Abstract
The optimal approach for stem cell transplantation in children with immunodeficiency has yet to be determined. Conditioning therapy is necessary for reliable engraftment and full immune reconstitution; however, the beneficial effect of cytoreductive conditioning is counterbalanced by increased short- and long-term treatment-related toxicity. Whether bone marrow transplantation with a nonmyeloablative preparative regimen was sufficient for the establishment of donor immune reconstitution, with the resultant correction of disease phenotype, was investigated. Eight patients with severe immunodeficiency states underwent T-cell replete bone marrow transplantation from a human leukocyte antigen-matched unrelated (n = 6) or sibling (n = 2) donor with nonmyeloablative conditioning using a fludarabine–melphalan–anti-lymphocyte globulin-based regimen. All patients had severe organ dysfunction that precluded transplantation with conventional conditioning. All patients were engrafted with predominantly donor hematopoiesis, and the duration of neutropenia was brief. Significant acute graft-versus-host disease (GVHD) did not develop, but one patient had limited chronic GVHD. One patient died of disease recurrence, and 3 have stable, mixed chimerism. At a median follow-up of 1 year, all patients have had good recovery of CD3+ T-cell numbers, and 6 of 7 evaluable patients have normal phytohemagglutinin stimulation indices. The rate of immune reconstitution is comparable with that of historical controls undergoing standard myeloablative protocols. Two patients with CD40 ligand deficiency now show significant expression, and a patient with adenosine deaminase deficiency has improved deoxy adenosine triphosphate metabolites. In summary, it has been demonstrated that nonmyeloablative stem cell transplantation permits rapid engraftment from both sibling and unrelated donors with minimal toxicity even in the presence of severe organ dysfunction. If long-term immune reconstitution of patients treated with this protocol is demonstrated, it is believed this approach might offer significant advantages compared with standard protocols by combining adequate immune reconstitution with reduced short- and long-term toxicity.
Introduction
Allogeneic stem cell transplantation (SCT) is curative for congenital immunodeficiency states. However, the optimal strategy for SCT in children with severe immunodeficiency who lack a human leukocyte antigen (HLA)-identical sibling donor remains controversial. One approach is SCT from an HLA-matched unrelated donor. Filipovich et al1 have reported excellent engraftment and long-term immune reconstitution after T-cell–replete unrelated donor bone marrow transplantation (BMT) with myeloablative conditioning therapy. The ability to use a T-cell–replete graft from an unrelated donor makes this an attractive approach from the point of view of immune reconstitution, though this must be counterbalanced by the increased risk for graft-versus-host disease (GVHD) and the potential for delay in transplantation because of the donor search. An alternative approach is to use bone marrow from a haploidentical donor who has been rigorously T-cell–depleted to prevent GVHD. The advantage of this approach is that the donor is readily available. However, in the absence of conditioning therapy, a significant incidence of graft failure has been reported in patients with T-B-NK+ SCID and adenosine deaminase (ADA) deficiency.2 Further, the time course of T-cell reconstitution may be prolonged in some patients, which may necessitate a subsequent bone marrow “boost” to improve immune recovery,3 and many patients have poor B-cell reconstitution.4 These problems have led many groups to use conditioning before HLA–haploidentical SCT,5 and there is preliminary evidence to suggest that this may improve B- though not T-cell recovery, particularly in patients with B-SCID.6 Overall, with the available SCT protocols for children with immunodeficiencies (particularly those with residual immune function) who lack an HLA-identical donor, intensive pretransplant conditioning appears to be a prerequisite for reliable engraftment and complete immune reconstitution. The beneficial effect of cytoreductive conditioning, however, is counterbalanced by increased short- and long-term toxicity. In particular, infection and end organ decompensation contribute to the high treatment-related mortality rates seen with conventionally conditioned SCT in older children who have acquired organ dysfunction. Similarly, late adverse effects, such as growth retardation, infertility, and secondary malignancy, are particularly difficult to justify in children with nonmalignant disorders.
Recently, some groups7-12 have reported SCT for hematologic malignancies, generally from HLA-identical sibling donors who were on highly immunosuppressive but nonmyeloablative regimens. Indeed, with such regimens, it appears possible to establish engraftment against major histocompatibility complex-mismatched barriers.13 This represents a shift from the paradigm that the main goal of SCT is to rescue hematopoiesis after maximally tolerated doses of chemotherapy and radiotherapy to one in which the aim is to establish a state of host tolerance to the effector cells of the GVHD response. With many of these regimens, it does seem that reliable myeloid and lymphoid engraftment can be achieved without myeloablation and with apparently reduced short-term toxicity. Although in general follow-up is too short and relapse rates are too high to assess long-term transplant-related toxicity, it is likely that this will also be reduced. In addition, preliminary reports of pregnancies after nonmyeloablative SCT are encouraging.
Given the reduced toxicity of SCT with nonmyeloablative conditioning, it seems an attractive approach to take for children with nonmalignant disorders in whom myeloablation per se plays no role in disease eradication and in whom a mixed chimeric state may be sufficient to correct the disease phenotype. We reasoned it might be particularly useful for severe immunodeficiency states in which, by virtue of the host's immunodeficiency, reduced conditioning may be sufficient to establish host tolerance to the donor immune cells responsible for immune reconstitution. Further, many children with immunodeficiency states acquire organ toxicities that preclude SCT with conventional conditioning regimens. SCT with nonmyeloablative conditioning may offer the potential for the more complete immune reconstitution seen with conditioned SCT without the toxicity of conventional conditioning. Woolfrey et al14 have reported stable mixed chimerism in 2 patients with T-cell immunodeficiency after sibling SCT using only post-transplant immunosuppression with mycophenolate mofetil and cyclosporin A. One of these patients showed evidence of improved T-cell immunity after SCT. Here we report the first series of nonmyeloablative SCT for childhood immunodeficiency states. In that series, 6 of 8 patients had no HLA-identical sibling donor and received SCT from HLA-matched unrelated donors. To balance the potential for better immune reconstitution with the risk for GVHD, we elected to use T-cell–replete grafts with partial in vivo T-cell depletion using rabbit anti-lymphocyte globulin (ALG). In view of the experimental nature of such an approach, our initial studies were performed in patients in whom SCT with conventional conditioning therapy was contraindicated because of severe organ dysfunction.
Patients and methods
Patients
This study involved patients with immunodeficiency who were ineligible for conventional myeloablative conditioning because of comorbidity. None of the patients were candidates for SCT without conditioning because of residual T- or natural killer-cell function that was likely to compromise engraftment. Patient characteristics and immunologic profiles before BMT are outlined in Table1. The median age was 6.5 years (range, 9 months to 18 years). All donors were fully HLA-compatible serologically at class 1 (A, B, C loci) and molecularly at class 2 (DRB1, DQB1 loci).
Patient and disease characteristics and pretransplant complications
| Patient . | Age/sex . | Diagnosis . | Lymphocytes/mm3 . | PHA stimulation S.I. (normal > 100) . | Complications . | Donor . |
|---|---|---|---|---|---|---|
| 1 | 10 y/M | CID | Abs. L 180 | 6.1 | Bronchiectasis | MUD |
| CD3 81 | Sclerosing cholangitis | |||||
| CD4 61 | ||||||
| CD19 18 | ||||||
| CD16 10.8 | ||||||
| 2 | 18 y/M | CD40L deficiency | Abs. L 1470 | 361.4 | Sclerosing cholangitis | MUD |
| CD3 975 | Orthotopic liver transplantation | |||||
| CD4 780 | ||||||
| CD19 397 | ||||||
| CD16 59 | ||||||
| 3 | 11 y/M | CD40L deficiency | Abs. L 3640 | 220.7 | Sclerosing cholangitis | MUD |
| CD3 2802 | Cryptosporidiosis | |||||
| CD4 1783 | ||||||
| CD19 510 | ||||||
| CD16 218 | ||||||
| 4 | 3.8 y/M | XLP/HLH | Abs. L 1990 | 197.2 | Pneumonitis | MSD |
| CD3 1444 | CNS HLH | |||||
| CD4 896 | ||||||
| CD19 438 | ||||||
| CD16 20 | ||||||
| 5 | 9 mo/F | ADA deficiency | Abs. L 630 | 1.1 | Pneumonitis | MUD |
| CD3 170 | Enteropathy | |||||
| CD4 151 | ||||||
| CD19 12 | ||||||
| CD16 384 | ||||||
| 6 | 11 mo/M | T+B−NK− | Abs. L 1020 | 445.4 | Pneumocystis carinii pneumonitis | MUD |
| SCID | CD3 979 | |||||
| CD4 765 | Enteropathy | |||||
| CD19 30 | ||||||
| CD16 0 | ||||||
| 7 | 14 mo/M | T_B+NK− | Abs. L 1430 | 8.9 | Meningoencephalitis | MUD |
| SCID | CD3 229 | RSV bronchiolitis | ||||
| CD4 57 | Failure to thrive | |||||
| CD19 1158 | Enteropathy | |||||
| CD16 10 | ||||||
| 8 | 10 y/M | CID | Abs. L 1070 | 47.3 | Autoimmune thrombocytopenia | MSD |
| CD3 963 | ||||||
| CD4 460 | Hepatitis B infection | |||||
| CD19 0 | Enteropathy | |||||
| CD16 64 |
| Patient . | Age/sex . | Diagnosis . | Lymphocytes/mm3 . | PHA stimulation S.I. (normal > 100) . | Complications . | Donor . |
|---|---|---|---|---|---|---|
| 1 | 10 y/M | CID | Abs. L 180 | 6.1 | Bronchiectasis | MUD |
| CD3 81 | Sclerosing cholangitis | |||||
| CD4 61 | ||||||
| CD19 18 | ||||||
| CD16 10.8 | ||||||
| 2 | 18 y/M | CD40L deficiency | Abs. L 1470 | 361.4 | Sclerosing cholangitis | MUD |
| CD3 975 | Orthotopic liver transplantation | |||||
| CD4 780 | ||||||
| CD19 397 | ||||||
| CD16 59 | ||||||
| 3 | 11 y/M | CD40L deficiency | Abs. L 3640 | 220.7 | Sclerosing cholangitis | MUD |
| CD3 2802 | Cryptosporidiosis | |||||
| CD4 1783 | ||||||
| CD19 510 | ||||||
| CD16 218 | ||||||
| 4 | 3.8 y/M | XLP/HLH | Abs. L 1990 | 197.2 | Pneumonitis | MSD |
| CD3 1444 | CNS HLH | |||||
| CD4 896 | ||||||
| CD19 438 | ||||||
| CD16 20 | ||||||
| 5 | 9 mo/F | ADA deficiency | Abs. L 630 | 1.1 | Pneumonitis | MUD |
| CD3 170 | Enteropathy | |||||
| CD4 151 | ||||||
| CD19 12 | ||||||
| CD16 384 | ||||||
| 6 | 11 mo/M | T+B−NK− | Abs. L 1020 | 445.4 | Pneumocystis carinii pneumonitis | MUD |
| SCID | CD3 979 | |||||
| CD4 765 | Enteropathy | |||||
| CD19 30 | ||||||
| CD16 0 | ||||||
| 7 | 14 mo/M | T_B+NK− | Abs. L 1430 | 8.9 | Meningoencephalitis | MUD |
| SCID | CD3 229 | RSV bronchiolitis | ||||
| CD4 57 | Failure to thrive | |||||
| CD19 1158 | Enteropathy | |||||
| CD16 10 | ||||||
| 8 | 10 y/M | CID | Abs. L 1070 | 47.3 | Autoimmune thrombocytopenia | MSD |
| CD3 963 | ||||||
| CD4 460 | Hepatitis B infection | |||||
| CD19 0 | Enteropathy | |||||
| CD16 64 |
Two children had CD40 ligand deficiency, 5 had combined or severe combined immunodeficiency (1 ADA deficiency, 4 uncharacterized), and one had X-linked lymphoproliferative disorder evolving into hemophagocytic lymphohistiocytosis. The patient with ADA deficiency was treated with PEG-ADA and had resultant improvement in lymphocyte counts. Treatment was discontinued 3 weeks before BMT to decrease the likelihood of graft rejection. Of the patients with undefined combined immunodeficiency, patient 1 had progressive T- and B-cell lymphopenia with a poor phytohemagglutinin (PHA) response, patient 6 had T+B−NK− SCID with extremely diminished immunoglobulin production suggestive of a primary T-cell defect, patient 7 had a T↓ B+NK− phenotype, with abnormal activation of JAK3 on IL-2 stimulation suggestive of a defect in this pathway, and patient 8 had a T↓ B−NK+ phenotype with absent specific antibody production and autoimmune thrombocytopenia.
Pretransplant co-morbidities are detailed in Table 1. Three patients had end-stage sclerosing cholangitis, and recurrent variceal bleeds necessitated liver transplantation 1 month before BMT in one patient. Patient 8 had chronic hepatitis B, which was treated with lamivudine. One child had severe bronchiectasis. Five others had histories of severe respiratory infections (including 2 Pneumocystispneumonitis, 1 RSV [respiratory syncytial virus] bronchiolitis), requiring ventilation in one patient. Two patients had presumed encephalitis and most (6 of 8) had significant enteropathy, resulting in weight loss that required either nasogastric or parenteral feeding.
For comparison with regard to engraftment and immune reconstitution, we used a series of 19 consecutive patients with congenital immunodeficiency (4 Omenn syndrome, 4 undefined CID, 2 Wiskotte-Aldrich syndrome, 2 CD40 ligand deficiency, 1 CVID, 1 dyskeratosis congenita, 1 ADA, 1 PNP, 1 major histocompatibility complex class II deficiency, 1 RAG, 1 IFN-γ receptor) who underwent SCT from HLA-matched sibling (n = 10) or unrelated donors (n = 9) at our institute with conventional myeloablative conditioning using standard busulphan/cyclophosphamide protocols.
Preparative regimen, transplantation, and supportive care
Conditioning therapy consisted of the following: fludarabine 30 mg/m2 × 5 (days −7 to −3), melphalan 140 mg/m2 (day −2), and ALG 2.5 mg/kg × 5 (day −2 to +2 for unrelated donor transplants, day −4 to 0 for sibling transplants). Two patients (patients 5 and 6) younger than 1 year had a reduced melphalan dose of 125mg/m2. Patient 5 was also given a reduced dose of fludarabine (30 mg/m2 × 4). The total number of unfractionated allogeneic bone marrow mononuclear cells infused on day 0 ranged from 1.3 to 6.6 × 108/kg (median, 2.8 × 108/kg). No T-cell depletion or stem cell selection procedures were used. Prophylaxis against GVHD was with cyclosporin A 1.5 mg/kg twice daily intravenously starting on day −1 (except for patient 2, who was on tacrolimus after liver transplantation) and methylprednisolone 1 mg/kg intravenously starting on day 3. When patients were able to take oral medication, they were switched to oral cyclosporine and prednisolone. In the absence of GVHD, prednisolone was tapered from discharge over 2 to 4 weeks, and cyclosporine was continued to day 100 and then tapered over 1 month with careful monitoring of chimerism. All patients received G-CSF 5 μg/kg per day from day 8 until neutrophil counts recovered to more than 1 × 109/L. Antimicrobial prophylaxis during the transplantation period consisted of acyclovir 750 to 1500 mg/m2 per day intravenously from day −3 and then 2400 mg/m2 per day orally to 6 months, itraconazole 5 mg/kg per day until neutrophil recovery, co-trimoxazole adjusted to body surface area from neutrophil recovery to 6 months or normalization of the PHA stimulation index, penicillin V 250 to 500 mg/d from discharge to 2 years after transplantation. Patients at high risk for fungal infection, those unable to tolerate itraconazole, and those with severe enteropathy received AmBisome 1 mg/kg daily rather than itraconazole. Additionally, 3 patients with sclerosing cholangitis received anti-cryptosporidial prophylaxis with paromomycin 30 mg/kg per day orally or 16 mg/kg per day intravenously and oral azithromycin 10 mg/kg per day. Patient 8, who had chronic hepatitis B infection before BMT, continued lamivudine therapy throughout the transplantation period. Blood and urine samples of all patients were screened by DNA PCR and the DEAFF test, respectively, each week to check for cytomegalovirus; those who tested positive were treated with ganciclovir. All patients received intravenous immunoglobulin 0.5 g/kg per week during the transplantation period and then every 3 weeks until the CD4 count was more than 300 per μL and serum immunoglobulin A and M levels had normalized.
Study endpoints
The primary endpoints of the study were to evaluate engraftment, toxicity, incidence of acute GVHD, and day 100 transplant-related mortality rates. Engraftment was defined by neutrophil recovery to more than 0.5 × 109/L in conjunction with FISH/VNTR studies demonstrating donor hematopoiesis. Engraftment was evaluated monthly for the first 3 months and then every 3 months using Y-chromosome–specific fluorescent in situ hybridization (FISH) in the presence of a sex mismatch between host and donor or by VNTR microsatellite analysis. Lineage-specific chimerism was analyzed by separating peripheral blood mononuclear cells over a Ficoll density gradient, with isolation of the granulocytes from the red cell pellet followed by red cell lysis. Chimerism was quantitated from the percentage of donor cells in FISH or VNTR studies by calculating the percentage of Y-chromosome–positive cells or by comparison with known mixtures of recipient and donor DNA, respectively. These assays are able to detect mixed chimerism if more than 5% recipient or donor DNA is present. Mixed chimerism was defined as the presence of more than 5% host-derived cells on more than one occasion in any of the whole blood, mononuclear cell, or granulocyte fractions. Acute GVHD was assessed using the Seattle criteria.15 Toxicity was assessed using the criteria of Bearman et al.16 Secondary endpoints included immune reconstitution and incidence of chronic GVHD, defined as GVHD occurring 100 days or more after BMT and graded as none, limited, or extensive. Immune reconstitution was studied at 1, 2, 3, 6, 9, 12, and 15 months by FACS analysis of peripheral blood mononuclear cells using fluorescein isothiocyanate–phycoerythrin-labeled antibodies against CD3, CD4, CD8, and CD19, immunoglobulin A and M levels, and assay of PHA stimulation index (defined as the ratio of baseline:maximal stimulated levels of3H-thymidine uptake in 3-day cultures of peripheral blood mononuclear cells stimulated with a range of concentrations of PHA 0 to 8 μg/mL).
Results
Toxicity and survival
In this cohort of patients at high risk, SCT with our nonmyeloablative protocol was extremely well tolerated. The median duration of admission was 52 days (range, 27-84 days), which is comparable to that for our historical cohort of children undergoing conventionally conditioned SCT for immunodeficiency at this center (median, 54 days; range, 35-180 days). At the time of writing, 7 of 8 patients are alive and well. Patient 4 died of multiorgan failure secondary to Pseudomonas aeruginosasepticemia after treatment for recurrent hemophagocytic lymphohistiocytosis. The incidence rate of common transplantation-related complications is summarized in Tables 2 and3. It can be seen that the major regimen-related toxicities observed (Table3) were gastrointestinal and hepatic. Hepatotoxicity is discussed below. Mucositis was generally mild to moderate; however, 6 of 8 patients had pre-existing enteropathy so that 7 patients required parenteral nutrition. Patient 2 had a generalized seizure on day 18 in association with hypertension and was found by magnetic resonance angiography to have 2 nonenhancing vasculitic lesions in the right parietal lobe. His seizure was easily controlled with anticonvulsants, he had no other neurologic deficit, and lesions found on computed tomography resolved on subsequent scans. The only other neurologic toxicity observed was a moderate degree of sensorineural hearing loss in patient 4, who had received intravenous paromomycin for Cryptosporidiosis. Idiopathic pneumonitis, veno-occlusive disease, and post-transplant lymphoproliferative disease were not seen.
Transplantation-related complications: incidence of infection
| Bacteremia | 6 /8 |
| Viral | |
| CMV (blood) | 1 /8 |
| Adenovirus (blood) | 0 /8 |
| Adenovirus (stool) | 3 /8 |
| Other (RSV, HSV) | 2 /8 |
| Fungal | 0 /8 |
| Bacteremia | 6 /8 |
| Viral | |
| CMV (blood) | 1 /8 |
| Adenovirus (blood) | 0 /8 |
| Adenovirus (stool) | 3 /8 |
| Other (RSV, HSV) | 2 /8 |
| Fungal | 0 /8 |
Transplantation-related complications: regimen-related toxicity (number of patients)
| Bearman score . | |||||
|---|---|---|---|---|---|
| . | 0 . | I . | II . | III . | IV . |
| Pulmonary | 8 | 0 | 0 | 0 | 0 |
| Cardiac | 8 | 0 | 0 | 0 | 0 |
| Hepatic | 2 | 3 | 2 | 1 | 0 |
| Renal | 4 | 1 | 3 | 0 | 0 |
| Stomatitis | 0 | 3 | 5 | 0 | 0 |
| Gastrointestinal | 1 | 4 | 3 | 0 | 0 |
| Bladder | 8 | 0 | 0 | 0 | 0 |
| Bearman score . | |||||
|---|---|---|---|---|---|
| . | 0 . | I . | II . | III . | IV . |
| Pulmonary | 8 | 0 | 0 | 0 | 0 |
| Cardiac | 8 | 0 | 0 | 0 | 0 |
| Hepatic | 2 | 3 | 2 | 1 | 0 |
| Renal | 4 | 1 | 3 | 0 | 0 |
| Stomatitis | 0 | 3 | 5 | 0 | 0 |
| Gastrointestinal | 1 | 4 | 3 | 0 | 0 |
| Bladder | 8 | 0 | 0 | 0 | 0 |
With regard to infection (Table 2), 7 of 8 patients had more than one episode of neutropenic fever, blood cultures were positive in 6 patients. In one child with CD40 ligand deficiency and sclerosing cholangitis, Cryptosporidial cholangitis developed on day 15. His symptoms resolved with engraftment and intravenous paromomycin/azithromycin. Patient 8 had severe herpes simplex virus oropharyngitis on day 28, recurrent cytomegalovirus (CMV) reactivations treated with foscarnet, and adenovirus infection at day 19 that was treated with ribavirin. No fungal infections were observed.
Given that 5 of 8 patients had significant antecedent liver disease (sclerosing cholangitis, chronic hepatitis B), it was not surprising that transient hepatotoxicity was frequently seen. Aside from the episode of cholangitis referred to above, the only major example of this occurred in patient 2, in whom transient biopsy-proven hepatitis—presumed to be viral in origin—developed in his transplanted liver on day 38. As seen in Table4, 6 of 8 patients had significant transient worsening of liver function (as defined by a doubling from pre-transplant values of one or more of the following parameters: bilirubin, alkaline phosphatase, aspartate transaminase, or alanine transaminase). However, all have improved, and 5 of 7 evaluable patients, including all 3 patients with the most severe pre-BMT liver disease, had normal liver function findings at the time of the most recent follow-up. The other 2 patients had mildly elevated alanine transaminase levels.
Transplantation-related complications: hepatotoxicity
| Patient no. . | Bilirubin (μM) . | Alanine transaminase (IU/L) . | Alkaline phosphatase (IU/L) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-SCT . | Peak . | Current . | Pre-SCT . | Peak . | Current . | Pre-SCT . | Peak . | Current . | |
| 1 | 7 | 11 | 7 | 70 | 205 | 26 | 483 | 1075 | 280 |
| 2 | 144 | 130 | 11 | 67 | 375 | 23 | 810 | 1113 | 141 |
| 3 | 15 | 203 | 7 | 130 | 207 | 45 | 443 | 1022 | 244 |
| 4 | 10 | 20 | 32 | 65 | 92 | 13 | 175 | 292 | 356 |
| 5 | 3 | 9 | 5 | 41 | 136 | 81 | 152 | 196 | 214 |
| 6 | 7 | 15 | 8 | 16 | 87 | 143 | 115 | 144 | 217 |
| 7 | 3 | 16 | 5 | 81 | 22 | 11 | 181 | 185 | 126 |
| 8 | 8 | 26 | 5 | 53 | 162 | 25 | 157 | 118 | 95 |
| Patient no. . | Bilirubin (μM) . | Alanine transaminase (IU/L) . | Alkaline phosphatase (IU/L) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-SCT . | Peak . | Current . | Pre-SCT . | Peak . | Current . | Pre-SCT . | Peak . | Current . | |
| 1 | 7 | 11 | 7 | 70 | 205 | 26 | 483 | 1075 | 280 |
| 2 | 144 | 130 | 11 | 67 | 375 | 23 | 810 | 1113 | 141 |
| 3 | 15 | 203 | 7 | 130 | 207 | 45 | 443 | 1022 | 244 |
| 4 | 10 | 20 | 32 | 65 | 92 | 13 | 175 | 292 | 356 |
| 5 | 3 | 9 | 5 | 41 | 136 | 81 | 152 | 196 | 214 |
| 6 | 7 | 15 | 8 | 16 | 87 | 143 | 115 | 144 | 217 |
| 7 | 3 | 16 | 5 | 81 | 22 | 11 | 181 | 185 | 126 |
| 8 | 8 | 26 | 5 | 53 | 162 | 25 | 157 | 118 | 95 |
Normal ranges: bilirubin, 2-18 μM; ALT, 10-35 IU/L; ALP, 30-350 IU/L.
Engraftment
In all patients, absolute neutrophil counts dropped to below 0.1 × 109/L, but all had prompt hematopoietic recovery. The median time to neutrophil recovery to more than 0.5 × 109/L was 13 days (range, 9 to 17 days), and to an unsupported platelet count of more than 20 × 109/L, it was 22 days (range, 10 to 37 days). This compares favorably to counts in a cohort of 19 patients with congenital immunodeficiency who underwent transplantation at this center with standard busulphan/cyclophosphamide conditioning regimes. Median time to neutrophil recovery was 16 days (range, 11 to 32 days), and median time to platelet recovery was 28 days (range, 8 to 112 days). The median number of blood transfusions was 5.5, and the median time of platelet transfusions was 11.5. As shown in Table5, at 1 month after transplantation, 7 of 8 patients had engrafted with full donor chimerism and 5 of 7 evaluable patients had maintained predominantly donor hematopoiesis. In contrast to previous reports,17 chimerism in the lymphoid and myeloid lineages was generally concordant. Patient 4 experienced a decline in the contribution of donor cells to hematopoiesis at 2.5 months after transplantation that was associated with fever, hepatosplenomegaly, decreased peripheral blood count, and biochemical, bone marrow, and cerebrospinal fluid evidence of recurrent hemophagocytic lymphohistiocytosis. This may have been precipitated by a passive transfer of donor Epstein-Barr virus EBV in the absence of adequate T-cell reconstitution because the donor was serologically positive for EBV, and the patient became EBV DNA positive at the time of disease recurrence. He was treated with anti-thymocyte globulin and etoposide and received a further bone marrow graft from his sibling at day 91, but he died of Pseudomonas aeruginosa septicemia on day 104 after BMT. Patient 5, who had ADA deficiency who was given reduced conditioning with fludarabine 120 mg/m2 and melphalan 125 mg/m2, had progressive decrease in donor chimerism from 1 month after transplantation, consistent with the recovery of recipient hematopoiesis. This was initially manifested in the lymphoid and subsequently in the myeloid lineage. However, this was stabilized with the withdrawal of immunosuppression therapy, and, at the time of writing, she has remained a mixed chimera.
Engraftment and chimerism after non-myeloablative stem cell transplantation
| Patient . | Cell dose (108/kg) . | Neut >0.5 (days) . | PI >20 (days) . | Months post-BMT . | % Donor whole blood . | % Donor PBMNC . | % Donor gran . |
|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 9 | 13 | 1 | 100 | 100 | 100 |
| 2 | 95 | 90-95 | 95 | ||||
| 3 | 95 | 95 | 95 | ||||
| 6 | 90 | 90 | 90 | ||||
| 9 | 85 | 90 | 85 | ||||
| 12 | 90 | 90 | 85 | ||||
| 16 | 75 | ||||||
| 2 | 1.3 | 13 | 14 | 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 | ||||
| 3 | 100 | 100 | 100 | ||||
| 6 | 100 | 100 | 100 | ||||
| 15 | 100 | 100 | 100 | ||||
| 3 | 6 | 13 | 10 | 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 | ||||
| 3 | 100 | 100 | 100 | ||||
| 6 | 100 | 100 | 100 | ||||
| 9 | 100 | 100 | 100 | ||||
| 14 | 100 | 100 | 100 | ||||
| 4 | 1.7 | 15 | 36 | 1 | 100 | 100 | 100 |
| 2 | 90-95 | ||||||
| 3 | 80 | 80 | |||||
| 5 | 4.4 | 13 | 36 | 1 | 75 | 95 | |
| 2 | 55 | 85 | |||||
| 3 | 15 | 10 | |||||
| 4 | 25 | 5 | |||||
| 5 | 30 | 5 | |||||
| 8 | 45 | 5 | |||||
| 12 | 50 | 5 | |||||
| 6 | 6.6 | 13 | 29 | 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 | ||||
| 3 | 100 | 100 | 100 | ||||
| 5 | 100 | 100 | 100 | ||||
| 8 | 100 | 100 | 100 | ||||
| 12 | 100 | 100 | 100 | ||||
| 7 | 3.1 | 17 | 15 | 1 | 100 | 100 | 100 |
| 2 | 90 | ||||||
| 3 | 80 | 80 | 75 | ||||
| 4 | 45 | 60 | 45 | ||||
| 5 | 55 | 50 | 60 | ||||
| 7 | 60 | 45 | 55 | ||||
| 9 | 55 | ||||||
| 8 | 1.4 | 13 | 37 | 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 | ||||
| 3 | 100 | 100 | 100 | ||||
| 6 | 100 | 100 | 100 | ||||
| X = 13 | X = 22 |
| Patient . | Cell dose (108/kg) . | Neut >0.5 (days) . | PI >20 (days) . | Months post-BMT . | % Donor whole blood . | % Donor PBMNC . | % Donor gran . |
|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 9 | 13 | 1 | 100 | 100 | 100 |
| 2 | 95 | 90-95 | 95 | ||||
| 3 | 95 | 95 | 95 | ||||
| 6 | 90 | 90 | 90 | ||||
| 9 | 85 | 90 | 85 | ||||
| 12 | 90 | 90 | 85 | ||||
| 16 | 75 | ||||||
| 2 | 1.3 | 13 | 14 | 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 | ||||
| 3 | 100 | 100 | 100 | ||||
| 6 | 100 | 100 | 100 | ||||
| 15 | 100 | 100 | 100 | ||||
| 3 | 6 | 13 | 10 | 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 | ||||
| 3 | 100 | 100 | 100 | ||||
| 6 | 100 | 100 | 100 | ||||
| 9 | 100 | 100 | 100 | ||||
| 14 | 100 | 100 | 100 | ||||
| 4 | 1.7 | 15 | 36 | 1 | 100 | 100 | 100 |
| 2 | 90-95 | ||||||
| 3 | 80 | 80 | |||||
| 5 | 4.4 | 13 | 36 | 1 | 75 | 95 | |
| 2 | 55 | 85 | |||||
| 3 | 15 | 10 | |||||
| 4 | 25 | 5 | |||||
| 5 | 30 | 5 | |||||
| 8 | 45 | 5 | |||||
| 12 | 50 | 5 | |||||
| 6 | 6.6 | 13 | 29 | 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 | ||||
| 3 | 100 | 100 | 100 | ||||
| 5 | 100 | 100 | 100 | ||||
| 8 | 100 | 100 | 100 | ||||
| 12 | 100 | 100 | 100 | ||||
| 7 | 3.1 | 17 | 15 | 1 | 100 | 100 | 100 |
| 2 | 90 | ||||||
| 3 | 80 | 80 | 75 | ||||
| 4 | 45 | 60 | 45 | ||||
| 5 | 55 | 50 | 60 | ||||
| 7 | 60 | 45 | 55 | ||||
| 9 | 55 | ||||||
| 8 | 1.4 | 13 | 37 | 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 | ||||
| 3 | 100 | 100 | 100 | ||||
| 6 | 100 | 100 | 100 | ||||
| X = 13 | X = 22 |
Graft versus host disease
Four of 8 patients (50%) had grade 1 acute GVHD of the skin, and 1 might have had mild hepatic involvement simultaneously. This generally occurred at approximately the time of engraftment; all patients responded well to prednisolone 1 to 2 mg/kg per day. No patient had acute GVHD higher than grade 1. Patient 8 had limited chronic GVHD of the mouth and lower gut at 5 months after BMT that responded well to prednisolone 2 mg/kg per day. He is now taking reducing steroids, though he remains on cyclosporin A.
Immune reconstitution
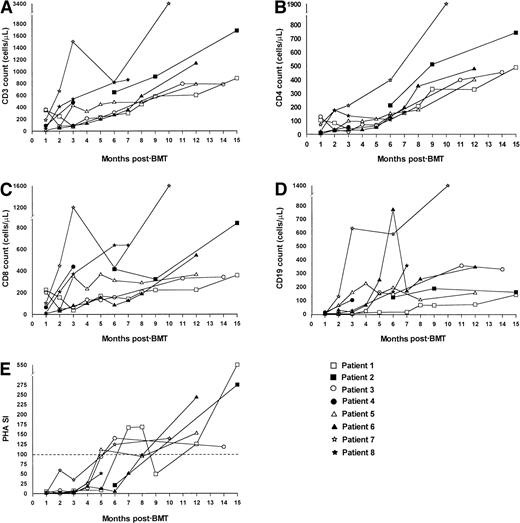
Figure 1 shows recovery of CD3+, CD4+, and CD8+ T cells, PHA stimulation index, and CD19+ B-cells after nonmyeloablative stem cell transplantation. At a median follow-up of 12 months, all 7 evaluable patients have had good recovery of T-cell numbers, and 5 of 7 have achieved normal age-related CD3 counts. All 4 patients with low CD3 counts before transplantation now have improved values. In all patients CD8 recovery preceded CD4 recovery. Four of 7 patients now have normal CD4 counts, and all have achieved CD4 counts of more than 300/μL. Three of 4 patients with low CD4 counts at presentation now have significantly improved values, and the remaining patient is still on immunosuppression for chronic GVHD. With regard to T-cell function, 6 of 7 evaluable patients now have normal PHA stimulation indices, and 3 of 4 patients with poor values at presentation show marked improvement. In our historical cohort, 12 of 17 evaluable patients achieved a normal CD3 count, 12 of 16 achieved a normal CD4 count, and 16 of 17 had a normal PHA stimulation index at a similar point in follow-up. In the current study, 4 of 7 evaluable patients achieved normal CD19+ counts (compared to 9 of 16 in our historical cohort), and all 4 patients with low CD19+counts at presentation have shown significant improvement. Four patients have normal immunoglobulin M levels, and 2 patients have normal immunoglobulin A levels (data not shown). Four patients continue to undergo prophylactic intravenous immunoglobulin replacement therapy. None of the patients have so far been vaccinated to assess specific antibody production. In patient 5, dATP levels improved to 169 μmol/L at 12 months after BMT compared with 652 μmol/L at presentation. Flow cytometric analysis of PMA- and ionomycin-stimulated CD40 ligand (CD154) expression on peripheral blood mononuclear cells from patients 2 and 4 at 12 months after BMT showed significant expression of CD40 ligand, whereas no expression was seen before BMT.
Immune reconstitution after nonmyeloablative stem cell transplantation.
Recovery of CD3+ (A), CD4+ (B), and CD8+ (C) T cells, CD19+ B cells (D), and the PHA stimulation index (E) are shown. The dotted line in E represents the lower limit of normal. The key shows which data set belongs to each patient using the nomenclature from Table 1.
Immune reconstitution after nonmyeloablative stem cell transplantation.
Recovery of CD3+ (A), CD4+ (B), and CD8+ (C) T cells, CD19+ B cells (D), and the PHA stimulation index (E) are shown. The dotted line in E represents the lower limit of normal. The key shows which data set belongs to each patient using the nomenclature from Table 1.
Quality of life
At the time of writing, 7 of 8 patients are alive and well and have Lansky scores of 90 to 100 at a median of 12 months after BMT (range, 7-17 months). Lansky scores in all 7 evaluable patients improved compared to what they were before BMT (Table6), and only one of the surviving patients has limited chronic GVHD.
Treatment outcome and current status
| Patient . | Months post-BMT . | GVH . | Lansky pre-BMT . | Score post-BMT . | Clinical status . |
|---|---|---|---|---|---|
| 1 | 17 | N | 80 | 100 | Well |
| 2 | 16 | N | 50 | 100 | Well |
| 3 | 15 | N | 90 | 100 | Well |
| 4 | 3 | N | 70 | — | Relapse/died |
| 5 | 12 | N | 90 | 100 | Well/mixed chimera |
| 6 | 12 | N | 80 | 90 | Well |
| 7 | 11 | N | 80 | 100 | Well/mixed chimera |
| 8 | 8 | Y | 80 | 90 | Well |
| Patient . | Months post-BMT . | GVH . | Lansky pre-BMT . | Score post-BMT . | Clinical status . |
|---|---|---|---|---|---|
| 1 | 17 | N | 80 | 100 | Well |
| 2 | 16 | N | 50 | 100 | Well |
| 3 | 15 | N | 90 | 100 | Well |
| 4 | 3 | N | 70 | — | Relapse/died |
| 5 | 12 | N | 90 | 100 | Well/mixed chimera |
| 6 | 12 | N | 80 | 90 | Well |
| 7 | 11 | N | 80 | 100 | Well/mixed chimera |
| 8 | 8 | Y | 80 | 90 | Well |
Discussion
This study provides a novel approach for allogeneic stem cell transplantation for patients with inherited immunodeficiency states. With the available SCT protocols for children with immunodeficiencies who lack an HLA-identical donor, particularly those with residual T- or natural killer-cell function, intensive pretransplantation conditioning appears to be a prerequisite for reliable engraftment and complete immune reconstitution (reviewed in Porta and Friedrich2). None of our patients were candidates for conventional myeloablative conditioning because of severe organ dysfunction. In those without HLA-identical family members, we used unrelated donors as the source of stem cells, in view of the ability to give a T-cell–replete graft, which may favor improved immune reconstitution. We have shown that reliable donor stem cell engraftment is possible without myeloablative conditioning in patients with severe organ dysfunction who would not be candidates for SCT with conventional conditioning. Our chimerism data demonstrate that patients engrafted with predominantly donor hematopoiesis initially, but there was subsequently a significant incidence of mixed chimerism approximating that reported previously,7,8 after nonmyeloablative SCT. The presence of mixed chimerism did not appear to correlate with mononuclear cell dose at transplantation. In most patients, chimerism in the lymphoid and myeloid lineages appeared to parallel each other, but in patient 5 the degree of donor chimerism was persistently significantly less in the myeloid than the lymphoid lineage. This may be of relevance in that analysis of ADA and dATP metabolite levels in the erythrocyte lineage may give results that do not reflect levels in the lymphoid lineage, where, by virtue of the higher degree of donor chimerism, better results would be predicted. Experiments to determine these parameters in lymphoid cells are underway. One patient experienced a relapse of his original disease. Three patients, including one who underwent reduced preparative chemotherapy, have had declines in the donor contribution to hematopoiesis that stabilized on withdrawal of immunosuppression in 2 of them, consistent with the recovery of host hematopoiesis rather than rejection. All remain mixed chimeras. In the immunodeficient host it may be possible to decrease conditioning intensity still further,14 but this may compromise eventual immune reconstitution. It is salutary to bear in mind as well that, at least in patients with hematologic malignancies, inadequate conditioning has been associated with graft rejection.7 12In view of this, particularly given that even patients with severe organ dysfunction experienced little toxicity with our conditioning regimen, we do not believe that further reduction in conditioning intensity is warranted.
Overall, BMT using our regimen was extremely well tolerated, and all 7 evaluable patients now have improved quality of life, as assessed by their Lansky scores. The duration of cytopenias was brief compared with our historical control cohort, and the incidence of infective complications was low. Despite this, however, the duration of hospitalization was not significantly different in our patients than in the historical cohort. This is not surprising given the high-risk nature of our patient group, and it was related to feeding issues in most patients, CMV reactivation in one patient, and orthopedic rehabilitation after hip fractures in another. Although our numbers are too small to draw firm conclusions, pneumonitis or veno-occlusive disease did not develop in any patients. This contrasts with the experience of the Jerusalem group,8 who noted some degree of veno-occlusive disease in 13 of 26 patients in their original series; this may reflect their use of busulphan as part of the conditioning, and it may reflect differences in the patient populations studied. One patient died of recurrent hemophagocytic lymphohistiocytosis, possibly precipitated by the transfer of EBV from a donor. EBV-associated post-transplant lymphoproliferative disease has been reported18 after nonmyeloablative transplantation, and we have recently observed this complication in a child with juvenile myelomonocytic leukemia who underwent transplantation according to the protocol presented here. Selective in vivo T-cell depletion with ALG may enhance the risk for EBV-related disease after transplantation. With regard to long-term complications of SCT, though it is predicted that the incidence of end-organ damage, infertility, growth retardation, and secondary malignancy may be lower with nonmyeloablative regimens than with standard conditioning regimens, demonstrating this will require long-term follow-up.
Although most groups8,11 using nonmyeloablative conditioning for SCT have reported an incidence of acute GVHD comparable to that seen with conventional SCT, we have not seen acute GVHD higher than grade 1. This is likely to reflect, at least in part, the younger age of our patients, but it may also relate to our use of ALG. It is interesting to note that the Jerusalem group19observed a similarly low incidence of severe acute GVHD in their patients with nonmalignant disease. The long-term incidence of chronic GVHD resulting from this approach remains to be determined. However, the fact that this complication developed in only 1 of 7 evaluable patients is encouraging, particularly in comparison with the 40% incidence of cGVHD 6 months after BMT that was observed in the EBMT retrospective analysis.6 This may be of considerable importance given that in patients undergoing haploidentical BMT for primary immunodeficiency, cGVHD at 6 months after BMT is significantly associated with impaired development of T-cell function and with reduced survival rates.6
In the evaluable patients, T-cell reconstitution has so far been good. At a median of 1 year after BMT, most patients have achieved normal CD3 and CD4 counts, and 6 of 7 have normal PHA stimulation indices. Significantly, there was marked improvement in CD3 counts and PHA stimulation indices in 4 patients and in CD4 counts in 3 of 4 patients with combined or severe combined immunodeficiency in whom these parameters were low before transplantation. The remaining patient underwent transplantation only 7 months ago and is currently undergoing reducing immunosuppression therapy for chronic GVHD. Similarly, 4 of 7 patients overall have normal B-cell numbers, and all patients with low CD19+ cell counts at presentation have shown significant improvement. Because we have observed stable donor myeloid engraftment in all patients, we anticipate B-cell recovery is likely in the remaining patients. In addition, the fact that some of our patients were able to clear viral infections (RSV, adenovirus) and Cryptosporidiosis attests to a significant recovery of functional immunity. Clearly, the determination of in vivo-specific antibody responses to immunization after longer follow-up will be necessary to confirm that B-cell function has been restored. We plan to vaccinate all patients at 18 months after BMT.
There are few published data concerning the kinetics of immune reconstitution in patients with immunodeficiency undergoing unrelated donor SCT and no controlled studies comparing immune reconstitution in children with immunodeficiency undergoing different methods of SCT from HLA nonidentical donors. Nevertheless, the pace of recovery of T-cell numbers and function in our study appears broadly to parallel that seen with matched historical immunodeficient controls in our patient cohort. Our results are similar to those seen in immunodeficient patients undergoing T-cell–depleted haploidentical SCT6 with conventional conditioning or in children with hematologic malignancy who received T-cell–depleted unrelated SCT.20 The pace of T-cell reconstitution is likely to reflect our use of ALG in the transplantation period, together with prednisolone and cyclosporin A after transplantation. Cyclosporin A may be particularly important in this regard because most patients showed marked improvement in their CD4 counts and PHA SI when cyclosporin A prophylaxis was discontinued (data not shown). Prolonged post-transplantation immunosuppression therapy may compromise long-term immune recovery by abrogating helper function from mature donor T cells. Determining the optimal timing of withdrawal of immunosuppression so as to accelerate immune reconstitution without increasing the likelihood of GVHD will be critical with this approach. If long-term immune recovery is poor, there may be a role for “top-up” donor lymphocyte infusion after transplantation to improve immunity; however, given the risk for GVHD, we do not believe that the routine prophylactic use of top-up donor lymphocyte infusion is indicated in patients with immunodeficiency.
In summary, we have demonstrated that nonmyeloablative SCT permits rapid engraftment from sibling and unrelated donors with minimal toxicity, even in the presence of severe organ dysfunction, thus establishing host tolerance to the donor immune cells responsible for immune reconstitution. We think this represents the treatment of choice for patients who are ineligible for conventional SCT. Long-term follow-up is needed before this approach is extended to patients at standard risk. If reduced long-term toxicity and full immune reconstitution of patients treated with such novel protocols are demonstrated, this approach may offer the possibility of adequate immune reconstitution with a reduction in the short- and long-term toxicity associated with conventional conditioning.
Acknowledgment
We thank Dr C. Craddock for helpful discussion and critical review of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul Veys, Department of Bone Marrow Transplantation, Great Ormond Street Hospital for Sick Children, London WC1N 3JH, United Kingdom; e-mail: paul.veys@gosh.tr-nthames.nhs.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal