mice with different surface markers, we could specifically assess the repopulation from each subset in vivo.
The observation that rapid engraftment of 38+34− cells appears to depend on the presence of 38−34+ cells (or their offspring) is intriguing (Figures 4 and 5). Do the 38−34+cells have only a general supportive role or do they have a specific facilitative role? Because 38+34− cells cannot begin to proliferate immediately when transplanted at the same time as 38−34+ cells (Figure 5), we asked whether an immediate proliferation of 38+34− cells would occur if 38−34+ cells were transplanted 7 days before the 38+34− cells to provide a proper environment. As shown in Figure 6, 38+34−cells did begin to proliferate immediately when 38−34+ cells were already present. Although a general supportive function of 38−34+cells cannot be excluded, a specific facilitative role (with an unknown mechanism) is more likely. Because the 38−34+cells were in the mice for 7 days, the facilitating effect might not have come directly from these cells but from cells derived from them. The specific cell type involved in the putative facilitating effect and the mechanism of this effect are currently being studied.
We are not certain what is the immediate progeny cell to the 38+34− cell, but our data suggest that the immediate pathway from the 38+34− cell is first the 38+34+ subset and then the 38−34+ subset. This pathway is based on the percentage of long-term reconstitution in both primary and secondary recipients and the CFU-S12 data from the 3 subsets of PHSC. We speculate that the next cells in the pathway after the 38−34+ subset are those with increasing lineage markers. Studies to determine the complete maturation pathway of PHSC are under way.
If the 38+34− cells are the most primitive stem cells with the highest LTR ability, should they be called the “true” stem cells? Because 38−34+ cells (or cells arising from them) appear to be required for 38+34− cells to engraft, should the 38−34+ subset of PHSC be considered support cells? We believe not, because 38+34+ and 38−34+ cells do have some LTR ability and it is possible that 38+34− cells also have some supporting ability (ie, an ability to support engraftment and proliferation). Therefore, the situation may be more like that proposed by Schofield17 and Lansdorp18 and reiterated by Donnelly et al,19 all of whom speculated that it is perhaps inaccurate to label one cell type a stem cell because sets of cells may have varying degrees of stem-cell potential. We propose that the PHSC subsets 38+34−, 38+34+, and 38−34+together form a stem-cell compartment.
Recently, Sato et al20 (with a covering analysis by Goodell21) suggested the intriguing possibility that murine CD34− and CD34+ stem cells may be able to convert into each other under some conditions. This hypothesis was based on data obtained from mice treated with 5-fluorouracil (5-FU). Although we did not examine the effect of 5-FU on individual subsets of PHSC, the concept that CD34+ cells can convert to CD34− cells in vivo is compatible with the model of a stem-cell compartment described here.
What are the implications of our work for the clinical applications of BMT and stem-cell gene therapy? Because the primary LTR cells in mice are in the CD34− fraction, this may also be the location of the primary LTR cells in humans, as was suggested previously by Goodell et al,11 Bhatia et al,12 and Zanjani et al.13 As pointed out by Donnelly et al,19CD34+ LTR cells in mice are 100 times more abundant than CD34− cells, but the CD34− cells are more efficient in long-term reconstitution after BMT than the more abundant CD34+ cells. Currently, clinical protocols using stem cells are based on purification of human CD34+ hematopoietic cells. If conditions for expanding CD34− PHSC in culture were identified, perhaps far fewer cells would need to be given to patients to effect reconstitution. It is also possible that for human CD34− PHSC, a different set of transduction conditions is required for gene transfer and that therefore the current difficulty in carrying out stem-cell gene therapy successfully may be partly due to targeting the wrong (or a less efficient) stem cell.
Acknowledgments
We thank Guoliang Li and Lujiang Zhu for excellent technical assistance, Sylvia Chavira and Mark Hechinger of the USC Flow Cytometry Laboratory for assistance with FACS analysis and sorting, Mike Astle of the Jackson Laboratory for breeding and providing mice, and Drs Esmail Zanjani, Donald Kohn, and Jan Nolta for helpful comments on the manuscript.
Supported in part by grants from SyStemix/Genetic Therapy Inc/Novartis and from the G. Harold and Leila Y. Mathers Charitable Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
W. French Anderson, Norris Cancer Center, Rm 6316, USC Keck School of Medicine, 1441 Eastlake Ave, Los Angeles, CA 90033; e-mail: sdiaz@genome2.hsc.usc.edu.

![Fig. 2. Repopulation kinetics of subsets of pluripotent hematopoietic stem cells (PHSC). / Bone marrow transplantation (BMT) was performed as follows: 100 Ly5.1 donor cells (from 1 of the 3 subsets, 38+34−[●], 38+34+ [■], or 38−34+ [▴]) and 2 × 105Ly5.2 whole BM competitor cells were transplanted into Ly5.2 lethally irradiated mice. Blood samples were collected at the indicated times after BMT and examined for the presence of Ly5.1 multilineage reconstitution (marked on y-axis). The data suggest that all 3 subsets have short-term multilineage reconstituting ability but that only the 38+34− subset of PHSC has marked long-term reconstituting ability. T indicates T lymphocytes; B, B lymphocytes; M, macrophages; and G, granulocytes. Data are from 2 independent experiments using 3 to 6 mice per group in each experiment; the values presented are means ± SE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/9/10.1182_blood.v96.9.3016/5/m_h82100317002.jpeg?Expires=1769150531&Signature=251EhoTBWvFtXSWaJ-DRrxrno8f-KGA7PAM-g6WqIPuywJ4ed7WLtlpByAUsnb7yDiMbQopKIMFr~qmczMK2rgOSS8xvQDuv6KcqoJ5amAVgqpZ26YZY0VO8eygSdj0WSUi0iJUAFkaQGQVU-Gmws~ATAfrJhLR0dfK91gOwuZNUWbIgTBjXp4k1rm-T243sTN2j7eLWUtk3Vrq2e3lf0R3sMZbl3m1UlCoD2By0f5a64Bl6CsJUE1pC-Uqxgfhmkif2H5T-fPfr3Ndj4FXfKHw1zIpqbpGb0BHdmWMszRCUafsbZ~VzcdM6mkRMwLtDP7VoTnWlAH~~iUpBH6WfYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Repopulation kinetics of 38+34loand 38−34lo cells. / BMT was performed as follows: 100 Ly5.1 donor cells (either 38+34lo [●]or 38−34lo [■] cells) and 4 × 105 Ly5.2 whole BM competitor cells were transplanted into Ly5.2 lethally irradiated recipient mice. Peripheral blood was examined periodically. The data for CD34lo cells, together with the data for CD34− cells, suggest that only CD38+ cells can provide long-term reconstitution efficiently. T indicates T lymphocytes; B, B lymphocytes; M, macrophages; and G, granulocytes. Data are from 2 independent experiments using 6 to 8 mice per group in each experiment; the values presented are means ± SE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/9/10.1182_blood.v96.9.3016/5/m_h82100317003.jpeg?Expires=1769150531&Signature=K2ZTVyfsbfvHzGy1P8tG4h6C28ShEYvD5z2tHGeNjLqUJBhJK2~joJERbymJumDjGwxlgiZV07peBwotpS-y757Akj65imMK0-c28GEGaJYVTPSddkgug-zOt-eiyU03BDpER9FtXbj6NyZLo7~GnG6ZS09-wtOnCXrJIskTE88oEBlHiSM6ZQcqdnLb4O95uEODSvg2RTLJotMZECLQz6~jEAw7BTCoTdKv8brwhmVbj9rjgs3dVsmm6~1vQnKr9yfe1ycZ6MWgksAF8WssI-sKSW5G3fRobbJwOJsdotQXdEZc-ZIJuGdPPhWHAIaLSQsWsSM4JbbBpovz3SS~7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal