Abstract
To evaluate the importance of the thymus for the reconstitution of immunity in recipients of a T-cell–depleted bone marrow, we measured the appearance of CD4+CD45RA+RO−naive T cells (thymic rebound), restoration of the diversity of the T-cell–receptor (TCR) repertoire and the response to vaccinations with tetanus toxoid (TT). Repopulation by CD4+CD45RA+RO− thymic emigrants varied among patients, starting at approximately 6 months after transplantation. Young patients reconstituted swiftly, whereas in older patients, the recovery of normal numbers of naive CD4+ T cells could take several years. Restoration of TCR diversity was correlated with the number of naive CD4+CD45RA+RO− T cells. Moreover, the extent of the thymic rebound correlated with the patient's capacity to respond to vaccinations. Patients without a significant thymic rebound at the moment of vaccination (CD4+CD45RA+RO− T cells less than 30 μL) did not respond, or responded only marginally even after 3 boosts with TT. We conclude that during the first year after transplantation, the absence of an immune response is due mainly to the loss of an adequate T-cell repertoire. Restoration of the repertoire can come only from a thymic rebound that can be monitored by measuring the increase of CD4+CD45RA+RO−naive T cells. This will allow postponing revaccinations to a moment when the patient will be able to respond more effectively. This may be particularly useful in the elderly patient who, owing to low thymic activity, might not yet be able to respond 1 year after transplant when revaccinations are usually scheduled.
Introduction
Stem cell transplantation (SCT) is an established treatment for various hematological disorders.1-6Unfortunately, the transplant procedure causes a severe immune deficiency, resulting in numerous, often life-threatening infections.7-10 Because during the first weeks after transplant, every hematological lineage is affected, initially all arms of the immune system are deficient. Late complications are usually due to malfunctioning of the T-cell compartment, and infectious morbidity correlates with low CD4+ T-cell counts.11Although the nature of infections in long-term survivors of SCT pointed clearly at a T-cell deficiency,7,11-15 the reason T-cell immunity remained impaired despite a rapid recovery of normal numbers of T lymphocytes has been uncovered only recently. It has been shown that once the T-cell compartment has been eradicated, it is reconstituted through 2 different pathways.16-20 One is thymus dependent and produces T cells with a very diverse repertoire.20 Initially however, the T-cell compartment is reconstituted through expansion of mature T cells. Because these cells are the progeny of only a limited number of precursors, their T-cell receptor (TCR) repertoire is of limited diversity.21
While unbalanced T-cell repertoires have been observed after several treatments affecting lymphocyte viability, the phenomenon is most evident in patients transplanted with a T-cell–depleted graft.21-23 Here, the number of mature T cells cotransfused with the graft is so low that some TCRBV (beta variable region) families may consist of only a single clone.20 In addition, when T-cell depletion is performed only on the graft, the number of donor T cells transfused is in the same order of magnitude as the number of recipient T cells that have survived the conditioning. Because the T-cell depletion also reduces the antihost activity of the graft, recipient T cells may expand to form a significant part of the peripheral T-cell pool.24 25 Because of the combination of these phenomena, it is very instructive to study recipients of T-cell–depleted allografts as a model for the rules of the reconstitution of T-cell immunity after transplant. Another consequence of the very low number of T cells at the time of transplant is that after the expansion phase, the expanded cells completely dominate the cells that have remained quiescent. The ensuing homogeneous expression of memory markers significantly facilitates the study of the reconstitution of the T-cell pool through the thymic pathway, since even a low thymic activity will be spotted by the reappearance of CD4+CD45RA+RO− naive T cells in the blood. Furthermore, because the recipient bone marrow has been eradicated, the pool of recipient T cells cannot be replenished through the thymic pathway. Therefore, the recipient T cells represent a true thymus-independent pool of memory cells that can be followed in time. In this report, we analyzed the restoration of T-cell immunity in 10 adults transplanted with an in vitro T-cell–depleted graft of their HLA-identical sibling. We found that in every patient, the CD4+CD45RA+RO− naive T cells of donor origin emerging with a lag time of approximately 6 months were responsible for the diversification of the T-cell repertoire as well as for the patient's capacity to respond to vaccinations. As a consequence, reconstitution of T-cell immunity depended entirely on the thymic rebound, which varied significantly between patients.
Patients, materials, and methods
Blood samples
Posttransplant peripheral blood cells were collected at the time points indicated. Mononuclear cells were harvested from Ficoll-Hypaque (Ficoll-Hypaque, Pharmacia, Uppsala, Sweden) density gradients and kept in liquid nitrogen until use.
Fluorescence-activated cell analysis/sorting
We performed 2- and 3-color immunofluorescence by incubating the cells with anti-CD4 (mouse-immunoglobulin [Ig]–G2a or mouse-IgG2b, biotinylated), anti-CD8 (mouse-IgG1), anti-CD3 (mouse-IgG2b, biotinylated), anti-CD45RA–fluorescein isothiocyanate (mouse-IgG1), or anti-CD45RO (mouse-IgG2a) and staining with either subclass-specific antisera (Southern Biotechnologies, Birmingham, AL) or streptavidin-red 670 (Life Technologies, Basel, Switzerland). Samples were analyzed on a FacsScan (Becton Dickinson, Mountain View, CA). To obtain BV2+ T cells, cells were stained with TCRBV2-specific antibodies and sorted by fluorescence-activated cell sorting (Becton Dickinson).
Proliferation assay
Cells (1 × 105/flat-bottom well) were cultured in 200 μL of RPMI 1640 medium, 100 IU/mL penicillin, 100 μg/mL streptomycin, 1 mmol/L sodium pyruvate, nonessential amino acids, and 2 mmol/L L-Glutamin (Life Technologies), supplemented with 5% human AB serum and 10−5 mol/L β-mercaptoethanol (Sigma, St. Louis, MO). We added 1/4000 tetanus toxoid (TT) (Institut Mérieux, Lyons, France) as a stimulus. After 6 days of culture, the proliferative responses were measured by the amount of 3H-thymidine (5 μCi/well, 25 mCi/mmol) (Amersham-Pharmacia, Uppsala, Sweden) incorporated after an additional 8-hour pulse. Responses are expressed as the stimulation index (cpm response/cpm background) normalized to that of a normal control. Cells lines were established after 10 days of culture. Cloning of antigen-specific cells was done as previously described.26
Analysis of the recipient/donor origin of the cells
The recipient/donor origin of cells was determined on phytohemagglutinin-stimulated (Murex Biotech Ltd, Dartford, UK) clones derived from the sorted TCRBV2 cells from patient number 9 at 6 and 24 months post-SCT as described previously.24 Briefly, high molecular weight DNA was prepared from nuclei solubilized in Triton-X100 (Sigma) and incubated with proteinase K (Promega Corporation, Madison, WI) followed by polymerase chain reaction (PCR) with the minisatellite 33.1 primers. The amplification mixtures were separated on 1% agarose gels, and the origin of the clones was determined by comparison with the recipient (pre-SCT) and donor-specific profiles.
Spectratype analysis
All procedures as well as the sequences of oligonucleotides corresponding to the 21 variable segments of the TCR beta chain used in this study have been published previously.20 In brief, total RNA and complementary DNA were prepared from 2000 and 8000 CD3+ cells with the use of RNeasy kits from Qiagen (Hilden, Germany). PCR was performed with 6-FAM–, HEX-, and TET-5′–labeled primers (Amplimmun, Madulain, Switzerland), which allows amplification and analysis of multiple TCRBV segments in the same reaction. Data analysis was performed with the Genescan analysis software (Perkin Elmer, Rotkreuz, Switzerland).
Results
Reconstitution of the T-cell compartment by CD4+ T cells in patients transplanted with an in vitro T-cell–depleted bone marrow
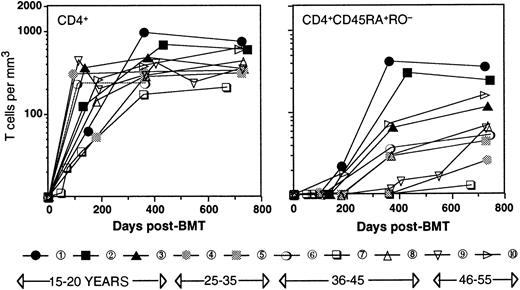
Table 1 shows the age, type of disease, conditioning, and post-transplant immune suppression regimen for the patients studied. All patients (ages 17 through 52, patient numbers in order of age) were transplanted for leukemia in first remission with a Campath-1M T-cell–depleted marrow27 from an HLA-identical sibling. Engraftment (more than 0.5 × 109/L polymorphonuclear leukocytes) occurred between 17 and 35 days after transplant, and all patients remained in complete remission during the time of the study. Two patients (patient number 7, patient number 8) showed mild signs of graft-versus-host disease (GVHD); patient number 7 suffered from chronic GVHD, for which this patient received immune suppression during 19 months posttransplant. Figure 1shows that with the exception of patient number 7, CD4+ T cells reconstituted rapidly, and no correlation between the number of CD4+ T cells and the age of the patient was observed. The reconstitution occurred in 2 phases. During the first 6 months after transplant, CD4+ T cells were generated exclusively through expansion of mature T cells, which was evident for 2 different reasons. First, no cells with a CD45RA+RO− naive phenotype of thymic emigrants were detected, confirming that after intense conditioning, the thymus activity recovers only with a considerable lagtime.18 Second, a significant percentage (28 ± 17; range, 5-60) of the T cells were of recipient origin.20,22,25 Because the increase in cell numbers took place in the absence of a recipient bone marrow24 and therefore, in the absence of T-cell precursors, it is evident that this increase must have occurred through peripheral expansion of the few cells that had survived the conditioning. At approximately 6 months after transplant, the first CD4+ T cells with a CD45RA+RO− naive phenotype appeared. These cells were very likely thymic emigrants because they were of 100% donor origin.28 As a consequence, the ratio between recipient and donor T cells (11 ± 10; range, 5-40 at 2 years after SCT) diminished significantly.22 Furthermore, as reported by others,18 29 naive CD4+ cells seemed to recover faster in younger patients, 2 of whom already had normal numbers (200/μL or greater) of naive CD4+ T cells in their circulation at 1 year after transplant. In contrast, the other patients reconstituted much more slowly. These differences could be very significant, with 3 patients being almost void of naive CD4+ T cells at 1 year after transplant.
Clinical characteristics of the patients
| Patient number . | Diagnosis . | Age . | Conditioning* . | Rejection prophylaxis† . | GVHD prophylaxis‡ CyA (months) . | GVHD a/c1-153 . |
|---|---|---|---|---|---|---|
| 1 | ALL | 17 | Cy-TBI (12) | TLI, MP | 2 | 0/0 |
| 2 | ALL | 17 | Cy-TBI (12) | TLI, MP | — | 0/0 |
| 3 | AML | 20 | Cy-TBI (13.5) | TLI, MP | 3 | 0/0 |
| 4 | AML | 29 | Cy-TBI (13.5) | TLI, MP | 1 | 0/0 |
| 5 | AML | 35 | Cy-TBI (12) | MP | 2 | 0/0 |
| 6 | AML | 44 | Cy-TBI (12) | — | 3 | 0/0 |
| 7 | AML | 44 | Bu-Cy | MP | 19 | 1/+ |
| 8 | MM | 45 | Cy-TBI (12) | MP | 3 | 1/0 |
| 9 | AML | 48 | Cy-TBI (12) | MP | 5 | 0/0 |
| 10 | AML | 52 | Cy-TBI (12) | MP | 3 | 0/0 |
| Patient number . | Diagnosis . | Age . | Conditioning* . | Rejection prophylaxis† . | GVHD prophylaxis‡ CyA (months) . | GVHD a/c1-153 . |
|---|---|---|---|---|---|---|
| 1 | ALL | 17 | Cy-TBI (12) | TLI, MP | 2 | 0/0 |
| 2 | ALL | 17 | Cy-TBI (12) | TLI, MP | — | 0/0 |
| 3 | AML | 20 | Cy-TBI (13.5) | TLI, MP | 3 | 0/0 |
| 4 | AML | 29 | Cy-TBI (13.5) | TLI, MP | 1 | 0/0 |
| 5 | AML | 35 | Cy-TBI (12) | MP | 2 | 0/0 |
| 6 | AML | 44 | Cy-TBI (12) | — | 3 | 0/0 |
| 7 | AML | 44 | Bu-Cy | MP | 19 | 1/+ |
| 8 | MM | 45 | Cy-TBI (12) | MP | 3 | 1/0 |
| 9 | AML | 48 | Cy-TBI (12) | MP | 5 | 0/0 |
| 10 | AML | 52 | Cy-TBI (12) | MP | 3 | 0/0 |
ALL indicates acute lymphocytic leukemia; AML, acute myelogenous leukemia; MM, multiple myeloma.
Cy indicates cyclophosphamide (120 mg/kg or 200 mg/kg in combination with busulfan (Bu) (4 daily doses of 4 mg/kg body weight, Mesna was used systematically). TBI indicates total body irradiation (Gy given in 6 doses).
TLI indicates total lymphoid irradiation (6 Gy at day −5 and −4); MP indicates methyl prednisolone (4 × 1 g/m2).
GVHD prophylaxis consisted of in vitro T-cell depletion with Campath 1M and Cyclosporin A (CyA) with a trough at 200 ng/mL.
a/c indicates acute/chronic.
Time course of the reconstitution of T cells.
CD4+ (left panel) and CD4+CD45RA+RO− (right panel) T cells. Patient numbering and black/gray/open symbols relate to the age of the patient.
Time course of the reconstitution of T cells.
CD4+ (left panel) and CD4+CD45RA+RO− (right panel) T cells. Patient numbering and black/gray/open symbols relate to the age of the patient.
Reconstitution of the TCR repertoire by thymic emigrants
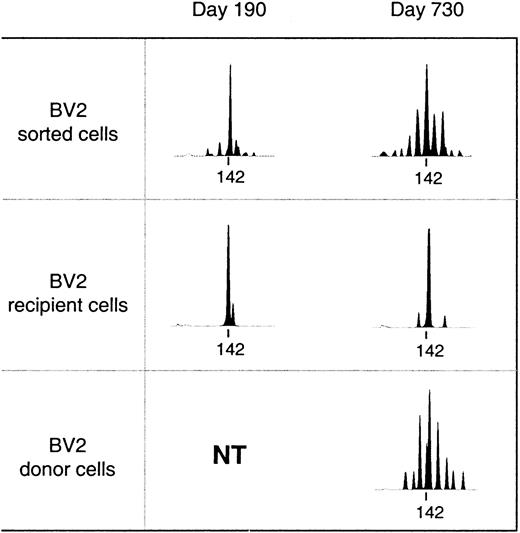
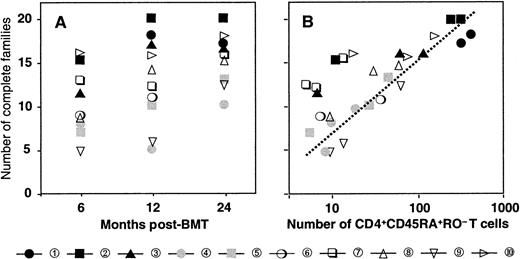
Reconstitution of the T-cell compartment by expansion restores T-cell numbers without reconstituting the diversity of the T-cell repertoire.21 This is true not only for patients transplanted with T-cell–depleted grafts, who receive low numbers of T cells at the time of transplant, but also for recipients of unmanipulated grafts.22 Consequently, reactivity against numerous antigens may be lost. TCR diversity can be assessed by spectratyping, a method of measuring the size heterogeneity of the TCR hypervariable CDR3 region. Spectratypes from normal repertoires are complex and show a Gaussian distribution of 8 to 10 bands, representing the different lengths of the respective TCR V-D-J regions. In contrast, spectratypes from individuals with a limited TCR repertoire show gaps owing to the absence of clones with V-D-J regions of the corresponding length. We have previously shown that these gaps in the repertoire disappear during the second year after SCT owing to the appearance of T cells with a CD45RA+RO− naive phenotype with a normal, diverse repertoire.20 If these cells are produced by the thymus, they should be exclusively of donor origin because no recipient stem cells exist anymore to produce the necessary precursors. Figure 2 shows a detailed analysis of the diversification of the BV2-family in patient number 9. In the upper panel, we performed a spectratype analysis on 2.103TCRBV2-sorted T cells from blood samples at 190 and 730 days after transplant. The results show that the repertoire at day 190, which was dominated by a band at 142 bp, had diversified at day 730 to the extent that the spectratype had become indistinguishable from that of a normal donor.20 The repertoire diversification of the T-cell pool that still contained significant numbers (more than 70%) of recipient T cells25 was due solely to the appearance of donor T cells with a random repertoire. This is shown in the middle and lower panel, where we separated the BV2-expressing T cells of donor and recipient origin by in vitro cloning of T cells under limiting dilution conditions. Clearly, the repertoire of the recipient T cells did not change because the spectratype of day 190 (15 clones pooled) was almost identical to that of day 730 (33 clones pooled). In contrast, the day-730 spectratype of the donor T cells (33 clones pooled) was almost as complete as that of the 2.103 TCRBV2 T cells sorted originally. Thus, the repertoire diversification between day 190 and day 730 was due entirely to donor T cells that were absent during the first 6 months after transplant. Holes in the repertoire can be repaired only through production of new T cells by the thymus. Therefore, the reconstitution of TCR diversity must initially correlate with the extent of the thymic rebound. Although this will be true for CD4+ as well as CD8+ T cells, thymic activity is most easily monitored by the production of CD4+CD45RA+RO− T cells18 because the phenotypes of naive CD8+ T cells are more complex.30 Quantification of the TCR diversity is possible by determining the percentage of complete spectratypes in samples of low numbers of T cells.20 We have shown that in samples of normal individuals containing as few as 8.103 T cells, the spectratypes of approximately 80% of the TCRBV families are complete. This percentage is significantly lower in patients with restricted T-cell repertoires.20 Figure3A shows the changes in TCR diversity by comparison of the number of complete TCRBV spectratypes generated by a sample of 8.103 T cells taken at different time points after transplant. It shows that after 6 months, the TCR repertoire of all patients was still significantly below normal. At 1 year, only the youngest patients (black symbols) showed repertoire complexities comparable to that of normal individuals. Other patients reconstituted much more slowly, and even at 2 years after transplant, 3 of 10 patients still had very limited repertoires. This was clearly due to the low rate at which their T-cell compartment was reconstituted by naive T cells (Figure 3B). With the exception of the samples in which the spectratypes reflected merely the expanded memory T-cell pool (fewer than 20 to 30/μL naive cells), TCR diversity correlated well with the number of CD4+CD45RA+RO− T cells in the blood. Because these naive T cells are exclusively of donor origin28 and do not emerge when the patient is thymectomized,31 it is evident that the restoration of T-cell repertoire depends on stem cells producing pre-T cells as well as on a functioning thymus.
Diversification of the T-cell repertoire occurs only in T cells of donor origin.
Upper panel: spectratypes of 2.103 T cells sorted on basis of BV2 expression. Recipient or donor spectratypes (middle and lower panel) are from independent T-cell clones pooled on the basis of their recipient/donor origin as determined by minisatellite analysis. The tag marks the position of a PCR-product of 142 base pairs. NT indicates not tested (only clones of recipient origin were obtained).
Diversification of the T-cell repertoire occurs only in T cells of donor origin.
Upper panel: spectratypes of 2.103 T cells sorted on basis of BV2 expression. Recipient or donor spectratypes (middle and lower panel) are from independent T-cell clones pooled on the basis of their recipient/donor origin as determined by minisatellite analysis. The tag marks the position of a PCR-product of 142 base pairs. NT indicates not tested (only clones of recipient origin were obtained).
Diversification of the repertoire in time is due to the appearance of CD4+CD45RA+RO−naive T cells.
(A) Number of families with a complete spectratype at 6, 12, and 24 months after transplant. (B) Relation between the number of families with a complete spectratype and the number of CD4+CD45RA+RO− naive T cells. Patient numbers and symbols as in Figure 1.
Diversification of the repertoire in time is due to the appearance of CD4+CD45RA+RO−naive T cells.
(A) Number of families with a complete spectratype at 6, 12, and 24 months after transplant. (B) Relation between the number of families with a complete spectratype and the number of CD4+CD45RA+RO− naive T cells. Patient numbers and symbols as in Figure 1.
A thymic rebound is essential to respond to vaccinations
After SCT, T-cell memory is lost and patients have to be revaccinated.8,10,32,33 Because patients respond poorly during the first year after transplant,32 vaccinations are usually postponed to the second or third year. An obvious explanation for this late recovery is that the lack of responsiveness is the effect of the holes in the T-cell repertoire. If so, unresponsiveness will last until the thymus has produced new antigen-specific T cells. Therefore, although the responding cell itself will express memory markers, the extent of the response will correlate with the size of the naive pool, which reflects the thymic rebound. Figure4 shows that for the 9 patients who had lost their in vitro anti-TT response completely (not shown), this was indeed the case. Of the 3 patients immunized at 1 year after transplant, only patient number 10, a 53-year-old patient whose thymic rebound was only slightly inferior to that of the young patients (Figure 1) responded. In contrast, for the 2 patients in whom the thymic rebound was less evident, even 3 boosts with TT remained ineffective. The same correlation between the response and the extent of thymic rebound existed for the patients immunized much later. Patient number 4 and patient number 7, the 2 patients with the lowest thymic rebound, responded less well than the patients in whom the number of CD4+CD45RA+RO− naive T cells was higher. Interestingly, the number of naive T cells and the resulting restoration of the T-cell repertoire correlated better with the patient's capacity to respond than, eg, the number of CD4+ T cells, age, or the moment of immunization. Therefore, the appearance of CD4+CD45RA+RO− T cells after SCT can be taken as a marker for the reconstitution of the immune response, which should be of use in scheduling the revaccination program.
The in vitro cellular response to TT is proportional to the number of CD4+CD45RA+RO− naive T cells.
●—●, Number of CD4+CD45RA+RO− naive T cells at the time of the first vaccination and at the time of the third vaccination (for the second time point, no data were available for P1 [patient number 1], whereas for P7 and P8 data show the values of approximately 6 months after the third vaccination). The bars show the stimulation index (cpm response/cpm background) of patient cells normalized to the stimulation index of a normal control stimulated with TT in the same experiment. In all experiments, the same 2 normal controls, who had not been recently immunized, were used. In different experiments, responses of the normal controls varied between 35.103 and 173.103 cpm (90 ± 60.103), whereas the cpm in cultures without antigen (patients as well as normal controls) never exceeded 1200.
The in vitro cellular response to TT is proportional to the number of CD4+CD45RA+RO− naive T cells.
●—●, Number of CD4+CD45RA+RO− naive T cells at the time of the first vaccination and at the time of the third vaccination (for the second time point, no data were available for P1 [patient number 1], whereas for P7 and P8 data show the values of approximately 6 months after the third vaccination). The bars show the stimulation index (cpm response/cpm background) of patient cells normalized to the stimulation index of a normal control stimulated with TT in the same experiment. In all experiments, the same 2 normal controls, who had not been recently immunized, were used. In different experiments, responses of the normal controls varied between 35.103 and 173.103 cpm (90 ± 60.103), whereas the cpm in cultures without antigen (patients as well as normal controls) never exceeded 1200.
Discussion
T-cell immune deficiency is responsible for a significant number of late deaths after SCT.34 Apparently, it may take years before T-cell immunity is restored, and particularly in the elderly patient, the pretransplant level may never be achieved. There are several reasons T-cell immunity can be insufficient when all other hematopoietic lineages are functional. First, stem cells produce only T-cell precursors, and in contrast to other hematopoietic lineages, T cells still have to undergo an extensive postmarrow maturation process in the thymus. Most of the T-cell pool is produced around birth, and it is not known whether the adult thymus still has the capacity to regenerate a new repertoire.20,35,36 The process of understanding why immunity is not restored by the T cells that repopulate the patient after SCT has been slow. To date, it has been established that T-cell reconstitution after SCT is not a recapitulation of T-cell ontogeny, but that initially, the T-cell compartment is restored through expansion of mature T cells16 that have survived the conditioning18or that are cotransfused with the graft.17 Because the expanding T cells do not contain the repertoire needed to respond to common antigens, the risk of infections remains significant notwithstanding the normal number of T cells in the blood.
The loss of memory is evident not only in the frequent occurrence of infections but perhaps even more so in the incapacity of the patient to respond to vaccinations against classical recall antigens. Once memory has been lost, it is obvious that a response can be recruited only from naive cells that have the capacity to recognize the antigen. In this report, we show that in recipients of a T-cell–depleted graft, who lack such naive T cells after transplantation, the response to vaccinations with TT depends entirely on the thymic rebound. This is further supported by the fact that TT-specific clones were never found before the appearance of CD4+CD45RA+RO− T cells in the blood and that in 3 patients studied, the T cells participating in the anti-TT response are entirely of donor origin (work in progress). It is not known to what extent this is also true for patients who receive substantial quantities of T cells with their stem-cell graft. Although one might argue that recipients of unmanipulated grafts receive enough donor memory T cells and possibly enough naive cells to carry through the donor's immune system, they still respond poorly to vaccinations. Moreover, in such patients, restoration of immunity appears also to be correlated with markers of thymic activity,37 showing that the transferred naive cells do not contain enough repertoire to respond to all antigenic challenges after SCT.
We have assessed the response to vaccinations with TT by the in vitro proliferative response of TT-specific T cells. This assay gives a good quantitative impression of the response, because it directly reflects the frequency of antigen-specific T cells in the blood. After SCT, this has an advantage over measuring the humoral response, because before vaccination, more than half of the patients still have anti-TT antibodies in the serum.38 In addition, antibody titers may vary more than 50-fold, making it difficult to weigh the significance of the very weak responses. In our group of patients, this also made the interpretation of anti-TT titers difficult. Nevertheless, we observed some correlations between the antibody response and the extent of the thymic rebound at the time of immunization. The 3 patients with the highest numbers of naive cells (patients number 1, 2, and 6) also had the highest antibody titers. In contrast, when the numbers of naive T cells were low and no proliferative response was detected (patients number 8 and 9), even 3 boosts of TT had little effect. Patient number 8 did not respond to the first vaccination, and after the third boost, this patient's titer was the lowest of all the samples tested. Furthermore, the 3 boosts did not induce any change in the antibody titer in patient number 9, who was already positive before vaccination.
In conclusion, we have shown that the thymic rebound as measured by the reappearance of CD4+CD45RA+RO− naive T cells in the blood correlates with the capacity to respond to vaccinations. Although our data have to be confirmed in a larger group of patients, we believe that measuring the thymic activity could become a very valuable method to estimate the patient's posttransplant immune capacity. Clearly, the number of naive cells were more predictive for the response to the vaccination than other parameters, such as total numbers of CD4+ T cells, the moment of vaccination, or the age of the patient. Only with the steadily growing impact of the thymic production on the total number of T cells, the response to the vaccinations correlated with the number of CD4+ cells as well.
It has been shown that in long-term survivors, low numbers of CD4+ cells correlate to high infectious morbidity.11 It will be interesting to see whether the increase in the number of CD4+CD45RA+RO− T cells during the first year could serve as an even better parameter for the general immune status of the patient. If so, this could be used to identify the patients with a slow immune reconstitution so that prophylactic regimens and revaccination programs could be adjusted accordingly.
Acknowledgments
The authors thank Dr Nathalie Rufer for critical reading of the manuscript and Solange Vischer for excellent technical assistance.
Supported by a grant from the Swiss National Science Foundation (#31-53774.98) and by the Dr Henri Dubois-Ferrière-Dinu Lipatti Foundation.
E.R. and F.D.-G. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eddy Roosnek, Unité d'Immunologie de Transplantation, Hôpital cantonal universitaire de Genève, 24 rue Micheli-du-Crest, CH-1211 Genève 14, Switzerland; e-mail:roosnek@cmu.unige.ch.

![Fig. 4. The in vitro cellular response to TT is proportional to the number of CD4+CD45RA+RO− naive T cells. / ●—●, Number of CD4+CD45RA+RO− naive T cells at the time of the first vaccination and at the time of the third vaccination (for the second time point, no data were available for P1 [patient number 1], whereas for P7 and P8 data show the values of approximately 6 months after the third vaccination). The bars show the stimulation index (cpm response/cpm background) of patient cells normalized to the stimulation index of a normal control stimulated with TT in the same experiment. In all experiments, the same 2 normal controls, who had not been recently immunized, were used. In different experiments, responses of the normal controls varied between 35.103 and 173.103 cpm (90 ± 60.103), whereas the cpm in cultures without antigen (patients as well as normal controls) never exceeded 1200.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/6/10.1182_blood.v96.6.2299/5/m_h81800152004.jpeg?Expires=1769106271&Signature=B3hn4px96iBLONAEkjlYuSArykniSPr0eqlMU7EF8UkpVfYZoGZSb8AUs5kUbNmiNjirutA2V2939MMn3oXm7K3K5mwLsOStmiDfba3qBg2HuNqmlHmVe4ksIn3ovL8S6dNXRMfHNuz71pOGUh6b0pDdcvnypbyUThUidHswOZkCgqGiufZlKMJ61R~Wv2K3k5c7Nt3Pbpo-GSQPJ-B17VHlS04xEplvUxYyXOVdtWwhLJSJ3lfkhwJe7ap3Iuv5E4B-M3tp8XOAakdNm-ZACosGLOpdWPuYitUHemVfO2b~aMoCNd2x~2Rg6n17-GgzqXZg11H~RB1ldtlVpxmYfQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal