Abstract
Idiopathic myelofibrosis (MF) is a myeloproliferative syndrome characterized by an increase in bone marrow collagen. Megakaryocytes (Mks), which store growth factors in their α granules, are known to be involved in the pathogenesis of MF. Previously, mice given bone marrow grafts infected with a retrovirus carrying murine thrombopoietin (TPO) complementary DNA developed a disease resembling human idiopathic MF. In this study, we used this murine model (TPO mice) to determine whether release of α granules is responsible for fibroblast activation and development of fibrosis. The intracellular trafficking of several α-granule proteins (von Willebrand factor, fibrinogen, and transforming growth factor β (TGFβ), which are stored in the granule matrix; and αIIbβ3 integrin and P-selectin (CD62p), which are located in the α-granule membrane) was studied with immune electron microscopy in bone marrow Mks from TPO mice. P-selectin immunolabeling increased consistently and was occasionally found lining the demarcation membrane system. Evidence of extensive emperipolesis was also found in TPO mouse Mks, involving almost exclusively neutrophil and eosinophil polymorphonuclear (PMN) cells with altered morphologic features. In parallel, the host Mks had myeloperoxidase-positive granules scattered in their cytoplasm, associated with marked ultrastructural cytoplasmic alterations and ruptured α-granule membranes. Similar observations were made in bone marrow biopsy specimens from 12 patients with idiopathic MF; indeed, there was an increased rate of emperipolesis involving mostly PMN cells, abnormal P-selectin expression, and mutual subcellular PMN and Mk alterations. This study indicates that in idiopathic MF, abnormal P-selectin distribution in Mks induces selective sequestration of PMN cells. This results in a release of α-granular proteins and growth factors, which in turn induces fibroblast activation and fibrosis deposition.
Introduction
Idiopathic myelofibrosis (MF), also known as primary MF or agnogenic myeloid metaplasia, is a clonal myeloproliferative disorder characterized by bone marrow fibrosis, extramedullary hematopoiesis, splenomegaly, and an elevated number of peripheral blood hematopoietic precursors.1-3 MF can also be associated with other myeloproliferative syndromes (disorders of megakaryocytes [Mks]) and with cancer.4,5 In both instances, the bone marrow contains excessive deposition of extracellular-matrix proteins and collagen. It was shown that an abnormal release of growth factors such as transforming growth factor β (TGF-β) and platelet-derived growth factor (PDGF) can be responsible for the development of medullary fibrosis.6 7
Because Mks and platelets are considered to be the major cellular sources of PDGF and TGF-β8 and abnormal populations of Mks have been observed in bone marrow from patients with MF,4 it is likely that these cells are involved in the pathogenesis of MF.9 However, the mechanism by which a pathologic release of growth factors from Mks may occur in MF is poorly understood.10
Remarkably, mice given bone marrow grafts of cells infected with a retrovirus carrying thrombopoietin (TPO) complementary DNA (cDNA) developed a lethal myeloproliferative disorder that resembles human idiopathic MF.11,12 TPO is the main physiologic stimulator of megakaryocytopoiesis and thrombocytopoiesis.13 14 “TPO mice” have prominent medullary fibrosis and therefore constitute a unique model for studying the possible role of Mks in the development of MF. In this study, we documented the ultrastructural features of Mks in bone marrow from TPO mice and correlated our findings with pathophysiologic features of human idiopathic MF. In the mouse model, there was a markedly abnormal subcellular P-selectin distribution that appeared to correlate with excessive and pathologic emperipolesis of polymorphonuclear (PMN) leukocytes in the Mks. The same types of abnormalities were observed in bone marrow from patients with idiopathic MF. Therefore, this study provides insight into the possible pathophysiologic explanation for the genesis of MF.
Materials and methods
Mice
A fragment encoding murine TPO cDNA was inserted into a virus based on the MPZen-2 vector containing the myeloproliferative sarcoma virus long terminal repeat. A fragment encoding a neomycin phosphotransferase cDNA (Neo) was inserted into the MPZen-2 plasmid. After packaging in the GP+E-86 cell line, the G418-resistant clones were isolated and expanded. The most efficient TPO producer clones were tested for virus production. Four days after treatment with 5-fluorouracil, bone marrow cells from mouse femurs were cocultivated with MPZen TPO and MPZen Neo virus–producing GP+E-86 cells. Nonadherent cells were removed and injected into lethally irradiated female C57/Bl6J mice. Mice given grafts of bone marrow cells with a separate infection with MPZen TPO were studied 3 weeks, 7 weeks, and 2 months after transplantation, in parallel with mice given bone marrow cells infected with MPZen Neo virus (controls).11
Cells
Peripheral blood samples were collected from the retro-orbital plexus of mice. Blood was drawn into acid citrate–dextrose buffer (6.8 mmol/L citric acid, 11.2 mmol/L trisodium citrate, and 24 mmol/Lglucose; pH 6.5) and centrifuged (100g for 10 minutes). Isolated platelets overlaying the buffy coat were removed and fixed. Bone marrow cells were removed by flushing the femur with 1% glutaraldehyde (Taab Laboratories Equipment Ltd, United Kingdom) in phosphate buffer (0.1 mol/L; pH 7.4).11
Bone marrow biopsy specimens (n = 12) obtained from patients with idiopathic MF for diagnostic purposes at the Department of Hematology, Hôpital Henri Mondor, Créteil, France, were studied after informed consent was obtained. Biopsy specimens obtained in a search for malignancy that yielded normal results were used as controls (n = 9). A 1-μL fragment from 3 biopsy specimens was routinely fixed for examination with electron microscopy (EM).
Electron microscopy
Platelets and bone marrow cells were fixed for 1 hour in 3% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4), washed, and embedded in Epon.15 Alternatively, cells were incubated in Graham and Karnovsky medium containing 3, 3′ diaminobenzidine (Sigma Chemical Co, St Louis, MO) for cytochemical detection of myeloperoxidase.16 17
Immuno–electron microscopy
Platelets and bone marrow cells were fixed in 1% glutaraldehyde in phosphate buffer (0.1 mol/L; pH 7.4) for 1 hour, washed, and embedded in glycolmethacrylate. Postembedding immunolabeling was performed on thin sections and was followed by detection with goat antirabbit IgG conjugated with 10-nm gold particles (British BioCell International, Cardiff, United Kingdom).18
Rabbit antibodies for von Willebrand factor (vWF) and fibrinogen (Dakopatts, Denmark) were used at a concentration of 100 μg/mL. Antimouse TGF-β polyclonal rabbit IgG (Santa Cruz, CA) was used at a concentration of 50 μg/mL. Polyclonal antihuman rabbit antibodies for αIIbβ318 and P-selectin, were kindly provided by Dr Michael Berndt (Vascular Biology Laboratory, Baker Medical Research Institute, Prahran, Victoria, Australia) and were used at a concentration of 10 μg/mL. Thin sections were examined with an electron microscope (CM 10; Philips, Eindoven, The Netherlands) after staining with uranyl acetate and lead citrate.15
Results
Murine model
Semithin sections were observed with use of light microscopy to conduct a comparative study of the whole bone marrow. In bone marrow from normal mice, Mks had a mean diameter of 70 μm and an evenly circular shape. They were scarce, occurring at an average of 2 Mks/mm2 of the section (Figure1A). In bone marrow from TPO mice, Mks had several striking features: a general increase in number (up to 10-fold the control amount), formation of clusters composed of 3 to 10 cells, an increase in size (2- or 3-fold the normal size), and increased lobularity of the nucleus (Figure 1A,B).
Microscopic view of control and TPO-mouse bone marrow.
(Ai) Sample from a normal mouse. This is a representative light-microscopic field with respect to the frequency and appearance of the normal megakaryocyte (Mk) population. Mks are easily distinguished from the other bone marrow cells by their large size and lobulated nuclei (arrowheads). (Aii) Light-microscopic view of sample from a thrombopoietin (TPO) mouse obtained 7 weeks after transplantation shows that Mks are more numerous and larger and have more lobulated nuclei than in those in normal samples. Mks are often arranged in clusters (arrowheads). (B) Average electron-microscopic appearance of an Mk from a TPO mouse. The cell is large, with a large nucleus of increased lobularity (N). α-Granules and demarcation membranes are scarce. Other characteristics of cytoplasmic immaturity are present, eg, conspicuous rough endoplasmic reticulum (er) and numerous ribosomes (r) (original magnification ×3300). (C) Electron-microscopic view of a polymorphonuclear (PMN) cell in the demarcation membrane system (dm) of a TPO mouse Mk. In the left corner, a PMN cell (PN1) is penetrating the cytoplasm of the Mk. Another PMN cell (PN2) is totally surrounded by demarcation membranes (dm) and shows signs of activation (pseudopods on the plasma membrane and cytoplasmic vacuole [arrowhead]). This figure illustrates the selectivity of the cell types retained in Mks. The cytoplasm organelles of the host Mk show alterations and vacuolation (v) (original magnification ×3300). (D) Electron-microscopic view of 3 PMN cells (1, 2, and 3) in the cytoplasm of a TPO mouse Mk that have important signs of alteration. An empty membrane seems to be all that is left of a fourth PMN cell (4). An intact PMN cell is on the left (5). The Mk boundary is indicated by a dotted line. The cytoplasm of the host Mk shows alterations, with the organelle limits poorly recognizable (original magnification ×3300).
Microscopic view of control and TPO-mouse bone marrow.
(Ai) Sample from a normal mouse. This is a representative light-microscopic field with respect to the frequency and appearance of the normal megakaryocyte (Mk) population. Mks are easily distinguished from the other bone marrow cells by their large size and lobulated nuclei (arrowheads). (Aii) Light-microscopic view of sample from a thrombopoietin (TPO) mouse obtained 7 weeks after transplantation shows that Mks are more numerous and larger and have more lobulated nuclei than in those in normal samples. Mks are often arranged in clusters (arrowheads). (B) Average electron-microscopic appearance of an Mk from a TPO mouse. The cell is large, with a large nucleus of increased lobularity (N). α-Granules and demarcation membranes are scarce. Other characteristics of cytoplasmic immaturity are present, eg, conspicuous rough endoplasmic reticulum (er) and numerous ribosomes (r) (original magnification ×3300). (C) Electron-microscopic view of a polymorphonuclear (PMN) cell in the demarcation membrane system (dm) of a TPO mouse Mk. In the left corner, a PMN cell (PN1) is penetrating the cytoplasm of the Mk. Another PMN cell (PN2) is totally surrounded by demarcation membranes (dm) and shows signs of activation (pseudopods on the plasma membrane and cytoplasmic vacuole [arrowhead]). This figure illustrates the selectivity of the cell types retained in Mks. The cytoplasm organelles of the host Mk show alterations and vacuolation (v) (original magnification ×3300). (D) Electron-microscopic view of 3 PMN cells (1, 2, and 3) in the cytoplasm of a TPO mouse Mk that have important signs of alteration. An empty membrane seems to be all that is left of a fourth PMN cell (4). An intact PMN cell is on the left (5). The Mk boundary is indicated by a dotted line. The cytoplasm of the host Mk shows alterations, with the organelle limits poorly recognizable (original magnification ×3300).
A significant rise in the frequency of emperipolesis, ie, entry of hematopoietic cells into the Mk cytoplasm, was also observed (Figure1C). Emperipolesis, the passage of a cell into the cytoplasm of another cell,19-21 has been shown to be associated with myeloproliferative disorders22 23 and implicate bone marrow cells. In the TPO mice model, there was a correlation between the progression of the syndrome and the degree of emperipolesis, eg, between the number of Mks and the percentage of Mks showing emperipolesis (Table 1). Moreover, the type of cells undergoing emperipolesis in Mks is usually reflective of the cellular composition of the marrow. In TPO mice, the cells undergoing emperipolesis were predominantly PMN neutrophils (86%) and eosinophils (13%).
Emperipolesis in and alteration of megakaryocytes (Mks) in bone marrow from control mice and from thrombopoietin (TPO) mice after transplantation
| Mouse group . | Mks showing emperipolesis (%) . | Alteration of cells . |
|---|---|---|
| Controls | 0 | No |
| TPO mice (3 weeks) | 25-50 | No |
| TPO mice (7 weeks) | >60 | Yes/No |
| TPO mice (2 months) | 50 | Yes |
| Mouse group . | Mks showing emperipolesis (%) . | Alteration of cells . |
|---|---|---|
| Controls | 0 | No |
| TPO mice (3 weeks) | 25-50 | No |
| TPO mice (7 weeks) | >60 | Yes/No |
| TPO mice (2 months) | 50 | Yes |
Ultrastructural studies of samples obtained from TPO mice 2 months after grafting showed marked alterations in both the host Mks and the PMN cells in the Mks (Figure 1D). First, the PMN cells appeared to be damaged, with apoptotic-like nuclei and disrupted plasma membranes. Myeloperoxidase-positive lytic granules from the PMN cells appeared free, often scattered in the Mk cytoplasm. The host Mks also had numerous cytoplasmic alterations, vacuoles, dystrophic demarcation membrane systems (DMS), and ruptured α-granules (Figure2A). Contact fibrosis was found in association with disrupted Mks (Figure 2B).
Views of altered Mks and results with labeling for P-selectin.
(A) Myeloperoxidase-positive PMN granules (arrowheads) are free in the cytoplasm of an altered Mk (original magnification ×3135). (B) Myelofibrosis (f) develops at contact with an altered Mk. The Mk has a dysmorphic and heterogenous demarcation membrane system (dm). Some well-preserved α granules are scattered in the cytoplasm (arrows), as are swollen and disrupted α granules (arrowheads) (original magnification ×11 700). (C) Results of immunogold labeling for P-selectin done on frozen thin section from a TPO mouse Mk. P-selectin (arrowheads) is detected in the vicinity of a PMN cell (PN) entering the Mk demarcation membrane system (dm) by means of emperipolesis. Labeling appears in the α-granular (A) membrane, in cytoplasmic vacuolar structures (V), and along some demarcation membranes (dm) (original magnification ×38 750). The inset shows a lower-magnification view of the PMN cell (PN) in the Mk (original magnification ×12 400).
Views of altered Mks and results with labeling for P-selectin.
(A) Myeloperoxidase-positive PMN granules (arrowheads) are free in the cytoplasm of an altered Mk (original magnification ×3135). (B) Myelofibrosis (f) develops at contact with an altered Mk. The Mk has a dysmorphic and heterogenous demarcation membrane system (dm). Some well-preserved α granules are scattered in the cytoplasm (arrows), as are swollen and disrupted α granules (arrowheads) (original magnification ×11 700). (C) Results of immunogold labeling for P-selectin done on frozen thin section from a TPO mouse Mk. P-selectin (arrowheads) is detected in the vicinity of a PMN cell (PN) entering the Mk demarcation membrane system (dm) by means of emperipolesis. Labeling appears in the α-granular (A) membrane, in cytoplasmic vacuolar structures (V), and along some demarcation membranes (dm) (original magnification ×38 750). The inset shows a lower-magnification view of the PMN cell (PN) in the Mk (original magnification ×12 400).
P-selectin is the specific platelet receptor for leukocytes and a component of the α-granule membrane24,25 and is also associated with the dense granule membrane.26 In TPO mice, the overall degree of labeling for P-selectin was increased and localized in the membranes of numerous small intracytoplasmic vacuolar structures in addition to the α-granules. P-selectin also appeared to line the DMS near engulfed PMN cells (Figure 2C). Labeling for other α-granular proteins, including vWF, fibrinogen, αIIbβ3, and TGF-β, showed virtually no differences between control and TPO mice in the pattern and density of labeling (not shown).
Patients
Bone marrow biopsy specimens from 12 patients with idiopathic MF and 9 control subjects were examined with light microscopy for the presence of emperipolesis. Emperipolesis was significantly greater in Mks in specimens from the patients (mean values, 13.7% in patients and 2.8% in controls; Table 2). Moreover, EM examination of bone marrow from patients (n = 3) showed abnormalities that were also found in marrow from the TPO mice (Figures3A and 3B): an increase in emperipolesis, morphologic alterations in cells that had entered the Mks, myeloperoxidase-positive lytic granules from PMN cells free in the cytoplasm of Mks, cytoplasmic damage in Mks, apoptotic-like features, and contact fibrosis. Finally, P-selectin labeling showed increased expression and abnormal localization of this antigen, similar to the pattern observed in samples from TPO mice (Figure 3C).
Findings in bone marrow biopsy specimens from patients with and without idiopathic myelofibrosis (IMF)
| Patient . | Diagnosis . | Mks showing emperipolesis (%) . | Reticulin density (myelofibrosis) . | Richness . |
|---|---|---|---|---|
| 1 | No IMF | 0.3 | Normal | + |
| 2 | No IMF | 1.6 | Normal | + |
| 3 | No IMF | 1.7 | Normal | + |
| 4 | No IMF | 3.3 | Normal | + |
| 5 | No IMF | 3.9 | Normal | ++ |
| 6 | No IMF | 4.1 | Normal | + |
| 7 | No IMF | 4.2 | Normal | + |
| 8 | No IMF | 4.9 | Normal | + |
| 9 | No IMF | 2.2 | Normal | + |
| 10 | IMF | 11.0 | + | ++ |
| 11 | IMF | 11.4 | + | + |
| 12 | IMF | 10.7 | ++ | ++ |
| 13 | IMF | 10.7 | ++ | + |
| 14 | IMF | 12.4 | +++ | + |
| 15 | IMF | 12.7 | +++ | + |
| 16 | IMF | 13.0 | +++ | + |
| 17 | IMF | 13.7 | +++ | + |
| 18 | IMF | 16.5 | +++ | ++ |
| 19 | IMF | 17.1 | +++ | ++ |
| 20 | IMF | 17.2 | +++ | + |
| 21 | IMF | 18.0 | +++ | + |
| Patient . | Diagnosis . | Mks showing emperipolesis (%) . | Reticulin density (myelofibrosis) . | Richness . |
|---|---|---|---|---|
| 1 | No IMF | 0.3 | Normal | + |
| 2 | No IMF | 1.6 | Normal | + |
| 3 | No IMF | 1.7 | Normal | + |
| 4 | No IMF | 3.3 | Normal | + |
| 5 | No IMF | 3.9 | Normal | ++ |
| 6 | No IMF | 4.1 | Normal | + |
| 7 | No IMF | 4.2 | Normal | + |
| 8 | No IMF | 4.9 | Normal | + |
| 9 | No IMF | 2.2 | Normal | + |
| 10 | IMF | 11.0 | + | ++ |
| 11 | IMF | 11.4 | + | + |
| 12 | IMF | 10.7 | ++ | ++ |
| 13 | IMF | 10.7 | ++ | + |
| 14 | IMF | 12.4 | +++ | + |
| 15 | IMF | 12.7 | +++ | + |
| 16 | IMF | 13.0 | +++ | + |
| 17 | IMF | 13.7 | +++ | + |
| 18 | IMF | 16.5 | +++ | ++ |
| 19 | IMF | 17.1 | +++ | ++ |
| 20 | IMF | 17.2 | +++ | + |
| 21 | IMF | 18.0 | +++ | + |
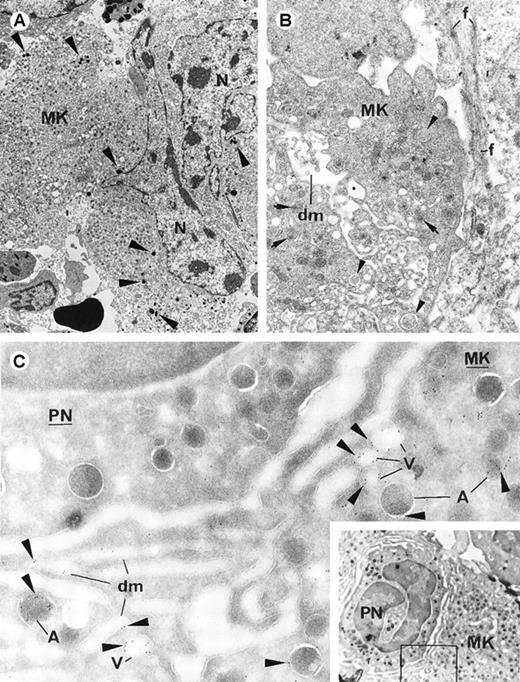
Electron-microscopic views of bone marrow from patients with idiopathic myelofibrosis.
(A) Several cells have entered the Mk. The host Mk shows ultrastructural alterations (vacuoles [V] and poorly delimited organelles [arrows]). Myeloperoxidase-positive PMN (PN) granules (arrowheads) are free in the Mk intracellular space (original magnification ×2760). (B) A damaged PMN cell is engulfed in the paranuclear area (N) of a Mk. The Mk cytoplasm has numerous vacuoles (V) and poorly delimited organelles, and myeloperoxidase-positive PMN granules are scattered in it. The engulfed PMN cell (surrounded by a dotted line; nucleus indicated by n) has plasma membrane alterations and apoptotic-like chromatin texture, suggesting its destruction when sequestrated in the host. Contact fibrosis (f) is present (original magnification ×3960). (C) Results of immunogold labeling for P-selectin done on frozen sections of human bone marrow. Labeling (arrowheads) is present in the Mk subcellular organelles in a distribution similar to that in samples from TPO mice: in the α-granule membrane (A), numerous small vacuolar structures (V), and occasionally along the demarcation membrane system (dm) (original magnification ×39 100).
Electron-microscopic views of bone marrow from patients with idiopathic myelofibrosis.
(A) Several cells have entered the Mk. The host Mk shows ultrastructural alterations (vacuoles [V] and poorly delimited organelles [arrows]). Myeloperoxidase-positive PMN (PN) granules (arrowheads) are free in the Mk intracellular space (original magnification ×2760). (B) A damaged PMN cell is engulfed in the paranuclear area (N) of a Mk. The Mk cytoplasm has numerous vacuoles (V) and poorly delimited organelles, and myeloperoxidase-positive PMN granules are scattered in it. The engulfed PMN cell (surrounded by a dotted line; nucleus indicated by n) has plasma membrane alterations and apoptotic-like chromatin texture, suggesting its destruction when sequestrated in the host. Contact fibrosis (f) is present (original magnification ×3960). (C) Results of immunogold labeling for P-selectin done on frozen sections of human bone marrow. Labeling (arrowheads) is present in the Mk subcellular organelles in a distribution similar to that in samples from TPO mice: in the α-granule membrane (A), numerous small vacuolar structures (V), and occasionally along the demarcation membrane system (dm) (original magnification ×39 100).
Discussion
Mks have been clearly implicated in the development of MF.1 A pathologic release of growth factors stored in Mk granules has been proposed to result in activation and proliferation of fibroblasts, leading to fibrosis.7 However, the subcellular events that cause the inappropriate release of growth factors from Mks into the extracellular environment are still unknown.27 28
In this study, we investigated the ultrastructure of Mks and the intracellular trafficking of their α-granule proteins during MF development by using mice with overexpression of TPO11 29and a disease resembling human idiopathic MF. Immune EM of proteins stored in α granules (vWF, fibrinogen, αIIbβ3, and TGF-β) showed no apparent differences between normal and TPO mice in labeling intensity and subcellular localization. These results, especially as they pertain to TGF-β, suggest that the disease did not originate from abnormal protein synthesis and storage but may have instead been caused by an inappropriate release of the stored substances. However, because we collected no data on PDGF levels in this study, we cannot rule out the possibility that secretion of PDGF is increased in MF.
We did find a significant difference in expression of P-selectin in TPO mice and controls; this was characterized by an atypical intracellular distribution of the antigen and increased cytoplasmic immunogold labeling. In the TPO mice, in addition to the normal α-granular membrane labeling observed in control mice, P-selectin appeared to be located in numerous intracytoplasmic vacuoles and in DMS membranes.
In samples from TPO mice, we found a marked increase in the number of cells undergoing emperipolesis into Mks compared with results in samples from controls. Emperipolesis, a rare but interesting phenomenon, is defined as the random passage of different types of bone marrow cells through the Mk cytoplasm without any important effects on either the host or invading cells. In our study, the intensity of emperipolesis also appeared to be related to the severity of the disease, findings that were supported by observation of a correlation between the rate of fibrosis and the intensity of emperipolesis in samples from patients with idiopathic MF. The emperipolesis in our murine model was different in numerous ways from normal emperipolesis as described in the literature. First, the emperipolesis in the TPO mice appeared not to be a random phenomenon but selectively involved the uptake of both neutrophils and eosinophils. Second, Mk cytoplasm showed important signs of alteration. Third, the engulfed PMN cells had morphologic damage in association with liberation of their peroxidase-positive granules into the Mk cytoplasm. The apparent selectivity of the engulfed cells and the alterations in both host and invading cells indicate clearly that the phenomenon observed in the TPO murine model is pathological.
Neutrophilic and eosinophilic PMN cells are known to express the P-selectin ligand 1 (PSGL-1).30,31 Expression of endothelial P-selectin results in binding of PSGL-1 and is the first step leading to the rolling of neutrophils32,33 on the vascular wall. We hypothesize that when PMN cells penetrate the DMS of Mks in TPO mice, the binding of PSGL-1 to the abnormally distributed P-selectin may result in the eventual trapping of PMN cells in the DMS. PMN cells that are abnormally retained in the Mk DMS become progressively activated, undergoing cell death and releasing their lytic granules34 into the cytoplasm of the Mk. This leads to the progressive destruction of the host Mk, with degradation and lysis of α-granules and subsequent release of α-granular growth factors. This in turn results in marked fibroblast activation and infiltration leading eventually to the development of MF.
The destruction of Mks appeared to be limited (< 20% of Mks had intracellular PMN cells) and the numbers of remaining, intact Mks and the platelet production were still considerably increased. Thus, Mk and platelet levels remained extremely elevated in the animal model. It can be hypothesized that this rise induces an elevated growth-factor level that is responsible for MF; however, an increase in platelets and Mks also occurs in other myeloproliferative syndromes, such as polycythemia vera and essential thrombocythemia, and is not necessarily associated with MF.
Finally and most important, the findings in the TPO mouse model were similar to observations in samples from patients with idiopathic MF. Mks in bone marrow from those patients also showed an increase in emperipolesis. The presence of cells in the cytoplasm of Mks was previously observed in myeloproliferative disorders,22 23but no correlation with the disease or pathologic implications were described. In this study, however, the rate of emperipolesis in patients with MF correlated with the amount of reticulin deposition. Moreover, consequent mutual alterations in Mks and PMN cells were observed in parallel with abnormal P-selectin expression and subcellular distribution. In conclusion, our study indicates that a specific and pathogenic interaction between PMN leukocytes and Mks plays a role in the development of MF.
Acknowledgments
We thank Dr Michael Berndt for generously providing antibodies to P-selectin and αIIbβ3, Pr Michel Tulliez for providing human biopsy specimens and useful comments, Dr Paul Harrison for carefully reviewing the English of the manuscript, Dr Paul-Henri Roméo for constant support, and Mr Jean-Marc Massé for skillful photographic assistance.
Supported partly by La Ligue Nationale contre le Cancer and by La Fondation pour la Recherche Médicale. A.S. is a fellowship recipient from La Ligue Nationale contre le Cancer (Comité du Val de Marne).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elisabeth Cramer, INSERM U.474, Maternite-Port-Royal, 5eme étage, 123 Boulevard de Port-Royal, 75014 Paris, France; e-mail: emcramer@cochin.inserm.fr.

![Fig. 1. Microscopic view of control and TPO-mouse bone marrow. / (Ai) Sample from a normal mouse. This is a representative light-microscopic field with respect to the frequency and appearance of the normal megakaryocyte (Mk) population. Mks are easily distinguished from the other bone marrow cells by their large size and lobulated nuclei (arrowheads). (Aii) Light-microscopic view of sample from a thrombopoietin (TPO) mouse obtained 7 weeks after transplantation shows that Mks are more numerous and larger and have more lobulated nuclei than in those in normal samples. Mks are often arranged in clusters (arrowheads). (B) Average electron-microscopic appearance of an Mk from a TPO mouse. The cell is large, with a large nucleus of increased lobularity (N). α-Granules and demarcation membranes are scarce. Other characteristics of cytoplasmic immaturity are present, eg, conspicuous rough endoplasmic reticulum (er) and numerous ribosomes (r) (original magnification ×3300). (C) Electron-microscopic view of a polymorphonuclear (PMN) cell in the demarcation membrane system (dm) of a TPO mouse Mk. In the left corner, a PMN cell (PN1) is penetrating the cytoplasm of the Mk. Another PMN cell (PN2) is totally surrounded by demarcation membranes (dm) and shows signs of activation (pseudopods on the plasma membrane and cytoplasmic vacuole [arrowhead]). This figure illustrates the selectivity of the cell types retained in Mks. The cytoplasm organelles of the host Mk show alterations and vacuolation (v) (original magnification ×3300). (D) Electron-microscopic view of 3 PMN cells (1, 2, and 3) in the cytoplasm of a TPO mouse Mk that have important signs of alteration. An empty membrane seems to be all that is left of a fourth PMN cell (4). An intact PMN cell is on the left (5). The Mk boundary is indicated by a dotted line. The cytoplasm of the host Mk shows alterations, with the organelle limits poorly recognizable (original magnification ×3300).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/4/10.1182_blood.v96.4.1342/4/m_h81600131001.jpeg?Expires=1769086545&Signature=tTEd8O0XA59IGA0YkKetAq05cColrpRYkueYUneACPCoWx69syFpc~Z27KEMOGu6AeGEcEL7TTqoKHTPtgw2GJu0miiTIOgShCg8Y985XIlRqExnHtOXYidsK44Yuqw26oQwrLhhQ7beeqTP1K24onOUiiczUykcalexgPj37fqgSxVGMZocJYu8jgIieZrlW7meuFgECtW1GvZRSD4jdy-AaarZRX1ZGbGGUNdnMK9dvMMmCsF5xkyEWVsRSf1T3jy8Fh9wjPdqd1-zd9Xea0UD3Q470N4kbzrotXDLe9S6tL2G0hr3txmo5jKyMW6YvUzrW7wBOkU880BYxTIfOA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Electron-microscopic views of bone marrow from patients with idiopathic myelofibrosis. / (A) Several cells have entered the Mk. The host Mk shows ultrastructural alterations (vacuoles [V] and poorly delimited organelles [arrows]). Myeloperoxidase-positive PMN (PN) granules (arrowheads) are free in the Mk intracellular space (original magnification ×2760). (B) A damaged PMN cell is engulfed in the paranuclear area (N) of a Mk. The Mk cytoplasm has numerous vacuoles (V) and poorly delimited organelles, and myeloperoxidase-positive PMN granules are scattered in it. The engulfed PMN cell (surrounded by a dotted line; nucleus indicated by n) has plasma membrane alterations and apoptotic-like chromatin texture, suggesting its destruction when sequestrated in the host. Contact fibrosis (f) is present (original magnification ×3960). (C) Results of immunogold labeling for P-selectin done on frozen sections of human bone marrow. Labeling (arrowheads) is present in the Mk subcellular organelles in a distribution similar to that in samples from TPO mice: in the α-granule membrane (A), numerous small vacuolar structures (V), and occasionally along the demarcation membrane system (dm) (original magnification ×39 100).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/4/10.1182_blood.v96.4.1342/4/m_h81600131003.jpeg?Expires=1769086545&Signature=B8ZuLgPLcTQWyuDX2xQa0fF2g8XIZ8mERKr2r6coFFnfNwMmmcKqgo6WKXf6rneRMCnnzWdnFM0LZiBwGQWwDVQx~thyOzHNQ2deORf1A~3s925t-IhBHbcXVAW~CSyHUhL3U1TE8qyumtXZpXphdX~giQGPI5SewlEdIAPiXjcamR48F8PkzPMuNVq072~6dTBiMqzeaaRdH0uWSsaGBAuRIki5uHWBVNzDBnTBkh7M8weLXTA6K0jVL617s-KATDQHbBwqy2Xd3MmDd1V6o4nIwJ5cSriSBeZEAjVYhERpNzbgEY~u9AzQx5l0rH8XYQmFsUhF7sTnzVybHobz5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal