Abstract
In unstimulated conditions osteoclast renewal occurs as a result of the stromal cell production of the key osteoclastogenic factors, receptor activator of NFkB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF). Inflammation is known to cause increased osteoclastogenesis; however, the mechanisms responsible for this phenomenon are poorly understood. We now show that interleukin-1 (IL-1) and tumor necrosis factor alpha (TNFα), cytokines typically produced in inflammatory conditions, increase the stromal cell production of IL-7. This factor, in turn, up-regulates production of osteoclastogenic cytokines by T cells leading to stimulation of osteoclast (OC) formation. Although T cells were found to produce soluble forms of both RANKL and M-CSF, saturating concentrations of osteoprotegerin failed to inhibit approximately 40% of the OC formation, suggesting that IL-7 acts via both RANKL-dependent and RANKL-independent pathways. Despite the identification of T-cell–secreted M-CSF, this cytokine was not essential for either RANKL-dependent or -independent OC formation, suggesting that T cells secrete other cytokines capable of substituting for M-CSF action. On the basis of our data, we propose a novel mechanism for inflammatory bone loss in which induction of IL-7 from stromal cells by IL-1 and TNFα leads to the production of soluble osteoclastogenic cytokines by T cells. Thus, the mechanism by which IL-7 causes bone resorption involves the activation of T cells and the T-cell–dependent augmentation of osteoclastogenesis.
Introduction
Activated T cells have long been associated with the increased osteoclast (OC) formation and the accelerated bone resorption characteristic of inflammatory conditions.1-3Recently, evidence has accumulated that suggests that T cells stimulate OC formation by producing the key osteoclastogenic cytokine, receptor activator of NFkB ligand (RANKL),4,5 also known as ODF,6 OPGL,7 and TRANCE.8However, the mechanism(s) leading to up-regulation of T-cell RANKL production under inflammatory conditions remains unknown, although it is likely induced by other inflammatory cytokines. One such factor is interleukin 7 (IL-7),9,10 a cytokine that has previously been demonstrated to induce bone loss through increased osteoclastogenesis when administered in mice.11 Further attesting to the relevance of IL-7 for the regulation of bone resorption is the finding that IL-7 receptor-knockout mice show greatly increased femoral trabecular bone volume compared with wild-type and heterozygous littermates.11 The mechanism by which IL-7, a product of bone marrow stromal cells (SCs),12 leads to increased osteoclastogenesis and bone resorption remains to be established. IL-7 is known to be a powerful inducer of both B- and T-lymphopoiesis.11,13 As B-lymphopoiesis is also up-regulated by E2 deficiency, a condition known to lead to bone loss, it has been suggested that increased B-lymphopoiesis may be responsible for the elevated osteoclastogenesis and bone loss associated with IL-7.11 In support of this hypothesis is the recent report that B220+ pre-B cells may be capable of differentiating into OC precursors.14 However, as yet, no formal demonstration that this phenomenon accounts for IL-7–induced bone loss has been published. The mechanism of IL-7–induced OC formation and the cell populations involved thus remain uncertain.
In this study, we demonstrate that IL-1 and tumor necrosis factor alpha (TNFα) induce the production of IL-7 from human stromal cells and osteoblasts. We also show that IL-7 induces osteoclastogenesis through T cells by RANKL-dependent and -independent mechanisms. Despite the identification of soluble macrophage colony-stimulating factor (M-CSF) in the supernatants of IL-7–induced T cells, neither RANKL-dependent nor RANKL-independent OC formation was reliant on M-CSF, suggesting the presence of other cytokines capable of substituting for M-CSF activity.
These findings suggest that IL-7 may be an important mediator of the osteoclastogenesis and bone loss in inflammatory conditions such as rheumatoid arthritis and periodontitis.
Materials and methods
All reagents were purchased from the Sigma Chemical Corporation (St Louis, MO), unless otherwise indicated. The rhIL-1β was kindly provided by Dompe pharmaceutical, (L'Aquila, Italy) and rhIL-3, rhM-CSF, and rhGM-CSF by Genetics Institute (Boston, MA). Polyclonal antibodies directed against the human integrin subunits αV (L230) and β3 (AP3), were generous gifts of Dr S. D. Blystone (Washington University, St Louis, MO). K10C7, an affinity purified murine monoclonal antibody, recognizing the mature form of cathepsin K was a kind gift of Dr M. Gowen, (SmithKline Beecham, King of Prussia, PA). Anti-pp60c-src (Antibody 327) was kindly provided by Dr Y. Abu-Amer (Washington University, St Louis, MO). The antiosteoclast antibody 121F was a kind gift of Dr P. Osdoby (Washington University, St Louis, MO).
Generation of human osteoclasts
Human OCs were generated as previously described15with modifications. Briefly, human peripheral blood stem cells (PBSCs), a blood product enriched in CD34+ stem cells, was prepared by a single leukapheresis of healthy donors, treated with granulocyte colony-stimulating factor (G-CSF) for 3 to 5 days. After purification of mononuclear cells by Histopaque density gradient centrifugation, PBSCs were plated at a density of 5 × 105 cells per well in a final volume of 0.5 mL, using 48-well plates and cultured in αMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco BRL), penicillin (50 U/mL), and streptomycin (50 μg/mL). Cultures were stimulated with 2.5 ng/mL rhIL-7 (R&D Systems, Minneapolis, MN) and were fed by replacing half the medium twice weekly with the addition of fresh IL-7. After 7 to 10 days of culture, cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP), using a commercial kit (Sigma) according to the manufacturer's instructions. As our OC characterization (see below) demonstrated a close correlation between TRAP expression on multinucleated (more than or equal to 3 nuclei) cells, with expression of both calcitonin receptor (more than 90%) and cathepsin K (more than 98%), 2 very specific markers of authentic OCs, we have thus defined multinucleated (more than or equal to 3 nuclei) TRAP positive cells as mature OCs.
Isolation and depletion of specific cell populations
T-cell, B-cell, and monocyte (early OC precursor) populations were isolated or depleted from PBSC cultures using anti-CD14 (monocytes/macrophages)–, anti-CD19 (B cells)–, or anti-CD3 (T cells)–coated immunomagnetic Dynabeads (Dynal, Lake Success, NY), according to the manufacturer's instructions. T cells positively selected using Dynabeads were 100% pure and more than 95% viable after isolation. Dynabeads were detached from purified CD3+T cells, after 6 hours, by gentle pipetting. As the CD3 antibody can activate the T cells, for some experiments purified unactivated T cells were obtained using negative selection columns (R&D Systems). T-cell purity of greater than or equal to 95% (as assessed by flow cytometry), was achieved by predepletion of adherent cell populations (monocytes and B cells) by 3 hours adhesion to plastic, followed by double negative selection on T-cell selection columns.
As immunomagnetic beads are phagacytosed by monocytes and cannot be removed, monocytes (a source of early OC precursors) were semipurified, after immunomagnetic antibody depletion of B cells (an adherent cell population), by adhesion onto plastic dishes for 3 hours, and nonadherent cells removed by 3 washes with phosphate-buffered saline (PBS). These populations were found to respond in the same manner as monocytes positively selected by CD14 immunomagnetic bead and were found to be greater than or equal to 64% CD14+ and greater than or equal to 99% T cells and B cells depleted as assessed by flow cytometry.
Purified monocytes were plated at a density of 1 × 105cells per well alone or with 1 × 105 purified T cells or B cells in a final volume of 0.5 mL using 48-well plates and cultured in αMEM, supplemented with 10% heat-inactivated FBS (Gibco BRL), penicillin (50 U/mL), and streptomycin (50 μg/mL). Where indicated, cultures were stimulated with 2.5 ng/mL rhIL-7 (R&D Systems, Minneapolis, MN) and were fed by replacing half the medium twice weekly with the addition of fresh IL-7. After 7 to 10 days of culture, cells were fixed and stained for TRAP.
In some experiments, purified monocytes (1 × 105 cells per well) received T-cell–conditioned medium that was derived from a 4× concentration of T cells (4 × 105 cells/500 μL) and added at 25% final volume (125 μL) representing a 1× final dose.
Immunohistochemistry
Immunohistochemical staining for αV and β3 integrin subunits, pp60c-src, cathepsin K, and for antigen to the anti-OC antibody 121F was conducted as previously described.16 Briefly, cells were grown in 48-well plates and washed 3 times with PBS before fixation. Cells were fixed in acetone:methanol (50%:50%, v/v) for 5 minutes and washed twice with PBS. Fixed cells were incubated with primary antibody (5 μg/mL) in PBS containing 3% bovine serum albumin (BSA) at 4°C for 18 hours. Wells were washed 3 times in PBS for 5 minutes with shaking. Cells were incubated with secondary antibody (antimouse IgG-biotin conjugate), in PBS containing 3% BSA for 2 hours at room temperature, washed 3 times, and incubated with avidin-peroxidase, in PBS containing 3% BSA for 40 minutes at room temperature. After 3 washes, color was developed using 4-chloronapthol, (0.03% in 0.05 mol/L Tris-HCl, pH 7.6, 0.1% H2O2). Wells were washed with water after several minutes to stop the staining. IgG isotype control was used to assess nonspecific staining.
Calcitonin receptor analysis
Expression of calcitonin receptors was assessed by autoradiography using 125I-labeled salmon calcitonin (7.4 × 107 MBq/mmol [2000 Ci/mmol]; Amersham Pharmacia Biotech, Arlington Heights, IL), as previously described.15 Briefly, mature OCs were cultured as described above, washed twice in PBS to remove nonadherent cells and labeled with 0.2 nmol/L 125I-calcitonin for 1 hour at room temperature. Two hundred-fold excess unlabeled salmon calcitonin was used to assess nonspecific binding. After washing, the cells were fixed, stained for TRAP and coated with LM-1 photographic emulsion (Amersham International, Arlington Heights, IL), and stored for 3 weeks at 4°C before developing.
Resorption pit assays
The ability of OCs to resorb pits was assessed by generating mature OCs (as described above) and transplanting onto whale dentine slices, in 48-well plates. After 2 to 5 days of culture, dentine slices were washed with PBS and incubated for 18 hours in 0.25 mol/L ammonium hydroxide, followed by sonication in PBS for 30 seconds. Dentine slices were washed with PBS and stained in toluidine blue (2% wt/vol) in PBS for 5 minutes, and pits photographed under light microscopy using an inverted phase contrast microscope (Olympus Optical Co, Tokyo, Japan).
Measurement of human interleukin-7 production by purified stromal cells and osteoblasts
Human stromal cells and osteoblasts were purified and cultured as previously described.17 Cultures were stimulated with IL-1 (1 ng/mL), TNFα (20 ng/mL), or IL-1 + TNFα. Cytokine concentrations were selected on the basis of maximal stimulation of IL-6 production by these cells, as determined previously.17 IL-7 secreted into the culture supernatant was measured by commercial enzyme-linked immunosorbent assay (ELISA) (Quantikine HS, R&D Systems).
Macrophage colony-stimulating enzyme-linked immunosorbent assay
M-CSF was measured in unstimulated, IL-7 (2.5 ng/mL) stimulated and IL-1 + TNFα (10 ng/mL each) stimulated culture supernatants from purified T cells after 48 hours of incubation, using a commercial ELISA (Quantikine, R&D Systems)
Semiquantitative reverse transcriptase-polymerase chain reaction for receptor activator of NFkB ligand
Total RNA from unstimulated and IL-7–stimulated T cells was isolated using Trizole Reagent (Gibco BRL, Gaithersburg, MA), according to the manufacturer's instructions. Semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) was conducted using Ready-To-Go RT-PCR beads (Amersham Pharmacia Biotech, Piscataway, NJ), according to the manufacturer's instructions. Briefly, 2 μg of total RNA was reverse transcribed using a pd(T)12-18 first strand primer for 30 minutes at 42°C, before PCR. The forward RANKL primer used was 5′-ATGCGCCGCGCCAGCAGAGACTACA, and the reverse primer 5′-ATCTATATCTCGAACTTTAAAAGCC. Commercial human GAPDH amplimer primers (Clontech, Palo Alta, CA) were used as internal standards. Genomic contamination was assessed by heat inactivation of the reverse transcriptase before RT-PCR. RANKL was subjected to 40 cycles of PCR and found to be in the exponential amplification range up to 45 cycles. GAPDH was cycled for 30 rounds and was exponential up to 35 cycles. PCR products were separated on 1% agarose gels and photographed under ultraviolet excitation after ethidium bromide staining.
Metabolic labeling for detection of soluble receptor activator of NFkB ligand
T cells were metabolically labeled as previously described.18 Briefly, cells were labeled overnight with 1.85 MBq (50 μCi) 35S-labeled methionine and cysteine (EXPRESS label, Amersham Pharmacia Biotech) and then stimulated for 24 hours with IL-7 (2.5 ng/mL). Supernatant was immunoprecipitated using antibody against human RANKL (R&D Systems) and protein G beads. The immunoprecipitated material was recovered by boiling in sample loading buffer, and separated by SDS-PAGE. Gels were dried and exposed to autoradiography film (Kodak XAR; Eastman Kodak Company, Rochester, NY).
Macrophage colony-stimulating factor neutralization
For M-CSF neutralization experiments, 20 μg/mL of polyclonal M-CSF antibody (R&D Systems) was added to wells before addition of IL-7–stimulated T-cell supernatant. This concentration of antibody was found to completely neutralize OC formation induced by RANKL and 500 ng/mL of rhM-CSF, a dose 50-fold higher than the concentration of soluble M-CSF estimated to be present in T-cell supernatants by ELISA. In addition, monocytes treated with M-CSF and anti–M-CSF antibody were found to be dead after 7 days in culture compared with greater than 95% viability with M-CSF–stimulated cells alone, assessed by trypan blue dye exclusion.
Osteoprotegerin neutralization
For osteoprotegerin (OPG) neutralization experiments, OPG (R&D Systems) was added to wells before the addition of IL-7–stimulated T-cell supernatant at a concentration ranging from 1 to 4 μg/mL. The lowest concentration of inhibitor (1 μg/mL) was found to completely neutralize OC formation induced by a saturating dose of rhRANKL (100 ng/mL) and 50 ng/mL of rhM-CSF, verifying its biologic activity.
Statistical analysis
Group mean values were compared by unpaired 2-tailed Student t test.
Results
To investigate the enhanced production of IL-7 as occurs under inflammatory conditions, we measured the secretion of IL-7 by purified human bone marrow stromal cells and in purified human osteoblasts before and after IL-1 and TNFα stimulation. The data (Figure1) show that both IL-1 and TNFα induce the secretion of IL-7 from both bone marrow stromal cells and osteoblasts. In addition, combined treatment with both IL-1 and TNFα resulted in no further stimulation of IL-7 production. (Figure1).
IL-1 and TNFα up-regulate the stromal cell and osteoblast production of IL-7.
Purified human bone marrow stromal cells (▪) and osteoblasts (■) (see “Materials and methods”) were stimulated with IL-1 (1 ng/mL) and TNFα (20 ng/mL), alone or in combination. IL-1 and TNFα stimulation of IL-7 secretion was measured by ELISA. Data points are shown as average + SD of 3 replicate samples measured in duplicate.
IL-1 and TNFα up-regulate the stromal cell and osteoblast production of IL-7.
Purified human bone marrow stromal cells (▪) and osteoblasts (■) (see “Materials and methods”) were stimulated with IL-1 (1 ng/mL) and TNFα (20 ng/mL), alone or in combination. IL-1 and TNFα stimulation of IL-7 secretion was measured by ELISA. Data points are shown as average + SD of 3 replicate samples measured in duplicate.
To evaluate the mechanism of IL-7–mediated osteoclastogenesis, in the absence of the confounding influence of stromal cells, we used human PBSCs, a mixed population of mononuclear cells containing monocytes/macrophages, early OC precursors of the monocytic lineage, and T- and B-lymphocytes. PBSCs do not contain SCs or SC precursors.15 When cultured in vitro with IL-1, IL-3, and GM-CSF, PBSCs generate large numbers of authentic bone resorbing OCs within 21 days of culture.15
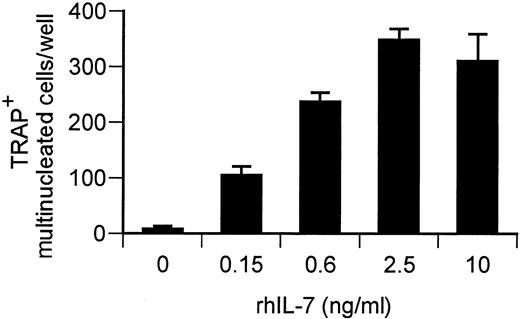
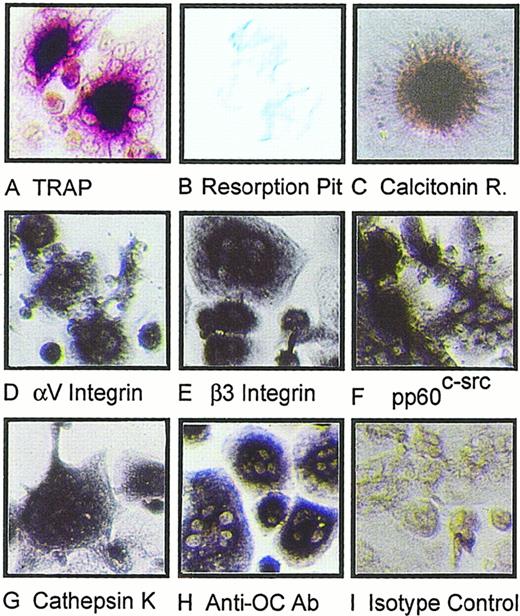
IL-7 has previously been reported to induce proliferation of T-lymphocytes13,19 and to induce bone loss in mice11 through an undefined mechanism. We thus tested the effects of rhIL-7 on OC formation in PBSC cultures. The data show that rhIL-7 stimulates formation of OC dose dependently and within 7 to 10 days of culture (Figure 2). These cells were found to display numerous markers of mature OCs, including TRAP, pit resorption, calcitonin receptor, the integrin subunits αV and β3, pp60c-src, and mature cathepsin K, and react with the anti-OC antibody 121F, which recognizes both avian and human OCs20 (Figure 3). These cells thus satisfy the current criterion for authentic OCs.
IL-7 induces OC formation from PBSCs in a dose-dependent manner.
PBSC cultures were stimulated with rhIL-7 (0 to 10 ng/mL) and TRAP+ multinucleated cells counted after 7 to 10 days of culture. Data points are shown as average + SD of 3 replicate wells. Data are representative of 3 independent experiments.
IL-7 induces OC formation from PBSCs in a dose-dependent manner.
PBSC cultures were stimulated with rhIL-7 (0 to 10 ng/mL) and TRAP+ multinucleated cells counted after 7 to 10 days of culture. Data points are shown as average + SD of 3 replicate wells. Data are representative of 3 independent experiments.
Characterization of human osteoclasts generated by IL-7 stimulation of PBSCs.
The multinuclear cells formed after IL-7 stimulation were analyzed for markers of authentic human OCs (see “Materials and methods”). These cells were found to express tartrate resistant acid phosphatase (TRAP) (A); resorp pits on dentine slices (B); express calcitonin receptors (C), which were competed out by 200-fold excess unlabeled calcitonin (data not shown); express the integrin subunits αV (D) and β3 (E); express pp60c-src (F), mature cathepsin K (G), and react with the anti-OC antibody 121F (H). Nonspecific binding was assessed by IgG isotype control (I). Magnification = 200 × for all panels.
Characterization of human osteoclasts generated by IL-7 stimulation of PBSCs.
The multinuclear cells formed after IL-7 stimulation were analyzed for markers of authentic human OCs (see “Materials and methods”). These cells were found to express tartrate resistant acid phosphatase (TRAP) (A); resorp pits on dentine slices (B); express calcitonin receptors (C), which were competed out by 200-fold excess unlabeled calcitonin (data not shown); express the integrin subunits αV (D) and β3 (E); express pp60c-src (F), mature cathepsin K (G), and react with the anti-OC antibody 121F (H). Nonspecific binding was assessed by IgG isotype control (I). Magnification = 200 × for all panels.
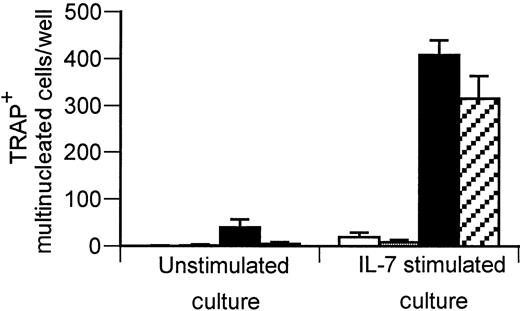
As T cells,13,19 B cells,13 and monocytes21 are all known to respond to IL-7, we examined which cell population(s) were responsible for transducing the osteoclastogenic activity of IL-7. To achieve this aim, the ability of IL-7 to induce OCs in purified monocytes alone or with monocytes containing purified B cells or T cells was tested. The data show that neither monocytes alone nor monocytes with B cells were capable of supporting significant OC formation in either the presence or absence of IL-7 (Figure 4). However, in the presence of IL-7, mixed monocytes and T cells resulted in a large induction of OC formation. In addition, conditioned medium (CM) from IL-7–stimulated T cells (25% vol/vol) was also capable of inducing substantial OC formation. The data thus suggest that the ability of IL-7 to generate OCs in this system is related to T-cell–secreted factors rather than to cells derived from the B-lineage, as has previously been suggested.11 Identical results were obtained using T cells purified from unmobilized human PBMCs rather than from PBSCs (data not shown).
IL-7 induces OC formation in monocytes via actions on T cells.
Purified monocytes alone (■), monocytes with T cells replaced (▪), and monocytes with B cells replaced (░) were cultured with or without rhIL-7 (2.5 ng/mL) or CM from purified T cells (▨) cultured in the presence or absence of rhIL-7. After 7 to 10 days, TRAP+ multinucleated cells were counted. Only IL-7–stimulated T-cell replete cultures and IL-7–stimulated T-cell CM were able to stimulate appreciable OC formation. Data points are shown as average + SD of 3 replicate wells. Data are representative of 3 independent experiments.
IL-7 induces OC formation in monocytes via actions on T cells.
Purified monocytes alone (■), monocytes with T cells replaced (▪), and monocytes with B cells replaced (░) were cultured with or without rhIL-7 (2.5 ng/mL) or CM from purified T cells (▨) cultured in the presence or absence of rhIL-7. After 7 to 10 days, TRAP+ multinucleated cells were counted. Only IL-7–stimulated T-cell replete cultures and IL-7–stimulated T-cell CM were able to stimulate appreciable OC formation. Data points are shown as average + SD of 3 replicate wells. Data are representative of 3 independent experiments.
To investigate possible mechanisms by which IL-7–stimulated T cells could induce OC formation, we evaluated the ability of IL-7 to induce the key osteoclastogenic factor RANKL on T cells isolated from PBSCs. Using semiquantitative RT-PCR, IL-7 was found to up-regulate RANKL expression (Figure 5A). No RANKL expression was observed by unstimulated T cells. Identical results were obtained using T cells purified from unmobilized human PBMCs rather than from PBSCs (data not shown).
IL-7 up-regulates RANKL mRNA and RANKL secretion in T cells.
(A) Negatively isolated T cells from PBSCs were stimulated with IL-7 for 24 hours and RNA isolated. Semiquantitative RT-PCR was conducted as described in the “Materials and methods” section. IL-7–stimulated T cells showed up-regulation of RANKL expression. No RANK message was detectable in unstimulated T cells. (B) Secretion of RANKL into the culture supernatant was measured by metabolic labeling of purified T cells, followed by RANKL immunoprecipitation and SDS-PAGE (see “Materials and methods”). Soluble RANKL was detected in IL-7–stimulated cells but not in unstimulated cells. Data are representative of 2 independent experiments.
IL-7 up-regulates RANKL mRNA and RANKL secretion in T cells.
(A) Negatively isolated T cells from PBSCs were stimulated with IL-7 for 24 hours and RNA isolated. Semiquantitative RT-PCR was conducted as described in the “Materials and methods” section. IL-7–stimulated T cells showed up-regulation of RANKL expression. No RANK message was detectable in unstimulated T cells. (B) Secretion of RANKL into the culture supernatant was measured by metabolic labeling of purified T cells, followed by RANKL immunoprecipitation and SDS-PAGE (see “Materials and methods”). Soluble RANKL was detected in IL-7–stimulated cells but not in unstimulated cells. Data are representative of 2 independent experiments.
Because we have identified that substantial osteoclastogenic activity is present in IL-7 stimulated T-cell CM, we conducted a metabolic labeling experiment to identify the presence of soluble RANKL. The data show (Figure 5B) the immunoprecipitation by anti-RANKL antibody, of a 26 kd protein from IL-7–stimulated T-cell CM. This protein corresponds exactly to the reported size of soluble RANKL.7 18 RANKL was not detected in unstimulated T-cell CM.
Because RANKL stimulates OC formation only when OC precursors are also stimulated with M-CSF,7 22 T cells could facilitate OC formation by secreting M-CSF. To investigate this possibility, we measured M-CSF in the supernatants of T-cell CM by ELISA. IL-7 stimulation of T cells resulted in M-CSF secretion of approximately 9 ng/mL per 106 cells in a 48-hour period, a concentration that is physiologically relevant, as we have found that 10 ng/mL of rhM-CSF is sufficient to support RANKL-mediated OC formation in vitro (data not shown). No M-CSF secretion was detected in unstimulated T cells. Stimulation of T cells by IL-1α and TNFα failed to induce detectable levels of M-CSF, thus demonstrating that these cytokines do not directly induce T-cell M-CSF expression but act indirectly via IL-7.
To verify that the enhanced osteoclastogenic activity induced by IL-7 CM was due to the presence of secreted RANKL and M-CSF, we added the RANKL inhibitor OPG or neutralizing antibody to M-CSF, into cultures of monocytes treated with IL-7–stimulated T-cell CM. A high dose of OPG (1 μg/mL), a concentration that completely inhibited OC formation induced by 100 ng/mL rhRANKL (data not shown), was found to reduce OC formation in IL-7–stimulated T-cell cultures by approximately 60% (Figure 6). This confirms that biologically active RANKL is secreted by T cells into the medium. However, because OPG did not completely block the stimulation of OC formation induced by T cells, even at concentrations up to 4 μg/mL (data not shown), the data suggest that other unidentified factors distinct from RANKL also contribute to the stimulation of OC formation. M-CSF neutralization failed to block either the RANKL-dependent or RANKL-independent OC formation induced by IL-7–stimulated T-cell CM, using concentrations of antibody found to completely inhibit OC formation induced by rhRANKL and 500 ng/mL of rhM-CSF (a 50-fold higher dose than the concentration of M-CSF measured in T-cell supernatants by ELISA). This result suggests the presence of additional factors capable of substituting for M-CSF. The identity of such factors remains to be determined.
The RANKL inhibitor OPG blunts OC formation induced by IL-7 stimulated T-cell CM.
Purified monocytes from PBSCs were induced to form OCs by stimulation with one quarter volume (final) of IL-7–stimulated T-cell CM (4 × concentrated) (■), in the presence or absence of the RANKL inhibitor OPG (1 μg/mL). (▪, unstimulated.) After 7 to 10 days in culture, TRAP+ multinucleated cells were counted. Data representative of 3 independent experiments. P < .01 with respect to IL-7–stimulated T-cell CM control.
The RANKL inhibitor OPG blunts OC formation induced by IL-7 stimulated T-cell CM.
Purified monocytes from PBSCs were induced to form OCs by stimulation with one quarter volume (final) of IL-7–stimulated T-cell CM (4 × concentrated) (■), in the presence or absence of the RANKL inhibitor OPG (1 μg/mL). (▪, unstimulated.) After 7 to 10 days in culture, TRAP+ multinucleated cells were counted. Data representative of 3 independent experiments. P < .01 with respect to IL-7–stimulated T-cell CM control.
Discussion
In this study, we have investigated the hypothesis that the enhanced production of IL-1 and TNFα, characteristic of inflammation, is able to stimulate the stromal cell production of IL-7 and promote osteoclastogenesis via actions on T cells. We show that IL-7 leads to enhanced osteoclastogenesis and increased bone resorption in vitro, by RANKL-dependent and -independent mechanisms.
Of considerable interest is the demonstration of substantial osteoclastogenic activity in IL-7–stimulated T-cell CM. Osteoclastogenesis has heretofore been thought to require physical contact between stromal cells and OC precursors of the hematopoietic lineage.23 However, the finding that T cells secrete soluble M-CSF and the confirmation that they secrete soluble RANKL4 18 provide a mechanism to explain how T cells can promote OC formation in the absence of cell-cell contact. This enables these lymphocytes not only to impact local sites of inflammation by means of membrane- bound factors but also to play a crucial role in the systemic bone loss characteristic of rheumatoid arthritis through the production of secreted factors.
Previous reports suggest that, in the mouse, increased production of soluble RANKL is the major, if not the exclusive, mechanism by which inflammation induces bone loss in vivo.4 In contrast, the finding that saturating concentrations of OPG (4 μg/mL) did not completely inhibit the formation of OCs induced by IL-7–stimulated T-cell CM suggests that human T cells can secrete factors that can support OC formation independently of RANKL.
Previous reports have also suggested that the osteoclastogenic effects of IL-7 are a result of its ability to promote B-lymphopoeisis.11 Our findings do not support this hypothesis, as the data demonstrate that IL-7 fails to induce osteoclastogenesis in the absence of T cells. Thus, the osteoclastogenic activity of IL-7 results from its ability to activate the T cells production of osteoclastogenic cytokines. However, we cannot exclude the possibility that effects of IL-7 on other cell populations may contribute to increase OC formation. For example, B220+ pre-B cells, (a population known to be induced by IL-7 treatment11), have recently been reported to be capable of differentiating into OC precursors. Thus, IL-7 may support osteoclastogenesis by redirecting pre-B cells into an OC lineage.14 However, because OC formation requires the action of osteoclastogenenic cytokines on the osteoclast precursors, our data suggest that T cells are crucial to this process as well.
Our study measuring IL-7 production was conducted using concentrations of IL-1 and TNFα previously determined to induce maximal stimulation of IL-6 production from SCs and osteoblasts.17 Confirming that our observations reflect maximal target cell stimulation, combined SC stimulation with IL-1 and TNFα failed to stimulate more IL-7 production over treatment with TNFα alone.
The demonstration that IL-1 and TNFα induce stromal cell and osteoblast production of IL-7 extends previous observations demonstrating that these cytokines increase IL-7 production by fibroblastic cell lines derived from rheumatoid arthritic patients.24 Together, the data from us and others suggest that IL-7 may be a potential mediator of the bone loss in conditions characterized by augmented production of IL-1 and TNFα, such as inflammation.
The differentiation of OC precursors into mature OCs is reported to require the coordinated effects of RANKL and M-CSF.7,22M-CSF is also a critical enhancer of OC life span. Thus, stimuli that enhance the production of M-CSF in the bone marrow are likely to enhance OC formation and stimulate bone resorption. In accordance with this hypothesis, we have previously reported that IL-1 and TNFα stimulate OC formation by inducing the formation of SCs characterized by increased M-CSF production.25,26 We now show that, although IL-1 and TNFα do not directly stimulate the production of M-CSF in T cells, they do so indirectly by enhancing the SC production of IL-7. Although in vivo additional M-CSF may contribute to enhanced OC production by supporting RANKL-stimulated osteoclastogenesis, and by enhancing survival of macrophages and OC precursors, the T-cell–derived M-CSF was not necessary for OC formation in vitro. These data suggest that T cells are capable of producing additional factors capable of substituting for M-CSF. A number of recent studies have elucidated a number of cytokines, including VEGF,27 GM-CSF, and IL-3,28 which are capable of substituting for M-CSF. IL-7–stimulated T cells may thus secrete one or more of these factors.
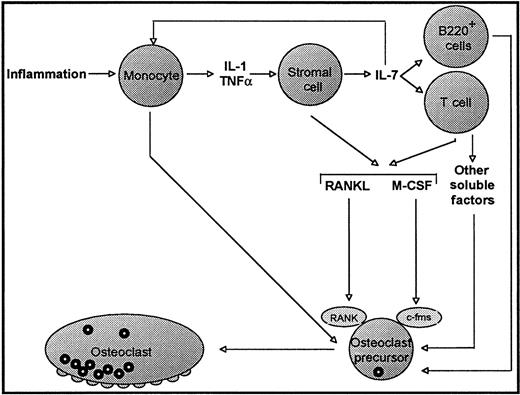
On the basis of our data and those of others, we now propose a new mechanism (Figure 7), which may account for the accelerated bone loss observed in inflammatory conditions such as rheumatoid arthritis and periodontitis.
Model for IL-7–mediated OC formation under conditions of inflammation.
Under inflammatory conditions, increased production of the inflammatory cytokines IL-1 and TNFα leads to production of IL-7 from bone marrow stromal cells and osteoblasts. IL-7 is also known to feedback on monocytes, inducing further IL-1 and TNFα secretion.21The effects of increased IL-7 production are 2-fold. First, increased IL-7 results in induction of B-lymphopoiesis and an increase in the number of B220+ pre-B cells, a population reported to be capable of acting as OC precursors.14 Second, IL-7 has direct effects on T cells, resulting in the production of the osteoclastogenic cytokines M-CSF and RANKL, as well as other unidentified osteoclastogenic factors. Thus, a combination of an increased OC precursor pool, along with the production of osteoclastogenic cytokines by T cells, leads to the enhanced OC production and increased bone resorption characteristic inflammatory processes such as rheumatoid arthritis and periodontitis.
Model for IL-7–mediated OC formation under conditions of inflammation.
Under inflammatory conditions, increased production of the inflammatory cytokines IL-1 and TNFα leads to production of IL-7 from bone marrow stromal cells and osteoblasts. IL-7 is also known to feedback on monocytes, inducing further IL-1 and TNFα secretion.21The effects of increased IL-7 production are 2-fold. First, increased IL-7 results in induction of B-lymphopoiesis and an increase in the number of B220+ pre-B cells, a population reported to be capable of acting as OC precursors.14 Second, IL-7 has direct effects on T cells, resulting in the production of the osteoclastogenic cytokines M-CSF and RANKL, as well as other unidentified osteoclastogenic factors. Thus, a combination of an increased OC precursor pool, along with the production of osteoclastogenic cytokines by T cells, leads to the enhanced OC production and increased bone resorption characteristic inflammatory processes such as rheumatoid arthritis and periodontitis.
Supported in part by grants from the National Institutes of Health (AG 13534 from the National Institute on Aging to R.P. and AR-99-001 to M.N.W. and AR46370 to L.R from the National Institute of Arthritis and Musculoskeletal and Skin Disease), and a grant from the Barnes-Jewish Hospital Research Foundation to M.N.W.
M.N.W. and S.C. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Roberto Pacifici, Division of Bone and Mineral Diseases, Barnes-Jewish Hospital, North, 216 S Kingshighway Blvd, St Louis, MO 63110; e-mail: pacifici@imgate.wustl.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal