Abstract
Determining in vitro drug resistance may reveal clinically relevant information in childhood leukemia. Using the methyl-thiazol-tetrazolium assay, the resistance of untreated leukemic cells to 21 drugs was compared in 128 children with acute myeloid leukemia (AML) and 536 children with acute lymphoblastic leukemia (ALL). The differences between 3 French-American-British (FAB) types (M1/M2, M4, and M5) were also compared. AML was significantly more resistant than ALL to the following drugs, as noted by the median resistance: glucocorticoids (greater than 85-fold), vincristine (4.4-fold), l-asparaginase (6.9-fold), anthracyclines (1.8- to 3.4-fold), mitoxantrone (2.6-fold), etoposide (4.9-fold), platinum analogues (2.4- to 3.4-fold), ifosfamide (3.5-fold), and thiotepa (3.9-fold). For cytarabine and thiopurines, the median LC50 values (the drug concentration that kills 5% of the cells) were equal. Also, busulfan, amsacrine, teniposide, and vindesine showed no significant differences, but the numbers were smaller, and the median LC50 values were 1.3- to 5.2-fold higher in AML. None of the drugs demonstrated greater cytotoxicity in AML. FAB M5 was significantly more sensitive than FAB M4 to most drugs frequently used in AML, as indicated by the following ratios of median sensitivities: the anthracyclines (2.6- to 3.2-fold), mitoxantrone (12.5-fold), etoposide (8.7-fold), and cytarabine (2.9-fold). For etoposide and cytarabine (5.4- and 3.4-fold, respectively) FAB M5 was also significantly more sensitive than FAB M1/M2. FAB M5 was equally sensitive tol-asparaginase and vincristine as ALL. Only 15% of the AML samples were “intermediately” sensitive to glucocorticoids, mainly in FAB M1/M2. The poorer prognosis of childhood AML is related to resistance to a large number of drugs. Within AML, FAB M5 had a distinct resistance pattern. These resistance profiles may be helpful in the rational design of further treatment protocols.
Introduction
In childhood acute lymphoblastic leukemia (ALL) prognosis has improved dramatically over the past decades, and survival has currently reached 70%-80%.1,2 Children with acute myeloid leukemia (AML) experience a worse prognosis, with overall survival rates of 40%-60% despite intensive chemotherapy.3-6 Also, the complete remission (CR) rate differs, with 5%-10% induction failures due to refractory disease and toxicity in AML versus only 1%-2% induction failures in ALL.
Differences in prognosis may reflect differences in cellular drug resistance as one of the important determinants of treatment outcome. This can be studied using a total cell kill assay such as the methyl-thiazol-tetrazolium (MTT) assay. For childhood AML, clinical outcome and the results of cellular drug resistance testing showed good correlations.7 Other studies have shown the relation between in vitro drug resistance and disease status as well as cell biological features.7-9 For childhood ALL, the independent prognostic significance of the results obtained by in vitro drug resistance testing was demonstrated in several studies.10-13 Also, cellular drug resistance was able to, at least partially, explain differences in clinical outcome between the immunophenotypic subgroups.14 15
In the present study, we analyzed the differences in cellular resistance to 21 drugs in 128 AML samples versus 536 ALL samples, which might explain the differences in prognosis between these patient groups. The LC50 value, that is, the drug concentration needed to kill 50% of the cells, was used as a measure of resistance. Also, as AML is a heterogeneous disease, we compared the drug resistance profiles of different FAB-type subgroups: FAB M1, FAB M2, FAB M4, and FAB M5. Numbers in the other FAB-type subgroups were too small for separate analysis. A preliminary analysis, published in 1994, compared cellular resistance of 25 AML cases with 125 ALL cases.16 Part of the data on ALL that were published previously are used here as reference data only.10 13-16
Materials and methods
Patient samples
We tested either bone marrow or peripheral samples, which were taken at initial diagnosis, from children (aged 0-18 years) diagnosed with AML or ALL. Three different collaborative groups participated in the study: the AML-BFM Study Group (AML-BFM SG, Münster, Germany), the CoALL Study Group (Hamburg, Germany), and the Dutch Childhood Leukemia Study Group (DCLSG, Den Haag, The Netherlands). ALL samples collected between January 1989 and April 1998 (DCLSG/CoALL) and AML samples collected between February 1990 and June 1999 (DCLSG/AML-BFM SG) were included. Central review of the diagnosis, as well as characterization of the disease by immunophenotype (ALL) or FAB classification (AML), was done by reference laboratories of these groups.17
Cells
Mononuclear cells were separated by density gradient centrifugation. In the case of ALL samples with a low (less than 90%) blast percentage (determined morphologically on May-Grünwald-Giemsa [MGG] cytospin preparations), we used monoclonal antibodies linked to magnetic beads to remove nonleukemic cells, as previously described.18 In AML cases a low blast percentage (less than 80%) may be due to the presence of mature granulocytes (greater than 10%) and/or lymphocytes (greater than 10%). To eliminate granulocytes we performed freezing in liquid nitrogen and thawing. Lymphocytes were removed using immunomagnetic beads. In some samples, phagocytosis of these beads by myeloid leukemia cells was observed. Therefore, the procedure was carried out at room temperature.
The immunophenotype of ALL cells was determined either by immunocytochemistry or by flow cytometry. Precursor B-ALL was defined as surface immunoglobulin–negative/terminal deoxynucleotidal transferase (TdT) terminal-positive/human leukocyte antigen-Dr+/CD19+(sIg−/TdT+/HLA-Dr+/CD19+) and subdivided into 2 groups: common/pre-B ALL (CD10+and/or cμ+) and pro-B ALL (CD10−/cμ−). T-ALL was defined as cytoplasmic CD3+/CD7+. Patients with mature B-ALL (sIg+) were excluded from the study.
MTT assay
In vitro drug resistance of leukemia samples was assessed using a 4-day cell culture assay based on the principle that only viable cells are able to reduce 3-(4,5-dimethylthiazol-2,5-diphenyl) tetrazolium bromide (MTT) to a colored formazan product, which can be determined spectrophotometrically at 562 nm.19 Data from bone marrow and peripheral blood samples were evaluated together, as were data from fresh and cryopreserved samples, because this does not influence the results of in vitro drug resistance testing.8,20 A panel of 21 drugs was tested, each at 6 different concentrations in duplicate in 96-well microculture plates. The optical density (OD) is linearly related to the number of viable cells.19,20 Control wells, which are used to determine the control cell survival (CCS), contain leukemic cells with culture medium but without drugs; to blank the spectrophotometer, wells with culture medium only were used. Cytotoxicity was calculated at each drug concentration by the equation (OD treated well/mean OD control wells) × 100%, after correction for the background OD of the blank wells. The results were considered evaluable only if the control wells contained 70% leukemic cells (as determined by morphology after MGG staining) after 4 days of culture.16 Also, the mean control OD after correction for the background at day 4 must have exceeded 0.05 arbitrary units for valid results. The LC50 value, which is the drug concentration needed to kill 50% of the leukemia cells, was used as a measure of resistance. This is calculated with the following equation: ([% leukemic cell survival > 50%] − 50) / ([% leukemic cell survival > 50%] − [leukemic cell survival < 50%]) * (drug concentration above 50% leukemic cell survival − drug concentration below 50% leukemic cell survival) + (drug concentration below LCS 50%).
Drugs
The following drugs were tested at the given concentrations (brand names in parentheses): 0.008-8.0 μg/mL aclarubicin (Lundbeck A/S, Copenhagen, Denmark); 0.006-20 μg/mL amsacrine (Amsidine; Parke-Davis, Hoofddorp, The Netherlands); 1.23-300 μg/mL busulfan (Myleran, Glaxo Wellcome, Zeist, The Netherlands), 15.6-500 μg/mL 6-mercaptopurine (Puri-Nethol, Glaxo Wellcome), and 1.56-50 μg/mL 6-thioguanine (Lanvis; Glaxo Wellcome); 0.49-500 μg/mL carboplatin (Paraplatin, Bristol-Myers Squibb, Woerden, The Netherlands), 0.39-12.5 μg/mL cisplatin (Platinol, Bristol-Myers Squibb), 0.05-50 μg/mL etoposide (Vepesid, Bristol-Myers Squibb), and 0.003-8 μg/mL teniposide (Vumon, Bristol-Myers Squibb); 0.002-2.5 g/mL cytarabine (Cytosar, Pharmacia & Upjohn, Woerden, The Netherlands); 0.002-2 μg/mL daunorubicin (Cerubidine, Rhône-Poulenc Rorer, Amstelveen, The Netherlands); 0.0002-6 μg/mL dexamethasone disodium phosphate and 0.008-250 μg/mL prednisolone disodium phosphate (Bufa Pharmaceutical Products, Uitgeest, The Netherlands); 0.008-8 μg/mL doxorubicin (Adriablastina, Pharmacia & Upjohn) and 0.002-2 μg/mL idarubicin (Zavedos, Pharmacia & Upjohn); 0.1-100 μg/mL 4-hydroperoxy-ifosfamide (active metabolite of ifosfamide) (Asta-Medica, Dieman, The Netherlands); 0.003-10 IU/mLl-asparaginase (Paronal; Christiaens, Breda, The Netherlands); 0.001-1 μg/mL mitoxantrone (Novantrone, AHP Pharma Weyth Lederle, Hoofddorp, The Netherlands) and 0.03-100 μg/mL thiotepa (Lerdertepa, AHP Pharma Weyth Lederle); and 0.05-50 μg/mL vincristine (Oncovin, Eli Lilly, Nieuwegein, The Netherlands) and 0.049-50 μg/mL vindesine (Eldisine, Eli Lilly).
Statistical analysis
Differences in the distribution of the LC50 values were analyzed using the Mann-Whitney U test. The correlations were calculated using the Spearman rank correlation coefficient (rho). For statistical comparisons of categorical variables, the χ2test or the Fisher exact test, in case of small numbers, was used. Using the 2-tailed test, P ≤ .01 was considered statistically significant. If the number of samples in a specific subgroup was less than 4, a statistical analysis was not performed.
For childhood ALL, we previously defined the LC50 value threshold levels for prednisolone sensitivity, as those values showed a rather skewed distribution: highly sensitive (less than 0.1 μg/mL), intermediately sensitive (0.1-150 μg/mL), and resistant (greater than 150 μg/mL).10 We will also refer to these levels to describe prednisolone resistance in childhood AML.
Results
Patient characteristics
In the study period we received material from 183 childhood AML patients. When we compared the clinical data from these patients with published data in the literature4 (patients included in the AML-BFM 87 Protocol), there were no significant differences found. This included age, white blood cell (WBC) count, sex, and FAB-type distribution. In 128 AML samples (70%) at least one drug was successfully tested. Patients with an unsuccessful assay (n = 55, 30%) did not differ from patients with a successful assay regarding age and sex distribution, but they had a significantly lower WBC count (P < .001). Also, the FAB-type distribution was different, with higher assay failure rates in FAB M6 (100%), FAB M7 (75%), and unclassified samples (57%), and lower failure rates in FAB M3 (0%) and FAB M4 (17%) (P < .001). Data are shown in Table 1.
Characteristics of 183 children with newly diagnosed AML
| AML . | All patients . | Assay, unsuccessful . | Assay, successful . | P . |
|---|---|---|---|---|
| Patients, no. | 183 | 55 | 128 | |
| Age, median (P25-P75), y | 6.9 (2.4-11.8) | 5.0 (1.8-10.3) | 7.5 (3.1-12.6) | .02 |
| WBC count, median (P25-P75), ×109/L | 23.5 (6.9-72.9) | 8.7 (3.8-39.2) | 28.3 (12.5-88.4) | < .001 |
| Sex, % M:F ratio | 55.2:44.8 | 56.4:43.6 | 54.6:45.4 | .83 |
| FAB distribution, no. (%) | < .001 | |||
| M0 | 8 (4.4) | 2 (3.6) | 6 (4.7) | |
| M1 | 22 (12.0) | 8 (14.5) | 14 (10.9) | |
| M2 | 43 (23.5) | 12 (21.8) | 31 (24.2) | |
| M3 | 7 (3.8) | 0 (0) | 7 (5.5) | |
| M4 | 47 (25.7) | 8 (14.5) | 39 (30.6) | |
| M4Eo+ | 20 | 2 | 18 | |
| M5 | 34 (18.6) | 9 (16.4) | 25 (19.5) | |
| M6 | 3 (1.6) | 3 (5.5) | 0 (0) | |
| M7 | 12 (6.6) | 9 (16.4) | 3 (2.3) | |
| Unclassified | 7 (3.8) | 4 (7.3) | 3 (2.3) |
| AML . | All patients . | Assay, unsuccessful . | Assay, successful . | P . |
|---|---|---|---|---|
| Patients, no. | 183 | 55 | 128 | |
| Age, median (P25-P75), y | 6.9 (2.4-11.8) | 5.0 (1.8-10.3) | 7.5 (3.1-12.6) | .02 |
| WBC count, median (P25-P75), ×109/L | 23.5 (6.9-72.9) | 8.7 (3.8-39.2) | 28.3 (12.5-88.4) | < .001 |
| Sex, % M:F ratio | 55.2:44.8 | 56.4:43.6 | 54.6:45.4 | .83 |
| FAB distribution, no. (%) | < .001 | |||
| M0 | 8 (4.4) | 2 (3.6) | 6 (4.7) | |
| M1 | 22 (12.0) | 8 (14.5) | 14 (10.9) | |
| M2 | 43 (23.5) | 12 (21.8) | 31 (24.2) | |
| M3 | 7 (3.8) | 0 (0) | 7 (5.5) | |
| M4 | 47 (25.7) | 8 (14.5) | 39 (30.6) | |
| M4Eo+ | 20 | 2 | 18 | |
| M5 | 34 (18.6) | 9 (16.4) | 25 (19.5) | |
| M6 | 3 (1.6) | 3 (5.5) | 0 (0) | |
| M7 | 12 (6.6) | 9 (16.4) | 3 (2.3) | |
| Unclassified | 7 (3.8) | 4 (7.3) | 3 (2.3) |
The samples received were for in vitro cellular drug resistance testing. The P values represent the statistical differences between patients with and without a successful drug resistance assay. P25 indicates the 25th percentile; P75, the 75th percentile; y, years.
Unsuccessful assays in AML were due to true assay failures, which occurred in 31 samples, or to samples that were not suitable for testing, which occurred in 24 cases. True assay failures were caused by: (1) low OD in control wells (n = 7), (2) low blast percentage after 4 days of culture (n = 22), (3) infection (n = 1), and (4) poor quality of duplicate measurements (n = 1). Unsuitable samples were the result of a lack of cells or a low percentage of leukemic cells at day 0. There were no significant differences (data not shown) between these 2 failure groups regarding age (P = .18), sex (P = .42), WBC count (P = .26), or FAB-type distribution (P = .71).
Patients with AML were divided into 3 subgroups: FAB M1/M2, FAB M4, and FAB M5. FAB M1/M2 were taken together because there were no significant differences (data not shown) in drug resistance, age, sex, or WBC count between FAB M1 and M2. There were no significant differences among these 3 subgroups regarding sex and WBC count. Patients with FAB M5 were significantly younger than patients with FAB M1/M2 (P = .001), whereas the differences between FAB M1/M2 and FAB M4, as well as between FAB M4 and M5, were not significant.
We also successfully tested 536 childhood ALL samples consisting of 430 c/pre-B ALL, 87 T-ALL, and 19 pro-B ALL samples. The distribution of the immunophenotypic subgroups in this study is representative of ALL patients in The Netherlands and Germany.2,10,21 Data on the success rates of ALL samples (including immunophenotypic subgroups) have been published in this journal before.10 Patients with AML were significantly older (P = .001) and had a higher WBC count at diagnosis (P = .01) than patients with ALL. Sex distribution did not differ significantly (P = .68).
MTT assay
The median CCS after 4 days, expressed as the percentage of plated control cells at day 0, was significantly higher (P < .001) in AML than in ALL patients. For AML, 49% of the samples had a CCS of greater than 100%, whereas for ALL, this was the case in only 11%. The median OD/105 cells (a measure of the metabolic activity of viable cells to convert MTT) for AML was significantly higher than for ALL (P < .001). The data are shown in Table 2. Comparing the 3 FAB-type subgroups (FAB M1/M2, M4, and M5), there were no significant differences between the percentage of blasts after 4 days of culture and the CCS. FAB M1/M2 did have a significantly lower median OD/105 cells than FAB M4 and M5 (Table 2).
Cellular drug resistance assay characteristics showing differences between AML and ALL and the several AML FAB-type subgroups
| Assay characteristics . | AML . | ALL . | P* . | FAB M1/2 . | FAB M4 . | FAB M5 . | P† M1/2-M4 . | P†M1/2-M5 . | P† M4-M5 . |
|---|---|---|---|---|---|---|---|---|---|
| Blasts d4, % | 87 | 89 | .02 | 83 | 85 | 89 | .43 | .02 | .06 |
| CCS, median | 99 | 64 | < .001 | 91 | 106 | 87 | .07 | .75 | .15 |
| (range), % | (66-211) | (13-201) | (45-168) | (48-211) | (28-184) | ||||
| OD/105cells, | 0.51 | 0.23 | < .001 | 0.45 | 0.62 | 0.62 | < .001 | .003 | .40 |
| median (range), % | (0.12-1.16) | (0.05-0.66) | (0.12-0.73) | (0.29-1.16) | (0.28-1.11) |
| Assay characteristics . | AML . | ALL . | P* . | FAB M1/2 . | FAB M4 . | FAB M5 . | P† M1/2-M4 . | P†M1/2-M5 . | P† M4-M5 . |
|---|---|---|---|---|---|---|---|---|---|
| Blasts d4, % | 87 | 89 | .02 | 83 | 85 | 89 | .43 | .02 | .06 |
| CCS, median | 99 | 64 | < .001 | 91 | 106 | 87 | .07 | .75 | .15 |
| (range), % | (66-211) | (13-201) | (45-168) | (48-211) | (28-184) | ||||
| OD/105cells, | 0.51 | 0.23 | < .001 | 0.45 | 0.62 | 0.62 | < .001 | .003 | .40 |
| median (range), % | (0.12-1.16) | (0.05-0.66) | (0.12-0.73) | (0.29-1.16) | (0.28-1.11) |
The percentage of blasts d4 and CCS indicate the percentage of blasts and the median CCS, respectively, in the control wells after 4 days of culture.
Statistical differences between AML and ALL.
Statistical differences between the FAB-type subgroups in AML.
We correlated CCS and OD/105 cells with the results of in vitro drug resistance testing. For AML there was no significant correlation between CCS and cellular drug resistance. Also, if we divided the AML samples in 2 groups according to the median CCS, no significant differences in drug resistance were found. The OD/105 cells showed statistically significant, but only weak, correlations with the LC50 values of 2 drugs in AML: amsacrine (rho = 0.27, P = .01) and daunorubicin (rho = 0.28,P = .006). In the ALL samples there was a statistically significant correlation between CCS and LC50 values for cytarabine only (rho = 0.34, P < .001). The OD/105 cells in ALL only correlated with the LC50 values for l-asparaginase (rho = −0.45, P < .001).
Cellular drug resistance: AML versus ALL
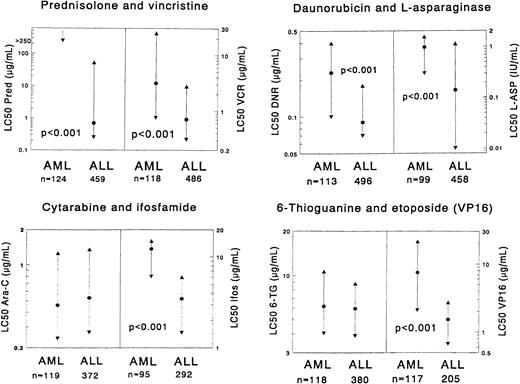
Results of in vitro drug resistance testing are summarized in Table 3 and illustrated in Figure1. In general, dose-response curves could be obtained for all drugs. Marked differences between individual patients were found, both in ALL cases as well as in AML cases. However, glucocorticoids often were not able to kill more than 50% of the leukemic cells, even not at the highest concentrations. For prednisolone, this occurred in 81% of the AML samples and in 21% of the ALL samples.
Comparisons of drug resistance in vitro between samples from children with AML and ALL
| Drug . | LC50 values . | AML . | ALL . | RR . |
|---|---|---|---|---|
| Cytarabine | Median | 0.46 | 0.53 | 0.9 |
| P25-P75 | 0.24-1.26 | 0.27-1.36 | ||
| n | 119 | 372 | ||
| Daunorubicin | Median | 0.23 | 0.09 | 2.63-150 |
| P25-P75 | 0.10-0.40 | 0.07-0.18 | ||
| n | 113 | 496 | ||
| Idarubicin | Median | 0.17 | 0.05 | 3.43-150 |
| P25-P75 | 0.09-0.29 | 0.02-0.09 | ||
| n | 76 | 185 | ||
| Doxorubicin | Median | 0.54 | 0.30 | 1.83-150 |
| P25-P75 | 0.31-0.89 | 0.14-0.42 | ||
| n | 81 | 247 | ||
| Aclarubicin | Median | 0.28 | 0.11 | 2.53-151 |
| P25-P75 | 0.13-0.49 | 0.08-0.20 | ||
| n | 21 | 71 | ||
| Mitoxantrone | Median | 0.13 | 0.05 | 2.63-150 |
| P25-P75 | 0.03-0.39 | 0.01-0.10 | ||
| n | 102 | 256 | ||
| Etoposide | Median | 7.45 | 1.52 | 4.93-150 |
| P25-P75 | 2.08-21.25 | 0.67-2.70 | ||
| n | 117 | 205 | ||
| Teniposide | Median | 1.00 | 0.26 | 3.8 |
| P25-P75 | 0.24-1.56 | 0.18-0.61 | ||
| n | 12 | 299 | ||
| 6-Thioguanine | Median | 6.18 | 5.95 | 1.0 |
| P25-P75 | 4.06-10.66 | 3.90-8.80 | ||
| n | 118 | 380 | ||
| 6-Mercaptopurine | Median | 93.8 | 104.2 | 0.9 |
| P25-P75 | 55.3-187.5 | 51.3-250.0 | ||
| n | 20 | 359 | ||
| Amsacrine | Median | 0.57 | 0.11 | 5.2 |
| P25-P75 | 0.14-1.36 | 0.07-0.26 | ||
| n | 92 | 6 | ||
| 4-Hydroperoxyifosfamide | Median | 12.22 | 3.48 | 3.53-150 |
| P25-P75 | 6.06-14.99 | 1.46-5.98 | ||
| n | 95 | 292 | ||
| Thiotepa | Median | 7.61 | 1.96 | 3.93-150 |
| P25-P75 | 2.48-10.93 | 0.70-2.70 | ||
| n | 23 | 27 | ||
| Busulfan | Median | 37.78 | 28.24 | 1.3 |
| P25-P75 | 25.57-59.01 | 21.29-30.34 | ||
| n | 91 | 8 | ||
| Cisplatin | Median | 5.96 | 1.76 | 3.43-151 |
| P25-P75 | 2.72-7.84 | 1.33-2.39 | ||
| n | 20 | 20 | ||
| Carboplatin | Median | 31.49 | 13.22 | 2.43-151 |
| P25-P75 | 19.06-81.313-152 | 7.39-13.22 | ||
| n | 4 | 57 | ||
| L-Asparaginase3-153 | Median | 0.97 | 0.14 | 6.93-150 |
| P25-P75 | 0.30-1.60 | 0.01-1.13 | ||
| n | 99 | 458 | ||
| Vincristine | Median | 3.13 | 0.71 | 4.43-150 |
| P25-P75 | 0.76-24.6 | 0.31-2.76 | ||
| n | 118 | 486 | ||
| Vindesine | Median | 6.88 | 2.68 | 2.6 |
| P25-P75 | 2.04-21.74 | 0.77-9.23 | ||
| n | 15 | 103 | ||
| Prednisolone | Median | >250 | 0.69 | >3603-150 |
| P25-P75 | >250->250 | 0.25-52.14 | ||
| n | 124 | 459 | ||
| Dexamethasone | Median | >6 | 0.07 | >853-150 |
| P25-P75 | >6->6 | 0.02-1.02 | ||
| n | 18 | 310 |
| Drug . | LC50 values . | AML . | ALL . | RR . |
|---|---|---|---|---|
| Cytarabine | Median | 0.46 | 0.53 | 0.9 |
| P25-P75 | 0.24-1.26 | 0.27-1.36 | ||
| n | 119 | 372 | ||
| Daunorubicin | Median | 0.23 | 0.09 | 2.63-150 |
| P25-P75 | 0.10-0.40 | 0.07-0.18 | ||
| n | 113 | 496 | ||
| Idarubicin | Median | 0.17 | 0.05 | 3.43-150 |
| P25-P75 | 0.09-0.29 | 0.02-0.09 | ||
| n | 76 | 185 | ||
| Doxorubicin | Median | 0.54 | 0.30 | 1.83-150 |
| P25-P75 | 0.31-0.89 | 0.14-0.42 | ||
| n | 81 | 247 | ||
| Aclarubicin | Median | 0.28 | 0.11 | 2.53-151 |
| P25-P75 | 0.13-0.49 | 0.08-0.20 | ||
| n | 21 | 71 | ||
| Mitoxantrone | Median | 0.13 | 0.05 | 2.63-150 |
| P25-P75 | 0.03-0.39 | 0.01-0.10 | ||
| n | 102 | 256 | ||
| Etoposide | Median | 7.45 | 1.52 | 4.93-150 |
| P25-P75 | 2.08-21.25 | 0.67-2.70 | ||
| n | 117 | 205 | ||
| Teniposide | Median | 1.00 | 0.26 | 3.8 |
| P25-P75 | 0.24-1.56 | 0.18-0.61 | ||
| n | 12 | 299 | ||
| 6-Thioguanine | Median | 6.18 | 5.95 | 1.0 |
| P25-P75 | 4.06-10.66 | 3.90-8.80 | ||
| n | 118 | 380 | ||
| 6-Mercaptopurine | Median | 93.8 | 104.2 | 0.9 |
| P25-P75 | 55.3-187.5 | 51.3-250.0 | ||
| n | 20 | 359 | ||
| Amsacrine | Median | 0.57 | 0.11 | 5.2 |
| P25-P75 | 0.14-1.36 | 0.07-0.26 | ||
| n | 92 | 6 | ||
| 4-Hydroperoxyifosfamide | Median | 12.22 | 3.48 | 3.53-150 |
| P25-P75 | 6.06-14.99 | 1.46-5.98 | ||
| n | 95 | 292 | ||
| Thiotepa | Median | 7.61 | 1.96 | 3.93-150 |
| P25-P75 | 2.48-10.93 | 0.70-2.70 | ||
| n | 23 | 27 | ||
| Busulfan | Median | 37.78 | 28.24 | 1.3 |
| P25-P75 | 25.57-59.01 | 21.29-30.34 | ||
| n | 91 | 8 | ||
| Cisplatin | Median | 5.96 | 1.76 | 3.43-151 |
| P25-P75 | 2.72-7.84 | 1.33-2.39 | ||
| n | 20 | 20 | ||
| Carboplatin | Median | 31.49 | 13.22 | 2.43-151 |
| P25-P75 | 19.06-81.313-152 | 7.39-13.22 | ||
| n | 4 | 57 | ||
| L-Asparaginase3-153 | Median | 0.97 | 0.14 | 6.93-150 |
| P25-P75 | 0.30-1.60 | 0.01-1.13 | ||
| n | 99 | 458 | ||
| Vincristine | Median | 3.13 | 0.71 | 4.43-150 |
| P25-P75 | 0.76-24.6 | 0.31-2.76 | ||
| n | 118 | 486 | ||
| Vindesine | Median | 6.88 | 2.68 | 2.6 |
| P25-P75 | 2.04-21.74 | 0.77-9.23 | ||
| n | 15 | 103 | ||
| Prednisolone | Median | >250 | 0.69 | >3603-150 |
| P25-P75 | >250->250 | 0.25-52.14 | ||
| n | 124 | 459 | ||
| Dexamethasone | Median | >6 | 0.07 | >853-150 |
| P25-P75 | >6->6 | 0.02-1.02 | ||
| n | 18 | 310 |
The results are expressed in LC50 values given as μg/mL. Median indicates median LC50 values; P25, the 25th percentile; P75, the 75th percentile; n, the number of samples. RR (resistance ratio) indicates the median LC50 for AML divided by the median LC50 for ALL. RR > 1.0, indicates that for the given drug, AML is more resistant than ALL.
P ≤ .001, as determined by the Mann-WhitneyU test.
P > .001 and P ≤ .01, as determined by the Mann-Whitney U test.
The minimum to maximum range is given, rather than P25-P75.
l-asparaginase is given as IU/mL, not as μg/mL.
Each graph shows the comparison of AML samples versus ALL samples for 2 particular drugs.
The median LC50 value (dot) and the 25th and 75th percentile (triangles) are shown for each drug. The number of patients (n) for each group is shown at the bottom of the graph. P values, determined by the Mann-Whitney U test, are shown for statistically significant differences between AML and ALL. LC50 values are given as μg/mL, and values for l-asparaginase are given as IU/mL. In the prednisolone graph for AML, the 25th percentile is depicted as 250 μg/mL for practical reasons, but in reality it is greater than 250 μg/mL, which is above the highest concentration used in our assay. The median and P75 are not depicted for the same reason.
Each graph shows the comparison of AML samples versus ALL samples for 2 particular drugs.
The median LC50 value (dot) and the 25th and 75th percentile (triangles) are shown for each drug. The number of patients (n) for each group is shown at the bottom of the graph. P values, determined by the Mann-Whitney U test, are shown for statistically significant differences between AML and ALL. LC50 values are given as μg/mL, and values for l-asparaginase are given as IU/mL. In the prednisolone graph for AML, the 25th percentile is depicted as 250 μg/mL for practical reasons, but in reality it is greater than 250 μg/mL, which is above the highest concentration used in our assay. The median and P75 are not depicted for the same reason.
AML cells were found to be significantly more resistant in vitro to most of the drugs than ALL cells. This was most obvious for the glucocorticoids with very high resistance ratios. When the threshold levels for prednisolone sensitivity, as defined in childhood ALL,10 were used for the AML samples, the sensitivity varied as follows: there were no AML samples highly sensitive to prednisolone (LC50 value less than 0.1μg/mL); 19 of 124 (15.3%) samples showed intermediate sensitivity (0.1-150 μg/mL); and all other samples were resistant to prednisolone (more than 150μg/mL).
Several other drugs showed significant differences between AML and ALL, with moderate to high resistance ratios. These includedl-asparaginase, vincristine, all anthracyclines, mitoxantrone, etoposide, the platinum analogues, and the alkylating drugs ifosfamide and thiotepa. No statistically significant differences in cellular drug resistance were found between AML and ALL for cytarabine, 6-mercaptopurine, 6-thioguanine, busulfan, amsacrine, teniposide, and vindesine. For cytarabine and the thiopurines, the resistance ratios between ALL and AML were 0.9-1.0. However, for the other 4 drugs these ratios were 1.3-5.2, and the sample sizes were relatively small (n = 6-15, either for AML or ALL). None of the drugs tested demonstrated greater cytotoxicity in AML.
Cellular drug resistance in AML: FAB-type subgroups M1/M2, M4, and M5
Due to low numbers we did not compare the different FAB subgroups for resistance to carboplatin and teniposide. FAB M0, M3, M6, and M7 were not studied separately because of inadequate numbers. FAB M1 and FAB M2 were analyzed together because they did not differ in drug resistance. The results are shown in Table4 and illustrated in Figure2.
Comparisons of in vitro drug resistance between samples from different FAB subtypes of children with newly diagnosed AML
| Drug . | LC50 values . | FAB M1/M2 . | FAB M4 . | FAB M5 . | RR4-150 M4-M1/2 . | RR4-151M1/2-M5 . | RR‡ M4-M5 . |
|---|---|---|---|---|---|---|---|
| Cytarabine | Median | 0.51 | 0.43 | 0.15 | 0.8 | 3.44-153 | 2.94-153 |
| P25-P75 | 0.33-1.64 | 0.30-0.63 | 0.12-0.44 | ||||
| n | 42 | 37 | 22 | ||||
| Daunorubicin | Median | 0.14 | 0.35 | 0.11 | 2.54-153 | 1.3 | 3.24-155 |
| P25-P75 | 0.09-0.35 | 0.25-0.45 | 0.05-0.22 | ||||
| n | 40 | 37 | 20 | ||||
| Idarubicin | Median | 0.13 | 0.26 | 0.10 | 2.0 | 1.3 | 2.64-155 |
| P25-P75 | 0.09-0.27 | 0.16-0.31 | 0.03-0.13 | ||||
| n | 26 | 26 | 12 | ||||
| Doxorubicin | Median | 0.49 | 0.81 | 0.27 | 1.7 | 1.8 | 3.04-153 |
| P25-P75 | 0.36-0.81 | 0.52-1.02 | 0.11-0.43 | ||||
| n | 31 | 27 | 16 | ||||
| Aclarubicin | Median | 0.17 | 0.28 | 0.33 | 1.6 | NC | NC |
| P25-P75 | 0.10-0.38 | 0.28-0.46 | 0.03-0.914-154 | ||||
| n | 11 | 5 | 3 | ||||
| Mitoxantrone | Median | 0.08 | 0.25 | 0.02 | 3.1 | 4.0 | 12.54-153 |
| P25-P75 | 0.02-0.19 | 0.05-0.50 | 0.00-0.11 | ||||
| n | 35 | 38 | 14 | ||||
| Etoposide | Median | 7.36 | 11.96 | 1.37 | 1.6 | 5.44-155 | 8.74-155 |
| P25-P75 | 2.62-27.85 | 6.73-24.78 | 0.54-3.02 | ||||
| n | 40 | 37 | 23 | ||||
| 6-Mercaptopurine | Median | 55.6 | 93.8 | 134.6 | 1.7 | NC | NC |
| P25-P75 | 47.6-103.0 | 70.8->500 | 15.0-145.84-154 | ||||
| n | 8 | 5 | 3 | ||||
| 6-Thioguanine | Median | 5.20 | 8.98 | 6.18 | 1.7 | 0.8 | 1.5 |
| P25-P75 | 3.34-9.69 | 5.57-12.64 | 3.50-9.08 | ||||
| n | 43 | 36 | 22 | ||||
| Amsacrine | Median | 0.24 | 0.94 | 0.15 | 3.9 | 1.6 | 6.34-155 |
| P25-P75 | 0.13-1.00 | 0.53-1.47 | 0.08-0.36 | ||||
| n | 28 | 35 | 14 | ||||
| Ifosfamide | Median | 11.60 | 12.95 | 11.76 | 1.1 | 1.0 | 1.1 |
| P25-P75 | 5.93-13.76 | 11.37-15.04 | 4.86-13.89 | ||||
| n | 28 | 34 | 19 | ||||
| Thiotepa | Median | 4.00 | 8.51 | 0.70 | 2.1 | NC | NC |
| P25-P75 | 1.87-8.27 | 7.08-12.00 | 0.51-8.444-154 | ||||
| n | 9 | 9 | 3 | ||||
| Busulfan | Median | 33.33 | 38.49 | 25.60 | 1.2 | 1.3 | 1.5 |
| P25-P75 | 25.59-67.58 | 27.93-51.19 | 9.72-58.21 | ||||
| n | 29 | 34 | 13 | ||||
| Cisplatin | Median | 3.79 | 7.03 | 2.30 | 1.9 | 1.6 | 3.1 |
| P25-P75 | 2.86-8.53 | 5.37-9.42 | 1.41-6.254-154 | ||||
| n | 7 | 7 | 4 | ||||
| L-Asparaginase# | Median | 0.63 | 1.09 | 0.30 | 1.7 | 2.1 | 3.64-153 |
| P25-P75 | 0.18-1.58 | 0.65-1.59 | 0.01-1.18 | ||||
| n | 31 | 36 | 17 | ||||
| Vincristine | Median | 2.99 | 21.55 | 0.53 | 7.2 | 5.6 | 40.74-160 |
| P25-P75 | 1.81-18.02 | 2.64-35.94 | 0.18-23.96 | ||||
| n | 43 | 37 | 20 | ||||
| Vindesine | Median | 3.14 | 17.66 | ND | 5.6 | NC | NC |
| P25-P75 | 2.04-20.02 | 14.85-36.77 | 0.68-8.754-154 | ||||
| n | 6 | 5 | 2 | ||||
| Prednisolone | Median | >250 | >250 | >250 | NC | NC | NC |
| P25-P75 | 213.5->250 | >250->250 | >250->250 | ||||
| n | 43 | 39 | 24 | ||||
| Dexamethasone | Median | >6 | >6 | >6 | NC | NC | NC |
| P25-P75 | >6->6 | >6->6 | >6->64-154 | ||||
| n | 8 | 5 | 3 |
| Drug . | LC50 values . | FAB M1/M2 . | FAB M4 . | FAB M5 . | RR4-150 M4-M1/2 . | RR4-151M1/2-M5 . | RR‡ M4-M5 . |
|---|---|---|---|---|---|---|---|
| Cytarabine | Median | 0.51 | 0.43 | 0.15 | 0.8 | 3.44-153 | 2.94-153 |
| P25-P75 | 0.33-1.64 | 0.30-0.63 | 0.12-0.44 | ||||
| n | 42 | 37 | 22 | ||||
| Daunorubicin | Median | 0.14 | 0.35 | 0.11 | 2.54-153 | 1.3 | 3.24-155 |
| P25-P75 | 0.09-0.35 | 0.25-0.45 | 0.05-0.22 | ||||
| n | 40 | 37 | 20 | ||||
| Idarubicin | Median | 0.13 | 0.26 | 0.10 | 2.0 | 1.3 | 2.64-155 |
| P25-P75 | 0.09-0.27 | 0.16-0.31 | 0.03-0.13 | ||||
| n | 26 | 26 | 12 | ||||
| Doxorubicin | Median | 0.49 | 0.81 | 0.27 | 1.7 | 1.8 | 3.04-153 |
| P25-P75 | 0.36-0.81 | 0.52-1.02 | 0.11-0.43 | ||||
| n | 31 | 27 | 16 | ||||
| Aclarubicin | Median | 0.17 | 0.28 | 0.33 | 1.6 | NC | NC |
| P25-P75 | 0.10-0.38 | 0.28-0.46 | 0.03-0.914-154 | ||||
| n | 11 | 5 | 3 | ||||
| Mitoxantrone | Median | 0.08 | 0.25 | 0.02 | 3.1 | 4.0 | 12.54-153 |
| P25-P75 | 0.02-0.19 | 0.05-0.50 | 0.00-0.11 | ||||
| n | 35 | 38 | 14 | ||||
| Etoposide | Median | 7.36 | 11.96 | 1.37 | 1.6 | 5.44-155 | 8.74-155 |
| P25-P75 | 2.62-27.85 | 6.73-24.78 | 0.54-3.02 | ||||
| n | 40 | 37 | 23 | ||||
| 6-Mercaptopurine | Median | 55.6 | 93.8 | 134.6 | 1.7 | NC | NC |
| P25-P75 | 47.6-103.0 | 70.8->500 | 15.0-145.84-154 | ||||
| n | 8 | 5 | 3 | ||||
| 6-Thioguanine | Median | 5.20 | 8.98 | 6.18 | 1.7 | 0.8 | 1.5 |
| P25-P75 | 3.34-9.69 | 5.57-12.64 | 3.50-9.08 | ||||
| n | 43 | 36 | 22 | ||||
| Amsacrine | Median | 0.24 | 0.94 | 0.15 | 3.9 | 1.6 | 6.34-155 |
| P25-P75 | 0.13-1.00 | 0.53-1.47 | 0.08-0.36 | ||||
| n | 28 | 35 | 14 | ||||
| Ifosfamide | Median | 11.60 | 12.95 | 11.76 | 1.1 | 1.0 | 1.1 |
| P25-P75 | 5.93-13.76 | 11.37-15.04 | 4.86-13.89 | ||||
| n | 28 | 34 | 19 | ||||
| Thiotepa | Median | 4.00 | 8.51 | 0.70 | 2.1 | NC | NC |
| P25-P75 | 1.87-8.27 | 7.08-12.00 | 0.51-8.444-154 | ||||
| n | 9 | 9 | 3 | ||||
| Busulfan | Median | 33.33 | 38.49 | 25.60 | 1.2 | 1.3 | 1.5 |
| P25-P75 | 25.59-67.58 | 27.93-51.19 | 9.72-58.21 | ||||
| n | 29 | 34 | 13 | ||||
| Cisplatin | Median | 3.79 | 7.03 | 2.30 | 1.9 | 1.6 | 3.1 |
| P25-P75 | 2.86-8.53 | 5.37-9.42 | 1.41-6.254-154 | ||||
| n | 7 | 7 | 4 | ||||
| L-Asparaginase# | Median | 0.63 | 1.09 | 0.30 | 1.7 | 2.1 | 3.64-153 |
| P25-P75 | 0.18-1.58 | 0.65-1.59 | 0.01-1.18 | ||||
| n | 31 | 36 | 17 | ||||
| Vincristine | Median | 2.99 | 21.55 | 0.53 | 7.2 | 5.6 | 40.74-160 |
| P25-P75 | 1.81-18.02 | 2.64-35.94 | 0.18-23.96 | ||||
| n | 43 | 37 | 20 | ||||
| Vindesine | Median | 3.14 | 17.66 | ND | 5.6 | NC | NC |
| P25-P75 | 2.04-20.02 | 14.85-36.77 | 0.68-8.754-154 | ||||
| n | 6 | 5 | 2 | ||||
| Prednisolone | Median | >250 | >250 | >250 | NC | NC | NC |
| P25-P75 | 213.5->250 | >250->250 | >250->250 | ||||
| n | 43 | 39 | 24 | ||||
| Dexamethasone | Median | >6 | >6 | >6 | NC | NC | NC |
| P25-P75 | >6->6 | >6->6 | >6->64-154 | ||||
| n | 8 | 5 | 3 |
The results are expressed in LC50 values given as μg/mL.
NC indicates that the RR could not be calculated.
RR is the median LC50 for FAB M4 divided by the median LC50 for FAB M1/M2. RR > 1.0 indicates that for the given drug, FAB M4 is more resistant than FAB M1/M2.
RR is the median LC50 for FAB M1/M2 divided by the median LC50 for FAB M5. RR > 1.0 indicates that for the given drug, FAB M1/M2 is more resistant than FAB M5.
RR is the median LC50 for FAB M4 divided by the median LC50 for FAB M5. RR > 1.0 indicates that for the given drug, FAB M4 is more resistant than FAB M5.
P > .001 and P ≤ .01, as determined by the Mann-Whitney U test.
P ≤ .001, as determined by the Mann-WhitneyU test.
The minimum to maximum range is given, rather than P25 and P75.
# l-asparaginase is given as IU/mL, not as μg/mL.
P = .016.
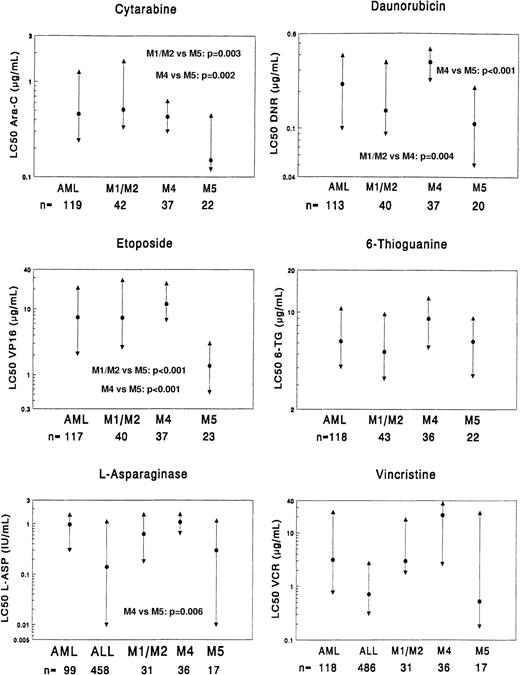
Comparison of the 3 AML FAB-type subgroups (FAB M1/M2, FAB M4, and FAB M5) as well as the total group of AML (including the other FAB types) as a reference group.
The data for ALL are depicted in the graph for both vincristine and l-asparaginase. The median LC50 value (dot) and the 25th and 75th percentiles (triangles) are shown for each drug. The number of patients (n) for each group is shown at the bottom of the graph. P values, as determined by the Mann-WhitneyU test, are shown only for statistically significant differences between the FAB-type subgroups (Table 4). LC50 values are given as μg/mL, and l-asparaginase values are given as IU/mL.
Comparison of the 3 AML FAB-type subgroups (FAB M1/M2, FAB M4, and FAB M5) as well as the total group of AML (including the other FAB types) as a reference group.
The data for ALL are depicted in the graph for both vincristine and l-asparaginase. The median LC50 value (dot) and the 25th and 75th percentiles (triangles) are shown for each drug. The number of patients (n) for each group is shown at the bottom of the graph. P values, as determined by the Mann-WhitneyU test, are shown only for statistically significant differences between the FAB-type subgroups (Table 4). LC50 values are given as μg/mL, and l-asparaginase values are given as IU/mL.
When comparing FAB M1/M2 with FAB M4, FAB M4 was 2.5-fold more resistant (P = .004) to daunorubicin than FAB M1/M2. For the other drugs, no significant differences were found. FAB M5 was significantly more sensitive to etoposide (5.4-fold,P < .001) and cytarabine (3.4-fold,P = .003) than FAB M1/M2, but these FAB types did not differ significantly from the other drugs. FAB M4, when compared with FAB M5, showed significant resistance to all anthracyclines (2.6- to 3.2-fold) as well as to mitoxantrone (12.5-fold), amsacrine (6.3-fold), etoposide (8.7-fold), cytarabine (2.9-fold), andl-asparaginase (3.6-fold). The sensitivity between FAB M4 and FAB M5 for vincristine did not reach statistical significance (greater than 40-fold, P = .016), but it is noteworthy.
ALL is considered clinically sensitive to vincristine andl-asparaginase. Therefore, we compared FAB M5 with ALL for vincristine and l-asparaginase. FAB M5 was more sensitive to vincristine than ALL (median, 1.3-fold), but this difference was not statistically significant (P = .88). Forl-asparaginase, FAB M5 was 2.1-fold more resistant than ALL, but again this did not reach statistical significance (P = .96). Of the 19 AML samples that showed intermediate prednisolone sensitivity, 10 samples were classified as FAB M1/M2. The other FAB types were: FAB M4, 5 samples; FAB M5, 1 sample; FAB M7, 2 samples; and unclassified, one sample.
Discussion
In 1994 we published a preliminary comparison of cellular drug resistance between childhood AML and ALL. We then concluded that AML was significantly more resistant to glucocorticoids and vincristine.16 In the present, much larger study we found that childhood AML was highly resistant in vitro to a large number of drugs (14 of 21). This primarily involved typical “ALL drugs” such as the glucocorticoids, vincristine, and l-asparaginase. However, several other drugs also showed significantly less cytotoxicity in AML than in ALL including the anthracyclines, mitoxantrone, etoposide, some alkylating agents, and the platinum analogues. Very high resistance ratios were found, especially for the glucocorticoids, but AML cells were also more than 4-fold resistant tol-asparaginase, vincristine, and etoposide when compared with ALL cells. None of the drugs demonstrated greater cytotoxicity in AML samples when compared with ALL. These data are in good agreement with clinical practice and may contribute to the difference in prognosis between childhood ALL and AML. Because AML was found to be resistant to many different classes of drugs, this might reflect a defect in the final common pathway of cytotoxicity, possibly in the drug-induced apoptotic pathways.
For 7 drugs, childhood AML was not significantly more resistant than childhood ALL. However, for 4 of these 7 drugs (vindesine, busulfan, amsacrine, and teniposide), the number of samples was relatively low, and their median LC50 values were still 1.3- to 5.2-fold higher in AML than in ALL. For the other 3 drugs (cytarabine, 6-thioguanine, and 6-mercaptopurine), equal sensitivity was found for the AML and ALL samples (resistance ratios, 0.9-1.0) that were studied in adequate numbers. This equal cytotoxicity corresponds well with the important therapeutic role of cytarabine in childhood AML. The intensive use of this drug has certainly led to a better prognosis for children with AML.22 Another drug, 6-thioguanine is also extensively used in childhood AML, both in induction3 as well as in consolidation and/or maintenance therapy.4,22 Our in vitro results support the frequent use of 6-thioguanine in childhood AML. Apart from their effectiveness in vitro, another advantage is that these drugs are relatively nontoxic. There have been no long-term effects described, although a recent paper by Relling et al23 pointed out that there was an increased incidence of secondary brain tumors after prophylactic cranial radiotherapy and simultaneous antimetabolite-based antileukemia therapy.
In a previous study we demonstrated that AML cells were as equally sensitive to the antimetabolite methotrexate (MTX) as ALL cells, but only after continuous exposure to MTX.24 In that study we used the in situ thymidylate synthase inhibition assay because resistance to MTX cannot be evaluated with the MTT assay.25 Based on these results, the AML-BFM SG recently started a phase II clinical trial using MTX in an up-front window as a continuous intravenous infusion in children with relapsed or refractory AML.
We never observed high sensitivity to prednisolone, and we found intermediate sensitivity only in a minority (15%) of the AML samples. Interestingly, there was an overrepresentation of FAB M1/M2 leukemias (53%, 10 of 19 samples) in this intermediately prednisolone-sensitive subgroup. Intermediate sensitivity to prednisolone was seen in 22% of the FAB M1/M2 samples, 13% of the FAB M4 samples, and only 4% of the FAB M5 samples. Hongo et al26 also reported that the FAB subtypes M1/M2 were relatively sensitive to prednisolone and described this to be associated with t(8;21).
When comparing the 3 FAB-type subgroups for further differences in resistance we found FAB M5 to be significantly more sensitive than FAB M4 to most drugs frequently used in AML treatment, ie, the anthracyclines, etoposide, and cytarabine. For the latter 2 drugs, FAB M5 was also significantly more sensitive than FAB M1/M2. Clinically, FAB M5 is characterized by younger age (often infants), high WBC count, central nervous system disease, and extramedullary leukemia, with an increased risk of early deaths due to metabolic and bleeding complications.27,28 The BFM group reports that FAB M5 is a high-risk FAB type associated with poor prognosis,4 but this is not supported by others and therefore probably treatment dependent.29-33 In the current MRC AML12 trial, resistant disease, as determined by lack of CR, has not occurred in children with FAB M5 to date, whereas the overall incidence of resistant disease in the trial is 5% (B. Gibson, Glasgow, Scotland; personal communication, ). The sensitivity of FAB M5 to etoposide has been previously published.34-36 Interestingly, FAB M5 was also 3.6-fold more sensitive to l-asparaginase than FAB M4, and there was no significant difference in l-asparaginase sensitivity between FAB M5 and ALL. This can be explained by the remarkably low level of asparagine-synthetase in FAB M5.37 38 It is also noteworthy that FAB M5 samples, as a group, are as sensitive to vincristine as ALL, whereas some FAB M5 samples are relatively vincristine-resistant (Figure 2). This explains why the very high resistance ratios we observed between the 3 FAB-type subgroups for vincristine are not significant. Based on these data we conclude that FAB M5 has a specific drug resistance profile that is different from the other FAB types studied here. Such results may support the idea of further subgroup-specific therapy in AML.
Although there were clear differences in drug resistance between the groups, the differences in LC50 values within groups (between individual samples) were often larger, leading to considerable overlap between the groups. This demonstrates that actual resistance or sensitivity should always be defined on the level of the individual patient sample, but differences between groups may allow us to rationally design further treatment protocols.
When interpreting these data, it has to be taken into account that our results for AML may not be representative of all children with AML. Due to a higher percentage of assay failures in FAB M6, FAB M7, and unclassified samples (Table 1), we tested a selection of samples, with an overrepresentation of FAB M3 and FAB M4. Also, the success rate is lower in samples taken from patients with a lower WBC count. It is difficult to estimate how this selection has influenced our drug resistance data. The WBC count is a well-known risk factor in AML, and we found FAB M4 to be a relatively resistant FAB type; therefore our results on AML may overestimate the extent of resistance. Both the CCS after 4 days of culture and the OD/105 cells (the MTT specific activity) of AML cells were higher than those parameters of ALL cells (Table 2). Within AML, FAB M1/M2 had a significantly lower median OD/105 cells than FAB M4 and FAB M5; however, there were no significant differences regarding CCS. These parameters were only very weakly correlated with resistance to some of the tested drugs, without a clear pattern of positive or negative correlations. Therefore, the differences in CCS and MTT specific activity do not seem to account for the observed differences in drug resistance between AML and ALL or the subgroups studied.
In conclusion, the poor prognosis of childhood AML in comparison with childhood ALL can, at least partially, be attributed to a general pattern of cellular drug resistance. For cytarabine and the thiopurines we found equal sensitivity between AML and ALL. Therefore, our results support the current use of those drugs in the treatment of childhood AML. In general, the use of glucocorticoids is not supported by our data, with the possible exception of some patients with FAB M1/M2. FAB M5 is relatively sensitive in vitro to all drugs frequently used in AML treatment, but it is also equally as sensitive tol-asparaginase and vincristine as childhood ALL samples. These results can be used in the rational design of further chemotherapy protocols.
Acknowledgments
The authors wish to thank all hospitals participating in the German AML-BFM SG, the German COALL Study Group, and the DCLSG. Board members of the DCLSG are H. van den Berg, J. P. M. Bökkerink, S. S. N. de Graaf, B. Granzen, P. M. Hoogerbrugge, W. A. Kamps, F. A. E. Nabben, R. Pieters, J. A. Rammeloo, T. Révész, and A. J. P. Veerman. We would also like to thank Mrs A. Heus for her secretarial support.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christian M. Zwaan, Department of Pediatric Hematology/Oncology, University Hospital Vrije Universiteit, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; e-mail:cm.zwaan@azvu.nl.