Abstract
Arsenic trioxide (As2O3) has recently been used successfully in the treatment of acute promyelocytic leukemia and has been shown to induce partial differentiation and apoptosis of leukemic cells in vitro. However, the mechanism by which As2O3 exerts its antileukemic effect remains uncertain. Emerging data suggest that the endothelium and angiogenesis play a seminal role in the proliferation of liquid tumors, such as leukemia. We have shown that activated endothelial cells release cytokines that may stimulate leukemic cell growth. Leukemic cells, in turn, can release endothelial growth factors, such as vascular endothelial growth factor (VEGF). On the basis of these observations, we hypothesized that As2O3 may interrupt a reciprocal loop between leukemic cells and the endothelium by direct action on both cell types. We have shown that treatment of proliferating layers of human umbilical vein endothelial cells (HUVECs) with a variety of concentrations of As2O3results in a reproducible dose- and time-dependent sequence of events marked by change to an activated morphology, up-regulation of endothelial cell adhesion markers, and apoptosis. Also, treatment with As2O3 caused inhibition of VEGF production in the leukemic cell line HEL. Finally, incubation of HUVECs with As2O3 prevented capillary tubule and branch formation in an in vitro endothelial cell–differentiation assay. In conclusion, we believe that As2O3 interrupts a reciprocal stimulatory loop between leukemic cells and endothelial cells by causing apoptosis of both cell types and by inhibiting leukemic cell VEGF production.
Introduction
Arsenic has been used for centuries to treat a wide variety of illnesses. Most recently, arsenic trioxide (As2O3) has been used successfully in the treatment of patients with acute promyelocytic leukemia (APL) who relapsed after initial therapy with chemotherapy and all-trans retinoic acid.1-6 As2O3 has been shown to induce dose- and time-dependent apoptosis in vitro in a variety of malignant myeloid,7-13 lymphoid,14-18 and megakaryocytic19 cells. Recent investigations have also found that treatment with As2O3 induces apoptosis in plasma cell lines and primary myeloma cells,20 esophageal carcinoma cells,21 and immortalized human cervical epithelial cells.22 In vivo, arsenic appears to induce apoptosis of APL cells without significant myelosuppression or serious systemic toxicity.2 3
The mechanism by which arsenic exerts its antileukemic effect remains uncertain. In APL cells, arsenic targets PML onto nuclear bodies and induces degradation of the PML/RAR-α fusion protein.8,11,23 In non-APL cells, arsenic has been shown to recruit several nuclear body antigens onto nuclear bodies, but causes degradation of only the PML protein.23 Other work, however, suggests that arsenic-induced apoptosis is independent of PML and PML/RAR-α,12 and several alternative mechanisms of action have been proposed, including mitotic arrest and inhibition of GTP-dependent polymerization and microtubule formation10 and modulation of the intracellular glutathione redox system.24 25
Emerging evidence suggests that the endothelium and angiogenesis may be critical for the proliferation of liquid tumors, such as leukemia.26-28 It is well established that angiogenesis, the formation of new capillaries from preexisting blood vessels, plays a critical role in the growth of solid tumors by facilitating delivery of nutrients to and removal of waste products from the tumor bed.29 Tumor growth also requires expansion of existing endothelium; an increase in tumor mass requires an equivalent increase in endothelial cell mass. The ultimate endothelial mass is dependent upon a delicate balance between proliferative and apoptotic signals within the tumor microenvironment. A similar system may be required for the development of liquid tumors. Our laboratory and others have shown that activated endothelial cells can release a variety of cytokines that may stimulate leukemic cell growth.30,31 Leukemic cells, in turn, have the capacity to release endothelial growth factors, such as vascular endothelial growth factor (VEGF), and may also express growth factor receptors on their surface, such as VEGF receptor-2 (VEGFR-2).31,32 Therefore, leukemic blasts may be able to support their own development both by promoting neoangiogenesis and via an autocrine loop.31 32
On the basis of these observations, we speculated that arsenic may interrupt a reciprocal loop between leukemic cells and the endothelium by direct action on both cell types. We have found that As2O3 causes dose- and time-dependent activation and apoptosis of human endothelial cells, but not fibroblasts, in vitro. Also, As2O3 inhibits VEGF-induced capillary tubule formation and decreases leukemic cell VEGF production. These data suggest that As2O3may exert its antileukemic effect in part through inhibition of angiogenesis.
Materials and methods
Reagents
All reagents were obtained from Sigma Chemical Co, St Louis, MO, unless otherwise noted. A clinical grade 5 × 10−2mol/L As2O3solution2 was diluted to 5 × 10−4mol/L in phosphate-buffered saline (PBS) and stored at 4°C. The stock solution was free of endotoxin (Bio-Whittaker, Walkersville, MD). Further dilutions to working concentrations were made before use. The concentrations of selected dilutions were confirmed using inductively coupled plasma mass spectrometry (Medtox Laboratories, St Paul, MN).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated according to methods previously described.33 The cells were cultured in M199 medium supplemented with 18% fetal bovine serum, 2% pooled human serum, basic fibroblast growth factor (FGF 5 ng/mL) (R&D Systems, Minneapolis, MN), VEGF165 (5 ng/mL) (Peprotech, Rocky Hill, NJ), endothelial cell growth supplement (Intracel, Rockville, MD), heparin, L-glutamine, amphotericin-B, penicillin, and streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37°C. Samples of cells were obtained, mixed with an equal volume of 0.4% trypan blue, and manually counted in triplicate by means of a hemocytometer to determine the number of viable cells. Human foreskin fibroblasts and the fibroblast cell line MRC-5 (kindly provided by Dr M. A. S. Moore, Sloan Kettering Institute) were grown in RPMI medium supplemented with 10% fetal calf serum. The leukemia cell lines HL60 and HEL (American Type Culture Collection, Rockville, MD) were also grown in RPMI medium supplemented with 10% fetal calf serum.
Flow cytometry
To characterize the activated endothelial cells, the following fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies were used: intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), E-selectin, and immunoglobulin (Ig)G isotype control (Immunotech, Marcella, France). For flow cytometry, 100 000 cells were incubated with the respective antibodies, fixed with 1% formalin, washed, and analyzed by means of a Coulter Elite flow cytometer.
Annexin-V staining
Cells were incubated with FITC-conjugated Annexin-V according to the recommendations of the manufacturer (Immunotech). Nuclei were counterstained with propidium iodide (PI) and screened by flow cytometry (approximately 10 000 events). Early apoptosis was estimated by the relative amount of FITC+PI− cell populations. FITC+PI+ cells represented either secondary necrotic or postapoptotic populations.
Protein extraction and Western blotting
Total cell extracts from HUVECs were obtained by lysing the cells in cold RIPA buffer (50 mmol/L Tris, 5 mmol/L EDTA, 1% Triton X-114, 0.4% sodium cacodylate, and 150 mmol/L NaCl) in the presence of protease inhibitors (1 mg/mL aprotinin, 10 mg/mL leupeptin, 1 mmol/L β-glycerophosphate, and 1 mmol/L phenylmethylsulfonyl fluoride). After centrifugation to remove cell debris, supernatants were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (using 10% acrylamide gels) under reducing conditions (in the presence of 250 mmol/L β-mercaptoethanol). Proteins were subsequently blotted onto a nitrocellulose membrane (Schleicher and Schuell, Keene, NH) following conventional protocols. The membranes were stained with 0.2% ponceau S red to assure equal protein loading. Finally, blots were blocked in 1% bovine serum albumin/PBS with 1% Tween-20 for 1 hour at room temperature and incubated with primary and secondary antibodies. Monoclonal antibodies against human Bcl-2 (Calbiochem, Cambridge, MA) and Bax (Santa Cruz Biotechnology, Santa Cruz, CA) were used at dilutions of 1:500 and 1:1000, respectively. The ECL chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) detection system and ECL film (Amersham Pharmacia Biotech) were used to visualize the presence of specific proteins in the nitrocellulose blots.
TUNEL assay
HUVECs were grown to 70% confluence on poly-D-lysine–coated coverslips and incubated for 24 hours with varying concentrations of As2O3. The TUNEL (terminal deoxynucleotidyl transferase–-mediated deoxyuridine triphosphate nick end labeling) assay was performed with the use of the In Situ Cell Death Detection Kit, AP (Boehringer Mannheim, Mannheim, Germany), according to the manufacturer's instructions. Fast red tablets (Boehringer Mannheim) were used as the chromogenic substrate.
Matrigel-induced capillary tube formation
The Matrigel assay has been widely used as an in vitro measurement of endothelial cell differentiation and was performed as described previously.34 35 Briefly, 24-well plates (Costar, Cambridge, MA) were coated with 300 μL/well growth-factor–reduced Matrigel (Becton Dickinson, San Jose, CA) and allowed to stand for 30 minutes at 37°C to form a gel layer. After gel formation, HUVECs (1 × 105 cells) in a medium containing X-Vivo (Bio-Whittaker) and endothelial cell growth supplement were applied to each well in the absence or presence of VEGF165 20 ng/mL and various concentrations of As2O3. Each treatment was performed in triplicate wells. The plates were incubated at 37°C for 48 hours with 5% CO2 and photographed with the use of inverted phase-contrast microscopy (Nikon, Tokyo, Japan).
Enzyme-linked immunosorbent assay for VEGF
The VEGF concentration in samples of conditioned medium was measured by means of a human VEGF immunoassay that recognizes the soluble isoforms VEGF121 and VEGF165 (R&D Systems). For cell culture supernatants, a sensitivity of 5 pg/mL could be achieved.
Statistical analysis
All experiments were performed in triplicate unless otherwise noted; results are expressed as mean ± standard deviation. Comparison of means was performed by means of the analysis of variance procedure (Dunnett's t test, SAS for Windows version 6.12, SAS Institute, Cary, NC).
Results
Effect of As2O3 on viability and proliferation of HUVECs
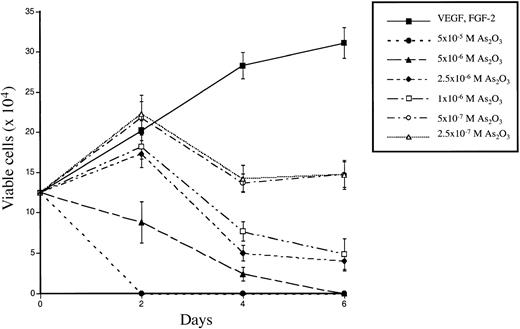
To investigate the effects of As2O3 on the survival and proliferation of HUVECs, 70% to 80% confluent monolayers of rapidly proliferating cells were grown for 1 to 6 days in the presence of endothelial cell growth supplement, 20% serum, FGF-2 (5 ng/mL), VEGF (5 ng/mL), and various concentrations of As2O3, ranging from 2.5 × 10−7mol/L to 5 × 10−5 mol/L. Each condition was performed and counted in triplicate. As shown in Figure1, As2O3significantly decreased cell viability and proliferation at all concentrations tested in comparison with control cells. The curves were reproducible using several different cell passages, from number 1 to 3 (data not shown). At the highest concentrations tested (5 × 10−5 mol/L and 5 × 10−6 mol/L), As2O3 superseded the effects of the endothelial growth factors and prevented cell proliferation, causing a rapid decline in the number of viable cells; after 6 days, no viable cells remained in either condition. At intermediate doses of As2O3 (2.5 × 10−6 mol/L and 1 × 10−6 mol/L), cell proliferation was completely inhibited after 48 hours exposure, and the number of viable cells steadily decreased to approximately 30% of control cells by day 6. At low concentrations of As2O3(5 × 10−7 mol/L and 2.5 × 10−7 mol/L), cell proliferation was initially increased compared with controls, but subsequently declined, leaving approximately 50% fewer viable cells than controls at day 6.
Effect of As2O3 on the viability and proliferation of HUVECs.
Rapidly proliferating, 70% to 80% confluent HUVECs were incubated in culture medium with different concentrations of As2O3 for the time period indicated. The number of viable cells was counted in triplicate by means of the trypan blue exclusion assay. The results show the mean ± SD of 3 independent experiments.
Effect of As2O3 on the viability and proliferation of HUVECs.
Rapidly proliferating, 70% to 80% confluent HUVECs were incubated in culture medium with different concentrations of As2O3 for the time period indicated. The number of viable cells was counted in triplicate by means of the trypan blue exclusion assay. The results show the mean ± SD of 3 independent experiments.
HUVECs appeared not to develop resistance to the effects of arsenic: more than 75% of cells treated with low or intermediate concentrations of As2O3 for 2 to 3 days were able to recover and proliferate if exposure to arsenic was discontinued, and they were subsequently susceptible to its effects upon retreatment (data not shown). Even at low concentrations, all cells eventually died after continuous exposure. In contrast, human foreskin fibroblasts and MRC-5 embryonic fibroblasts were minimally affected even by high concentrations of As2O3; after longer than 12 days of continuous exposure to 5 × 10−6 mol/L As2O3, the number of viable cells was still 70% of control cells (data not shown).
Treatment of HUVECs with As2O3 increases apoptosis in a dose- and time-dependent manner
To show whether the growth inhibition on endothelial cells by As2O3 was caused by induction of apoptosis, rapidly proliferating, early passage HUVECs were incubated with different concentrations of As2O3. All conditions were performed in triplicate. The cells were dual-stained with Annexin-V–FITC and PI and analyzed by flow cytometry. After 72 hours, there was a dose-dependent increase in early and late apoptotic cells (Figure 2). At the highest concentration tested (5 × 10−6 mol/L), nearly 40% of the cells showed signs of early apoptosis, and approximately 80% of the population was undergoing either early or late apoptosis after 72 hours, compared with 12% of the control cells (growth-factor treated), 18% of the 5 × 10−7 mol/L, and 34% of the 2.5 × 10−6 mol/L groups.
Induction of apoptosis in HUVECs treated with As2O3.
Early passage, rapidly proliferating HUVECs were incubated with different concentrations of As2O3, dual-stained with Annexin-V–FITC and PI and analyzed by flow cytometry. After 72 hours, there was a dose-dependent increase in early and late apoptotic cells, as shown in the second and fourth quadrants of the plots. Results are representative of 3 similar independent experiments.
Induction of apoptosis in HUVECs treated with As2O3.
Early passage, rapidly proliferating HUVECs were incubated with different concentrations of As2O3, dual-stained with Annexin-V–FITC and PI and analyzed by flow cytometry. After 72 hours, there was a dose-dependent increase in early and late apoptotic cells, as shown in the second and fourth quadrants of the plots. Results are representative of 3 similar independent experiments.
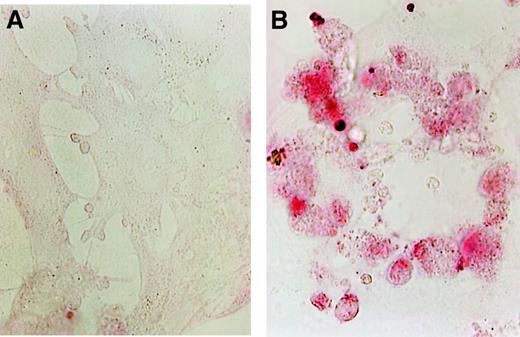
To further characterize the apoptosis observed in arsenic-treated HUVECs, we assessed Bcl-2 and Bax expression using Western blotting (Figure 3) and DNA strand breaks using the TUNEL assay (Figure 4). HUVECs constitutively express Bcl-2, even under serum-free conditions. As expected, Bcl-2, an antiapoptotic signal, is increased after treatment with VEGF, the most potent growth and survival factor for endothelium. Bcl-2 expression decreased in a dose-dependent manner after treatment with As2O3. Bax expression was not affected by treatment with As2O3 as compared with control cells, but the Bcl-2/Bax ratio decreased after treatment, thus correlating with the observations of increased Annexin-V staining and apoptosis. TUNEL staining of HUVECs was markedly increased after only 24 hours of treatment with 5 × 10−6 mol/L As2O3; representative photographs are shown in Figure 4.
Western blot analysis for Bcl-2 and Bax proteins in HUVECs after treatment with varying concentrations of As2O3 in the absence and presence of exogenous VEGF.
Equal amounts of protein were loaded onto each lane.
Western blot analysis for Bcl-2 and Bax proteins in HUVECs after treatment with varying concentrations of As2O3 in the absence and presence of exogenous VEGF.
Equal amounts of protein were loaded onto each lane.
Rapidly proliferating HUVECs were grown to 70% confluence on poly-D-lysine–coated coverslips and incubated in culture medium with and without As2O3 for 24 hours.
(A) Control cells. (B) The photographs (original magnification 500 ×) show strongly increased TUNEL staining after treatment with 5 × 10−6 mol/L As2O3 in comparison with control cells.
Rapidly proliferating HUVECs were grown to 70% confluence on poly-D-lysine–coated coverslips and incubated in culture medium with and without As2O3 for 24 hours.
(A) Control cells. (B) The photographs (original magnification 500 ×) show strongly increased TUNEL staining after treatment with 5 × 10−6 mol/L As2O3 in comparison with control cells.
Treatment of HUVECs with As2O3 results in endothelial cell activation and increased binding of leukemic cells
Prior to undergoing apoptosis, HUVECs treated with endotoxin-free As2O3 were observed to attain the spindle-shaped morphology of activated endothelium, as opposed to the characteristic cobblestone appearance of resting HUVECs grown in tissue culture (Figure 5). FACS analysis was performed to determine whether this activated morphology corresponded with up-regulation of 3 molecular markers of endothelial cell activation: E-selectin, ICAM, and VCAM. There was significantly increased expression of all 3 markers after 5 days' incubation with 5 × 10−7 mol/L As2O3 (Figure5). To investigate whether leukemic cells would demonstrate increased binding to arsenic-activated endothelial cells, 1 × 105HEL cells were added to HUVECs that had become spindle-shaped in the presence of 2 different concentrations of As2O3; HUVECs activated with interleukin (IL)–1β were used as a positive control. All conditions were performed in triplicate. The coculture system was allowed to incubate for 1 hour, and the adherent population was quantitated with the use of phase-contrast microscopy. Treatment with As2O3or IL-1β resulted in an approximately 15-fold increase in the binding of HEL cells (Figure 6).
Comparison of the characteristic cobblestone-pattern morphology of resting HUVECs grown in tissue culture with the spindle-shaped, activated morphology observed after treatment with As2O3.
The representative fluorescence-activated cell sorter (FACS) plots demonstrate that As2O3-induced up-regulation of the adhesion molecules VCAM, ICAM, and, to a lesser extent, E-selectin.
Comparison of the characteristic cobblestone-pattern morphology of resting HUVECs grown in tissue culture with the spindle-shaped, activated morphology observed after treatment with As2O3.
The representative fluorescence-activated cell sorter (FACS) plots demonstrate that As2O3-induced up-regulation of the adhesion molecules VCAM, ICAM, and, to a lesser extent, E-selectin.
HUVECs were grown in culture medium in the presence and absence of 2 different concentrations of As2O3and IL-1β and incubated with 1 × 105 HEL cells for 1 hour.
The adherent population was quantified in triplicate with the use of phase-contrast microscopy.
HUVECs were grown in culture medium in the presence and absence of 2 different concentrations of As2O3and IL-1β and incubated with 1 × 105 HEL cells for 1 hour.
The adherent population was quantified in triplicate with the use of phase-contrast microscopy.
Effect of As2O3 on leukemic cell VEGF production
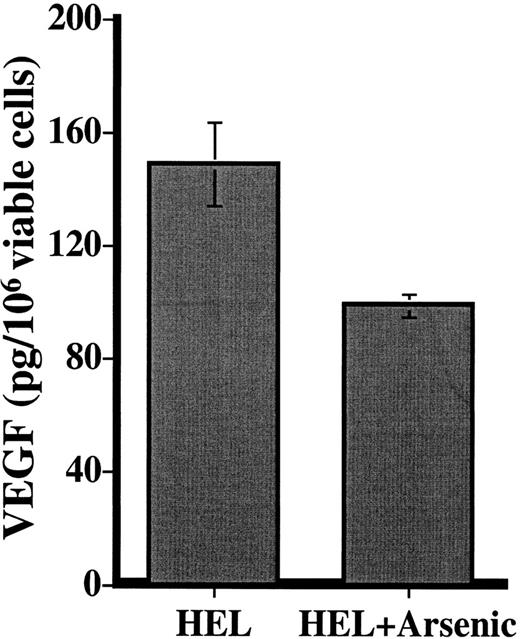
We investigated the effects of As2O3treatment on VEGF production by 2 different leukemia cell lines: HL60, which is refractory to the effects of As2O3,19,24 and HEL, which undergoes dose- and time-dependent apoptosis upon exposure to As2O3.19 In 2 independent experiments, treatment of HEL cells with 5 × 10−6 mol/L As2O3 for 48 hours resulted in a 34% reduction in leukemic cell VEGF (Figure 7). In contrast, VEGF production by HL60 cells was unaffected under the same treatment conditions (data not shown).
VEGF production by the cell line HEL in the presence and absence of 5 × 10−6 mol/L As2O3for 48 hours.
Results (pg/106 viable cells) are expressed as the mean ± SD of 2 independent experiments, in which each condition was tested in triplicate at 2 different dilutions.
VEGF production by the cell line HEL in the presence and absence of 5 × 10−6 mol/L As2O3for 48 hours.
Results (pg/106 viable cells) are expressed as the mean ± SD of 2 independent experiments, in which each condition was tested in triplicate at 2 different dilutions.
Effect of As2O3 on endothelial cell differentiation in vitro
To observe the effects of As2O3 on tubule formation in vitro, HUVECs were plated onto growth-factor–reduced Matrigel in the presence and absence of additional VEGF165and varying concentrations of As2O3. All conditions were performed in triplicate. The HUVECs adhered to the Matrigel surface within 18 to 24 hours and formed a branching, anastomosing network of capillarylike tubules with multicentric junctions over 24 to 48 hours, consistent with published observations.34 The largest number of tubules was produced with the addition of exogenous VEGF165 (Figure8). As2O3inhibited VEGF-induced tubule and branch formation in a dose-dependent manner, as shown in Figure 8.
Effect of As2O3 on Matrigel-induced tube formation of HUVECs in vitro.
HUVECs (1 × 105 cells/well) were plated on growth-factor–reduced Matrigel in the presence of a culture medium. (A) Culture medium contained X-Vivo and endothelial cell growth supplement (ECGS). (B) Culture medium contained X-Vivo, ECGS, and VEGF (20 ng/mL). (C) Culture medium contained X-Vivo, ECGS, VEGF (20 ng/mL), and 5 × 10−7 mol/L As2O3. (D) Culture medium contained X-Vivo, ECGS, VEGF (20 ng/mL), and 5 × 10−6 mol/L As2O3.
Effect of As2O3 on Matrigel-induced tube formation of HUVECs in vitro.
HUVECs (1 × 105 cells/well) were plated on growth-factor–reduced Matrigel in the presence of a culture medium. (A) Culture medium contained X-Vivo and endothelial cell growth supplement (ECGS). (B) Culture medium contained X-Vivo, ECGS, and VEGF (20 ng/mL). (C) Culture medium contained X-Vivo, ECGS, VEGF (20 ng/mL), and 5 × 10−7 mol/L As2O3. (D) Culture medium contained X-Vivo, ECGS, VEGF (20 ng/mL), and 5 × 10−6 mol/L As2O3.
Discussion
Emerging data suggest that the proliferation of both solid and liquid tumors may be dependent upon angiogenesis. In the present work, we sought to investigate the potential antiangiogenic activity of As2O3. Treatment of HUVECs with As2O3 at a variety of different concentrations results in a reproducible sequence of events marked by a change to an activated morphology, up-regulation of endothelial cell adhesion markers, and apoptosis, as measured by increased Annexin-V staining and a decreased Bcl-2–to–Bax ratio. This sequence may be unique to vascular endothelial cells, as As2O3 did not similarly affect fibroblasts treated with identical concentrations. The activation of HUVECs was not caused by endotoxin, as both the endothelial cell medium and the arsenic preparations were free of endotoxin. Also, treatment with As2O3 induced up-regulation of adhesion molecules 48 hours after exposure, whereas endotoxin-mediated up-regulation of adhesion molecules should take place within a few hours. It is possible that As2O3-induced apoptosis of endothelial cells results in the release of inflammatory cytokines, such as IL-1β or tumor necrosis factor, which may in turn activate the surrounding cells. Alternatively, activation may be a direct consequence of As2O3 itself. Activation of HUVECs was characterized by up-regulation of E-selectin, ICAM, and VCAM. It has been shown that adhesion of hematopoietic progenitor cells to bone marrow stroma and fibronectin is important for their anchoring in the bone marrow microenvironment and may result in decreased proliferation.36 We are investigating whether increased adhesion of leukemic blasts to activated endothelium decreases their proliferation or, perhaps, promotes differentiation.
Our data show that As2O3 induces dose- and time-dependent apoptosis of HUVECs. There is ample evidence that As2O3 probably does not simply cause nonspecific cytotoxicity. The drug has few immediate toxicities at clinically effective doses, suggesting that it does not induce generalized apoptosis or necrosis. In vitro, it causes minimal apoptosis of fibroblasts and is not active against a number of malignant cell lines, even at high concentrations. Pharmacokinetic analyses of clinical samples have shown peak plasma arsenic concentrations of 5.5 to 7.3 μmol/L per liter, and the steady state is believed to be between 1 and 2 μmol/L.4The concentrations of As2O3 achieved in bone marrow may be significantly higher than those in plasma, as accumulation of As2O3 is greatest in tissues rich in sulfhydryl-group–containing proteins, such as bone marrow.4 Arsenic is also known to bind hemoglobin and can be distributed rapidly, especially to highly vascular organs. These properties, in combination with the observations described in this work, suggest that As2O3 may exert its antileukemic effect through direct action against both leukemic cells and the endothelium.
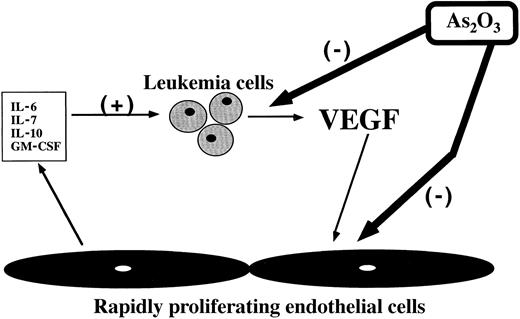
Perez-Atayde et al26 have noted increased blood vessel density in the bone marrow of children with acute lymphoblastic leukemia. Data from our laboratory also show increased vascularity in bone marrow biopsy specimens from adult patients with different types of acute leukemia (manuscript in preparation). It is known that impaired production or expression of VEGF will disrupt angiogenesis.37 We believe that As2O3 interrupts a reciprocal stimulatory loop between leukemic cells and endothelial cells by causing apoptosis of both cell types and by inhibiting leukemic cell VEGF production (Figure9). Release of VEGF by leukemic blasts may be an important stimulus for neoangiogenesis in the bone marrow. Endothelium is exquisitely sensitive to the dose of VEGF to which it is exposed, and it is possible that the 34% reduction in VEGF production observed after treatment with arsenic is physiologically significant. In our in vitro endothelial cell differentiation assay, used as a measure of angiogenesis, treatment with As2O3prevents capillary tubules and branch formation even in the presence of additional VEGF.
Schema illustrating proposed sites of action of As2O3 in interrupting a reciprocal feedback loop between leukemic cells and endothelium.
Schema illustrating proposed sites of action of As2O3 in interrupting a reciprocal feedback loop between leukemic cells and endothelium.
A combination of direct toxicity against leukemic cells with antiangiogenic activity may be the optimum characteristic of a successful antileukemic agent. Future experiments are planned to establish the role of the endothelium in leukemic cell proliferation using coculture systems and to combine As2O3with other specific antiangiogenic factors, such as neutralizing monoclonal antibodies to VEGF and VEGFR-2. Finally, we plan to evaluate the role of As2O3 in inhibiting angiogenesis in murine leukemia models.
Acknowledgments
We thank Alice Hafner, MS, for her help with statistical analysis, and Barbara Ferris for expert technical assistance.
S.R. supported by the American Heart Association Grant-In-Aid; NHLBI grants R01-HL-58707 and R01-HL-61849; the Dorothy Rodbell Foundation for Sarcoma Research; and the Rich Foundation. G.J.R. supported by the Susan Murphy Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shahin Rafii, Division of Hematology and Oncology, Department of Medicine, Weill Medical College of Cornell University, 1300 York Ave, Rm C-606, New York, NY 10021; e-mail:srafii@mail.med.cornell.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal