Abstract
Highly active retroviral therapy has been associated with a decline in the frequency of cytopenia in patients with human immunodeficiency virus (HIV) infection. This may result from lower hematologic toxicity of newer antiviral drugs and their increased efficacy against HIV-1. Protease inhibitors, in addition to their effects on HIV replication, appear to affect various cellular functions. Recently, it was reported that ritonavir inhibited caspase-1 expression in normal CD4+ cells. It was hypothesized that protease inhibitors may improve hematopoietic function owing to their direct effects on the bone marrow progenitor cells. When ritonavir was added to methylcellulose cultures of bone marrow cells from HIV-infected patients and normal controls, colony formation increased 2.4-fold (n = 5) in control cultures and 4-fold (n = 5) in cultures of cells from HIV-infected patients. In the presence of ritonavir, cultures of CD34+ cells showed markedly decreased apoptosis in comparison with untreated cultures (45% decrease in apoptotic cell number; n = 6). A synthetic inhibitor of caspase 1 (Ac-Tyr-Val-Ala-Asp-aldehyde [single-letter amino acid codes]), which inhibits activation of several caspases including CPP32 and interleukin 1β–converting enzyme (ICE or caspase 1), also decreased the rate of apoptosis and enhanced colony formation by progenitor cells derived from HIV-infected patients (3-fold; n = 5). In ritonavir-treated samples derived from HIV-infected individuals, the number of cells expressing ICE also decreased. In conclusion, HIV protease inhibitors may, by blocking the caspase-dependent apoptotic pathway, overcome inhibition of hematopoiesis seen in patients with HIV infection, an effect unrelated to their antiviral activity.
Introduction
Advanced HIV infection is associated with cytopenias that are, in part, related to bone marrow (BM) failure. Although direct infection of progenitor cells may be possible under some circumstances in vitro,1 CD34+cells from human immunodeficiency virus (HIV)–infected patients rarely demonstrate evidence of viral infection.2,3 Although the stem cell compartment appears to be relatively well preserved early in the disease in asymptomatic HIV-infected individuals, in patients with low CD4 counts or with opportunistic infections, there is a marked deficiency in primitive progenitors as measured by reduction in long-term colony-initiating cell (LTCIC) numbers.4Depletion of progenitor cells may be related a number of reasons including infection with HIV or apoptosis, related to changes in cytokine expresssion or other immunological factors. Data from our laboratory failed to demonstrate any evidence of infection of the most immature progenitors, the LTCICs, after exposure to HIV-1 or HIV-2 isolates.5 On the other hand, hematopoietic progenitor cells derived from BM of HIV-infected patients have been reported to undergo apoptosis at an increased rate.6 Apoptosis of hematopoietic progenitor cells, through the Fas-ligand (Fas-L)/Fas-receptor (Fas-R) pathway, is a major mechanism by which activated T cells kill virus-infected cells.7 It is likely that Fas-L and other cytokine products of activated T cells contribute to the hematopoietic inhibition of HIV-1 infection.8,9Infection with HIV or exposure of BM accessory cells to gp120 increases production of tumor necrosis factor–α (TNF-α),6 a cytokine that increases expression of Fas-R on CD34+cells.8,9 Furthermore, increased levels of Fas-L have been reported in patients with acquired immunodeficiency syndrome (AIDS), and as a general mechanism, triggering the Fas-R on hematopoietic cells results in apoptosis.8,9 This effect has been clearly demonstrated for lymphocytes derived from HIV-1 infected patients.10 TNFα and interferon-γ (IFN-γ) are both produced in increased amounts during HIV-1,11 and these cytokines up-regulate Fas expression on hematopoietic cells.8 Increased expression of Fas on CD34+cells has been found in immune-mediated BM-failure syndromes, including aplastic anemia and myelodysplastic syndrome.12Cross-linking the Fas-R by either soluble or membrane-bound Fas-L results in activation of cysteine proteases called caspases. Caspase-1 (ICE), caspase 3 (CPP32), and caspase 8 (FICE) appear to be important checkpoints leading to activation of several effector proteins and apoptosis.13 Although synthetic inhibitors of caspases block the apoptotic pathway in lymphocytes,14,15it is unclear which of the caspases is the key enzyme involved in this effect.16 Similar mechanisms have been shown to operate in hematopoietic progenitor cells.17 For example, caspase 1 is activated in CD34+ cells by Fas triggering, and synthetic inhibitors of caspases can block Fas-mediated apoptosis of hematopoietic cells.17 We and others have demonstrated that caspase-1 messenger RNA (mRNA) is constitutively produced by CD34+ cells in both normal and HIV-1–infected individuals. Although caspase 1 is not present in freshly isolated CD34+cells, Fas triggering and exposure of CD34+ cells to IFN-γ and hematopoietic growth factors result in generation of the active form of caspase-1 protein in these cells.17
HIV protease inhibitors have led to increased CD4+ cell numbers and decreased viral loads, resulting in decreased mortality rate and clinical improvement of AIDS patients. Previously, we demonstrated that HIV protease inhibitors blocked apoptosis and caspase-1 expression in culture of CD4+ cells from normal, uninfected patients and HIV-infected persons; HIV-infected patients receiving protease inhibitor combinations also demonstrated decreases in CD4+ cell apoptosis and caspase-1 content.18 On the basis of these findings, we hypothesized that a similar mechanism may also operate in early hematopoietic cells. In this study, we attempted to determine if ritonavir, a protease inhibitor, could block apoptosis, decrease ICE expression, and increase hematopoietic colony formation in BM cells derived from HIV-infected patients and normal controls.
Patients, materials, and methods
Patient selection
BM samples were obtained from patients with HIV and normal volunteers after obtaining informed consent according to protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute, Bethesda, MD, and the Georgetown University Medical Center, Washington, DC. For HIV-infected persons, BM examinations were performed for clinical indications, usually to exclude infection (5 patients) or for evaluation of cytopenias (3 patients). Only 3 of the 8 HIV-infected patients were taking protease inhibitors at the time of BM aspiration. Of the remaining patients, 5 were taking alternate nucleoside analogs. None had active cytomegalovirus infection; all had CD4+ counts below 200 cell/μL.
Cell preparation
BM was obtained by aspiration from the posterior iliac crest into syringes containing media supplemented 1:10 with heparin (O'Neill and Feldman, St Louis, MO). BM mononuclear cells (BMMCs) were isolated by density gradient centrifugation with the use of lymphocyte separation medium (Organon, Durham, NC). Cells were washed in Hank balanced salt solution (Life Technologies, Gaithersburg, MD), resuspended in Iscove modified Dulbecco medium (Life Technologies), and supplemented with 20% fetal calf serum (Life Technologies).
Flow cytometric analysis
Phycoerythrin (PE)-conjugated monoclonal antibody (mAb) directed against human CD34+ antigen (Becton Dickinson, Mountain View, CA) was used for phenotypic analysis of BM progenitor cells. Samples were analyzed by means of an Epics Elite flow cytometer (Coulter, Hialeah, FL).
Intracellular staining
Intracellular staining for ICE expression was performed with the PharMingen Intracellular Staining Kit (Franklin Lakes, NJ).19 BMMCs or peripheral blood mononuclear cells (PBMCs) were stained with PE-conjugated CD34+antibody, fixed, and permeabilized and stained with anti-ICE antibody and fluorescein isothiocyanate-labeled (FITC)–conjugated antirabbit antibody. Permeabilized isotypic controls were stained with secondary antibodies. Previous data17 18 demonstrated correlation of intracellular staining for ICE with immunoblot.
Separation of CD34+ cells
After washing with phosphate-buffered saline (PBS) (Life Technologies) supplemented with 2% human albumin, cells were applied to an affinity column containing biotin-coated beads, and the CD34+ cell fraction was eluted with PBS. An aliquot of eluted cells was stained with PE-conjugated anti-CD34+ mAb (Becton Dickinson) to assess purity; usually, 70% to 90% of separated cells were positive for CD34 antigen. For preparations of higher purity, cells were further fractionated: column-purified cells were stained with FITC anti-CD34 mAb, washed with PBS, and sorted by flow cytometry (Epics V; Coulter); by this combined method, the purity of cells was 97% to 99%.
Hematopoietic cell culture
Numbers of hematopoietic colony-forming cells were measured in methylcellulose colony cultures under standard conditions. Freshly isolated BMMCs were plated in methylcellulose (Stem Cell Technology, Vancouver, BC, Canada) in the presence of 50 ng/mL interleukin-3 (IL-3; Genzyme, Boston, MA), 20 ng/mL granulocyte-macrophage colony stimulating factor (Boehringer, Indianapolis, IN), 50 ng/mL stem cell factor (Amgen, Thousand Oaks, CA), and 2 U/mL erythropoietin (Amgen). Total BMMCs were plated at a density of 1 × 105 in 1 mL of medium in 35-mm dishes. CD34+ cells were plated at a density of 1 × 103 cells/0.5 mL methylcellulose in 48-well 11-mm plates. Colonies on replicate plates were counted, and the average number of colonies per 105 cells was calculated. When appropriate, 100 μg/mL of ICE inhibitor (Ac-Tyr-Val-Ala-Asp-aldehyde) (Calbiochem, Cambridge, MA) was added to culture of BM obtained from HIV-infected patients (this concentration was previously found to be optimal17 for increasing colony formation in normal donors). This ICE inhibitor has demonstrated specificity for inhibition of caspase 1, 8, and 3.20
Long-term BM culture for the determination of LTCIC numbers was performed. We used 10 × 106 BMMCs to initiate stromal culture. Allogeneic stroma was grown to confluence. Culture medium consisted of stem cell medium (Stem Cell Technology) supplemented with 1 × 10−6 mol/L hydrocortisone succinate (Sigma, St Louis, MO) and was replaced weekly. After 3 weeks of culture, stromal cells were briefly treated with trypsin, washed, and placed in 48-well plates. After reestablishment of a confluent cellular layer, the plates were irradiated (15 Gy of 250-kV x-rays) and used for the measurement of LTCIC content in mononuclear cell fractions derived from BM and PB of patients and healthy volunteers. At least 2 (if cell numbers were low) but more often 3 or more cell concentrations were applied to preestablished, irradiated stromal feeder layers and cultured at 33°C for 5 weeks. Media changes were performed weekly. After 5 weeks, the adherent cells were harvested by treatment with trypsin (Life Technologies), washed, and replated in duplicate in methylcellulose in order to estimate the numbers of cells able to form secondary colonies.
Apoptosis assays
Apoptotic cells were quantitated by means of the annexin assay.21 BMMCs were prepared as described above. After culture, cells were washed with PBS and stained with annexin and propidium iodide as previously described.17 21Samples were analyzed by means of flow cytometry. Gates were drawn to exclude dead, propidium-iodide–staining cells, and the number of apoptotic cells staining with annexin were quantitated.
Statistical analysis
Statistical significance was calculated by means of a nonparametric Wilcoxon 2-sample or 1-sample test.
Results
Effect of ritonavir on hematopoietic colony formation in short-term methylcellulose colony assay and long-term BM culture
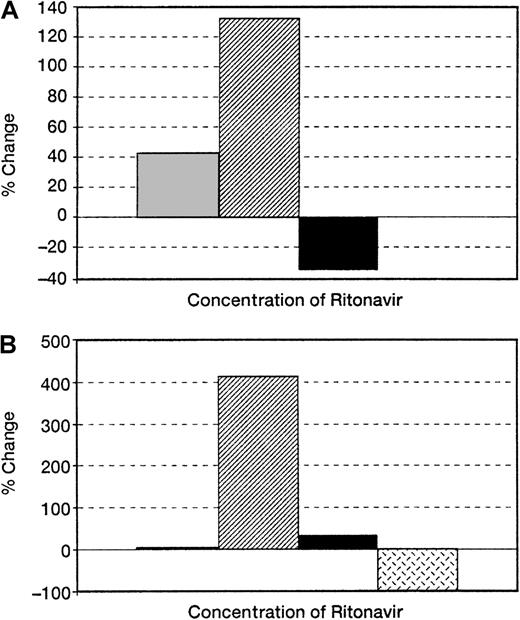
First, we determined the effect of ritonavir on colony formation measured in short-term culture. When ritonavir was added to methylcellulose cultures of BM cells from HIV-infected patients and normal controls, colony formation was augmented; in cultures from uninfected normal controls, the number of colonies was increased 2.4-fold (n = 5; P < .01) (Figure1) at concentrations of 5 nm; in cultures of cells from HIV-infected patients there was a 4-fold increase (n = 3) (P < .05). Both myeloid and erythroid colonies were equally increased.
Effect of ritonavir on colony formation of BM derived from normal and HIV-infected persons.
BMMCs obtained from (A) normal uninfected controls (n = 5) and (B) HIV-infected patients (n = 5) were plated in methylcellulose as previously described. Ritonavir increased colony formation in a dose-dependent fashion in cultures of cells from both HIV-infected patients and normal controls, but to a greater extent in the patient samples. Samples of BMMCs plated with 3 nm ritonavir (n = 3) showed increases in colony growth between 2 nm and 5 nm (20% in normal samples and 200% in HIV-infected persons). ░ indicates a 2-nmol/L concentration of Ritonavir; ▨, 5-nmol/L concentration; ▪, 10-nmol/L concentration;  , 20-nmol/L concentration.
, 20-nmol/L concentration.
Effect of ritonavir on colony formation of BM derived from normal and HIV-infected persons.
BMMCs obtained from (A) normal uninfected controls (n = 5) and (B) HIV-infected patients (n = 5) were plated in methylcellulose as previously described. Ritonavir increased colony formation in a dose-dependent fashion in cultures of cells from both HIV-infected patients and normal controls, but to a greater extent in the patient samples. Samples of BMMCs plated with 3 nm ritonavir (n = 3) showed increases in colony growth between 2 nm and 5 nm (20% in normal samples and 200% in HIV-infected persons). ░ indicates a 2-nmol/L concentration of Ritonavir; ▨, 5-nmol/L concentration; ▪, 10-nmol/L concentration;  , 20-nmol/L concentration.
, 20-nmol/L concentration.
To assess the effect of ritonavir on the most primitive progenitor cells, we also placed BM of normal donors with ritonavir (5 nm) in long-term culture, and determined progenitor number by replating experiments performed after 5 weeks. There was a 2-fold increase in the yield of secondary colonies in cultures containing ritonavir (Table1).
Effect of ritonavir on LTCIC of normal BM
| Ritonavir concentration . | Colonies/105 PBMCs . |
|---|---|
| 0 nm | 2 ± 3 |
| 5 nm | 15 ± 2 |
| 10 nm | 0 |
| Ritonavir concentration . | Colonies/105 PBMCs . |
|---|---|
| 0 nm | 2 ± 3 |
| 5 nm | 15 ± 2 |
| 10 nm | 0 |
Ritonavir was added to long-term bone marrow (BM) culture at a concentration of 5 nm. Culture medium consisted of stem cell medium, supplemented with hydrocortisone succinate with or without retonavir, and was replaced weekly. Colonies were assessed after 5 weeks as described in “Materials and methods” (n = 2).
Effect of a caspase inhibitor on colony formation of PBMCs obtained from HIV-infected patients
We previously demonstrated that CD34+ cells demonstrated up-regulated expression of capsase-1 during in vitro culture and that the level of caspase-1 expression correlated with apoptosis of these cells.17 In addition, a caspase-1 inhibitor also increased colony growth in normal BM culture. We now determined whether addition of a synthetic caspase inhibitor can also affect colony formation in BM obtained from HIV-infected patients. A caspase-1 inhibitor, in concentrations similar to those reported to increase normal BM growth, increased colony growth of BM progenitor cells obtained from HIV-infected patients17(Figure 2).
Effect of caspase inhibitor on colony formation of BM obtained from HIV-infected persons.
Effects of ICE inhibitor on hematopoietic colony formation by BMMCs obtained from HIV-infected patients (n = 3). Numbers of erythroid (▨) and myeloid (▪) colonies are demonstrated as bars seen after 105 BMMCs. Results are similar to those observed in normal controls.
Effect of caspase inhibitor on colony formation of BM obtained from HIV-infected persons.
Effects of ICE inhibitor on hematopoietic colony formation by BMMCs obtained from HIV-infected patients (n = 3). Numbers of erythroid (▨) and myeloid (▪) colonies are demonstrated as bars seen after 105 BMMCs. Results are similar to those observed in normal controls.
Effect of ritonavir on apoptosis and ICE expression of CD34+ cells
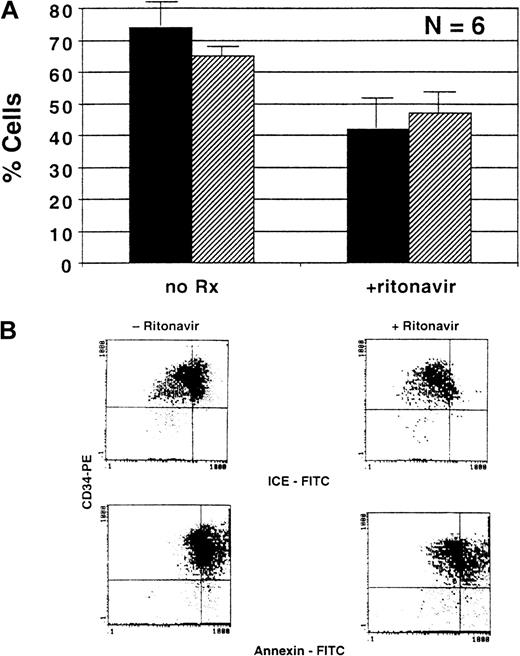
CD34+ cells obtained from HIV-infected BM reportedly show increased apoptosis.6 Because of the increase in colony formation in both long-term and short-term cultures performed in the presence of ritonavir, we studied the effect of ritonavir on apoptosis and ICE expression of purified CD34+ cells. We also measured expression of caspase 1 to determine whether the caspase-mediated transduction cascade was involved in the effect of ritonavir. When CD34+ cells were cultured in the presence of ritonavir, there was a 45% decrease in the number of apoptotic cells as determined in the annexin assay (n = 6;P < .05) (Figure 3). This effect was dose-related up to the concentration of 5 nm; higher concentrations of ritonavir resulted in toxicity and drastically decreased cell viability (data not shown). Fas-R expression was unaffected by the addition of ritonavir to CD34+ cells (data not shown); the expression of caspase 1, as measured by double-staining with CD34 mAb and intracellular staining for ICE, was decreased after 2 days of culture (n = 5; P < .05) (Figure 3).
Effect of ritonavir on apoptosis and cell death in normal BM.
Samples of purified CD34+ cells, obtained from normal uninfected controls, were cultured with and without ritonavir and stained with annexin V or ICE antibody after 72 hours. (A) Summary of results from 5 experiments performed to measure the number of apoptotic cells. ▪ Indicates annexin; ▨, ICE. (B) Examples of flow cytometry scattergrams showing annexin-stained cells cultured with and without ritonavir.
Effect of ritonavir on apoptosis and cell death in normal BM.
Samples of purified CD34+ cells, obtained from normal uninfected controls, were cultured with and without ritonavir and stained with annexin V or ICE antibody after 72 hours. (A) Summary of results from 5 experiments performed to measure the number of apoptotic cells. ▪ Indicates annexin; ▨, ICE. (B) Examples of flow cytometry scattergrams showing annexin-stained cells cultured with and without ritonavir.
Discussion
The HIV protease inhibitors decrease viral replication and result in reduced viremia and slowed progression to AIDS. Clinically, these agents have significantly improved the quality of life of patients with HIV-1 infection and have led to remarkably decreased mortality due to AIDS. Following the introduction of treatment with protease inhibitor combinations, symptomatic cytopenias and the transfusion requirements in HIV-infected patients have concurrently fallen. It is likely that decreased viremia in patients successfully treated with antiviral combination therapy also results in diminished levels of cytokines with inhibitory properties on the hematopoietic system, such as IFN-γ, TNF-α, or Fas-L.19 High levels of IFN-γ and TNF-α not only are associated with a poor prognosis in HIV infection,22 but are correlated with the degree of anemia.23 Furthermore, IFN-γ and TNF-α up-regulate Fas-R expression on CD34+ cells and result in apoptosis of CD34+ cells and inhibition of hematopoiesis in vitro. Infection of progenitor cells with HIV-1 has been reported infrequently in patients with advanced disease2; furthermore, infection of progenitor cells alone does not generally result in significant suppression of BM function.5 Therefore, it seemed possible that protease inhibitors decreased the incidence of cytopenias by a mechanism independent of its effects on HIV replication in early hematopoietic cells.
We hypothesized that protease inhibitors must have a direct effect on hematopoietic colony formation by inhibiting apoptosis and, more specifically, caspase expression in CD34+ cells. In our study, we have shown that ritonavir increased colony formation of normal BM progenitors and progenitor cells derived from HIV-1–infected persons. This positive effect was paralleled by decreased apoptosis and caspase expression in the CD34+ cell compartment. In agreement with these results, we have previously reported that normal unmanipulated CD34+ cells expressed caspase-1 mRNA, though we did not detect significant expression of ICE protein in these cells.17 However, most CD34+ cells expressed ICE protein in tissue culture and underwent apoptosis that could be blocked by addition of a synthetic caspase inhibitor. IFN-γ up-regulated expression of ICE inhibitor and promoted apoptosis. Increased apoptotic CD34+ cells have been reported in patients with HIV infection with cytopenias.6 The overexpression of IFN-γ and TNF-α by lymphocytes in the HIV-infected patients11 may increase expression of Fas-R and ICE expression in CD34+ cells and thus be responsible for the more exaggerated effect of ritonavir on BM from HIV-infected patients when compared with uninfected marrow.
The beneficial effect of caspase blockade on normal marrow may be related to inhibitory cytokines produced constitutively in the BM microenvironment. It is important to note that we measured ICE (caspase 1) expression, other caspases may also have been involved.16 We recently demonstrated that caspase 1 is most important in modulating CD34+ cell apoptosis.24
In summary, ritonavir increased colony growth of BM obtained either from HIV-infected patients with advanced disease or from normal individuals. Apoptosis and ICE expression in purified CD34+were also decreased during treatment in vitro. Protease inhibitors may improve hematopoiesis in HIV-infected patient by a mechanism that is independent of their effect on HIV viremia. Whether this class of drugs could be used in other marrow failure states needs further research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elaine M. Sloand, National Heart, Lung, and Blood Institute, National Institutes of Health, 31 Center Dr, MSC 2490, Bldg 31, Rm 4A11, Bethesda, MD 20892-2490.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal