Abstract
Effective engraftment of hematopoietic cells targeted for gene transfer is facilitated by cytoreductive preconditioning such as high-dose total body irradiation (TBI). To minimize the adverse side effects associated with TBI, experiments were conducted to determine whether sublethal doses of TBI would allow sufficient engraftment of MTX-resistant hematopoietic cells to confer survival on recipient mice administered MTX. FVB/N animals were administered 1, 2, or 4 Gy TBI (lethal dose, 8.5 Gy), transplanted with 107 FVB/N transgenic marrow cells expressing an MTX-resistant dihydrofolate reductase (DHFR) transgene, and then administered MTX daily for 60 days. Control mice administered 1 Gy with or without subsequent transplantation of normal marrow cells succumbed to MTX toxicity by day 45. In contrast, nearly all animals transplanted with transgenic marrow survived MTX administration, regardless of the TBI dose used for preconditioning. The donor DHFR transgenic marrow engraftment level was proportional to the preconditioning dose of TBI but was surprisingly reduced in animals given 2 or 4 Gy TBI and subsequently administered MTX when compared with control animals administered phosphate-buffered saline. Animals preconditioned with 1 Gy were also protected from MTX toxicity when transplanted with reduced amounts (5 × 106 and 1 × 106 cells) of DHFR transgenic donor marrow, resulting in low-level (approximately 1%) engraftment. In conclusion, very mild preconditioning allows sufficient low-level engraftment of genetically modified stem cells for in vivo manifestation of the modified phenotype, suggesting the usefulness of mild preconditioning regimens in human gene therapy trials targeting hematopoietic stem cells.
Introduction
Hematopoietic stem cells (HSC) are an attractive target population for gene transfer and gene therapy because of their multilineage differentiation potential and capacity for self-renewal.1,2 HSC are readily accessed from the bone marrow or from the peripheral blood after mobilization with cytokines or other agents for ex vivo genetic modification.3 On reinfusion, engraftment of HSC is greatly facilitated by prior cytoreductive preconditioning, such as total body irradiation (TBI), or by the administration of cytoreductive agents such as cytoxan or busulfan.4-7 The risks for such preconditioning are justifiable in HSC transplantation for malignant disease because the cytoreductive treatment serves the dual purpose of antitumor chemotherapy as well as creating “hematopoietic space” for transplanted HSC to engraft.5,6 Cytoreductive preconditioning is also used to promote engraftment of allogeneic HSC in the treatment of genetic deficiencies.7 However, in the case of gene transfer into autologous HSC with subsequent transplantation for the treatment of genetic deficiencies, the risk for cytoreductive preconditioning has not been considered justifiable when such treatment would serve no other purpose than to facilitate engraftment of donor material of unproved efficacy.8 Thus, several human trials evaluating therapeutic gene transfer into HSC have been conducted without the benefit of any cytoreductive preconditioning, rendering more improbable the likelihood of engrafting a small proportion of stem cells that have been successfully modified genetically.9-13
Gene therapy trials would be benefited by the establishment of procedures for effectively engrafting a small proportion of transduced HSC in a population that has undergone transplantation, with reduced risk resulting from cytoreductive preconditioning. Engraftment of HSC without prior cytoreductive preconditioning has been demonstrated in experimental animals but has required multiple infusions of large stem cell doses.14-16 Malech and coworkers17recently reported significant engraftment in animals transplanted with donor marrow stem cells after the administration of very low doses of TBI. Here we show that the engraftment achievable after low-dose TBI and bone marrow transplantation (BMT) is sufficient to confer a distinct pharmacologic outcome, ie, methotrexate resistance of recipient animals mediated by the expression of a drug-resistant form of dihydrofolate reductase (DHFR) in donor HSC. The pharmacologic effectiveness of DHFR gene expression was exemplified by drug resistance observed in animals engrafted with as little as 1% donor DHFR transgenic HSC. These experiments model the idea of using a minimally invasive preconditioning regimen to facilitate partial engraftment of genetically engineered HSC, which are then capable of mediating a physiological outcome in the recipient. Use of the DHFR gene in these studies is particularly pertinent in light of recent evidence for the in vivo expandability of DHFR-expressing HSC,18 which could allow an increase in the representation of hematopoietic cells engineered to express DHFR and other therapeutic genes.
Materials and methods
Animals and bone marrow transplantation
Eight-week old FVB/N mice were obtained from the National Institutes of Health (Frederick, MD) and provided food and water ad libitum. At 12 weeks of age, recipient mice received total body irradiation (doses are specified in the figure legends and in the tables) from a cesium 137 source one day before bone marrow transplantation. Donor transgenic mice expressing the murine arg22 DHFR (line 04 or Tg04) were established and characterized as described by Morris et al.19 Donor transgenic mice carrying a nonexpressing amyloid precursor protein gene (TgAPP or normal donor) were described by Hsiao et al.20 Marrow was flushed from the hind limbs of donor mice, processed to a single-cell suspension in Iscove's modified Dulbecco's medium as previously described21 22 and transplanted in 0.5-mL volumes through the tail vein. The numbers of marrow cells transplanted are specified in the tables and the figure legends. Mice that received MTX ([+] Amethopterin; Sigma, St. Louis, MO) were injected intraperitoneally with 1 mg/kg MTX on days 1 to 4, 2 mg/kg MTX on days 5 to 8, and 4 mg/kg MTX on days 9 to 60. Control mice were injected with equivalent volumes of PBS. General health and survival were monitored daily, and hematocrit was determined weekly. Moribund mice were killed and tissues collected for histopathologic analysis. In some experiments, MTX was withdrawn and surviving primary transplant recipients were allowed to recover for an additional 60 days, at which point they were killed for marrow and spleen harvest. Marrow harvested from primary transplant recipients was transplanted into lethally irradiated secondary recipients, which were allowed to recover for 4 months before their marrow and spleen were harvested for quantitative Southern analysis. All procedures were reviewed and approved by the University of Minnesota Animal Care Committee.
Quantitative Southern analysis
Bone marrow and spleen were harvested from surviving primary and secondary transplant recipients 4 months after transplantation. Genomic DNA was isolated, digested withBglII, electrophoresed through 1% agarose/TAE, and blotted onto Nytran as previously described.22 Blots were probed for DHFR sequences using a 485-bp polymerase chain reaction product that included exons 1 and 2 and intron 1 of the murine DHFR gene.23 The murine Y probe was a 720-bp insert from plasmid pY2 (gift of Dr I. Lemischka).24 The 1.3-kb human amyloid precursor protein (APP) probe was a gift from Dr K. Hsiao.20 Probes were labeled with 32P by random priming (Oligolabeling Kit; Pharmacia, Piscataway, NJ). Blots were hybridized and washed as previously described.22Resultant signals were quantified using a 445SI PhosphorImager (Molecular Dynamics, Sunnyvale, CA). The Student t test was used for statistical analysis of differences in engraftment levels between groups of animals.
Histopathologic analysis
Animals were killed and tissue samples were harvested, including femur, sternum, ileum, liver, kidney, lung, heart, and brain. All samples were fixed in 10% phosphate-buffered formalin (further decalcifying bone samples in 1% formic acid), embedded in paraffin, sectioned, mounted, stained (hematoxylin and eosin), and analyzed microscopically without prior knowledge of sample identity.
Results
Reduced total body irradiation dose allows engraftment of MTX-resistant stem cells
It was previously demonstrated that normal FVB/N mice administered high-dose TBI (lethal or near lethal; 8.5 Gy) and subsequently transplanted with 107 DHFR transgenic marrow cells were resistant to lethal doses of MTX (4 mg/kg per day).21,22Under these conditions, a high degree of donor cell engraftment was observed in recipient animals.21 22 We sought to determine whether less severe doses of TBI could be used for preconditioning and still provide sufficient engraftment to confer drug-resistance on recipient animals. Animals were administered 1, 2, or 4 Gy TBI (approximately one eighth, one quarter, and one half lethal dose, respectively), transplanted with 107 line 04 DHFR transgenic marrow cells, and subsequently administered either PBS or MTX up to 4 mg/kg daily for 60 days. As shown in Figure1, animals in all 3 groups were resistant to MTX; all but one animal survived the entire 60 days of drug administration (Figure 1A). In contrast, we have observed that unirradiated FVB/N mice transplanted with line 04 DHFR transgenic marrow are not protected from MTX toxicity (Belur et al,manuscript in preparation). Protection from hematologic toxicity of methotrexate in all 3 radiation-dose groups was evidenced by the maintenance of hematocrit levels between 30 and 40 during the entire course of drug administration (Figure 1B). Control animals administered PBS exhibited nearly 100% survival and maintenance of normal hematocrit levels (more than 40) throughout the course of the experiment (data not shown).
Methotrexate resistance of sublethally irradiated mice transplanted with DHFR transgenic marrow.
Normal male mice were given 1, 2, or 4 Gy TBI one day before they underwent transplantation with 1 × 107 marrow cells from female line 04 (Tg04) DHFR transgenic donors. They were then administered either PBS or MTX (up to 4 mg/kg) daily for 60 days (n = 10 for each group). (A) Kaplan–Meier plot showing the fraction of surviving mice per time in the MTX- administered groups. (B) Mean weekly hematocrit of surviving mice in the MTX-administered groups at each time point.
Methotrexate resistance of sublethally irradiated mice transplanted with DHFR transgenic marrow.
Normal male mice were given 1, 2, or 4 Gy TBI one day before they underwent transplantation with 1 × 107 marrow cells from female line 04 (Tg04) DHFR transgenic donors. They were then administered either PBS or MTX (up to 4 mg/kg) daily for 60 days (n = 10 for each group). (A) Kaplan–Meier plot showing the fraction of surviving mice per time in the MTX- administered groups. (B) Mean weekly hematocrit of surviving mice in the MTX-administered groups at each time point.
Donor cell engraftment level was determined in all recipient animals 2 months after transplantation by quantitative Southern analysis of DNA extracted from the marrow and spleen, probing for the DHFR transgene (Figure 2). The level of engraftment observed was proportional to the radiation dose administered before transplantation. For PBS-administered animals, the level of marrow engraftment increased from approximately 10% in mice preconditioned with 1 Gy, to approximately 20% in mice given 2 Gy, to more than 60% when preconditioned with 4 Gy. Lower levels of engraftment were observed in the spleen. Surprisingly, mice administered MTX exhibited consistently lower levels of donor cell engraftment that were statistically significant in either marrow or spleen (1 Gy, 4 Gy) or in both marrow and spleen (2 Gy). These results suggest that MTX administration does not result in selective regeneration of donor-derived, MTX-resistant hematopoietic cells over endogenous, normal cells, but rather is associated with some toxicity that impedes engraftment of donor marrow.
TBI dose-dependent engraftment of transgenic marrow cells in sublethally irradiated BMT recipients.
Survivors from Figure 1 were killed 120 days after transplantation. Genomic DNA was isolated from bone marrow and spleen and subjected to quantitative Southern analysis as described in “Materials and Methods” (see Figure 4A for an example of the Southern hybridization signals used to quantitate DHFR transgenic cell engraftment levels). Values are presented as mean ± SD of between 5 and 9 samples. Results of statistical analyses between PBS- and MTX-administered animals are given as P values, displayed above each pair of groups.
TBI dose-dependent engraftment of transgenic marrow cells in sublethally irradiated BMT recipients.
Survivors from Figure 1 were killed 120 days after transplantation. Genomic DNA was isolated from bone marrow and spleen and subjected to quantitative Southern analysis as described in “Materials and Methods” (see Figure 4A for an example of the Southern hybridization signals used to quantitate DHFR transgenic cell engraftment levels). Values are presented as mean ± SD of between 5 and 9 samples. Results of statistical analyses between PBS- and MTX-administered animals are given as P values, displayed above each pair of groups.
Because sublethal doses of TBI as low as 1 Gy provided sufficient DHFR transgenic cell engraftment (as little as 10%) to confer MTX-resistance in recipient animals, we chose this dose (1 Gy) for further studies to verify these results under conditions controlled for MTX toxicity. Animals were given a dose of 1 Gy TBI, transplanted with either no cells, 1 × 107 normal FVB/N marrow cells, or 1 × 107 line 04 DHFR transgenic marrow cells, and subsequently administered either PBS or MTX at a final dose of 4 mg/kg per day. Male mice were used as recipients to allow quantitation of host cell reconstitution using a Y chromosome probe, and normal marrow cells were obtained from FVB/N animals transgenic for APP sequences to facilitate the quantitation of non-DHFR donor marrow engraftment using an APP probe (see below). All PBS-administered animals survived the entire period of study, and hematocrit levels were maintained above 40 (data not shown). Figure 3A shows that animals administered 1 Gy TBI and then transplanted with line 04 DHFR transgenic marrow were largely resistant to methotrexate, exhibiting nearly 70% survival over the 60-day period of drug administration. Protection of these animals from MTX toxicity was also apparent from the maintenance of hematocrit levels during MTX administration. In contrast, nearly all of the untransplanted animals or animals transplanted with normal (APP transgenic) marrow suffered from severe MTX toxicity and succumbed by day 45 after transplantation. These mice had impaired hematopoiesis, evidenced by declining hematocrit values that reached a nadir of 9% immediately before their demise. Histopathologic analysis showed mild to severe marrow hypoplasia in 9 of 11 samples examined in addition to mild to severe atrophy of the ilei, as characterized by blunting of villi, loss of crypts, and infiltration of lymphocytes and macrophages in the lamina propria. Two mice also showed atrial thromboses, and 3 other mice showed myocardial necrosis characterized by 1 or 2 mineralized myofiber segments. No such lesions were found in animals transplanted with line 04 DHFR transgenic marrow or in animals administered PBS, demonstrating that transplantation with DHFR transgenic marrow protects recipient animals from these toxicities.
Methotrexate resistance of mice preconditioned with 1 Gy TBI and transplanted with line 04 DHFR transgenic marrow.
Male mice were given 1 Gy TBI one day before transplantation with no marrow, 1 × 107 female normal (APP) marrow cells, or 1 × 107 female transgenic DHFR (Tg04) marrow cells. The animals were then administered either PBS or MTX (final dose of 4 mg/kg) intraperitoneally daily for 60 days (n = 10 for each group). (A) Kaplan–Meier plot showing the fraction of surviving mice per time in the MTX-administered groups. (B) Mean weekly hematocrit of surviving mice in the MTX-administered groups at each time point.
Methotrexate resistance of mice preconditioned with 1 Gy TBI and transplanted with line 04 DHFR transgenic marrow.
Male mice were given 1 Gy TBI one day before transplantation with no marrow, 1 × 107 female normal (APP) marrow cells, or 1 × 107 female transgenic DHFR (Tg04) marrow cells. The animals were then administered either PBS or MTX (final dose of 4 mg/kg) intraperitoneally daily for 60 days (n = 10 for each group). (A) Kaplan–Meier plot showing the fraction of surviving mice per time in the MTX-administered groups. (B) Mean weekly hematocrit of surviving mice in the MTX-administered groups at each time point.
The engraftment level of donor DHFR transgenic or normal (APP transgenic) marrow in these BMT recipients was determined by Southern hybridization analysis of DNA extracted from bone marrow and spleen 4 months after transplantation, probing for DHFR, APP, and Y-chromosome sequences. Figure 4A shows that the spleens of mice transplanted with either normal or line 04 cells contained donor-derived cells, as indicated by the presence of the APP band (lanes 3 and 4) or Tg DHFR band (lanes 5 to 8), respectively, verifying that 1 Gy TBI was sufficient to allow the engraftment of transplanted cells. Untransplanted animals contained only endogenous cells, as indicated by the presence of the Y band (lanes 1 and 2) and the absence of APP or DHFR transgene bands. Substantial host cell repopulation was observed in DHFR- and APP-transgenic marrow transplant recipients (lanes 3 to 8), consistent with the mild preconditioning used. Quantitation of DHFR and APP signals in marrow and spleen (Figure4B) indicated engraftment levels of 8% to 20%, similar to those previously observed for animals preconditioned with 1 Gy TBI. The mean engraftment levels in marrow and spleen were not significantly different (P > .05) for MTX-administered animals vs. PBS-administered controls.
Engraftment of donor-derived cells in bone marrow and spleen of primary BMT recipients preconditioned with 1 Gy TBI.
Surviving animals (depicted in Figure 3) were killed 120 days after transplantation. Genomic DNA samples isolated from bone marrow and spleen were subjected to quantitative Southern analysis as described in “Materials and methods.” (A) Representative Southern image of selected spleen samples. A DHFR probe was used to quantitate total DNA (En DHFR) and donor-derived DHFR transgenic DNA (Tg04 DHFR). An APP probe was used to quantitate donor-derived normal cells (APP), and a Y probe was used to quantitate the percentage of host cells (Y). (B) Levels of engrafted donor-derived cells. Signals from the Southern image were quantified using a PhosphorImager. Ratios of APP/En DHFR and Tg DHFR/En DHFR were used to calculate the percentage of donor-derived normal and Tg04 cells, respectively, from a standard curve. Values are presented as mean ± SD of 5 to 9 samples.
Engraftment of donor-derived cells in bone marrow and spleen of primary BMT recipients preconditioned with 1 Gy TBI.
Surviving animals (depicted in Figure 3) were killed 120 days after transplantation. Genomic DNA samples isolated from bone marrow and spleen were subjected to quantitative Southern analysis as described in “Materials and methods.” (A) Representative Southern image of selected spleen samples. A DHFR probe was used to quantitate total DNA (En DHFR) and donor-derived DHFR transgenic DNA (Tg04 DHFR). An APP probe was used to quantitate donor-derived normal cells (APP), and a Y probe was used to quantitate the percentage of host cells (Y). (B) Levels of engrafted donor-derived cells. Signals from the Southern image were quantified using a PhosphorImager. Ratios of APP/En DHFR and Tg DHFR/En DHFR were used to calculate the percentage of donor-derived normal and Tg04 cells, respectively, from a standard curve. Values are presented as mean ± SD of 5 to 9 samples.
Secondary transplants were conducted as a more stringent test for the stem cell character of donor-derived material engrafting in primary BMT recipients. Marrow cells (5 × 106) from each primary recipient that survived to 120 days were transplanted into each of 3 lethally irradiated secondary recipients, allowing the recipients to recover for an additional 4 months. Bone marrow and spleen were harvested from these mice for quantitative Southern analysis as described above. Table 1shows that the average level of donor-derived cells in each of the 3 groups of secondary transplant recipients was below 10% in the bone marrow and slightly higher in the spleen. Donor cell engraftment levels were significantly reduced in secondary transplant recipients in comparison with primary recipients (compare Figure 4B with Table1) for all groups except animals transplanted with normal (APP transgenic) marrow. As observed in the primary recipients for this experiment, the mean engraftment levels for both marrow and spleen of secondary recipients were not significantly different (P > .05) for PBS-administered animals vs. MTX-administered animals.
Engraftment of donor-derived cells in secondary BMT recipients
| Primary donor . | % donor-derived cells . | |
|---|---|---|
| Bone marrow . | Spleen . | |
| Normal donor marrow PBS | 6.5 ± 4.8 | 11.9 ± 3.2* |
| 1 | 2.3 ± 1.1 | 8.9 ± 4 |
| 2 | 13.1 ± 6.9 | 16.3 ± 3.5 |
| 3 | — | 9.1 |
| 4 | 7 ± 5.7 | 14.2 ± 2.1 |
| 5 | 3.7 ± 0.9 | 11.2 ± 2.2 |
| Tg04 donor marrow PBS | 4.2 ± 3.6† | 5.4 ± 2.8† |
| 6 | 10.2 ± 2.7 | 7.1 ± 3.2 |
| 7 | 2.1 ± 1.1 | 4.3 ± 1.1 |
| 8 | 0.7 | 2.0 ± 0.9 |
| 9 | 1.3 ± 0.3 | 4.0 ± 2.3 |
| 10 | 4.6 | 4.9 |
| 11 | 6.2 | 9.9 |
| Tg04 donor marrow MTX | 2.9 ± 0.6‡ | 3.5 ± 1.11-153 |
| 12 | 3.5 | 3.4 |
| 13 | 2.5 | 2.0 |
| 14 | 2.7 ± 1 | 3.4 ± 0.2 |
| 15 | 2.3 ± 0.7 | 5.1 |
| 16 | 3.6 ± 1.5 | 3.7 ± 1.7 |
| Primary donor . | % donor-derived cells . | |
|---|---|---|
| Bone marrow . | Spleen . | |
| Normal donor marrow PBS | 6.5 ± 4.8 | 11.9 ± 3.2* |
| 1 | 2.3 ± 1.1 | 8.9 ± 4 |
| 2 | 13.1 ± 6.9 | 16.3 ± 3.5 |
| 3 | — | 9.1 |
| 4 | 7 ± 5.7 | 14.2 ± 2.1 |
| 5 | 3.7 ± 0.9 | 11.2 ± 2.2 |
| Tg04 donor marrow PBS | 4.2 ± 3.6† | 5.4 ± 2.8† |
| 6 | 10.2 ± 2.7 | 7.1 ± 3.2 |
| 7 | 2.1 ± 1.1 | 4.3 ± 1.1 |
| 8 | 0.7 | 2.0 ± 0.9 |
| 9 | 1.3 ± 0.3 | 4.0 ± 2.3 |
| 10 | 4.6 | 4.9 |
| 11 | 6.2 | 9.9 |
| Tg04 donor marrow MTX | 2.9 ± 0.6‡ | 3.5 ± 1.11-153 |
| 12 | 3.5 | 3.4 |
| 13 | 2.5 | 2.0 |
| 14 | 2.7 ± 1 | 3.4 ± 0.2 |
| 15 | 2.3 ± 0.7 | 5.1 |
| 16 | 3.6 ± 1.5 | 3.7 ± 1.7 |
Bone marrow samples (5 × 106 cells) from primary recipients (described in Figures 3 and 4) that survived 120 days after transplantation were each transplanted into 3 lethally irradiated secondary recipients. Genomic DNA was isolated from these mice 4 months after transplantation, and quantitative Southern analysis was carried out to determine the level of DHFR or APP donor cell engraftment as described in “Materials and methods.” Values are presented as mean ± SD of triplicate secondary recipients (when available) derived from individual primary recipients (listed), and as mean ± SD for all secondary recipients (at the top) for each group. Statistical analysis vs. primary recipients (Fig. 4B):
P < .05;
P < .0001;
P < .00001;
P < .01.
Reduced DHFR transgenic marrow cell doses confer resistance to MTX
The experiments described above demonstrate that 1 × 107 line 04 DHFR transgenic cells conferred MTX resistance to more than 70% of recipients preconditioned with 1 Gy TBI and engrafted with approximately 10% donor material. To determine whether lower levels of DHFR transgenic cell engraftment confer drug resistance after low-dose (1 Gy) TBI, animals were transplanted with 1 × 107, 5 × 106, or 1 × 106 line 04 DHFR transgenic marrow cells and subsequently administered MTX at 4 mg/kg daily for 60 days. A slightly reduced survival rate (60%) was observed in the group transplanted with 1 × 106 cells compared with animals transplanted with 5 × 106 cells (70%) or 1 × 107 cells (75%), although these differences were not statistically significant (Figure 5A). Animals transplanted with 1 × 106 cells also exhibited reduced hematocrit levels (below 30) 2 to 4 weeks after transplantation, whereas animals transplanted with 5 × 106 cells or 1 × 107 cells maintained hematocrit levels above 30 for the duration of the period of drug administration.
Methotrexate resistance of mice transplanted with varying amounts of line 04 DHFR transgenic marrow cells.
Normal male mice were given 1 Gy TBI the day before transplantation with 1 × 106, 5 × 106, or 1 × 107 marrow cells from female line 04 donors (as indicated). MTX was then administered at increasing doses (culminating at 4 mg/kg) daily for 60 days (n = 10 for each group). (A) Kaplan-Meier plot showing fraction of surviving mice. (B) Mean weekly hematocrit of surviving mice at each time point.
Methotrexate resistance of mice transplanted with varying amounts of line 04 DHFR transgenic marrow cells.
Normal male mice were given 1 Gy TBI the day before transplantation with 1 × 106, 5 × 106, or 1 × 107 marrow cells from female line 04 donors (as indicated). MTX was then administered at increasing doses (culminating at 4 mg/kg) daily for 60 days (n = 10 for each group). (A) Kaplan-Meier plot showing fraction of surviving mice. (B) Mean weekly hematocrit of surviving mice at each time point.
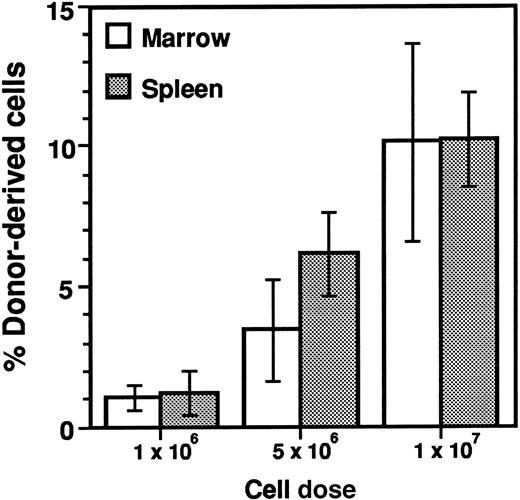
Engraftment of line 04 DHFR transgenic cells was determined 4 months after transplantation in the bone marrow and spleen by quantitative Southern analysis (Figure 6). Animals transplanted with 1 × 106 cells exhibited the lowest level of donor DHFR transgenic cell engraftment in bone marrow (1%) and spleen (1%). The level of donor-derived cells increased proportionately with the number of cells transplanted, resulting in approximately 5% and 10% engraftment in animals transplanted with 5 × 106 and 1 × 107 cells, respectively. Marrow samples from several of the primary transplant recipients were transplanted into lethally irradiated secondary transplant recipients to test for engraftment of hematopoietic stem cells in the primary recipients. DHFR transgenic donor cell engraftment levels were determined in bone marrow and spleen of secondary transplant recipients 4 months after transplantation (Table 2). Although the level of donor-derived cells in the marrow was increased in secondary recipients of animals transplanted with 106cells (P = .06) and decreased in secondary recipients of animals transplanted with 107 cells (P < .05) in comparison with primary recipients, these differences were not observed for the spleen. In general the engraftment levels observed in secondary transplant recipients paralleled the levels observed in the primary recipients, demonstrating that low-level engraftment of primitive HSC in primary recipients preconditioned with 1 Gy TBI was proportional to the DHFR transgenic marrow cell dose.
Dose-dependent engraftment of transgenic marrow cells in sublethally irradiated BMT recipients.
Survivors from the experiment described in Figure 5 were killed 120 days after transplantation. Genomic DNA was isolated from bone marrow and spleen, subjecting samples to quantitative Southern analysis as described in Figure 4. Values are presented as mean ± SD of 6 to 8 samples.
Dose-dependent engraftment of transgenic marrow cells in sublethally irradiated BMT recipients.
Survivors from the experiment described in Figure 5 were killed 120 days after transplantation. Genomic DNA was isolated from bone marrow and spleen, subjecting samples to quantitative Southern analysis as described in Figure 4. Values are presented as mean ± SD of 6 to 8 samples.
Engraftment of donor-derived cells in secondary BMT recipients
| Primary donor . | % donor-derived cells . | |
|---|---|---|
| Bone marrow . | Spleen . | |
| 1 × 106cells | 3.2 ± 1.8 | 0.43 ± 0.27 |
| 1 | 5.3 ± 2.3 | 0.33 ± 0.15 |
| 2 | 2.1 ± 0.4 | 0.74 ± 0.4 |
| 3 | 2.2 ± 0.9 | 0.22 ± 0.19 |
| 1 × 107cells | 5.0 ± 1.0 | 9.7 ± 2.2 |
| 4 | 4.0 ± 1.4 | 7.3 ± 3.1 |
| 5 | 4.9 ± 1.2 | 10.5 ± 0.4 |
| 6 | 6.0 ± 2.0 | 11.4 |
| Primary donor . | % donor-derived cells . | |
|---|---|---|
| Bone marrow . | Spleen . | |
| 1 × 106cells | 3.2 ± 1.8 | 0.43 ± 0.27 |
| 1 | 5.3 ± 2.3 | 0.33 ± 0.15 |
| 2 | 2.1 ± 0.4 | 0.74 ± 0.4 |
| 3 | 2.2 ± 0.9 | 0.22 ± 0.19 |
| 1 × 107cells | 5.0 ± 1.0 | 9.7 ± 2.2 |
| 4 | 4.0 ± 1.4 | 7.3 ± 3.1 |
| 5 | 4.9 ± 1.2 | 10.5 ± 0.4 |
| 6 | 6.0 ± 2.0 | 11.4 |
Bone marrow samples (5 × 106 cells) from primary recipients (described in Figures 5 and 6) that survived 120 days after transplantation were each transplanted into 3 lethally irradiated secondary recipients. Genomic DNA was isolated from these mice 4 months after transplantation, and quantitative Southern analysis was carried out to determine the level of DHFR or APP donor cell engraftment as described in “Materials and methods.” Values are presented as mean ± SD of triplicate secondary recipients (when available) derived from individual primary recipients (listed) and as mean ± SD for all secondary recipients (at the top) for each group.
We conclude that MTX resistance is observed in animals engrafted with an extremely low level (1%) of donor DHFR transgenic marrow, although a greater degree of sensitivity to MTX is observed than in animals engrafted at higher levels (5% and 10%).
Discussion
Syngeneic marrow transplant experiments were conducted to determine whether sublethal doses of total body irradiation would provide sufficient engraftment of DHFR transgenic marrow to confer drug-resistance in recipient animals. FVB/N mice administered as little as 1 Gy (approximately one eighth the lethal dose) of TBI and transplanted with 107 DHFR transgenic marrow cells were protected from MTX toxicity using a dosing schedule that was lethal for untransplanted animals or animals transplanted with nontransgenic marrow. Animals preconditioned with 1 Gy and subsequently transplanted with reduced doses of transgenic donor cells engrafted with as little as 1% donor cells; nonetheless, more than 60% of the recipients were resistant to MTX. We conclude that only a low level of drug-resistant DHFR transgenic cell engraftment is necessary to confer drug resistance in recipient animals.
Several gene therapy trials have been initiated to test the effectiveness of gene transfer into human hematopoietic cells as a means of treating genetic deficiencies. These include clinical trials for the treatment of adenosine deaminase deficiency,9,10chronic granulomatous disease,11 and Gaucher disease.12,13 In these trials, hematopoietic cells transduced with retroviral vector were infused into the patient without cytoablative preconditioning. Significant engraftment of transduced stem cells is unlikely under these conditions because the frequency of gene transfer into the total graft is likely to be low (1% or less), compounded by the absence of any cytoreductive preconditioning to provide “hematopoietic space” for the engraftment of donor cells. Schiffman et al16 reported low-level engraftment of transduced marrow cells in a mouse model of Gaucher disease, but this required multiple daily injections of large numbers of transduced cells. As an alternative to the lethal preconditioning often used in conjunction with bone marrow transplantation for hematopoietic malignancies, mild preconditioning has been tested in humans and in animals. Mardiney et al17 found that mice administered as little as 0.3 Gy TBI exhibited significant engraftment of congenic donor hematopoietic cells, whereas Tomita et al4 reported the absence of stem cell engraftment (as determined by serial transplantation) in mice preconditioned with 0.5 Gy irradiation.4 Huhn et al25 found that sublethal TBI (500 cGy) allowed effective engraftment of retrovirally marked HSC in rhesus monkeys. In the absence of any cytoreductive preconditioning, engraftment is favored by the repeated introduction of large numbers of donor cells.14,26 Nonmyeloablative preconditioning has also been investigated as a means of tolerizing allogeneic donor cells in animals27,28 and in humans.29 30 If used in conjunction with an ex vivo gene transfer procedure, such mild preconditioning of the recipient would thus increase markedly the likelihood of engrafting transduced donor material without subjecting the recipient to the risk of morbidity associated with more extensive preconditioning.
In the experiments described in this paper, we sought to determine conditions under which mild preconditioning could be administered while still allowing sufficient engraftment of DHFR transgenic marrow to confer MTX resistance in recipient animals. Our previously reported DHFR transgenic marrow transplant experiments were conducted under conditions (lethal TBI preconditioning, 107 transgenic donor cells) in which recipients became engrafted with a high proportion of donor material.21 22 In the current study, we observed a reduced level of donor cell engraftment that was proportional to the dose of TBI used for preconditioning (Figure 2) and the cell dose transplanted into partially ablated recipient animals (Figure 6). Using the mildest preconditioning dose tested (1 Gy) in combination with the fewest number of transplanted donor marrow cells (1 × 106), a 1% engraftment level was observed. The recipient animals were substantially resistant to MTX even at this low level of engraftment, demonstrating that it is not necessary for all, or even for a high proportion of hematopoietic cells, to express drug-resistant DHFR to confer drug resistance. However, at this level of engraftment (1%) there was a slight reduction in survival and in hematocrit levels compared with animals engrafted to a greater extent with donor DHFR transgenic marrow, perhaps indicating that this level of engraftment approaches the minimum necessary to render recipient animals drug resistant. These results thus demonstrate the application of mild preconditioning to establish low-level engraftment of donor marrow, which confers a distinct physiological characteristic on recipient animals (ie, MTX resistance).
The mechanism by which such a small proportion of drug-resistant cells protects recipient animals from MTX toxicity has yet to be determined. MTX administration is associated with myelotoxicity and gastrointestinal toxicity,31 both of which contribute to the demise of control animals not expressing drug-resistant DHFR activity (May et al,21 James et al,22 and this paper). Protection of hematopoietic cells was apparent from the maintenance of hematocrit during the course of drug administration (Figures 1, 3, and 5), and previously published results21 in animals more fully engrafted with DHFR transgenic marrow indicated substantial protection of gastrointestinal tissue as well. In recently conducted pharmacokinetic studies, we found that MTX does not accumulate to higher levels in the plasma or gastrointestinal tissues of normal animals or animals transplanted with normal marrow than in animals transplanted with DHFR transgenic marrow (Belur et al, manuscript in preparation). Systemic drug resistance, therefore, is most likely conferred through some cellular or molecular process mediated more directly by engrafted DHFR transgenic cells, such as growth factor production or regulation of immune/inflammatory reactions. We previously observed a substantial level of donor DHFR transgenic cell infiltration in recipient GI tissue (20% to 30% of total tissue)22 that could contribute to protection from MTX toxicity, not only in animals more fully engrafted with DHFR transgenic marrow21 22 but in animals exhibiting low-level engraftment as well.
MTX has been used extensively as an antitumor agent,32,33where its administration is limited by toxicity for normal tissues, most notably hematopoietic and gastrointestinal tissues.31Introduction and expression of variant, drug-resistant DHFR activity in normal, drug-sensitive tissues constitutes one potential way to counteract these toxic side effects. Numerous variant forms of mammalian DHFR have been generated and characterized that exhibit such drug-resistant character34 and, thus, might be used for this purpose. Retroviral transduction studies have demonstrated protection from MTX toxicity by introduction of the murine arg2235-37 or human ser3138 DHFR and protection from trimetrexate toxicity by the introduction of the human tyr22 DHFR.39 We have also found that transplantation with DHFR transgenic marrow expressing either murine arg2221 or tyr2222 DHFR rescues animals from lethal doses of methotrexate. Here we extend these results to show that only a small proportion of hematopoietic cells need express drug-resistant DHFR activity to render the recipient animal resistant to MTX. These results imply that a low level of ex vivo DHFR gene transfer (after retroviral transduction, for example) may be sufficient in the somatic modification of HSC to render recipient animals less sensitive to the toxic effects of MTX. Such drug resistance would require effective expression of the newly introduced DHFR gene in rapidly dividing hematopoietic cells that are acutely sensitive to MTX.
It was anticipated that the administration of MTX would provide a selective advantage for the engraftment of donor, drug-resistant, hematopoietic cells versus the regeneration of endogenous drug-sensitive host cells. However, we found no evidence for selectivity of DHFR transgenic cells under the conditions tested. On the contrary, in one experiment, MTX administration resulted in reduced levels of donor cell engraftment even though the transplanted donor cells were drug resistant. This inhibition of donor cell engraftment was observed in animals preconditioned at moderate doses of TBI. The lack of a selective advantage for engraftment of drug-resistant marrow cells is most likely explained by ineffective antifolate selection against the regeneration of normal host stem cells, previously reported for methotrexate40 and for trimetrexate.41However, the reduced DHFR transgenic cell engraftment observed in groups of animals given 2 or 4 Gy TBI and subsequently administered MTX suggests the existence of some MTX-sensitive host process that contributes to engraftment after transplantation but that is inhibited when MTX is administered, beginning immediately after transplantation and continuing during the process of engraftment. MTX has been used extensively for GVHD prophylaxis after allogeneic bone marrow transplantation,42,43 but this treatment has been associated with reduced rates of engraftment in human clinical trials.44 45 Our results suggest that this previously observed inhibitory effect of MTX on engraftment is partly the result of MTX toxicity for some host function that contributes to engraftment, in addition to toxicity for engrafting allogeneic cells. It may further be speculated that the expression of drug-resistant DHFR activity in donor stem cells would not be expected to overcome such a host-derived function until after the recipient has fully engrafted, assuming that this MTX-sensitive function is hematopoietic (ie, derived from the cellular products of hematopoietic stem cells).
Although a low level of DHFR transgenic cell engraftment may be sufficient for protection from MTX toxicity and application toward improved cancer chemotherapy, in other cases establishment of a higher proportion of transgene-expressing hematopoietic cells in the circulation would be therapeutically advantageous. For this purpose, the selective power of variant DHFR expression, demonstrated extensively in in vitro studies,35,46 could be harnessed to expand a small proportion of HSC expressing drug-resistant DHFR activity, thus increasing the proportion of cells containing the DHFR transgene plus any other therapeutic gene co-introduced along with the DHFR gene. Such selection of DHFR-expressing HSC has been confounded by the insensitivity of HSC toward antifolates (40,41; Warlick et al, manuscript in preparation; and this paper). However, the toxicity of antifolates for murine HSC was recently shown to be potentiated by the co-administration of nitrobenzylmercaptopurine riboside phosphate (NBMPR-P), a phosphorylated prodrug of the potent nucleoside transport inhibitor NBMPR.41 Allay et al18 also demonstrated the use of the antifolate trimetrexate in combination with NBMPR-P for in vivo selection of DHFR-expressing HSC. These results imply that the insensitivity of HSC for antifolates is caused in part by the ability of HSC to salvage nucleosides, but that inhibition of nucleoside transport restores differential antifolate sensitivity between normal versus DHFR-expressing HSC, as we have recently characterized for cultured mammalian cells in vitro.47 In this context, the results from the current study suggest an approach toward hematopoietic stem cell gene therapy in which the recipient is subjected to mild preconditioning and then transplanted with stem cells transduced using a vector designed for the expression of drug-resistant DHFR activity, with or without other genes to be co-introduced. By the administration of appropriate pharmacologic selective pressure, it may then be possible to increase the proportion of DHFR-virus–transduced cells present in the recipient, thus providing higher levels of transgene-expressing hematopoietic cells that may be necessary for the effective treatment of certain genetic deficiencies or other diseases.
Supported by research grant CA60803 from the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
R. Scott McIvor, Box 206 UMHC, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: mcivor@mail.med.umn.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal