Abstract
Chronic myelogenous leukemia (CML) is a clonal disorder of a pluripotent hematopoietic stem cells characterized by a chimericbcr-abl gene giving rise to a p210Bcr-Ablprotein with dysregulated tyrosine kinase activity. Radicicol, a macrocyclic antifungal antibiotic, binds to the N-terminal of heat shock protein 90 (Hsp90) and destabilizes Hsp90-associated proteins such as Raf-1. This study investigated the effect of radicicol, novel oxime derivatives of radicicol (KF25706 and KF58333), and herbimycin A (HA), a benzoquinoid ansamycin antibiotic, on the growth and differentiation of human K562 CML cells. Although KF25706 and KF58333 induced the expression of glycophorin A in K562 cells, radicicol and HA caused erythroid differentiation transiently. Cell cycle analysis showed that G1 phase accumulation was observed in K562 cells treated with KF58333. KF58333 treatment depleted p210Bcr-Abl, Raf-1, and cellular tyrosine phosphorylated proteins in K562 cells, whereas radicicol and HA showed transient depletion of these proteins. KF58333 also down-regulated the level of cell cycle–dependent kinases 4 and 6 and up-regulated cell cycle–dependent kinase inhibitor p27Kip1protein without an effect on the level of Erk and Hsp90 proteins. Immunoprecipitation analysis showed that p210Bcr-Abl formed multiple complexes with Hsp90, some containing p23 and others Hsp70; KF58333 treatment dissociated p210Bcr-Abl from Hsp90/p23 chaperone complexes. Furthermore, KF58333 induced apoptosis in K562 cells and administration of KF58333 prolonged the survival time of SCID mice inoculated with K562 cells. These results suggest that KF58333 may have therapeutic potential for the treatment of CML that involves abnormal cellular proliferation induced by p210Bcr-Abl.
Introduction
Chronic myelogenous leukemia (CML) is associated with a specific chromsomal translocation known as the Philadelphia (Ph) chromosome that results from a reciprocal translocation between the long arms of chromosome 9 and the long arms of chromosome 22.1 This t(9,22) translocation fuses bcrsequences upstream of the second exon of c-abl and generates 1 of 2 Bcr-Abl fusion proteins, the p185 or p210.2-5 The p210 form of Bcr-Abl (p210Bcr-Abl) is detected in 95% of the patients with CML, whereas the p185 form (p185Bcr-Abl) is observed in 5% of patients with childhood acute lymphocytic leukemia (ALL) and in more than 20% of adults with ALL.6,7 Furthermore, previous studies have shown that p210Bcr-Abl protein has a Src homology 2 domain (SH2) and that activated forms of p210Bcr-Abl tyrosine kinase require the SH2 for the transformation of fibroblasts.8
Because the tyrosine kinase activity of the Bcr-Abl proteins has been shown to be responsible for the expansion of leukemic cells at the blast stage and to be essential for their transforming ability, Bcr-Abl protein kinase has been thought to be a molecular target for chemotherapy of CML and other bcr-abl–positive leukemia. Under these circumstances, many investigators have reported on antagonists against the expression product of the bcr-ablchimeric gene. It has been reported that tyrphostin, a small molecule tyrosine kinase inhibitor, could inhibit Bcr-Abl tyrosine kinase and thus induce the differentiation of K562 CML cells.9 More recently Druker and colleagues10have reported that a selective inhibitor of Bcr-Abl tyrosine kinase, a 2-phenylaminopyrimidine class of compounds, could inhibit in vitro proliferation as well as in vivo tumor formation of Bcr-Abl–expressing CML cells. Honma and coworkers11 12 have also reported that herbimycin A (HA), a benzoquinoid ansamycin antibiotic originally isolated as a tyrosine kinase inhibitor, reduces tyrosine phosphorylation of K562 CML cells, thus inducing erythroid differentiation.
On the other hand, it has been shown that geldanamycin (GA), another benzoquinoid ansamycin antibiotic, could bind to the N-terminal site of heat shock protein 90 (Hsp90) and its homologue Grp94, both of which play an essential role in the folding and function of important cellular proteins including Raf-1 kinase, steroid hormone receptor, mutant p53, and p185ErbB2.13-18 Binding of GA to Hsp90 caused dissociation of Raf-1 or p185ErbB2 from the Hsp90 family of protein-containing complexes and consequently destabilized these kinases, leading to the attenuation of downstream signal transduction pathways.
Recently we have revealed that radicicol, a macrocyclic antifungal antibiotic originally isolated from the fungus Monosporium bonorden, could also bind to the N-terminal GA biding site of Hsp90 protein and thus destabilize Hsp90-associated proteins such as Raf-1 or p185ErbB2.19-21 Radicicol was also shown to possess many biologic activities, such as inhibition of tyrosine kinase, induction of differentiation, and reversion of oncogene-induced transformation.22 Radicicol lacks in vivo antitumor activity because of its chemical instability in animals; therefore, we have generated a novel series of oxime derivatives of radicicol that are more stable than the parent compound in vivo and share important biologic activities such as binding to Hsp90 and growth inhibitory activity in vitro (patent number: WO98/18780).
In this report, we show for the first time that both radicicol and its novel oxime derivatives, KF25706 or KF58333, induce differentiation of K562 CML cells into erythroid lineage through putative destabilization of p210Bcr-Abl protein, which might be mediated through its binding to Hsp90. In addition, KF58333 but not radicicol induced preferential G1-phase accumulation through putative down-regulation of cell cycle–dependent kinases 4 and 6 (Cdk4 and Cdk6) as well as up-regulation of the Cdk inhibitor p27Kip1, which was accompanied by induction of apoptosis of the cells. Furthermore consecutive intravenous (IV) injection of KF58333 prolonged the survival time of SCID mice inoculated with K562 cells.
Materials and methods
Reagents
Radicicol and HA were isolated and purified in our institute as previously described.19 Novel oxime derivatives of radicicol such as KF25706 or KF58333 were synthesized in our laboratories and this will be reported elsewhere. For in vitro assay, the stock solution of HA, radicicol, KF25706, and KF58333 was prepared as a 10 mmol/L dimethylsulfoxide (DMSO) solution and stored at −20°C until use. Cyclophosphamide (CY) was obtained from Shionogi Pharmaceuticals (Osaka, Japan).
Antibodies
Antityrosine phosphorylated protein monoclonal antibody was obtained from Kyowa Medex (Tokyo, Japan). Antihuman glycophorin A (GPA) monoclonal antibody, anti-p210Bcr-Abl monoclonal antibody, and anti-Bcl-2 monoclonal antibody were obtained from Pharmingen (San Diego, CA). Anti-Raf-1, anti-Cdk4, anti-Cdk6, and anti-p27Kip polyclonal antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Erk monoclonal antibody and anti-Cdk2 monoclonal antibody were obtained from Upstate Biotechnology Inc (Lake Placid, NY). Antiphosphorylated Erk monoclonal antibody was obtained from New England Biolabs (Beverly, MA). Anti-Hsp90 monoclonal antibody was obtained from Transduction Laboratories (Lexington, KY). Anti-Hsp70 monoclonal antibody and anti-p60Hop monoclonal antibody were purchased from StressGen Biotechnologies Corp (Victoria, British Columbia, Canada). Anti-p23 monoclonal antibody was a kind gift of Dr David Toft (Rochester, MN). Anti β-actin monoclonal antibody was obtained from Sigma (St Louis, MO). Anti-PARP monoclonal antibody was obtained from Enzyme System Products (Dublin, CA). Fluorescein isothiocyanate (FITC)-labeled goat antimouse IgG monoclonal antibody was obtained from Wako (Osaka, Japan).
Cell line and cell culture
The K562 cell line was originally established from a pleural effusion of a patient with CML in terminal crisis. K562 cell line was obtained from American Type Culture Collection. The cells were cultured in RPMI1640 (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; GIBCO), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (GIBCO) at 37°C in a humidified atmosphere of 5% CO2 in air. DMSO stock solutions of HA, radicicol, KF25706, and KF58333 were diluted to a final concentration of 0.1% (vol/vol) to minimize the biologic effect of DMSO. K562 cells (5×104cells/mL) were cultured in the presence of varying doses of drug for the indicated time periods and the cells were washed with calcium- and magnesium-free phosphate-buffered saline (PBS[−]) and used for further experiments. Control cells were incubated with 0.1% (vol/vol) of DMSO.
MTT assay
The K562 cells (5×104 cells/mL) were seeded in triplicate in RPMI1640 medium containing 10% FBS in 96-microwell plates (Nunc) and were incubated with various concentration of the drugs for 72 hours. After 10 μL of a 5 mg/mL stock solution of MTT (3-[4, 5-dimethylthiazol-2, 5-diphenyl tetrazolium bromide]) (Sigma) was added to each well for the last 4 hours of the culture, the formed formazan crystals were solibilized by adding 100 μL isopropanol-0.04 N HCl. Then the plates were measured for optical density by a dual-beam microplate reader (Wako) using a test wavelength of 570 nm with a reference of 630 nm. The concentration of drug required for 50% inhibition of cell growth (IC50) was determined by Soft max program (Wako).
Differentiation of K562
Expression of the erythroid-specific surface marker GPA was determined using a method previously described.22 Briefly, 1×106 cells were incubated at 4°C for 30 minutes with 100 μL (10 μg/mL) of mouse antihuman GPA monoclonal antibody (Pharmingen). Then the cells were washed twice with ice-cold PBS(−) to remove unbound antibody, resuspended in 10 μg/mL of FITC-labeled goat antimouse IgG (Wako), and incubated at 4°C for 30 minutes in the dark. The cells were washed twice with ice-cold PBS(−) and resuspended in 1% paraformaldehyde in PBS(−), pH7.4. Mouse IgG1 (Wako) was used as an isotype-matched negative control for each sample. Ten thousand events were analyzed for each sample by FACScan (Becton Dickinson, Bedford, MA). The cells treated with KF25706 or KF58333 were stained with benzidine (Sigma) as described previously.23 Briefly K562 cells were seeded in 24-well plates at a density of 104 cell/mL for the indicated time; the medium was carefully removed and the wells were overlaid with 100 μL bezidine solution. The percentage of bezidine-positive cells was determined after 15 minutes of incubation at room temperature.
Cell cycle analysis
The K562 cells were analyzed for nuclear DNA content using the method previously described. Briefly, 1×106 cells were suspended in a solution containing 0.1% Triton X-100 (Wako), 50 μg/mL of propidium iodide (PI; Wako), and 50 μg/mL of RNAse (Sigma). The cell cycle distribution was determined by the analysis of DNA content using FACScan and CellFIT system according to the manufacturer's protocol (Becton Dickinson).
Cell lysis and Western blotting
The K562 cells were lysed for 10 minutes by the addition of 50 μL of ice-cold lysis buffer (50 mmol/L Hepes-NaOH [pH 7.4], 250 mmol/L NaCl, 1 mmol/L EDTA, 1% Nonidet P-40, 1 mmol/L Dithiothreitol [Wako], 1 mmol/L phenylmethylsulfonyl fluoride [Sigma], 5 μg/mL leupeptin [Sigma], 2 mmol/L Na3VO4 [Wako], 1 mmol/L NaF [Wako], 10 mmol/L β-glycerophosphate [Wako]) on ice and the cell lysate was clarified by centrifugation. Ten micrograms of cell lysate of each sample was electrophoresed by 12.5% or 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (Dai-ichi Pure Chemicals, Tokyo, Japan), transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA), and immunoblotted with appropriate antibodies. For detection, the blots were incubated with the appropriate enhanced chemifluorescence (ECF) antibody (alkaline-phosphatase conjugated anti-IgG antibody; Bio-Rad Laboratories, Hercules, CA) and developed using the ECF detection system (Molecular Dynamics, Sunnyvale, CA), according to the instruction of the manufacturer. Membranes were scanned into Fluor Imager (Molecular Dynamics) and were analyzed by using Image Quant software (Molecular Dynamics).
Apoptosis
To detect early stage of apoptotic cells, an annexin V-FITC kit was used according to the manufacturer's instructions (Genzyme, Cambridge, MA). Briefly, K562 cells were cultured as indicated above for 48 hours, washed, and then stained with PI and annexin V-FITC in annexin-binding buffer at room temperature for 15 minutes in the dark. Samples were then diluted with binding buffer and were analyzed by FACScan within 1 hour.
Immunoprecipitation
Cells were lysed as detailed above and p210Bcr-Ablwas immunoprecipitated from approximately 1 mg of cellular proteins using 2 μg of p210Bcr-Abl monoclonal antibody as described previously.15 Following extensive washing, immunoprecipitates were resolved on 10% SDS-PAGE gels, transferred to PVDF membranes, and immunoblotted for Hsp90, Hsp70, p23, p60Hop, and p210Bcr-Abl as described above.
Half-life of Bcr-Abl determination
KF58333 (0.05 μmol/L) was added to K562 cells for 2 hours. Treated and untreated cells were washed and resuspended in methionine/cysteine-free RPMI containing 10% dialyzed FBS (Biofluids, Gaithersburg, MD). Exposure of treated cells to KF58333 was maintained. After 30 minutes, 35S-methionine/cysteine (Translabel, 100 μCi/mL; ICN Biochemical, Costa Mesa, CA) was added and incubation was continued for 1 hour. Cells were then washed and resuspended in nonradioactive, complete medium. Exposure to KF58333 was maintained in the treated cultures. At various times, cells were removed from culture, washed, and lysed and immunoprecipitated for Bcr-Abl protein as described previously. Immunoprecipitates were resolved by 7.5% SDS-PAGE. Gels were fixed, enhanced (Enlightening, Dupont/NEN, Boston, MA), and exposed to Kodak XAR-5 film (Eastman-Kodak, Rochester, NY). Bcr-Abl–specific bands were quantified by densitometric scanning/image analysis.
Animals
The SCID mice, at 5 weeks old, were purchased from Nippon SLC (Shizuoka, Japan), maintained on commercial food, water ad libitum, and housed at 23°C ± 5°C and 55% ± 5% relative humidity through the experiment.
Administration of KF58333
Ten SCID mice per group were intraperitoneally injected with CY (150 mg/kg) on day 0 and day 1 and inoculated intravenously with 107 K562 cells on day 2. KF58333 was dissolved in water containing 0.9% NaCl, 5% cremophore EL (Sigma), and 5% dimethylacetamide (Wako); 50 mg/kg KF58333 was administered in a volume of 0.25 mL/animal once a day from day 19 to day 23.
Statistical analysis
Statistical significance was determined using Mann-WhitneyU test; P values of less than .05 were considered to statistically significant.
Results
Antiproliferative activity of HA, radicicol, KF25706, and KF58333 against CML K562 cells
At first, we compared the growth inhibitory activity of HA, radicicol, KF25706, and KF58333 (Figure1) against cultured CML K562 cell line after 72 hours of exposure by the MTT method. As previously reported, HA showed concentration-dependent growth inhibitory activity against K562 cells, and both radicicol and its novel oxime derivatives, KF25706 or KF58333, exerted growth inhibitory activity against the cells (Figure 2). The concentrations of drug required for the IC50 of HA, radicicol, KF25706, and KF58333 against K562 cells were 0.27, 0.20, 0.30, and 0.033 μmol/L, respectively.
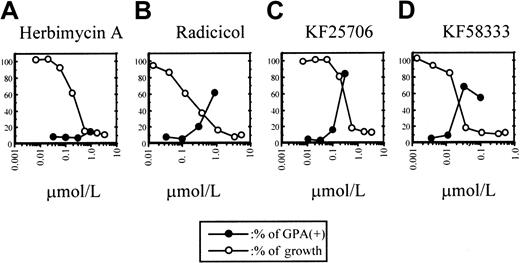
Differentiation and growth of K562 cells.
The relationship between differentiation and growth of K562 cells and treatment with HA (A), radicicol (B), KF25706 (C), and KF58333 (D). K562 cells were cultured with increasing concentrations of HA, radicicol, KF25706, and KF58333 for 72 hours and analyzed for differentiation and cell growth as described in “Materials and methods.” Percent of GPA-positive cells and relative percent of growth compared with untreated cells are plotted.
Differentiation and growth of K562 cells.
The relationship between differentiation and growth of K562 cells and treatment with HA (A), radicicol (B), KF25706 (C), and KF58333 (D). K562 cells were cultured with increasing concentrations of HA, radicicol, KF25706, and KF58333 for 72 hours and analyzed for differentiation and cell growth as described in “Materials and methods.” Percent of GPA-positive cells and relative percent of growth compared with untreated cells are plotted.
Erythroid differentiation of K562 by HA, radicicol, KF25706, and KF58333
It has been reported that GPA is selectively expressed on the cell surface of erythroblast or erythroleukemia cells thus suggesting that GPA could be a good marker for erythroid differentiation.20 The concentration dependency of the inhibition of cell growth and the induction of erythroid differentiation after 72 hours treatment of each drug was plotted in Figure 2. Although the percentage of GPA-expressing K562 cells (GPA positive) increased dramatically above the IC50 values of radicicol, KF25706, and KF58333, HA induced a relatively low level of expression of GPA in this condition.
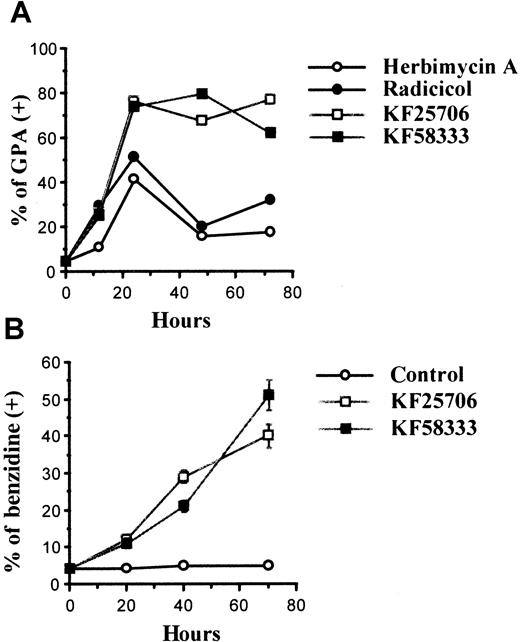
The K562 cells were treated with 0.3 μmol/L of HA, radicicol, KF25706, and 0.05 μmol/L of KF58333 for 12 to 72 hours and analyzed for the expression of GPA using FACScan. The time course of the percentage of GPA-positive expression after treatment with each drug is shown in Figure 3A. When K562 cells were treated with KF25706 (0.3 μmol/L) or KF58333 (0.05 μmol/L), more than 65% of cells became GPA positive after 24 hours of drug exposure and the effect lasted for at least 72 hours. HA (0.3 μmol/L) also induced GPA expression transiently at 24 hours (34%) and only 15% of the treated cells were GPA positive at 72 hours. Radicicol (0.3 μmol/L) also induced GPA expression transiently at 24 hours (43.6%) and 28.6% of the treated cells were GPA-positive at 72 hours. Furthermore, K562 cells treated with KF25706 (0.3 μmol/L) or KF58333 (0.05 μmol/L) were stained with benzidine to elucidate the possibility that radicicol derivatives simply increased the expression of GPA independent from erythroid differentiation. As shown in Figure3B, both KF25706 and KF58333 increased the percentage of benzidine-positive cells.
Time course of erythroid differentiation by HA, radicicol, KF25706, and KF58333.
K562 cells were cultured with 0.3 μmol/L of HA, radicicol, KF25706, and 0.05 μmol/L of KF58333 for the indicated times and analyzed for differentiation as described in “Materials and methods.” Percent of GPA-positive cells (A) and percent of benzidine-positive cells (B) are indicated.
Time course of erythroid differentiation by HA, radicicol, KF25706, and KF58333.
K562 cells were cultured with 0.3 μmol/L of HA, radicicol, KF25706, and 0.05 μmol/L of KF58333 for the indicated times and analyzed for differentiation as described in “Materials and methods.” Percent of GPA-positive cells (A) and percent of benzidine-positive cells (B) are indicated.
Effect of HA, radicicol, KF25706, and KF58333 on the cell cycle
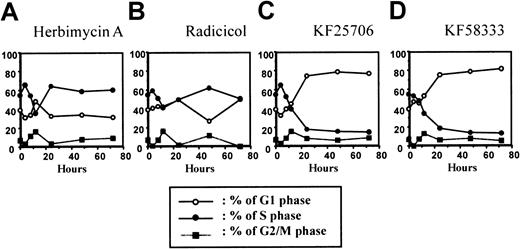
To further elucidate the mechanism(s) of differentiation induced by HA, radicicol, KF25706, and KF58333, we investigated the effect of these 4 drugs on cell cycle distribution of K562 cells using the PI single staining method. As shown in Figure4, HA (0.3 μmol/L) caused transient G1 phase accumulation at 12 hours after drug treatment; however, no further effects on the cell cycle were observed thereafter. KF25706 (0.3 μmol/L) or KF58333 (0.05 μmol/L) induced a time-dependent accumulation of the cells in G1 phase from 12 to 48 hours after drug exposure (Figure 4). Radicicol (0.3 μmol/L) showed little, if any, effect on cell cycle distribution of K562 in this condition (Figure 4).
Effect of treatment on the cell cycle in K562 cells.
The time course of cell cycle effect after treatment with HA (A), radicicol (B), KF25706 (C), and KF58333 (D) in K562 cells. K562 cells were cultured with 0.3 μmol/L of HA, radicicol, KF25706, and 0.05 μmol/L of KF58333 for indicated times and analyzed for cell cycle by FACScan as described in “Materials and methods.” Percents of G1 phase (○), S phase (●), and G2/M phase (▪) are plotted.
Effect of treatment on the cell cycle in K562 cells.
The time course of cell cycle effect after treatment with HA (A), radicicol (B), KF25706 (C), and KF58333 (D) in K562 cells. K562 cells were cultured with 0.3 μmol/L of HA, radicicol, KF25706, and 0.05 μmol/L of KF58333 for indicated times and analyzed for cell cycle by FACScan as described in “Materials and methods.” Percents of G1 phase (○), S phase (●), and G2/M phase (▪) are plotted.
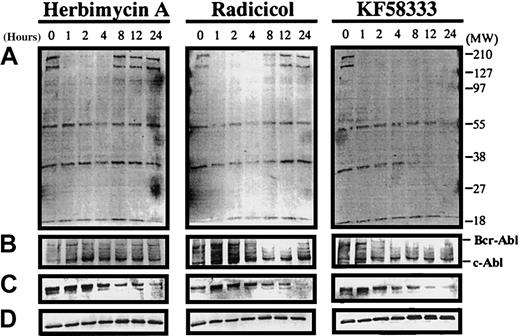
Destabilization of Hsp90-associated proteins by HA, radicicol, and KF58333
Recently, it has been revealed that radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with the ansamycin antibiotic, GA.21 To elucidate whether the induction of differentiation or cell cycle effects of these drugs would be mediated by their interaction with Hsp90, we examined the effect of HA, radicicol, and KF58333 on protein expression levels of p210Bcr-Abl, Raf-1, and tyrosine phosphorylated proteins in K562 cells using Western blotting analysis. When K562 cells were treated with HA (0.3 μmol/L), transient down-regulation of p210Bcr-Abl (Figure 5B), Raf-1 (Figure 5C), and tyrosine phosphorylated proteins (Figure 5A) was observed from 4 to 12 hours after treatment; however, the expression level of these proteins returned to basal levels thereafter. When K562 cells were treated with radicicol (0.3 μmol/L), transient depletion of p210Bcr-Abl, Raf-1, and tyrosine phosphorylated proteins was also observed from 4 to 24 hours after treatment. In sharp contrast, when K562 cells were treated with KF58333 (0.05 μmol/L), depletion of p210Bcr-Abl and tyrosine phosphorylated proteins began to be observed from 4 hours after treatment and down-regulation of Raf-1 protein was observed at 8 hours after treatment; these effects lasted for at least 72 hours after drug exposure. KF25706 (0.3 μmol/L) also showed similar activity (data not shown). No clear effect on Erk protein expression level was observed after HA, radicicol, and KF58333 treatment (Figure 5D), suggesting that the depletion of p210Bcr-Abl, Raf-1, and tyrosine phosphorylated proteins was not the result of nonspecific protein degradation. Depletion of p210Bcr-Abl and Raf-1 protein was also observed in KU812 CML cells treated with KF58333 (data not shown), suggesting that these effects of KF58333 are not restricted to one particular cell line.
Destabilization of p210Bcr-Abl, Raf-1, and tyrosine phosphorylated proteins by HA, radicicol, and KF58333 treatment.
K562 cells were cultured with 0.3 μmol/L of HA, radicicol, and 0.05 μmol/L of KF58333 for indicated times and analyzed by Western blotting as described in “Materials and methods.” Total cell lysates were subjected to Western blotting with antityrosine phosphorylated proteins antibody (A), anti-Abl antibody (B), anti-Raf-1 antibody (C), and anti-Erk antibody (D).
Destabilization of p210Bcr-Abl, Raf-1, and tyrosine phosphorylated proteins by HA, radicicol, and KF58333 treatment.
K562 cells were cultured with 0.3 μmol/L of HA, radicicol, and 0.05 μmol/L of KF58333 for indicated times and analyzed by Western blotting as described in “Materials and methods.” Total cell lysates were subjected to Western blotting with antityrosine phosphorylated proteins antibody (A), anti-Abl antibody (B), anti-Raf-1 antibody (C), and anti-Erk antibody (D).
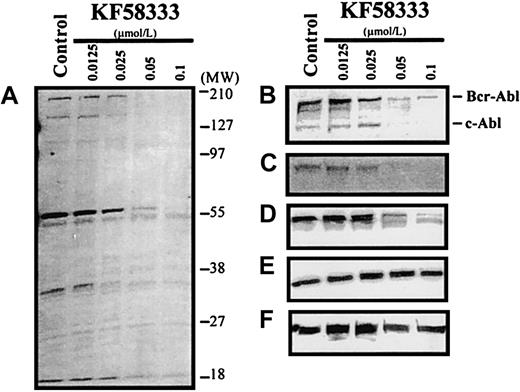
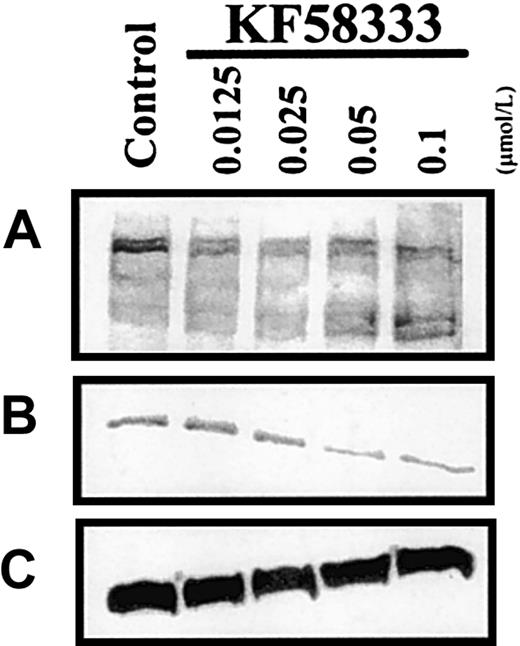
Concentration-dependent effect of KF58333 on expression level of Hsp90-associated proteins
When K562 cells were treated with various concentrations of KF58333 for 48 hours, the destabilization of tyrosine phosphorylated proteins, p210Bcr-Abl protein, and Raf-1 protein was also observed in a concentration-dependent manner (Figure6A-C). In contrast, the level of Erk protein and Hsp90 protein did not change after KF58333 treatment (Figure 6E,F). KF58333 also caused the down-regulation of phosphorylated Erk protein (Figure 6D) in a concentration-dependent manner, which might be secondary to depletion of Raf-1 or p210Bcr-Abl protein.
Destabilization of p210Bcr-Abl, Raf-1, phosphorylated Erk, Erk, Hsp90, and tyrosine phosphorylated proteins by KF58333 treatment.
K562 cells were cultured with increasing concentrations of KF58333 for 48 hours and analyzed as described in “Materials and methods.” Total cell lysates were subjected to Western blotting with antityrosine phosphorylated proteins antibody (A), anti-Abl antibody (B), anti-Raf-1 antibody (C), antiphosphorylated Erk antibody (D), anti-Erk antibody (E), and anti-Hsp90 antibody (F).
Destabilization of p210Bcr-Abl, Raf-1, phosphorylated Erk, Erk, Hsp90, and tyrosine phosphorylated proteins by KF58333 treatment.
K562 cells were cultured with increasing concentrations of KF58333 for 48 hours and analyzed as described in “Materials and methods.” Total cell lysates were subjected to Western blotting with antityrosine phosphorylated proteins antibody (A), anti-Abl antibody (B), anti-Raf-1 antibody (C), antiphosphorylated Erk antibody (D), anti-Erk antibody (E), and anti-Hsp90 antibody (F).
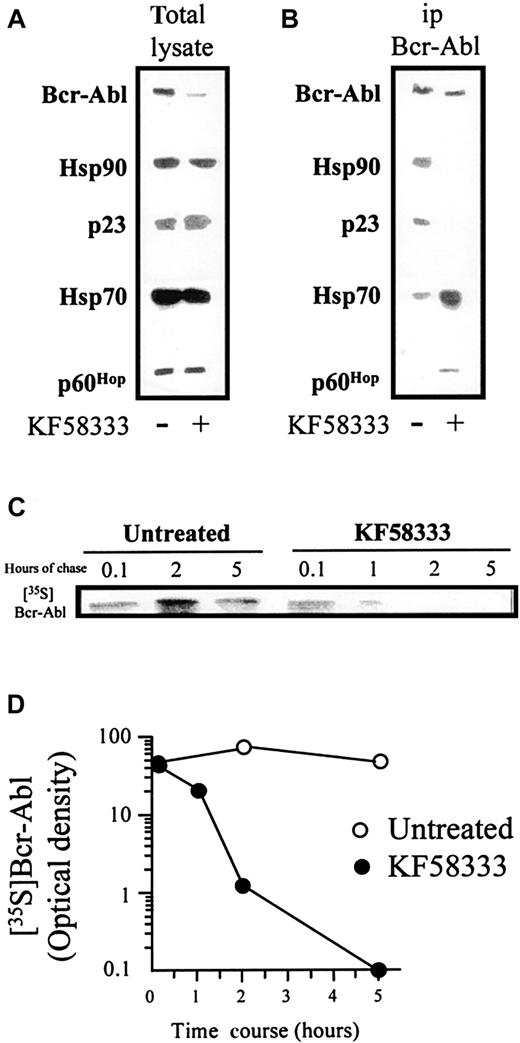
Effects of KF58333 on the Hsp90/p210Bcr-Ablcomplex
When K562 cells were treated with 0.05 μmol/L of KF58333 for 3 hours, total lysate was prepared, resolved by SDS-PAGE, and analyzed by Western blotting. Although KF58333 treatment induced obvious down-regulation of p210Bcr-Abl, the drug induced no clear decrease of Hsp90, p23, p60Hop, and Hsp70 (Figure7A). Next we immunoprecipitated p210Bcr-Abl from untreated cells or KF58333 (0.05 μmol/L) for 3 hours to examine the effects of brief drug exposure on association of p210Bcr-Abl with Hsp90 and its associated chaperones (Figure 7B). Exposure of K562 cells to KF58333 for 3 hours dissociated p210Bcr-Abl from Hsp90/p23 complexes, but drug treatment increased association of the kinase with Hsp70 and p60Hop. These data show that, at steady state in untreated cells, the primary Hsp90 complex associated with p210Bcr-Abl contains p23 and not Hsp70 and p60Hop. In contrast, following drug treatment, association with Hsp70 and p60Hop predominates.
KF58333 treatment destabilizes Hsp90/p210 Bcr-Abl complex.
The effects of KF58333 on Hsp90/p210Bcr-Abl complex (A and B) and the half-life of Bcr-Abl protein (C) are shown. (A) K562 cells were treated with 0.05 μmol/L of KF58333 for 3 hours, total lysate was prepared, resolved by SDS-PAGE, and analyzed by Western blotting as described previously. (B) About 1 mg of total cell lysate was immunoprecipitated with anti-p210Bcr-Abl monoclonal antibody and analyzed by Western blotting as described. (C) The half-life of Bcr-Abl protein in K562 cells treated with or without KF58333 (0.05 μmol/L) was determined as described in “Materials and methods.” 35S-methionine–labeled Bcr-Abl protein was immunoprecipitated and resolved by SDS-PAGE. Gels were fixed, enhanced, and exposed to Kodak XAR-5 film. (D) Bcr-Abl–specific bands were quantified by densitometric scanning/image analysis. 35S,35S-methionine; ip, immunoprecipitated.
KF58333 treatment destabilizes Hsp90/p210 Bcr-Abl complex.
The effects of KF58333 on Hsp90/p210Bcr-Abl complex (A and B) and the half-life of Bcr-Abl protein (C) are shown. (A) K562 cells were treated with 0.05 μmol/L of KF58333 for 3 hours, total lysate was prepared, resolved by SDS-PAGE, and analyzed by Western blotting as described previously. (B) About 1 mg of total cell lysate was immunoprecipitated with anti-p210Bcr-Abl monoclonal antibody and analyzed by Western blotting as described. (C) The half-life of Bcr-Abl protein in K562 cells treated with or without KF58333 (0.05 μmol/L) was determined as described in “Materials and methods.” 35S-methionine–labeled Bcr-Abl protein was immunoprecipitated and resolved by SDS-PAGE. Gels were fixed, enhanced, and exposed to Kodak XAR-5 film. (D) Bcr-Abl–specific bands were quantified by densitometric scanning/image analysis. 35S,35S-methionine; ip, immunoprecipitated.
Effects of KF58333 on the half-life of p210Bcr-Abl protein
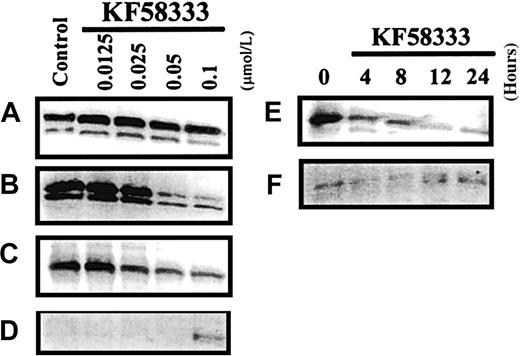
Effects of KF58333 on Cdks and Cdk inhibitors
To elucidate the mechanisms of G1 phase accumulation induced by KF58333, we investigated the effect of the drug on the expression level of several Cdks, which are thought to be responsible for progression of the cell cycle from G1 phase to S phase. As shown in Figure 8, an apparent decrease of Cdk6 protein and a moderate decrease of Cdk4 protein were observed after the treatment of K562 cells with KF58333 for 48 hours in a concentration-dependent manner (Figure 8B,C), whereas the level of Cdk2 protein remained unchanged 48 hours after drug exposure (Figure8A). We have also tested whether KF58333 would affect the expression level of Cdk inhibitors, which are thought to negatively regulate cell cycle progression form G1 to S phase. Interestingly, the expression level of Cdk inhibitor, p27Kip1 protein, was up-regulated by KF58333 (Figure 8D), whereas the expression level of p21Cip1 protein was not affected by the drug in K562 cells (data not shown). Time-course study with 0.1 μmol/L of KF58333 revealed that a moderate down-regulation of Cdk4 was observed as early as 4 hours after drug treatment (Figure 8E) and a clear up-regulation of p27Kip1 was obvious at 12 hours after drug treatment (Figure 8F).
KF58333 treatment decreased the expression level of Cdk4 and Cdk6.
K562 cells were cultured with indicated concentration of KF58333 for 48 hours or 0.1 μmol/L of KF58333 for the indicated time and analyzed as described in “Materials and methods.” Total cell lysates were subjected to Western blotting with anti-Cdk2 antibody (A), anti-Cdk4 antibody (B,E), anti-Cdk6 antibody (C), and anti-p27Kip1antibody (D,F).
KF58333 treatment decreased the expression level of Cdk4 and Cdk6.
K562 cells were cultured with indicated concentration of KF58333 for 48 hours or 0.1 μmol/L of KF58333 for the indicated time and analyzed as described in “Materials and methods.” Total cell lysates were subjected to Western blotting with anti-Cdk2 antibody (A), anti-Cdk4 antibody (B,E), anti-Cdk6 antibody (C), and anti-p27Kip1antibody (D,F).
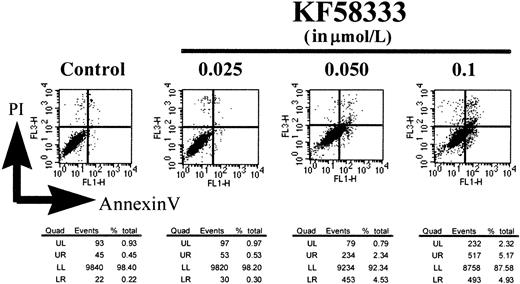
Induction of apoptosis of K562 cells by KF58333
To see whether KF58333 could activate the cell-death pathway in K562 cells, we assessed the cleavage of PARP protein, which is a known substrate for caspase 3, a key regulator of apoptotic cell death. As shown in Figure 9A, KF58333 induced an apparent cleavage of PARP protein at higher concentrations than its IC50 value. At the same time, the drug induced a slight decrease of Bcl-2 protein, which is a negative regulator of apoptotic cell death (Figure 9B). Annexin V binds phosphatidyl serine on the plasma membrane, allowing detection of the membrane disintegration, which is an early signature event in apoptosis.24 As shown in Figure 10, KF58333 increased the number of early apoptotic cells (annexin V–positive and PI-negative) in a concentration-dependent manner. In the presence of 0.1 μmol/L of KF58333, 4.9% early stage apoptotic cells was detected, whereas only 0.22% of those cells were detected in the absence of KF58333 (Figure10). These results suggest that KF58333 eventually induces apoptosis after 48 hours of culture in K562 CML cells.
Dose-dependent effects on PARP cleavage following KF58333 treatment.
K562 cells were cultured with increasing concentration of KF58333 for 48 hours and analyzed as described in “Materials and methods.” Total cell lysates were subjected to Western blotting with anti-PARP antibody (A), anti Bcl-2 antibody (B), and anti β-actin antibody (C).
Dose-dependent effects on PARP cleavage following KF58333 treatment.
K562 cells were cultured with increasing concentration of KF58333 for 48 hours and analyzed as described in “Materials and methods.” Total cell lysates were subjected to Western blotting with anti-PARP antibody (A), anti Bcl-2 antibody (B), and anti β-actin antibody (C).
Dose-dependent effects of KF58333 on induction of apoptosis.
K562 cells were cultured with increasing concentration of KF58333 for 48 hours and analyzed as described in “Materials and methods.” The number of annexin V–positive/PI-negative cells is indicated in the lower right quadrant. Quad, quadrant; UL, upper left; UR, upper right; LL, lower left; LR, lower right.
Dose-dependent effects of KF58333 on induction of apoptosis.
K562 cells were cultured with increasing concentration of KF58333 for 48 hours and analyzed as described in “Materials and methods.” The number of annexin V–positive/PI-negative cells is indicated in the lower right quadrant. Quad, quadrant; UL, upper left; UR, upper right; LL, lower left; LR, lower right.
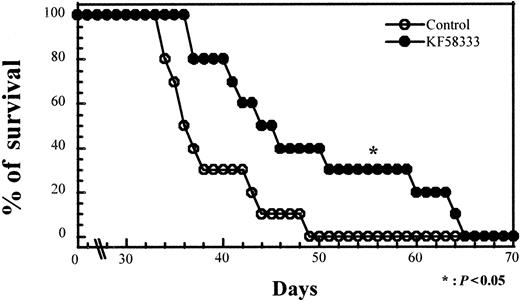
Effect of KF58333 on the survival time of SCID mice inoculated with K562 cells
To elucidate whether KF58333 could have a therapeutic potential in vivo, we examined the effect of KF58333 on survival of SCID mice inoculated with K562 cells. SCID mice were pretreated with 150 mg/kg CY for 2 consecutive days and were inoculated with 107 of K562 cells on day 2. All mice were randomized and separated into 2 groups. One group of 10 mice was treated with 50 mg/kg KF58333 for 5 consecutive days (from day 19 to day 23) and the other group was untreated. As shown in Figure 11, untreated mice inoculated with 107 K562 cells died of diffuse leukemia, as confirmed by necropsy, 34 to 49 days after treatment with CY (median survival, 37.4 days). Survival time of all the 10 mice treated with KF58333 was longer than that of untreated mice, ranging from 37 to 65 days after CY treatment (median survival, 47.8 days; P < .05, Mann-Whitney U test; compared with the survival of untreated mice). These results clearly demonstrated that KF58333 has in vivo antitumor activity in mice, thus suggesting that the compound may have therapeutic potential in the clinic.
KF58333 treatment prolonged the survival of SCID mice inoculated with K562 cells.
Ten SCID mice per group were injected intraperitoneally with 150 mg/kg of CY on day 0 and day 1 followed by inoculation of 107K562 cells on day 2. Mice were given an IV injection of KF58333 (50 mg/kg) from day 19 to day 23. Statistical significance was determined by Mann-Whitney U test; P values of less than .05 were considered statistically significant.
KF58333 treatment prolonged the survival of SCID mice inoculated with K562 cells.
Ten SCID mice per group were injected intraperitoneally with 150 mg/kg of CY on day 0 and day 1 followed by inoculation of 107K562 cells on day 2. Mice were given an IV injection of KF58333 (50 mg/kg) from day 19 to day 23. Statistical significance was determined by Mann-Whitney U test; P values of less than .05 were considered statistically significant.
Discussion
Recently we have revealed that radicicol could bind to the N-terminal site of Hsp90 at which GA also binds, thus destabilizing Hsp90 client proteins such as Raf-1, ErbB-2, and mutated p53.20,25 These results suggest that various biologic activities of radicicol could be mediated through the inhibition of function of Hsp90 and its family of proteins.26-29 In this study, we have revealed for the first time, at least to our knowledge, that both radicicol and its novel oxime derivatives, KF25706 or KF58333, could induce erythroid differentiation of K562 CML cells (Figure 2). Although the induction of GPA expression by HA and radicicol was transient and the number of GPA-positive cells returned to basal level 48 hours after drug exposure, KF25706 or KF58333 induced prolonged up-regulation of GPA-positive cells over 72 hours after the initial treatment (Figure 3A).
Western blot analysis revealed that HA, radicicol, or KF58333 depleted tyrosine phosphorylated proteins, Raf-1 protein, and p210Bcr-Abl protein from the cells. Again depletion of phosphorylated proteins, Raf-1 protein, and p210Bcr-Ablprotein by HA and radicicol was transient; however, the effect of KF58333 lasted as long as 72 hours after the initial treatment (Figure5). Interestingly, HA, radicicol, and KF58333 also induced the depletion of p210Bcr-Abl protein without any effect on expression level of Erk protein (Figure 5D). These data strongly suggest that destabilization of Raf-1 and p210Bcr-Ablprotein as well as depletion of tyrosine phosphorylated proteins are closely related to the erythroid differentiation in K562 cells. Further study showed that KF58333 also depleted phosophorylated Erk protein from K562 cells in a similar concentration range for the depletion of tyrosine phosphorylated proteins, p210Bcr-Abl protein, and Raf-1 protein, without any effects on the expression of Erk protein and Hsp90 protein (Figure 6) at the highest concentration tested (0.1 μmol/L). These results suggest that decrease of phosophorylated Erk protein might also contribute to the induction of differentiation and it is interesting to note that Erk protein might be activated (tyrosine phosphorylated) through both p210Bcr-Abl and Raf-1 proteins.
When we examined the direct inhibitory effect of KF58333 on p210Bcr-Abl kinase, there was no effect of KF58333 at 0.1, 1.0, and 10 μmol/L (data not shown) suggesting that KF58333 could destabilize p210Bcr-Abl protein. To date, no report shows that p210Bcr-Abl protein could form a complex with Hsp90 in cells. Under such circumstances, we have analyzed if it were the case using immunoprecipitation and primarily Western blotting. The results showed that p210Bcr-Abl protein formed a stable complex with Hsp90 and p23 in the absence of KF58333 (Figure 7A). More importantly, after the addition of 0.05 μmol/L of KF58333, p210Bcr-Abl protein was dissociated from an Hsp90/p23 chaperone complex and associated instead with Hsp70/p60Hop(Figure 7B). Disruption of Hsp90/p23/p210Bcr-Abl complexes is followed rapidly by the proteolytic degradation of p210Bcr-Abl; released p210Bcr-Abl proteins are likely to be degraded via an ubiquitin-dependent proteasome-mediated pathway because Raf-1 proteins have been reported to be degraded by the proteasome system.30 Furthermore, it has been reported that the normal half-life of p210Bcr-Abl protein is in excess of 24 hours31 32; our data showed that KF58333 treatment significantly decreased the half-life of p210Bcr-Abl protein (Figure 7C, D).
Although KF58333 induced persistent G1 phase accumulation in K562 cells, HA and radicicol induced transient G1 arrest at early time point (Figure 4). These data might suggest that persistent erythroid differentiation by KF58333 could be related to this persistent G1 phase accumulation. To elucidate the mechanism of G1 phase accumulation induced by KF58333, we investigated the expression of Cdk2, Cdk4, and Cdk6, which are known to play important roles in G1 to S phase progression of the cell cycle. Treatment with KF58333 resulted in an apparent decrease of Cdk6 protein and a moderate decrease of Cdk4 protein, without any effect on Cdk2 protein (Figure 8). It has been reported that Cdk4 is chaperoned by Hsp90 complex.33 34 Although it is unknown whether Hsp90 would play a role in folding and function of Cdk6 protein, our data suggest that Cdk6 could be stabilized by Hsp90 complex.
Because KF58333 actually exhibited in vivo tumor growth inhibitory activity in xenograft models (S.S. et al, manuscript in preparation), the effect of the compound was investigated in a K562 SCID mice model. As shown in Figure 11, KF58333 actually prolonged the life span of mice inoculated with K562 cells. These results demonstrated that KF58333 has in vivo antitumor activity in mice and may have therapeutic potential in the clinic. At this point, optimal dose and duration to prolong the survival of K562-inoculated SCID mice are unknown. It is our plan to examine the optimal dose schedule for antitumor effect of KF58333 in more detail as well as its effect on normal hematopoietic progenitor cells in vitro or in vivo.
In conclusion, KF58333, a novel derivative of radicicol, binds to the Hsp90 chaperone machinery, depletes p210Bcr-Abl and Raf-1 proteins followed by induction of erythroid differentiation and G1 phase accumulation, and induces apoptosis in human CML cells. Our results demonstrate the therapeutic possibility of KF58333 against CML.
Acknowledgments
We thank Miyoko Asano and Yasuyo Kumazawa for excellent technical assistance.
Kyowa Hakko Kogyo, Pharmaceutical Laboratories, Shizuoka, Japan; Medicine Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Submitted November 15, 1999; accepted May 23, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shiro Akinaga, 1188 Shimotogari Nagaizumi-cho Sunto-gun, Shizuoka, Japan 411-8731; e-mail:shiro.akinaga@kyowa.co.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal