Abstract
Plasmodium falciparum–infected erythrocytes (IRBCs) have been shown to interact with a number of endothelial adhesion molecules expressed on transfectants, on cell lines, and as immobilized purified receptor proteins under flow conditions. However, the experiments were designed in such a way that maximal numbers of adhesion molecules were provided as substratum. Whether the interactive events actually occur on microvascular endothelium, where the distribution and expression of adhesion molecules may be less, remains undetermined. In this study, the cytoadherance of IRBCs on human dermal microvascular endothelial cells (HDMECs) as a model of human microvasculature was examined. IRBCs were observed to tether, roll, and adhere on resting HDMECs, which constitutively expressed CD36 and intercellular adhesion molecule-1 (ICAM-1) at an optimal shear stress of 1 dyne/cm2. Stimulation of HDMECs with tumor necrosis factor–α for 5 and 24 hours, which resulted in up-regulation of ICAM-1 and induction of vascular cell adhesion molecule-1 expression, significantly increased the percentage of rolling cells that adhered without affecting the rolling flux. In contrast, P-selectin expression on HDMECs induced by oncostatin M led to an increase in both rolling flux and adhesion. Inhibition studies with receptor-specific monoclonal antibodies revealed that adhesion of IRBCs on HDMECs was largely CD36 dependent, whereas rolling could be mediated by any of the adhesion molecules studied. Collectively, these findings indicate that IRBCs interact synergistically with multiple adhesion molecules on vascular endothelium. The rolling of IRBCs may be the rate-limiting step in cytoadherance, since it can be modulated by cytokines to enhance CD36-mediated IRBC adhesion.
Introduction
Plasmodium falciparum malaria remains a major cause of morbidity and mortality in the tropics.1 The pathogenicity of the parasite results from its potential to multiply to high parasite burdens2 and its unique ability to adhere to capillary and postcapillary venular endothelium during the second half of its 48-hour life cycle, a process that is called cytoadherance.3 The resulting sequestration of infected erythrocytes (IRBCs) and microcirculatory obstruction have been proposed to be the basis of the multiorgan dysfunction seen in this infection.4
In the past few years, there has been increasing recognition that cytoadherance may be mediated sequentially by a number of endothelial adhesion molecules under physiological flow conditions.5-7IRBCs from clinical parasite isolates have been shown to tether and then roll on CD36, intercellular adhesion molecule-1 (ICAM-1), P-selectin, and vascular cell adhesion molecule (VCAM-1), but not E-selectin.6 Significant adhesion under shear is almost exclusive to CD36. Although some IRBCs bypass the rolling event and are arrested on CD36 immediately after tethering, there is strong evidence that the molecules that support rolling can contribute to CD36-dependent adhesion. On C32 melanoma cells, which coexpress CD36 and ICAM-1, inhibition of rolling by an anti–ICAM-1 antibody reduces the subsequent adhesion of some parasite isolates to CD36.6 Similarly, inhibition of rolling by an anti–P-selectin antibody reduces IRBC adhesion to CD36 on activated platelets.7 However, all these studies have been conducted with the use of cell lines and purified receptor proteins, which ensured that maximal numbers of adhesion molecules were provided as substratum. Whether the same synergistic adhesive interactions occur on microvascular endothelial cells, where the distribution and expression of adhesion molecules is less, has not been determined.
The current study examined the interactions of IRBCs with multiple adhesion molecules in a model of human microvascular endothelial cells under flow conditions in vitro. We used human dermal microvascular endothelial cells (HDMECs) because they express CD36, which is absent on the more commonly used human umbilical vein endothelial cells (HUVECs). Moreover, HDMECs constitutively express ICAM-1 and can be induced to express VCAM-1 and P-selectin by cytokines.8 9Our results indicate that up-regulation of ICAM-1 and induction of VCAM-1 expression by tumor necrosis factor-α (TNF-α) increased the percentage of rolling cells that adhered without increasing the rolling flux. In contrast, induction of P-selectin by oncostatin M (OSM) increased both the rolling flux and the number of adherent cells. These findings provide the first evidence that IRBCs interact synergistically with multiple adhesion molecules on microvascular endothelium. The rolling of IRBCs may determine the degree of sequestration and hence hypoxia in severe falciparum malaria, as rolling can be enhanced by proinflammatory cytokines to promote CD36-mediated IRBC adhesion.
Materials and methods
Tissue culture reagents and antibodies
Unless otherwise stated, all tissue culture reagents were obtained from Gibco BRL Life Technologies (Gaithersburg, MD).
Parasites
Cryopreserved parasite isolates from adult Thai patients with well-documented P falciparum malaria were thawed and studied during the first cycle in culture as described.6 7 The collection of specimens was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. A fresh aliquot was used for each of the experiments. IRBCs were harvested for flow studies when the parasites had reached the late trophozoite/early schizont stage by light microscopy. A total of 10 clinical parasite isolates were studied.
Endothelial cell isolation and culture
HDMECs were harvested from discarded neonatal human foreskin as described previously.10 The protocol was approved by the Ethics Committee of the University of Calgary. Briefly, foreskins were dissected into 2- to 3-mm2 segments, which were digested at 4°C for 16 hours in supplemented M199 containing 0.5 mg/mL collagenase type 1A (Boerhringer Mannheim Biochemicals, Indianapolis, IN). Microvessels were released from digested tissue segments by compressing the tissue segments gently with a spatula. To remove large debris, vessel preparations were passed through a 100-μm nylon mesh (Becton Dickinson, San Jose, CA). The cells were collected by centrifugation at 200g for 10 minutes, and the resulting cell pellets were resuspended in endothelial basal medium (EBM-2) with supplements supplied by the manufacturer (Clonetics, San Diego, CA). The cells were seeded in gelatin-coated tissue culture dishes. Only cell preparations that were greater than 95% positive for von Willebrand factor and platelet/endothelial cell adhesion molecule-1 (PECAM-1) expression by immunohistochemistry were maintained for flow experiments. The HDMECs were used from passage 1 to 7, during which adhesion molecule expression at rest and on stimulation was shown to be stable. Each set of experiments was performed on at least 3 different skin preparations.
Cytokines
Recombinant human TNF-α, interferon-γ (IFN-γ) and OSM were purchased from R & D Systems, Inc (Minneapolis, MN). The concentration of cytokines used for the stimulation of HDMECs in 35-mm tissue culture dishes (Corning, New York, NY) were as follows: TNF-α, 5 to 50 ng/mL; IFN-γ, 10 to 100 ng/mL; and OSM, 1 to 20 ng/mL.
Antibodies
The monoclonal antibodies (MoAbs) used were known to specifically inhibit the binding of IRBCs to the respective adhesion molecules.6 OKM5 (anti-CD36) was a kind gift of Ortho Diagnostics System (Raritan, NJ); 84H10 (anti–ICAM-1) was purchased from R & D Systems; 4B9 (anti–VCAM-1)11 was a kind gift of Dr R. Lobb, Biogen (Boston, MA). The anti–P-selectin MoAbs GA6, which inhibits neutrophil and HL-60 cell interaction with P-selectin12; AK-413; and AC1.2, which recognizes the short consensus repeat of P-selectin,14were provided by Dr H. K. Webster, Becton Dickinson Immunocytometry Systems (San Jose, CA). In inhibition studies, HDMEC monolayers were preincubated with the MoAbs at 10 μg/mL Hanks' balanced salt solution at 37°C for 30 minutes before being used in flow experiments.
Production and characterization of an inhibitory anti–P-selectin moAb
Monoclonal antibodies against human P-selectin were raised by immunizing Balb/c mice intraperitoneally with 20 μg of recombinant human P-selectin (rhP-selectin) (gift of Dr R. P. McEver, University of Oklahoma, Oklahoma City) in complete Freund's adjuvant (CFA), and boosted intraperitoneally 42 days later with 10 μg of rhP-selectin in incomplete CFA. After an additional 10 days, 4 days before fusion, 4 μg of rhP-selectin in phosphate-buffered saline was injected intraperitoneally. Splenocytes were fused with Sp2/mouse interleukin 6 (IL-6) myeloma cells with the use of polyethylene glycol 1500.15 Hybridomas were screened by means of an enzyme-linked immunosorbent assay. Cells from positive wells were cloned by limiting dilution. Supernatants from wells containing single clones were tested for their ability to inhibit IRBC rolling on immobilized P-selectin as previously described.7 A single inhibitory MoAb, TS10-6-6, was identified from 26 supernatants tested by this method. Clone TS10-6-6 cells were cultured in serum-free medium until the cells died. The spent supernatant was collected and frozen in aliquots at −20°C. The antibody was determined to be an immunoglobulin G1 (IgG1) and recognized nonreduced rhP-selectin in a Western blot. It also recognized cell-bound P-selectin expressed on Chinese hamster ovary (CHO) transfectants16 to the same extent as the commercial MoAb AK-4, but did not label untransfected CHO cells or HDMECs stimulated with TNF-α. Finally, TS10-6-6 inhibited the rolling of both leukocytes and IRBCs on immobilized P-selectin. In contrast, MoAb GA6 inhibited only leukocyte rolling. MoAb AC1.2 was not inhibitory for either cell type. Collectively, these findings confirmed the functional specificity of TS10-6-6 for P-selectin.
Determination of adhesion molecule expression on HDMECs
For resting and TNF-α–stimulated HDMECs, cells were detached from tissue culture plates with the use of 0.05% trypsin in 0.53 mmol/L EDTA. They were incubated with an unlabeled anti-CD36, anti–ICAM-1, anti–VCAM-1, or TS10-6-6 for 30 minutes at 4°C. The stained cells were washed, and a fluorescein-labeled antimouse IgG1 was added for 30 minutes at 4°C. Control cells were stained with the second antibody only. Washed cells were analyzed on a fluorescent cell sorter (Becton Dickinson). HDMECs stimulated with OSM were detached with EDTA alone (10 to 20 minutes at room temperature) and stained with phycoerythrin (PE)–labeled AC1.2 (Becton Dickinson). Control cells were stained with PE-labeled mouse IgG1 (Becton Dickinson).
Adhesive interactions of malaria under flow
IRBC-endothelial cell interactions at fluid shear stresses approximating those in the microvasculature were studied with the use of a parallel plate flow chamber as described.16 A suspension of IRBCs (1% hematocrit, 5% to 7% parasitemia in RPMI 1640, pH 7.2) was drawn through the flow chamber at varying rates with an infusion pump attached to the outlet. At this concentration of IRBCs, attached IRBCs and their motion could be clearly observed with phase contrast objectives (200 ×) and quantitated by analysis of videotaped images. The presence of intracellular parasites in interacting erythrocytes was confirmed by experiments in which IRBCs were labeled with rhodamine 6G (1:10 of 0.05%, 5 minutes), and the adhesive interactions were observed by epi-illumination at 510 to 560 nm, with the use of a 590-nm emission filter. Tethering referred to the initial contact between IRBCs and the endothelial monolayer. A rolling IRBC was defined as one that displayed a typical end-on-end rolling motion at a velocity of less than 150 μm/s, compared with a flow rate of red blood cells greater than 1000 μm/s, and a velocity greater than 150 μm/s for noninteracting cells in close proximity to the endothelial monolayer. The flux of rolling IRBCs was determined as the number that rolled past a fixed line on the monitor for the duration of the experiment, and expressed as the number of rolling IRBCs per minute per square millimeter. An IRBC was considered adherent if it remained stationary for more than 10 seconds, and the results were expressed as the number of adherent IRBCs per square millimeter of surface area.
Statistical analysis
All data are presented as mean ± SEM. Data were compared by ANOVA for paired samples, followed by post hoc analysis with Dunn's test for multiple comparisons. Probabilities of .05 or less were considered statistically significant.
Results
IRBC rolling and adhesion on resting HDMECs is shear dependent
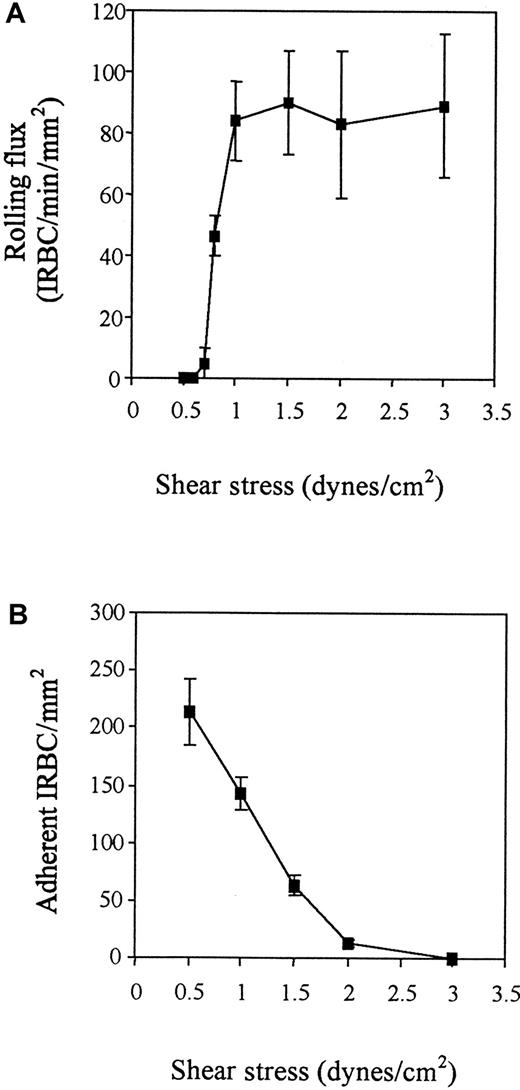
The interaction of IRBCs with resting HDMECs was studied at a shear stress of 0.5 to 3 dyne/cm2. IRBCs tethered to HDMECs at all shears tested, but rolling was absent until a threshold shear stress of 0.8 dyne/cm2 was reached (Figure1). Rolling flux quickly reached a peak at 1 dyne/cm2 and became essentially shear independent thereafter. In contrast, adhesion was highest at 0.5 dyne/cm2 and dropped very quickly to near zero by 2 dyne/cm2. However, once adherent, IRBCs could withstand an increase in shear stress of up to 10 dyne/cm2 (number of adherent IRBCs at 1 dyne/cm2 = 201/mm2 ± 14/mm2and at 10 dyne/cm2 = 188/mm2 ± 21/mm2 ; n = 3). All remaining experiments in this study were conducted at 1 dyne/cm2. At this shear stress, rolling IRBCs had a mean velocity of 41.79 μm/s ± 1.68 μm/s (based on 50 cells for each isolate).
Shear dependence of IRBC rolling and adhesion on resting HDMECs.
IRBCs were infused over HDMEC monolayers at different flow rates to generate the desired wall shear stress in the flow chamber. Results are the mean ± SEM of 3 parasite isolates studied. (A) Rolling. (B) Adhesion.
Shear dependence of IRBC rolling and adhesion on resting HDMECs.
IRBCs were infused over HDMEC monolayers at different flow rates to generate the desired wall shear stress in the flow chamber. Results are the mean ± SEM of 3 parasite isolates studied. (A) Rolling. (B) Adhesion.
Types of IRBC–endothelial-cell interactions
We observed 3 types of IRBC–endothelial cell interactions on resting and cytokine-stimulated HDMECs. Ninety percent to 100% of the adherent IRBCs from 9 of the 10 clinical isolates studied rolled for various distances before becoming arrested. In only 1 of 10 isolates, 40% ± 5.3% (4 experiments) of the adherent IRBCs bypassed the rolling event and adhered abruptly after tethering. The mean percentage of rolling cells that became adherent was 47% ± 8.8% for the 10 isolates, which meant that nearly half of the IRBCs that rolled did not adhere in the field of observation. The variations in the types of adhesive interaction at least in part reflect the genotypic and phenotypic heterogeneity of wild-type parasite isolates.
Adhesion molecule expression on TNF-α–stimulated HDMECs
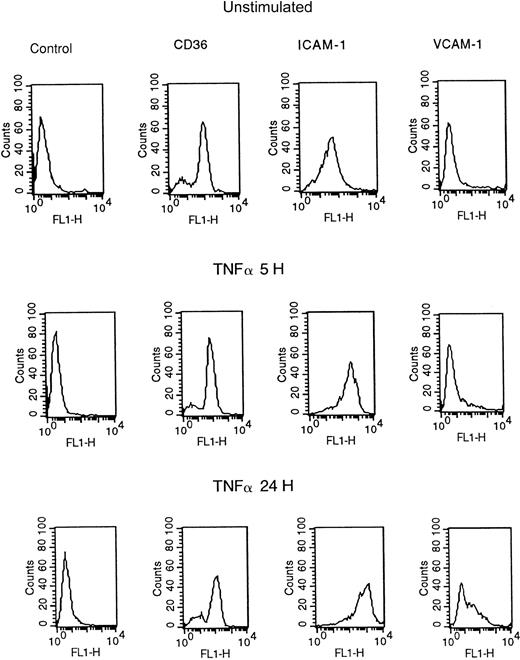
HDMECs constitutively express CD36 and ICAM-1 (Figure2). Stimulation with 5 ng/mL TNF-α for 5 or 24 hours resulted in up-regulation of ICAM-1 expression, in terms of both the percentage of positive cells and the mean fluorescent intensity. VCAM-1 expression was maximal at 24 hours. The percentage of CD36+ cells varied from 75% to 100% for different skin preparations, depending on the location of the microvessels,17 but was unchanged by the addition of 5 to 50 ng/mL TNF-α or 10 to 100 ng/mL IFN-γ for up to 30 hours. These results agree with previous reports on adhesion molecule expression on TNF-α–stimulated HDMECs.8 10
Flow cytometric analysis of adhesion molecule expression on resting HDMECs and HDMECs stimulated with 5 ng/mL of TNF-α for 5 and 24 hours.
The results are representative of 5 different HDMEC preparations. FL1-H indicates fluorescent intensity (fluorescein).
Flow cytometric analysis of adhesion molecule expression on resting HDMECs and HDMECs stimulated with 5 ng/mL of TNF-α for 5 and 24 hours.
The results are representative of 5 different HDMEC preparations. FL1-H indicates fluorescent intensity (fluorescein).
TNF-α enhances IRBC adhesion on HDMECs
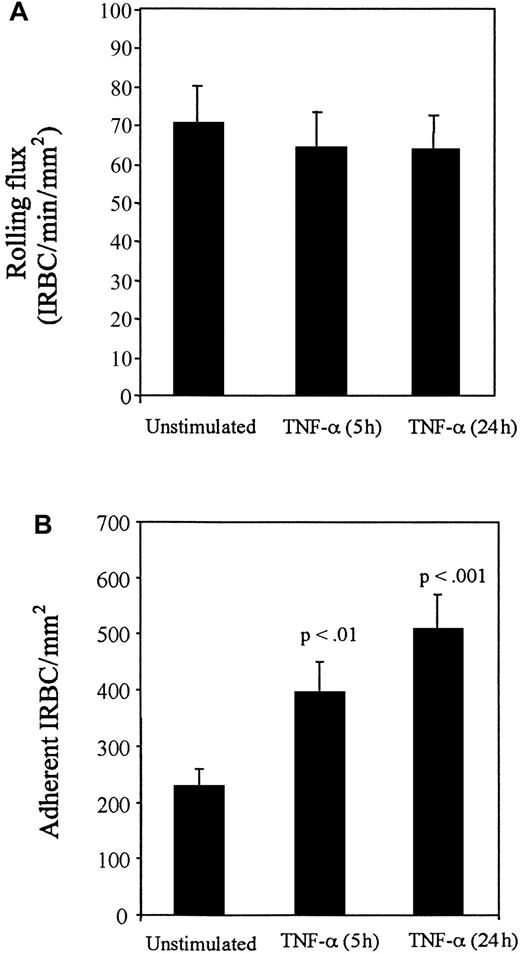
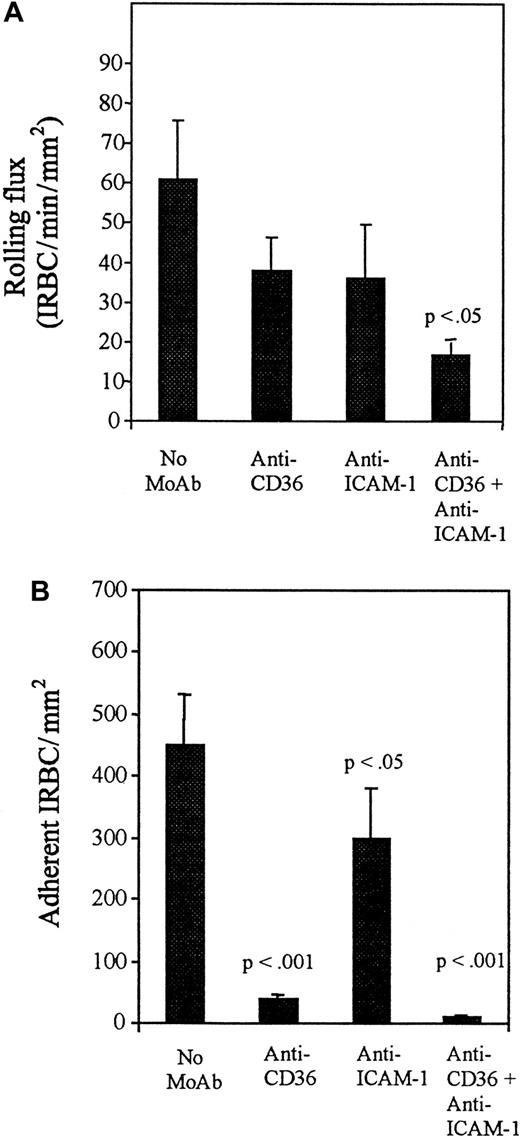
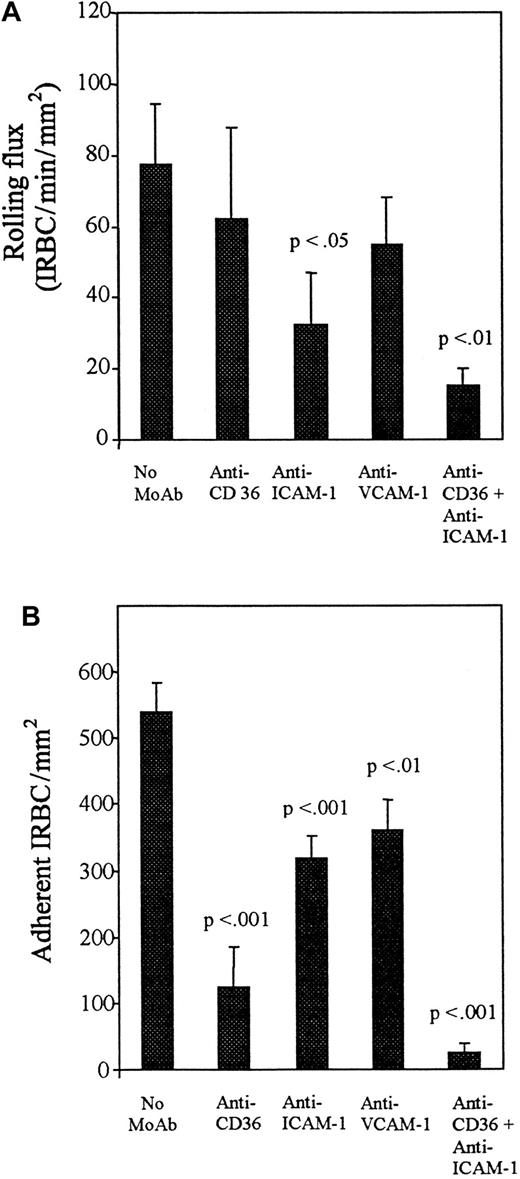
The addition of TNF-α to HDMECs for 5 hours and 24 hours resulted in no increase in the number of rolling cells compared with controls (Figure 3A), but a significantly greater number of IRBCs were adherent at both time points (P < .01 and .001, respectively) (Figure 3B). Accordingly, the percentage of rolling IRBCs that became adherent increased from 44% ± 8.8% on unstimulated HDMECs to 77% ± 6.5% after 5 hours and 97% ± 4.6% after 24 hours of TNF-α stimulation. To determine which adhesion molecules were involved in mediating IRBC rolling and adhesion on TNF-α–stimulated HDMECs, the inhibitory effect of various receptor-specific MoAbs was studied. The results are presented in Figures4 and 5. On HDMECs stimulated with TNF-α for 5 hours, the rolling flux was not significantly reduced by either anti-CD36 or anti–ICAM-1 alone, but was significantly reduced in the presence of both antibodies (P < .05). In contrast, adhesion was more than 90% inhibited by anti-CD36 (P < .001), whereas inhibition with anti–ICAM-1 was approximately 40% (P < .05). There was complete inhibition of adhesion when both antibodies were present (P < .001). A similar pattern of response to inhibitory antibodies was seen with HDMECs stimulated with TNF-α for 24 hours (Figure 5). Significant inhibition of rolling was seen only with anti–ICAM-1 (P < .05), whereas significant inhibition of adhesion was seen with anti-CD36 (P < .001), anti–ICAM-1 (P < .001), and anti–VCAM-1 (P < .01). Two irrelevant IgG1 MoAbs at 10 μg/mL had no effect on IRBC rolling or adhesion on TNF-α–stimulated HDMECs (data not shown).
Effect of TNF-α.
Monolayers were stimulated with TNF-α at 5 ng/mL for 5 and 24 hours before the flow experiments. Results are the mean ± SEM of 10 parasite isolates studied in 15 experiments. (A) Effect of TNF-α on the rolling of IRBC on HDMECs. (B) Effect of TNF-α on the adhesion of IRBCs on HDMECs.
Effect of TNF-α.
Monolayers were stimulated with TNF-α at 5 ng/mL for 5 and 24 hours before the flow experiments. Results are the mean ± SEM of 10 parasite isolates studied in 15 experiments. (A) Effect of TNF-α on the rolling of IRBC on HDMECs. (B) Effect of TNF-α on the adhesion of IRBCs on HDMECs.
Effect of blocking antibodies after 5 hours of TNF-α stimulation.
HDMECs were stimulated by TNF-α at 5 ng/mL for 5 hours. Stimulated HDMECs were preincubated with anti-CD36 (OKM5) and anti–ICAM-1 (84H10) at 10 μg/mL for 30 minutes at 37°C before the infusion of IRBCs. Results are the mean ± SEM of 5 parasite isolates studied. (A) Effect of blocking antibodies on the rolling of IRBCs on stimulated HDMECs. (B) Effect of blocking antibodies on the adhesion of IRBCs on stimulated HDMECs.
Effect of blocking antibodies after 5 hours of TNF-α stimulation.
HDMECs were stimulated by TNF-α at 5 ng/mL for 5 hours. Stimulated HDMECs were preincubated with anti-CD36 (OKM5) and anti–ICAM-1 (84H10) at 10 μg/mL for 30 minutes at 37°C before the infusion of IRBCs. Results are the mean ± SEM of 5 parasite isolates studied. (A) Effect of blocking antibodies on the rolling of IRBCs on stimulated HDMECs. (B) Effect of blocking antibodies on the adhesion of IRBCs on stimulated HDMECs.
Effect of blocking antibodies after 24 hours of TNF-α stimulation.
HDMECs were stimulated by TNF-α at 5 ng/mL for 24 hours. Experimental details are as for Figure 4 with the additional use of an anti–VCAM-1 MoAb (4B9). Results are mean ± SEM of 5 parasite isolates studied. (A) Effect of blocking antibodies on the rolling of IRBCs on stimulated HDMECs. (B) Effect of blocking antibodies on the adhesion of IRBCs on stimulated HDMECs.
Effect of blocking antibodies after 24 hours of TNF-α stimulation.
HDMECs were stimulated by TNF-α at 5 ng/mL for 24 hours. Experimental details are as for Figure 4 with the additional use of an anti–VCAM-1 MoAb (4B9). Results are mean ± SEM of 5 parasite isolates studied. (A) Effect of blocking antibodies on the rolling of IRBCs on stimulated HDMECs. (B) Effect of blocking antibodies on the adhesion of IRBCs on stimulated HDMECs.
OSM promotes IRBC rolling and adhesion to HDMECs
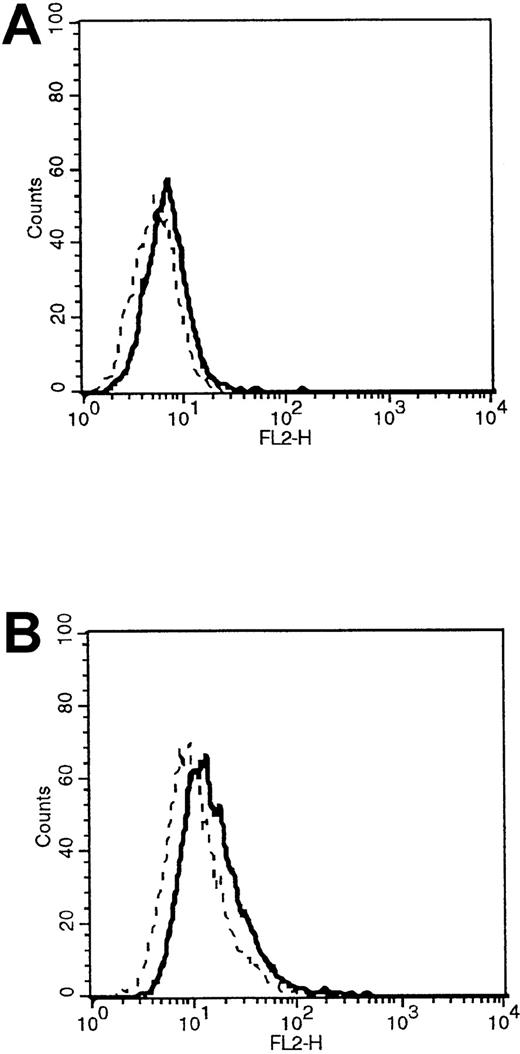
HDMECs were stimulated with OSM at 1, 10, and 20 ng/mL for 24 and 48 hours. A dose of 10 ng/mL for 48 hours most consistently induced a small but significant increase in P-selectin surface expression (Figure6) without affecting the expression of CD36, ICAM-1, or VCAM-1 (data not shown). The induced P-selectin supported the rolling of 184 ± 35 leukocytes per square millimeter (n = 7), which was inhibitable by the MoAb GA6, compared with 12 ± 2 leukocytes per square millimeter on unstimulated HDMECs (S Kerfoot et al, unpublished data).
Flow cytometric analysis of P-selectin expression.
(A) Analysis of P-selectin expression on resting HDMECs. (B) Analysis of P-selectin expression on HDMECs stimulated with 10 ng/mL of OSM for 48 hours. The thick line represents cells stained with a PE-labeled anti–P-selectin MoAb. The thin line represents cells stained with PE-labeled IgG1 control. The shift in mean fluorescent intensity for the unstimulated cells was from 7.38 to 9.29, and the shift for the OSM-stimulated cells was from 12.4 to 23.6. Results are representative of 3 HDMEC preparations. FL2-H indicates fluorescent intensity (phycoerythrin).
Flow cytometric analysis of P-selectin expression.
(A) Analysis of P-selectin expression on resting HDMECs. (B) Analysis of P-selectin expression on HDMECs stimulated with 10 ng/mL of OSM for 48 hours. The thick line represents cells stained with a PE-labeled anti–P-selectin MoAb. The thin line represents cells stained with PE-labeled IgG1 control. The shift in mean fluorescent intensity for the unstimulated cells was from 7.38 to 9.29, and the shift for the OSM-stimulated cells was from 12.4 to 23.6. Results are representative of 3 HDMEC preparations. FL2-H indicates fluorescent intensity (phycoerythrin).
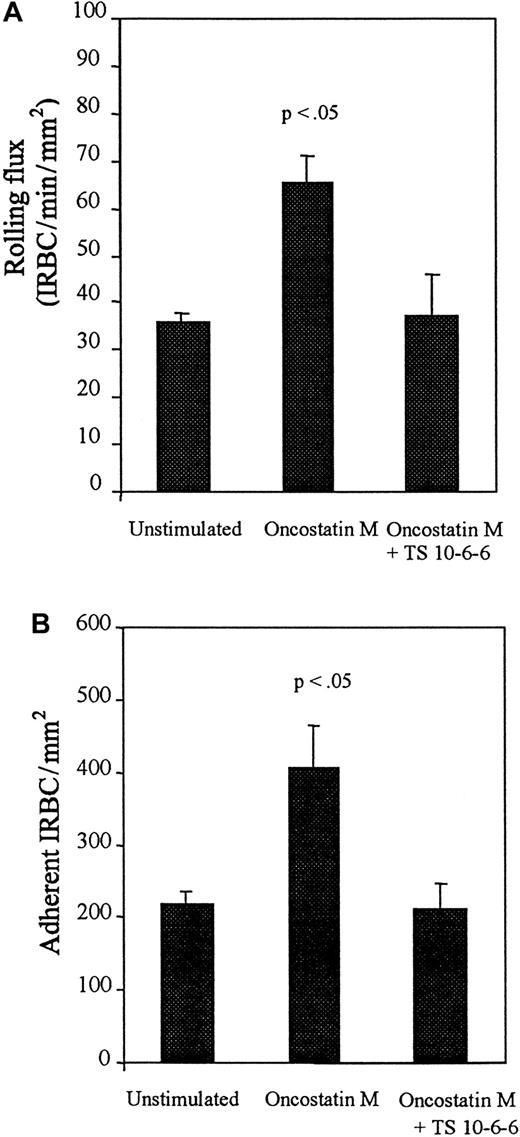
In contrast to the effect of TNF-α, OSM-stimulation resulted in a significant increase in both rolling and adherent IRBCs compared with unstimulated controls (Figure 7). The increase in IRBC interactions was mediated by P-selectin, as the effect of the cytokine was abrogated by preincubation of OSM-stimulated HDMECs with TS10-6-6.
Rolling and adhesion of IRBCs on resting HDMECs and HDMECs stimulated with 10 ng/mL of OSM for 48 hours.
Results are the mean ± SEM of 5 parasite isolates studied. (A) Rolling. (B) Adhesion.
Rolling and adhesion of IRBCs on resting HDMECs and HDMECs stimulated with 10 ng/mL of OSM for 48 hours.
Results are the mean ± SEM of 5 parasite isolates studied. (A) Rolling. (B) Adhesion.
Discussion
In this study, we tested the hypothesis that IRBCs interact with multiple adhesion molecules on microvascular endothelium under physiological flow conditions and that the different interactions are synergistic in promoting adhesion. We used dermal microvascular endothelial cells as a model system of human microvasculature. HDMECs are the only readily accessible cells that express or can be induced to express all the adhesion molecules (CD36, ICAM-1, P-selectin, and VCAM-1) that have so far been implicated in mediating cytoadherance under flow conditions. These adhesion molecules are of clinical significance as all of them have been demonstrated on the microvasculature of brain, liver, kidney, and lung tissues from patients who died from severe falciparum malaria.11 18
Our results indicate that IRBCs tethered, rolled, and adhered on both resting and cytokine-stimulated microvascular endothelial cells. Adhesion was largely via CD36. We have shown previously on CD36 transfectants that the molecule can in fact mediate all 3 phases of IRBC-endothelial cell interactions, ie, tethering, rolling, and adhesion.6 However, interaction with CD36 on microvascular endothelium, where the expression of the molecule was much lower than on the transfectants (mean fluorescent intensity at least a log lower), did not appear to be sufficient for the arrest of approximately two thirds of the rolling IRBCs. On the other hand, stimulation with TNF-α for 24 hours resulted in the adhesion of nearly 100% of the rolling IRBCs. Therefore, despite the fact that CD36 is the principal receptor for IRBCs on vascular endothelium, the degree of cyto-adherence in vivo may very well depend on secondary interactions with other adhesion molecules, such as ICAM-1 and VCAM-1, that accompany and enhance CD36-mediated IRBC adhesion. As a corollary, rolling interactions with other adhesion molecules may be a prerequisite for adhesion to CD36 for some, although not all, IRBCs, mimicking the adhesive cascade that is involved in the recruitment of leukocytes to sites of inflammation. The finding that IRBCs remained adherent despite increasing shear stress is physiologically relevant as the obstruction of blood flow in the microcirculation has been proposed as a major pathological mechanism in severe falciparum malaria.
In contrast to the pivotal role of CD36 in mediating IRBC adhesion, rolling of IRBCs appeared to be a more promiscuous event in terms of both the number of adhesion molecules that can support this interaction and the mechanisms by which rolling enhances subsequent adhesion to CD36. On TNF-α–stimulated HDMECs, rolling on ICAM-1 and VCAM-1 increased adhesion to CD36 without affecting rolling flux. The expression of these molecules appeared to increase the “stickiness” of HDMECs, so that a greater percentage of rolling IRBCs were arrested. The contribution of ICAM-1 and VCAM-1 in sustaining IRBC rolling was also seen in the experiments with inhibitory antibodies that did not significantly reduce the rolling flux but resulted in the arrest of fewer cells. A role for ICAM-1 in sustaining the rolling of leukocytes has also been suggested.19
The most novel obsevation in this present report is the demonstration that P-selectin expression increased the number of rolling as well as adherent cells, indicating an actual increase in the number of IRBCs recruited to the endothelial surface. This finding firmly establishes a major role for P-selectin in mediating cytoadherance to microvascular endothelium under flow conditions and is consistent with the key role of selectins in the initial tethering and rolling of leukocytes in their recruitment to sites of inflammation.
There is much evidence to support a proinflammatory role for OSM, a member of the IL-6 family of cytokines. On HUVECs, OSM induces P-selectin expression within 15 minutes and E-selectin expression in 4 hours through interaction with a high-affinity receptor.20 P-selectin induction through gene transcription is a slower but more sustained process, reaching a peak between 24 to 32 hours and maintaining it for up to 72 hours.9 As in previous studies involving HUVECs,9 P-selectin expression on OSM-stimulated HDMEC was difficult to demonstrate, but its presence was confirmed by the ability of the stimulated HDMECs, but not unstimulated cells, to support the rolling and adhesion of leukocytes. Although OSM has not been specifically studied in relation to human malaria, it is well documented that IL-6 levels, together with those of TNF-α, IL-1, and IFN-γ, are significantly associated with severity in falciparum malaria.21 The effect of IL-6 on IRBC–endothelial-cell interactions could not be studied in our experimental system because although endothelial cells possess functional glycoprotein (gp) 130, which is common to both OSM and IL-6 receptors, they lack the IL-6 receptor α-subunit that is necessary for IL-6 activity.22 During an inflammatory response, the α-subunit is shed by activated neutrophils and then combines with gp130 to constitute a functional IL-6 receptor on endothelial cells, resulting in the synthesis of E-selectin, ICAM-1, and VCAM-1 as well as IL-6 and IL-8. It is conceivable that IL-6 might indeed have a stimulatory role in cytoadherance in vivo where soluble IL-6 receptor α-subunit may be present in the circulation from a number of cellular sources.
The best studied cyto-adherent ligand on IRBC is P falciparum erythrocyte membrane protein 1 (PfEMP1), which is diverse in size (200 to 350 kd) and antigenically variant.23 The protein is exported from the parasite and linked to the erythrocyte cytoskeleton during the second half of the life cycle. Tryptic fragments of PfEMP1 cleaved from the surface of IRBCs bind to purified CD36, ICAM-1, and thrombospondin.24PfEMP1 also mediates the adhesion of IRBCs to chondroitin sulfate A (CSA) expressed on syncytiotrophoblasts of the human placenta.25 The domains on PfEMP1 that mediate interaction with CD36, ICAM-1, and CSA have been identified.26-28Using Western blot analysis and immunoprecipitation, we have recently demonstrated that PfEMP1 is also the ligand for P-selectin (Senczuk et al, unpublished data). Thus P falciparum appears to have evolved the capability of interacting with a number of adhesion molecules by the same variant surface protein.
The ultimate goal of any research on cytoadherance is the development of safe and effective methods of inhibiting or reversing the process. The synergism among multiple adhesion molecules in mediating cytoadherance described in this report could be exploited to develop antiadhesive strategies. There might even be other adhesion molecules such as PECAM-1 and αvβ3 that interact with IRBCs,29,30 but their roles in mediating the cytoadherance of wild-type parasites have not been fully assessed. It is likely that IRBCs will interact with as many adhesion molecules as are available in order to promote its own survival, as unattached IRBCs are trapped and removed by the spleen.31 32 Targeting the adhesion molecules will reduce cyto-adherence and potentially some of the complications seen in severe falciparum malaria.
Acknowledgments
We thank Dr Paul Kubes for reviewing the manuscript, Mrs. Laurie Robertson for the flow cytometry studies, and Dr Carolyn Lane and her colleagues at Valley View Family Practice Clinic, Calgary, Alberta, Canada, for providing the skin specimens.
Supported by the Medical Research Council of Canada; the Alberta Heritage Foundation for Medical Research, Alberta, Canada; and the Wellcome-Mahidol University, Oxford Tropical Medicine Research Programme, funded by the Wellcome Trust of Great Britain.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
May Ho, Department of Microbiology and Infectious Diseases, 3330 Hospital Dr NW, Calgary, Alberta, Canada T2N 4N1; e-mail: mho@ucalgary.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal