Abstract
It was hypothesized that during mammalian development, the extensive need for hematopoietic cells requires equal contribution to blood cell production from both quiescent and cycling hematopoietic stem cells (HSCs) while maintaining the stem cell pool. To investigate this hypothesis, the engraftment potential of umbilical cord blood (UCB) CD34+ cells residing in either G0(G0CD34+ cells) or G1(G1CD34+ cells) phases of the cell cycle was assessed in nonobese diabetic/severe combined immune-deficient (NOD/SCID) mice. Whereas the level of chimerism in mice transplanted with UCB G0CD34+ cells was 69.9% ± 24.0%, mice receiving equal numbers of G1CD34+ cells harbored 46.7% ± 21.3% human cells 8 weeks posttransplantation. Both groups of cells sustained multilineage differentiation and the production of CD34+cells in recipient animals. The relationship between the number of transplanted G0CD34+ or G1CD34+ cells and the level of chimerism was analyzed by a general linear models procedure. Although the initial level of chimerism following transplantation of G0CD34+ cells was higher than that sustained by G1CD34+ cells, the increment in the degree of chimerism obtained with each additional 103 cells of either phenotype was identical, suggesting that the reconstitution potential of these 2 types of cells was similar. Of interest is that human cells recovered from primary recipients of both G0CD34+ and G1CD34+cells engrafted in secondary NOD/SCID recipients, albeit at a substantially lower level, confirming the primitive nature of UCB CD34+ cells residing in G1.
Introduction
Lifelong production of all blood cells is sustained by a group of highly specialized cells known as hematopoietic stem cells (HSCs). Umbilical cord blood (UCB), bone marrow (BM), and mobilized peripheral blood (MPB) represent rich sources of HSCs, but primitive hematopoietic progenitor cells from these 3 tissues have been demonstrated to differ quantitatively and qualitatively.1-5 These differences raise the possibility that HSCs from these tissues may vary in their utility for clinical transplantation and somatic gene therapy. When engraftment of nonobese diabetic/severe combined immune-deficient (NOD/SCID) mice was used to quantitate the frequency of SCID repopulating cells (SRCs), vast discrepancies in the frequencies of HSCs were detected in UCB, BM, and MPB mononuclear cells.6,7 The SRC frequency among unfractionated UCB cells was 3 times higher than in BM and 6 times higher than in MPB (1 per 9.3 × 105, 1 per 3.0 × 106, and 1 per 6.0 × 106mononuclear cells in UCB, BM and MPB, respectively). Similar results were observed when purified CD34+ cells were examined.8,9 These differences in SRC frequency are in line with findings of in vitro studies in which the frequency of HSCs in UCB was higher than in adult BM and MPB.10 11
Hematopoietic stem cells capable of repopulating the marrow of transplant recipients are mainly in the quiescent phase of the cell cycle.12-15 However, in these studies, only a distinction between cells in G0/G1 versus S/G2+M was possible, leaving unanswered the question of whether the hematopoietic potential of cells in G0 is superior to that of cells residing in G1. Recently, we established a more scrutinizing relationship between the cell-cycle status of CD34+ cells from human MPB or adult BM and their SCID repopulating capacity.16 CD34+ SRCs from MPB were demonstrated to reside predominantly within the G0phase of the cell cycle. Transplantation of MPB G0CD34+ cells resulted on average in a 16-fold higher percentage of human cells in the BM of recipient mice than equal numbers of G1CD34+ cells, showing a direct relationship between hematopoietic potential of CD34+ cells and their mitotic quiescence. As in MPB, more than 97% of UCB CD34+ cells are in G0 and G1.2 We hypothesized that in contrast to adult sources of HSCs, the higher frequencies of SRCs in UCB6and the lower number of UCB CD34+ cells needed for engraftment in NOD/SCID mice17 might be due to the unique property of these fetal cells to engraft during the G1phase of their cell cycle. Therefore, we examined the marrow-repopulating potential of human UCB CD34+ cells fractionated on the basis of their position in the cell cycle. In this report, we demonstrate that UCB CD34+ cells residing in either G0 or G1 are equally effective in repopulating NOD/SCID mice, suggesting that, in this tissue, HSC activity may not be tightly associated with mitotic quiescence.
Materials and methods
Collection and purification of UCB CD34+ cells
UCB samples from full-term deliveries were collected in sterile tubes containing 20 U/mL preservative-free heparin. Samples were collected under the guidelines established by the Institutional Review Board of Indiana University (Indianapolis). Low-density cells were separated over Ficoll-Hypaque (Pharmacia, Piscataway, NJ). CD34+ isolation was performed by means of magnetic cell separation (MACS) columns (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and antibodies recognizing the CD34 epitope QBEND/10 according to the manufacturer's procedure. Purity of selected CD34+fraction was assessed by flow-cytometric analysis with the use of a phycoerythrin (PE)–conjugated monoclonal antibody recognizing a different CD34 epitope (HPCA-2) (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA).
Cell-cycle fractionation with Hoechst 33342 and Pyronin Y
To distinguish between cells in G0 or G1, which have the same DNA content but different RNA content, simultaneous DNA/RNA staining with Hoechst 33342 (Hst) (Molecular Probes, Eugene, OR) and Pyronin Y (PY) (Polysciences, Warrington, PA), respectively, was performed as previously described.18,19 Briefly, CD34+ cells were resuspended at a concentration of 5 × 106 or fewer cells per milliliter in Hst buffer containing 1 μg/mL Hst. Hst buffer consisted of Hanks Balanced Salt Solution (Biowhittaker, Walkersville, MD), 20 mmol/L Hepes (Biowhittaker), 1 g/L glucose, 10% fetal calf serum (FCS) (Hyclone, Logan, UT), and verapamil at 50 μmol/L (Sigma, St Louis, MO). After 45 minutes incubation at 37°C, PY was added at a final concentration of 1 μg/mL, and cells were further incubated for another 45 minutes at 37°C. Cells were washed once in chilled Hst buffer and incubated for 30 minutes with fluorescein isothiocyanate (FITC)–conjugated CD34 (Pharmingen, San Diego, CA) at 4°C. Cells were washed again, resuspended in Hst buffer, and analyzed or sorted on a FACStar Plus (BDIS) equipped with an argon laser providing the 488-nm excitation for PY and FITC and a krypton laser providing the 350-nm excitation for Hst. PY signal was selected with a 575 ± 13–nm bandpass filter, and Hst was detected with a 424 ± 22–nm bandpass filter. First gating was performed on CD34-positive cells. In this gate, cells were identified as being in G0 versus G1 on the basis of their relative PY staining. Cells in G0 were identified by their minimal RNA content, whereas cells traversing into G1 were defined as those with high or maximal RNA staining,20 thus allowing the isolation of viable CD34+ cells in G0(G0CD34+) or G1(G1CD34+) cells. Cell-sorting criteria for the identification of G0CD34+ and G1CD34+ cells were identical to those previously decribed,16 allowing us therefore to separate the upper limit of the G0 sort window from the lower limit of the G1 sort window by at least 150 fluorescence channels. During sorting, cells were kept on ice to minimize dye leaking and were protected from light. Viability of sorted cells always exceeded 98%. Postsort analysis was performed on sorted cells with the use of initial instrument settings employed during sorting. Purity of the sorted G0CD34+ and G1CD34+ cells consistently exceeded 90%. Given that all sorted cells were CD34+, contaminating cells were CD34+ cells falling adjacent to sort windows defining each sorted group but not within the sort window of the opposing phenotype.
High-resolution cell-cycle analysis
To verify the cell-cycle status of sorted G0CD34+ and G1CD34+cells, Ki-67 and 7-aminoactinomycin D (7-AAD) staining of cell suspensions was performed as described by Jordan et al,21with minor modification. This procedure confirms the position of sorted cells in the cell cycle using a combination of staining for DNA (with 7-AAD) and Ki-67, a nuclear antigen specifically expressed by cycling cells. Ki-67 expression should be higher or possibly restricted to PYhigh cells, whereas PYlow cells should be Ki-67 negative. Cells were washed and resuspended in 1 mL phosphate-buffered saline (PBS) with 0.4% formaldehyde. After 30 minutes at 4°C, 1 mL of PBS with 0.2% Triton X-100 was added, and cells were left overnight at 4°C. Cells were then washed twice in PBS with 1% bovine serum albumin (BSA) and stained with FITC anti–Ki-67 (clone MIB-1, Immunotech, Westbrook, ME) for 60 minutes at 4° C. Isotype controls were stained in parallel. Finally, cells were washed and resuspended in 0.5 mL PBS with 1% BSA containing 5 μg/mL 7-AAD (Sigma). After 3 hours of incubation on ice, samples were analyzed on a FACScan flow cytometer (BDIS) with the use of FL-1 and FL-3 channels for Ki-67 and 7-AAD, respectively.
Limiting dilution analysis for the estimation of the frequency of long-term culture initiating cells
To estimate the frequencies of long-term culture initiating cells (LTC-ICs) in G0CD34+ and G1CD34+ UCB cells, limiting dilution analysis assays were performed as previously described22 with some modifications.20 M2-10B4 cells were irradiated at 80 Gy (GammaCell 40, Nordion International, Kanata, Ontario, Canada) and plated in flat-bottomed 96-well plates at a concentration of 15 × 103 cells per well in 100 μL long-term culture medium (LTCM). LTCM consisted of Myelocult (Stem Cell Technologies, Vancouver, British Columbia, Canada) containing 10−6 mmol/L hydrocortisone (Sigma). After 24 hours, G0CD34+ or G1CD34+ UCB cells in 100 μL LTCM were added to the plated stromal cells in limiting dilution (64 to 8 cells per well, in a 2-fold dilution scheme using 48 wells per cell dose). Plates were maintained at 37°C in 100%-humidified atmosphere containing 5% CO2, with weekly half-medium changes. After 5 weeks, 120 μL medium was removed from each well followed by the addition of 150 μL of Iscove's modified Dulbecco's medium (IMDM) containing, at final concentration, 30% FCS, 1.3% methylcellulose, 5 × 10−5 mol/L 2-mercaptoethanol (Sigma), 50 ng/mL stem cell factor (SCF), 10 ng/mL interleukin-3 (IL-3), 10 ng/mL IL-6, 5 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), and 2 U/mL erythropoietin (EPO). All cytokines were a kind gift of Amgen (Thousand Oaks, CA). Plates were cultured at 37°C in 100%-humidified atmosphere containing 5% C02. After 2 weeks, wells were scored for the presence or absence of hematopoietic colonies, and the frequency of LTC-IC was calculated with the use of the maximum likelihood estimator.23
Transplantation of G0CD34+and G1CD34+ UCB cells into NOD/SCID mice
NOD/LtSz-scid/scid (NOD/SCID) mice24 used in these experiments were bred and housed at Indiana University and were kindly provided by Dr D. A. Williams (Indianapolis, IN). Mice were housed in micro-isolators under pathogen-free conditions and received autoclaved food and acidified water ad libitum. Animal experiments were performed in accordance with institutional guidelines approved by the Animal Care Committee of the Indiana University School of Medicine. Nine- to 12-week-old NOD/SCID mice were sublethally irradiated with 3 Gy from a 137Cs source. Mice received by intravenous injection as accessory cells, 107 nonadherent CD34− adult BM cells irradiated with 80 Gy. Mice were transplanted 2 hours later with 7 × 104 to 4 × 105 G0CD34+ or G1CD34+ UCB cells in equal numbers. After 8 weeks, blood was collected and the mice were killed by cervical dislocation, and the spleen and BM were collected. After single-cell suspensions were prepared from these tissues, red blood cells were lysed in 0.155 mol/L NH4Cl, 0.01 mol/L KHC03, and 0.1 mmol/L EDTA. Cell suspensions were washed twice and resuspended in IMDM with 10% FCS for further analysis.
Flow cytometric analysis of engraftment
To analyze the level of chimerism in recipient mice, the percentage of human CD45+ cells was determined. Single-cell suspensions of BM, spleen, or blood were incubated with mouse antihuman CD45 FITC (Pharmingen) or isotype control for 20 minutes at 4°C. Samples were analyzed on a FACScan (BDIS). Positive cells were identified by comparison with isotypic controls and with cells harvested from control (not transplanted) NOD/SCID mice stained with the same antibodies. To determine the frequencies of subsets of human cells, BM and spleen cell suspensions that contained more than 1% CD45+ cells were also stained with PE-conjugated CD19, CD20, CD33, CD34, and CD38 or FITC-conjugated CD61, CD3, CD4, and CD8 in different combinations and with CD45 Cy-5 (Pharmingen) as third color. Regardless of the level of chimerism, BM and spleen cells from chimeric secondary recipients were immunophenotyped as described above.
Transplantation of secondary recipients
In order to examine in vivo maintenance of the long-term repopulating potential of UCB G0 and G1CD34+ cells, human CD34+ cells contained in the marrow of primary NOD/SCID mice were transplanted into secondary recipients. BM cells from 3 to 4 primary recipients of G0CD34+ or G1CD34+cells were pooled and analyzed as described above for the expression of human CD45 and CD34. The percentage of CD45+CD34+ cells was then determined and used to calculate the number of murine BM cells containing 7.5 × 105 CD34+ cells. These cells were then transplanted into conditioned NOD/SCID mice as described above, and secondary recipients were analyzed for chimerism 8 weeks later. Secondary transplants were performed twice with donors from 2 separate primary transplants.
Clonogenic cell assay to determine human hematopoietic progenitor cell frequencies in recipient mice
To enumerate different hematopoietic progenitor cells contained in the BM of mice 8 weeks after transplantation with G0CD34+ or G1CD34+ UCB cells, murine BM cell suspensions that contained more than 1% human CD45+ cells were analyzed in progenitor cell assays. Total cell suspensions (between 1.1 × 104 and 7.5 × 104 total cells) containing 2 × 103CD45+CD34+ human cells (as determined by flow cytometric analysis of these samples) were assayed in duplicate in plastic 35-mm tissue culture dishes containing 1 mL IMDM containing, at final concentration, 1.3% methylcellulose, 30% FCS, 5 × 10−5 mol/L 2-mercaptoethanol, 50 ng/mL SCF, 10 ng/mL IL-3, 10 ng/mL IL-6, 5 ng/mL GM-CSF, and 2 U/mL EPO. Cultures were incubated at 37°C in 100%-humidified atmosphere containing 5% CO2. Hematopoietic colonies (burst-forming unit-erythroid [BFU-E]; colony-forming unit-granulocyte/macrophage [CFU-GM]; and colony-forming unit granulocyte/erythroid/macrophage/megakaryocyte [CFU-GEMM]) were scored after 2 weeks by means of an inverted microscope. An average of 2 CFU-GM–derived colonies were detected in 3 separate experiments when 4 × 104 control murine BM cells were plated under these conditions.
Statistical analysis
A general linear models procedure (analysis of variance) was used to examine the association between the percentage of chimerism and the position of cells in the cell cycle after adjustment of the number of cells infused. The interaction between cell-cycle status of graft cells and number of cells infused was also examined to assess whether the effect of cells infused on chimerism was similar between cells in different positions in the cell cycle. Where applicable, mean ± SD or mean ± SEM of multiple measurements is reported.
Results
Cell-cycle fractionation of UCB CD34+ cells
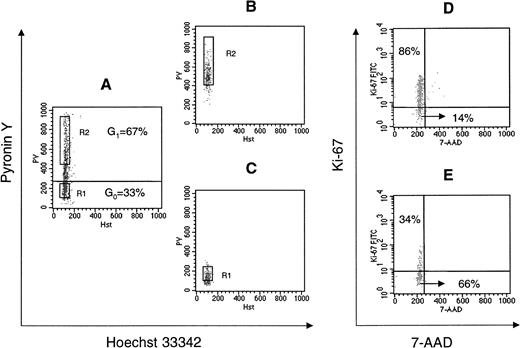
To separate CD34+ cells in G0 phase from cells in G1 phase of the cell cycle, cells were stained with Hst and PY to discriminate between quiescent cells (G0CD34+), which have 2n DNA and minimal RNA content, and those in early or late G1(G1CD34+), which are also Hstdimbut are Pybright owing to their higher RNA content. As illustrated in Figure 1A, 33% ± 2.9% of CD34+ cells were in G0, and 67 %± 2.9% were in G1. As previously reported,2 very few UCB CD34+ cells (less than 2%) displayed a staining pattern consistent with cells in S or G2 + M phases of cell cycle (Hstbright, PYbright). Postsort analysis performed directly after isolation of G0 and G1 fractions of CD34+ cells confirmed a purity of greater than 90% in both groups (Figure 1B,C). Validity of the cell-cycle status of sorted cells was confirmed by Ki-67 and 7-AAD staining. The majority of G0CD34+ cells in 4 separate experiments did not express Ki-67 (80.6% ± 10.0% of G0CD34+ cells were negative for the expression of Ki-67), confirming the quiescent nature of G0CD34+ cells. Results from one experiment are shown in Figure 1 where most PYbright cells (G1CD34+) expressed Ki-67 (86%), whereas only 34% of cells in G0 stained dim for Ki-67 (Figure1D,E).
DNA versus RNA staining of UCB CD34+ cells, postsort analysis of sorted G0 and G1CD34+ cells, and reanalysis of G0and G1CD34+ cells with 7-AAD and Ki-67 staining.
(A) Fluorescence analysis of UCB CD34+ cells stained with Hst and PY as described in “Materials and methods.” Quiescent cells residing in G0 (G0CD34+) have 2n DNA and minimal RNA content (cells falling below the horizontal line in dot plot A), whereas those in early or late G1 (G1CD34+) are also Hstdim but are Pybright owing to their higher RNA content (cells above the horizontal line). According to this definition, approximately 33% of UCB CD34+ cells were determined to be in G0. Sort windows to collect G0 and G1 cells were constructed as previously described16 for MPB and are shown in dot plot A as R1 and R2, respectively. In all sorts, at least 150 fluorescence channels separated the 2 sort windows. (B, C) Postsort analysis of sorted G1CD34+ cells (dot plot B) and G0CD34+ cells (dot plot C). Since PY fluorescence is lost with time, sorted cells “fall” below sort windows upon postsort analysis. (D,E) Cell-cycle status of sorted cells was confirmed by 7-AAD and Ki-67 staining. Most PYbrightcells (cells in G1; panel D) expressed Ki-67 (86%), whereas a substantially smaller fraction (34%) of cells in G0 (panel E) expressed low levels of Ki-67. Ki-67 and 7-AAD staining was repeated in 3 other experiments with similar results.
DNA versus RNA staining of UCB CD34+ cells, postsort analysis of sorted G0 and G1CD34+ cells, and reanalysis of G0and G1CD34+ cells with 7-AAD and Ki-67 staining.
(A) Fluorescence analysis of UCB CD34+ cells stained with Hst and PY as described in “Materials and methods.” Quiescent cells residing in G0 (G0CD34+) have 2n DNA and minimal RNA content (cells falling below the horizontal line in dot plot A), whereas those in early or late G1 (G1CD34+) are also Hstdim but are Pybright owing to their higher RNA content (cells above the horizontal line). According to this definition, approximately 33% of UCB CD34+ cells were determined to be in G0. Sort windows to collect G0 and G1 cells were constructed as previously described16 for MPB and are shown in dot plot A as R1 and R2, respectively. In all sorts, at least 150 fluorescence channels separated the 2 sort windows. (B, C) Postsort analysis of sorted G1CD34+ cells (dot plot B) and G0CD34+ cells (dot plot C). Since PY fluorescence is lost with time, sorted cells “fall” below sort windows upon postsort analysis. (D,E) Cell-cycle status of sorted cells was confirmed by 7-AAD and Ki-67 staining. Most PYbrightcells (cells in G1; panel D) expressed Ki-67 (86%), whereas a substantially smaller fraction (34%) of cells in G0 (panel E) expressed low levels of Ki-67. Ki-67 and 7-AAD staining was repeated in 3 other experiments with similar results.
LTC-IC content of UCB G0CD34+ and G1CD34+ cells
Whether fractionation of UCB CD34+ cells based on their position in cell cycle led to compartmentalization of primitive progenitor cells was first examined in vitro by assessing the frequency of LTC-IC in G0CD34+ cells relative to that of cells residing in G1. The frequency of LTC-IC was 47.6 ± 17.1 (mean ± SD) per 1 × 103 for G0CD34+ cells compared with 11.2 ± 8.1 per 1 × 103 for G1CD34+ cells (n = 2), demonstrating a 4-fold higher number of primitive cells in the G0 group, which did not reach statistical significance (P > .1).
NOD/SCID-repopulating ability of UCB CD34+ cells isolated in G0 or G1
The marrow-repopulating ability of G0CD34+and G1CD34+ cell populations from pooled (2 to 4 samples per experiment) UCB samples was assessed by transplanting equal numbers of either cell fraction into conditioned NOD/SCID recipients. In 9 separate experiments, a total of 36 mice were transplanted with UCB CD34+ cells separated into G0 (17 mice) and G1 (19 mice) using between 7 × 104 and 4 × 105 cells per animal. Human cell engraftment was evaluated after 8 weeks by flow cytometric determination of human CD45+ cells in cell suspensions of BM, spleen, and blood harvested from recipient mice. After transplantation of 4 × 105G0CD34+ or G1CD34+cells, high percentages of human CD45+ cells (up to 94% and 88%, respectively), were detected in the BM of transplanted mice (Figure 2). The mean percentage of human CD45+ cells detected in recipients of G0CD34+ cells was 69.9% ± 24.0%, whereas that documented in recipients of G1CD34+ cells was 46.7% ± 21.3%. When the mean values were adjusted for the number of cells injected, these chimerism levels were 71.8% and 44.7% for G0CD34+ and G1CD34+, respectively. Only 2 out of 36 mice, both transplanted with G1 cells, were negative for CD45+ cells. As measured by the frequencies of CD45+ cells in the BM of mice, the repopulating activity of G0CD34+ cells (n = 17) in BM was 1.6-fold higher compared with G1CD34+ cells (n = 19). The repopulating activity of G0CD34+ cells was only 1.2-fold higher than that of G1CD34+ cells when mice with more than 10% chimerism were considered for analysis (n = 16 for G0CD34+ and n = 14 for G1CD34+). The 1.6- and 1.2-fold differences in levels of engraftment between G0CD34+ and G1CD34+ cells were both statistically significant (P = .001 and P = .016, respectively). High levels of human cells were also detected in spleen and peripheral blood after transplantation of UCB CD34+cells in both phases of cell cycle (Figure 2). In spleen, the mean percentages of engraftment were 49.0% ± 23.3% and 27.5% ± 24.4% (P = .001) for G0CD34+ and G1CD34+, respectively, whereas chimerism levels of 22.2% ± 17.1% and 6.5% ± 5.5% (P = .001), respectively, were detected in peripheral blood.
Relationship between number of transplanted UCB G0CD34+ or G1CD34+cells and chimerism in NOD/SCID mice.
Chimerism was defined as the percentage of human CD45+cells detected in BM, spleen, or peripheral blood of recipient mice 8 weeks post-transplantation. Data were pooled from 9 separate experiments using 2 to 4 UCB samples per experiment. A total of 17 mice were transplanted with G0CD34+ cells and 19 with G1CD34+ cells. Each data point in every panel represents an individual mouse; however, 2 or more close points may overlap and appear as 1.
Relationship between number of transplanted UCB G0CD34+ or G1CD34+cells and chimerism in NOD/SCID mice.
Chimerism was defined as the percentage of human CD45+cells detected in BM, spleen, or peripheral blood of recipient mice 8 weeks post-transplantation. Data were pooled from 9 separate experiments using 2 to 4 UCB samples per experiment. A total of 17 mice were transplanted with G0CD34+ cells and 19 with G1CD34+ cells. Each data point in every panel represents an individual mouse; however, 2 or more close points may overlap and appear as 1.
Secondary transplants designed to investigate maintenance of long-term hematopoietic potential of UCB G0CD34+ and G1CD34+ cells revealed that proliferation of human HSCs in NOD/SCID mice most likely exhausts the repopulating capacity of these cells. Out of 4 secondary recipients of progeny of each of G0CD34+ and G1CD34+ cells, 1 in each group (25%) was chimeric at levels below 1% in the BM. Interestingly, chimerism in the spleen of these animals was slightly higher than that observed in the BM although it also remained below 1%. Multilineage differentiation in both secondary recipients was evident (data not shown).
Differentiation potential of repopulating cells present in UCB G0CD34+ and G1CD34+
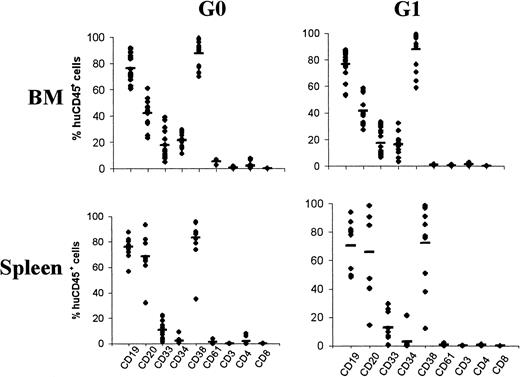
To compare the in vivo differentiation potential of engrafting UCB G0CD34+ and G1CD34+cells, the phenotypic profile of chimeric CD45+ cells in BM of recipient mice was determined by 3-color immunostaining. Data generated from these analyses are shown in Figure 3. In G0CD34+ transplanted mice, a mean of 21.4% ± 5.1% of CD45+ cells were CD34+, whereas in G1CD34+ transplanted mice 16.1% ± 6.9% were CD34+. Expression of CD19 and CD33 (76.2% ± 10.7% and 17.7% ± 10.2%, respectively, for G0CD34+ and 76.7% ± 11.4% and 17.1% ± 10.2% for G1CD34+ recipients) demonstrated the presence of repopulating cells with both lymphoid and myeloid potential. CD38 was expressed on most cells (88% ± 12%) at the same level in both groups. Expression of CD61, CD3, CD4, and CD8 on CD45+ cells was less than 5% in both groups of mice (Figure 3). The same phenotypic analysis method was used to characterize the differentiation of engrafted CD45+ cells in the spleens of recipient mice (Figure 3). The percentage of CD45+CD34+ cells was considerably lower in the spleen compared with the BM (2.4% ± 2.4% and 2.9% ± 6.9%, respectively, for G0CD34+ and G1CD34+ recipients). In addition to the comparable high expression of CD19 on spleen and BM CD45+cells, high expression of CD20 was also detected on spleen cells (68.5% ± 18.1% and 65.9% ± 31.7% for G0CD34+ and G1CD34+recipients, respectively), suggesting the presence of more primitive B cells in the BM. CD38 was also expressed by the majority of spleen cells in both groups (78% ± 24%), whereas expression of CD61, CD3, CD4, and CD8 was restricted to less than 5%.
Differentiation of human cells in BM and spleen of recipient mice transplanted with UCB G0CD34+ or G1CD34+ cells.
Each data point represents the percentage of human CD45+cells positive for the expression of CD antigens indicated on the x-axis in every recipient. BM (top panels; n = 17) and spleen (bottom panels; n = 11) cells from mice receiving G0CD34+ cells (left panels) and G1CD34+ (right panels) were analyzed for the expression of the 9 markers indicated.
Differentiation of human cells in BM and spleen of recipient mice transplanted with UCB G0CD34+ or G1CD34+ cells.
Each data point represents the percentage of human CD45+cells positive for the expression of CD antigens indicated on the x-axis in every recipient. BM (top panels; n = 17) and spleen (bottom panels; n = 11) cells from mice receiving G0CD34+ cells (left panels) and G1CD34+ (right panels) were analyzed for the expression of the 9 markers indicated.
Detection of human progenitor cells in the marrow of recipient NOD/SCID mice
To compare the different classes of progenitors in BM of recipient mice at 8 weeks after transplantation with G0CD34+ or G1CD34+ UCB cells, BM cell suspensions that contained more than 1% human CD45+ cells were assessed in progenitor cell assays (Figure4). The number of clonogenic progenitors contained in 2 × 103 CD45+CD34+cells derived from mice transplanted with UCB G0CD34+ cells was 34.7 ± 14.7 (Figure 4), whereas that detected in an equivalent number of BM cells from G1CD34+ recipients was 30.1 ± 26.5. Numbers of granulomonocytic, erythroid, or mixed colonies present in the marrow of chimeric animals were similar.
Mean numbers of clonogenic human progenitors ± SEM contained in the marrow of chimeric transplantation recipients.
From the BM of 17 mice receiving G0CD34+ cells and 15 mice transplanted with G1CD34+ cells, 2 × 103 CD45+CD34+ cells were assayed in methylcellulose as described in “Materials and methods.” Total number of colonies (■) represent the arithmetic sum of BFU-E– (▨); CFU-GM– (▤); CFU-GEMM– (▪) derived colonies. Only from 3 NOD/SCID recipients of G1CD34+ cells was it necessary to assay more than 105 total BM cells. In each case, the total number of detected hematopoietic colonies was within 2 SDs of the mean, demonstrating that these samples behaved similarly to those in which fewer than 105 cells were used.
Mean numbers of clonogenic human progenitors ± SEM contained in the marrow of chimeric transplantation recipients.
From the BM of 17 mice receiving G0CD34+ cells and 15 mice transplanted with G1CD34+ cells, 2 × 103 CD45+CD34+ cells were assayed in methylcellulose as described in “Materials and methods.” Total number of colonies (■) represent the arithmetic sum of BFU-E– (▨); CFU-GM– (▤); CFU-GEMM– (▪) derived colonies. Only from 3 NOD/SCID recipients of G1CD34+ cells was it necessary to assay more than 105 total BM cells. In each case, the total number of detected hematopoietic colonies was within 2 SDs of the mean, demonstrating that these samples behaved similarly to those in which fewer than 105 cells were used.
Mathematical model for engraftment of UCB G0CD34+ and G1CD34+ cells in NOD/SCID recipients
The relationship between the number of transplanted UCB G0CD34+ or G1CD34+cells and the level of chimerism detected 8 weeks later was analyzed by means of a general linear models procedure. Van der Loo et al25 previously demonstrated that a linear relationship exists between level of chimerism in NOD/SCID mice and number of CD34+ cells contained in a graft. Therefore, linear models were used to examine the interaction between the position of cells in the cell cycle and the number of cells infused, by means of the equation Y = α + β1X1 + β2X2, where Y is the estimated level of chimerism in transplanted mice, X1 is a variable equal to 0 if G0 cells are considered and equal to 1 if G1 cells are considered, and X2 = number of cells infused in thousands; α is a constant derived from the linear regression analysis and represents the Y intercept; β1 and β2 represent the null hypothesis that there is no relationship between level of chimerism and the cell-cycle status or number of transplanted cells.
Under these conditions, the plot of chimerism versus type or number of transplanted cells would be a horizontal line, and the correspondingP value would be insignificant (P > .05). However, if the analysis reaches statistical significance (P < .05), then the hypothesis is rejected, indicating that a relationship exists between chimerism and cell-cycle status or number of cells transplanted. Under these conditions, the value of β1 represents the expected difference between the levels of chimerism obtained with G0 versus G1 cells; β2, which is the slope, would then represent the increment in chimerism expected for every additional 103cells contained in the graft.
General linear models procedure analysis of chimerism revealed that a statistically significant relationship exists between chimerism and cell-cycle status of transplanted cells (P < .01 for β1 values). Furthermore, in the case of engraftment assessed in the BM, the increment in the level of chimerism per 1 × 103 cells transplanted was the same (0.179%) for both G0CD34+ and G1CD34+ cells, indicating that the reconstitution potential of these 2 types of cells, beyond the initial level of chimerism supported by each group, was similar. It is important to point out, however, that the difference between the initial level of chimerism attained in mice transplanted with G0CD34+ cells and that detected in mice receiving G1CD34+ cells was statistically significant (P = .01).
Discussion
Using a similar approach, we previously demonstrated16 that the marrow-repopulating potential of human MPB CD34+ cells, assessed in NOD/SCID recipients, was predominantly supported by mitotically quiescent cells, specifically those in G0 phase of cell cycle. Similarly, adult BM-derived G0CD34+ cells were superior to their counterparts residing in G1 in their ability to engraft and repopulate xenogeneic recipients.16 These findings were considered consistent with the prevailing dogma that in adults, HSCs reside in deep dormancy within the BM microenvironment.14,26 We hypothesized that the extensive need for hematopoietic cells in the developing fetus obligates all potential stem cells, regardless of their cell-cycle status, to actively contribute to blood cell production while maintaining the stem cell pool. Under such a scenario, during embryonic development, mitotically quiescent and cycling HSCs would be equally effective in their ability to engraft and sustain long-term hematopoiesis. However, in postnatal life, proliferation demands on HSCs decline, and these functions become restricted to resting cells, as is believed14,27 and experimentally demonstrated.13,16 28
The validity of this hypothesis was investigated by examining a late prenatal/neonatal source of HSCs, namely cord blood. Although CD34+ cells from BM, UCB, and MPB share several similar properties, progenitor cells from these 3 tissues have repeatedly been demonstrated to differ quantitatively and qualitatively from one another.2,5,11,29-33 Such differences clearly emanate from ontogeny-related properties and therefore provide a solid basis for using UCB cells in the first test of this hypothesis. If the proposed level of hematopoietic activity of cycling fetal HSCs declined during ontogeny, then cycling-UCB–derived stem cells may possess engrafting and repopulating potentials superior to those previously demonstrated for their counterparts from adult human tissues.16 Indeed our results demonstrate that the hematopoietic potential of UCB G1CD34+ cells was only 1.6-fold lower than G0CD34+ cells, a difference 10 times smaller than that previously observed for MPB cells,16 which, however, was also statistically significant (P = .001). In addition, when mice sustaining more than 10% chimerism were analyzed (total of 30 mice), only a 1.2-fold difference in engraftment was observed, which, interestingly, remained statistically significant (P = .016). Given the high levels of chimerism observed in the majority of transplanted mice, such a restricted comparison may be warranted. More importantly, when these data were analyzed by a general linear models procedure, it was noted that although G0cells have significantly higher chimerism values, the rate of increase in chimerism for each additional 1 × 103 cells transplanted (0.179%) was identical between mice receiving G0 cells and those transplanted with G1 cells. This observation suggests that UCB CD34+ cells residing in either G0 or G1 possess different initial marrow-repopulating capacities, but equivalent long-term proliferative potentials in vivo. The reason that G0CD34+cells provide an overall statistically significantly higher level of chimerism compared with G1CD34+ cells may be related to a higher engraftment potential or BM seeding efficiency of the former group of cells. Whether the enhanced engraftment potential of G0CD34+ cells is a function of enhanced proliferative capacity of these cells could not be gleaned from the present data. Studies aimed at examining this possibility and at investigating any preferential homing of G0CD34+ cells to the BM relative to their G1 counterparts are now underway in our laboratory. Also of interest is that secondary NOD/SCID recipients engrafted, albeit at low levels and frequencies, with human cells recovered from primary recipients of G1CD34+ cells, suggesting that a fraction of UCB long-term repopulating cells may be detected in the G1 phase of the cell cycle.
Cord blood HSC engraftment is clinically among the slowest when compared with BM and MPB.34-36 However, the number of transplanted UCB CD34+ cells in the majority of recipients is in general 1 log lower than that contained in BM grafts.37 This discrepancy in the number of CD34+ cells contained in grafts derived from either tissue is believed to play a critical role in the observed delay in engraftment following UCB transplantation. Our present results suggest that while possibly only G0CD34+ cells contained in a BM graft mediate long-term engraftment,16both G0CD34+ and G1CD34+ cells contained in UCB grafts contribute, almost similarly, to host marrow repopulation. Although it is difficult and highly speculative to assess the impact of these data on the clinical utility of UCB relative to that of BM and MPB, it may be suggested that comparisons between the number of transplantable HSCs contained in these 3 tissues should not be based simply on absolute CD34+ cell counts. Rather, a more detailed comparison of the engraftment potential of similar groups of cells within these tissues should also be considered.
Unfortunately, the relationship between human cells capable of sustaining xenogeneic engraftment in NOD/SCID mice for 7 to 10 weeks and those responsible for long-term marrow repopulation in humans is not well established. Although several studies have clearly identified phenotypic requirements of engrafting fractions of CD34+cells38,39 or even CD34− cells, in NOD/SCID and other xenogeneic transplantation models, the predicted in vivo function of these cells in a human setting cannot be evaluated. It is therefore difficult to ascertain from these studies whether in a clinical transplantation setting, UCB CD34+ cells in G0 or G1 differ in their long- versus short-term repopulating potential. In view of recent findings by Zijlmans et al40 concerning the nature of cells contributing to early phase engraftment in a murine model, it is unlikely that the hematopoietic activity observed for UCB G1CD34+ cells in our studies is short term.
When the frequency of LTC-IC among fractions of UCB CD34+cells was evaluated, a 4-fold difference in the frequency of these cells was noted between cells in G0 and those in G1. However, a smaller difference in the marrow-repopulating potential of these 2 cell fractions was observed, suggesting that, although primitive, LTC-IC measurements may not be indicative of the stem cell content of a graft. Such observations were previously reported by Larochelle et al39 and more recently experienced by Glimm and Eaves.41 Chimerism data from the limited number of secondary recipients examined in these studies imply that in vivo proliferation of graft cells is associated with loss of primitive function. Previous studies42,43illustrated the ability of human HSCs isolated from primary NOD/SCID mice transplanted with UCB CD34+ cells to engraft secondary recipients and establish multilineage hematopoiesis. Although the degree of chimerism in secondary recipients in these studies42,43 may have been higher than what was attained in our studies, distinct methodological procedures may explain these differences. It is possible that larger numbers of cells used in secondary transplants, harvesting of cells from primary recipients at 4 rather than 8 weeks posttransplantation, and use of growth factors during the primary transplant42,43 all contribute to the observed differences. However, it is important to note that as in our studies, between 0% and 25% of secondary recipients in the studies of Cashman and Eaves42 were chimeric after transplantation of cells derived from primary recipients not treated with human growth factors after transplantation.
Whether the enhanced ability of UCB G1CD34+cells to repopulate NOD/SCID recipients compared with their MPB counterparts is related to enhanced homing or seeding efficiency of these cells remains unknown. We previously reported differences between the adhesion molecule repertoire of MPB and BM cells residing in different phases of the cell cycle and speculated that these differences may be responsible for enhanced or decreased engraftment potential of classes of hematopoietic cells.44 Homing and seeding efficiencies of classes of hematopoietic progenitor cells as well as expression of specific adhesion molecules on these cells have been implicated by several groups in establishing and sustaining chimerism in vivo.42,43,45-48 Whether such differences are minimal between UCB G0CD34+ and G1CD34+ cells or are irrelevant to the hematopoietic potential of these cells is now under investigation. The impact of these results on gene transfer protocols is rather interesting. Unlike what we reported for MPB,16 it is possible that movement of UCB cells from G0 to G1 may not be as detrimental to their function as that documented for similar cells from MPB.16 Under such conditions, it may be possible to induce cell-cycle activation of quiescent UCB progenitor cells and initiate the required cell division process for efficient retroviral-mediated gene transfer49without altering or reducing the hematopoietic potential of these cells.
Supported by National Institutes of Health grant R01 HL55716 to E.F.S. The Herman B Wells Center for Pediatric Research is a Core Center of Excellence in Molecular Hematology (NIDDK P30 DK49218).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
E. F. Srour, Indiana University School of Medicine, 1044 W Walnut St, R4-202, Indianapolis, IN 46202-5254; e-mail: esrour@iupui.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal