Abstract
Elimination of tumor cells (“purging”) from hematopoietic stem cell products is a major goal of bone marrow–supported high-dose cancer chemotherapy. We developed an in vivo purging method capable of providing tumor-free stem cell products from most patients with mantle cell or follicular lymphoma and bone marrow involvement. In a prospective study, 15 patients with CD20+ mantle cell or follicular lymphoma, bone marrow involvement, and polymerase chain reaction (PCR)–detectable molecular rearrangement received 2 cycles of intensive chemotherapy, each of which was followed by infusion of a growth factor and 2 doses of the anti-CD20 monoclonal antibody rituximab. The role of rituximab was established by comparison with 10 control patients prospectively treated with an identical chemotherapy regimen but no rituximab. The CD34+ cells harvested from the patients who received both chemotherapy and rituximab were PCR-negative in 93% of cases (versus 40% of controls;P = .007). Aside from providing PCR-negative harvests, the chemoimmunotherapy treatment produced complete clinical and molecular remission in all 14 evaluable patients, including all 6 with mantle cell lymphoma (versus 70% of controls). In vivo purging of hematopoietic progenitor cells can be successfully accomplished in most patients with CD20+ lymphoma, including mantle cell lymphoma. The results depended on the activity of both chemotherapy and rituximab infusion and provide the proof of principle that in vivo purging is feasible and possibly superior to currently available ex vivo techniques. The high short-term complete-response rate observed suggests the presence of a more-than-additive antilymphoma effect of the chemoimmunotherapy combination used.
Myeloablative therapy followed by autologous hematopoietic progenitor cell rescue has an established, albeit not clearly defined, role in the management of non-Hodgkin lymphoma (NHL).1,2 One major obstacle to autologous stem cell transplantation in NHL is bone marrow and peripheral blood cell involvement with malignant cells. In fact, even if relapses after autologous transplantation usually result from residual cancer in the patient, cancer cells in bone marrow grafts contribute to relapse, as was demonstrated by gene-marking studies in patients with acute and chronic leukemia3,4 and patients with neuroblastoma.5 A role for bone marrow purging in autologous bone marrow transplantation for patients with NHL was suggested by studies that strongly indicated that residual lymphoma cells contribute to relapse.6 Whether these data also apply to peripheral blood cell grafts is unknown, although the presence of contaminating tumor cells in a product that is to be reinfused is of obvious concern.
With the aim of eliminating malignant cells from the graft, various ex vivo techniques involving the use of monoclonal antibodies or chemotherapeutic drugs have been developed.7 While generally effective, these purging techniques are labor intensive, delay hematopoietic recovery after transplantation, and substantially increase the costs of treatment.8
It was shown that hematopoietic progenitor cells harvested from the peripheral blood after high-dose conventional chemotherapy in patients with indolent lymphomas were tumor-free in a substantial proportion of cases9 and that in vivo administration of the monoclonal anti-CD20 antibody rituximab caused a rapid depletion of peripheral blood B cells in both cynomolgus monkeys10 and patients.11 This evidence was subsequently confirmed by the observation that when rituximab is given in combination with standard-dose chemotherapy, conversion of bone marrow and peripheral blood cells to negativity for tumor cells on polymerase chain reaction (PCR) assessment frequently occurs.12
In a study based on these findings, we investigated the ability of rituximab, given after 1 or 2 cycles of nonmyeloablative high-dose chemotherapy, to allow harvesting of peripheral blood progenitor cells free of contaminating lymphoma cells (in vivo purging) in 15 patients with either relapsed or refractory indolent lymphoma or mantle cell lymphoma (MCL). For each enrolled patient, we studied the extent of the mobilization and procurement of CD34+ cells, the presence of PCR-detectable lymphoma cells in the harvested product, and the kinetics of hematopoietic engraftment after reinfusion of the in vivo purged cells in patients who had myeloablation. The precise role of the antibody was assessed by comparison with results in a cohort of 10 consecutively seen similar patients who were treated with the same high-dose chemotherapy regimen but not rituximab.
Patients and methods
Patients
Between December 1996 and March 1999, 25 consecutively seen patients with either untreated mantle cell lymphoma or refractory or relapsed indolent NHL received high-dose sequential (HDS) chemotherapy with autologous hematopoietic progenitor cell support. Eligibility criteria included written informed consent; age, 60 years or younger; absence of severe organ dysfunction not due to tumor; no previous viral infections (hepatitis B or C or human immunodeficiency virus); a histologically confirmed diagnosis of mantle cell lymphoma or of indolent lymphoma, either refractory to or relapsed within 1 year after first-line polychemotherapy and requiring treatment; expression of CD20 by lymphoma cells; and availability of a molecular probe for PCR amplification of DNA to assess minimal residual disease (MRD).
Characteristics of patients given HDS alone and patients given rituximab with HDS (R-HDS) are shown in Table1. The study included 3 consecutive cohorts of patients whose treatment was dictated exclusively by the availability of rituximab. Thus, the first 10 and the last 5 enrolled patients received R-HDS, whereas the 10 patients seen consecutively between those groups (that is, enrolled after completion of the initial pilot study in 10 patients and before the antibody became commercially available) served as controls (HDS without rituximab).
Patient characteristics in the 2 therapy groups
| Characteristic . | High-dose sequential therapy (n = 10) . | Rituximab + high-dose sequential therapy (n = 15) . |
|---|---|---|
| Sex (male/female) | 5/5 | 11/4 |
| Median age, y (range) | 46 (36-53) | 43 (34-58) |
| Histologic findings | ||
| Follicular disease (grade 1 or 2) | 6 | 6 |
| Follicular disease (grade 3) | 1 | 1 |
| Mantle cell disease | 3 | 7 |
| Mucosa-associated lymphoid tissue lymphoma | — | 1 |
| Molecular rearrangement | ||
| Bcl1 | 3 | 3 |
| Bcl2 | 6 | 4 |
| IgH | 1 | 8 |
| Stage | ||
| III | 1 | 4 |
| IV | 9 | 11 |
| B-cell lymphoma symptoms | 0 | 4 |
| Mass greater than 10 cm | 3 | 0 |
| Sites of involvement | ||
| Nodal sites (including spleen) | 10 | 14 |
| Extranodal sites (excluding BM) | 3 | 2 |
| BM involvement (histologic) | 7 | 11 |
| BM involvement (PCR only) | 3 | 4 |
| Lymphoma cells in PB (morphologic) | 3 | 6 |
| Characteristic . | High-dose sequential therapy (n = 10) . | Rituximab + high-dose sequential therapy (n = 15) . |
|---|---|---|
| Sex (male/female) | 5/5 | 11/4 |
| Median age, y (range) | 46 (36-53) | 43 (34-58) |
| Histologic findings | ||
| Follicular disease (grade 1 or 2) | 6 | 6 |
| Follicular disease (grade 3) | 1 | 1 |
| Mantle cell disease | 3 | 7 |
| Mucosa-associated lymphoid tissue lymphoma | — | 1 |
| Molecular rearrangement | ||
| Bcl1 | 3 | 3 |
| Bcl2 | 6 | 4 |
| IgH | 1 | 8 |
| Stage | ||
| III | 1 | 4 |
| IV | 9 | 11 |
| B-cell lymphoma symptoms | 0 | 4 |
| Mass greater than 10 cm | 3 | 0 |
| Sites of involvement | ||
| Nodal sites (including spleen) | 10 | 14 |
| Extranodal sites (excluding BM) | 3 | 2 |
| BM involvement (histologic) | 7 | 11 |
| BM involvement (PCR only) | 3 | 4 |
| Lymphoma cells in PB (morphologic) | 3 | 6 |
BM indicates bone marrow; PCR, polymerase chain reaction; and PB, peripheral blood.
Treatment plan
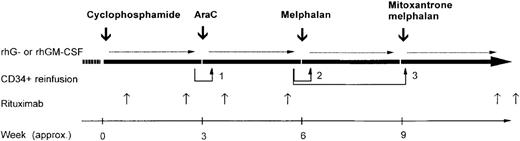
All patients who had not previously received doxorubicin were initially treated with 2 to 3 courses of this agent (75 mg/m2 of body-surface area given intravenously on day 1), prednisone (40 mg/m2 orally given from day 1 to day 21), and vincristine (1.4 mg/m2 given intravenously on day 1). Patients with refractory or relapsed disease who had received doxorubicin previously and patients with little or no response to the initial course described above received 2 to 3 cycles of cisplatin–high-dose cytarabine–dexamethasone chemotherapy.13 After the initial standard-dose phase, all patients received the 4-step high-dose sequence shown in Figure 1, including intravenous administration of high-dose cyclophosphamide (7 g/m2),14 high-dose cytarabine (either 1.5 or 2 g/m2 every 12 hours for 6 consecutive days),15high-dose melphalan (180 mg/m2),16 and high-dose mitoxantrone plus melphalan (60 and 180 mg/m2, respectively).17 The protocol entailed high-dose drug administrations every 3 weeks, depending on hematologic and nonhematologic toxicity. Patients with lesions larger than 5 cm at study entry or with documented or suspected residual disease after the end of treatment received consolidation radiotherapy according to procedures described previously.18
Overall treatment plan, including both the in vivo purging phase (cyclophosphamide and cytarabine) and subsequent myeloablative antilymphoma therapy (melphalan and mitoxantrone plus melphalan).
The doses were as follows: cyclophosphamide, 7 g/m2 of body-surface area; cytarabine, 1.5 to 2 g/m2 every 12 hours for 6 consecutive days; recombinant granulocyte colony-stimulating factor (CSF) or recombinant granulocyte-macrophage CSF, 5 μg/kg of body weight/day; reinfusion 1, 2 × 106CD34+ cells/kg, irrespective of polymerase chain reaction (PCR) status; and reinfusions 2 and 3, 5 × 106 and at least 8 × 106 CD34+ cells/kg, respectively, only if PCR negative. Intervals between cycles are approximate.
Overall treatment plan, including both the in vivo purging phase (cyclophosphamide and cytarabine) and subsequent myeloablative antilymphoma therapy (melphalan and mitoxantrone plus melphalan).
The doses were as follows: cyclophosphamide, 7 g/m2 of body-surface area; cytarabine, 1.5 to 2 g/m2 every 12 hours for 6 consecutive days; recombinant granulocyte colony-stimulating factor (CSF) or recombinant granulocyte-macrophage CSF, 5 μg/kg of body weight/day; reinfusion 1, 2 × 106CD34+ cells/kg, irrespective of polymerase chain reaction (PCR) status; and reinfusions 2 and 3, 5 × 106 and at least 8 × 106 CD34+ cells/kg, respectively, only if PCR negative. Intervals between cycles are approximate.
Rituximab (Roche, Milan, Italy) was diluted and administered as an intravenous infusion according to the manufacturer's instructions. Patients in the R-HDS group received 6 overall infusions of the antibody (375 mg/m2). Rituximab infusions 1 and 2 were administered 48 hours after cyclophosphamide and 24 hours before the first leukapheresis, respectively. Rituximab infusions 3 and 4 were given 24 hours after the last dose of cytarabine and 24 hours before the first planned leukapheresis after administration of cytarabine, respectively. Rituximab infusions 5 and 6 were given approximately 28 and 35 days, respectively, after the final stem cell autograft, after administration of mitoxantrone and melphalan.
The supportive care given during the entire course of HDS therapy (including administration of a hematopoietic growth factor) was described in detail previously.18 19 To hasten hematopoietic recovery and reduce hematologic toxicity, each patient received 3 progenitor/stem cell reinfusions, both after the nonmyeloablative course of high-dose cytarabine (reinfusion 1) and after the 2 subsequent submyeloablative/myeloablative courses of melphalan and mitoxantrone plus melphalan (reinfusions 2 and 3, respectively). The minimum target doses of CD34+ cells per kg of body weight to be reinfused after each of the 3 autografts were 2 × 106, 3 × 106, and 5 × 106, respectively. The small amount of CD34+ cells to be reinfused after cytarabine treatment was allowed to contain PCR-detectable disease, whereas autografting of products free of residual lymphoma was mandatory after administration of melphalan (reinfusion 2) and mitoxantrone plus melphalan (reinfusion 3). Each patient underwent the minimum amount of peripheral blood harvesting necessary to achieve this goal.
The timing and number of collections were prospectively guided by real-time assessment of circulating progenitor counts,20 as well as by results of overnight PCR analysis on a sample of leukapheresed cells. If the first harvest after cyclophosphamide was both quantitatively adequate for all 3 reinfusions (ie, it yielded ≥ 10 × 106 CD34+ cells/kg) and PCR negative, no additional leukaphereses were performed. If these criteria were not met , a second harvest was attempted during the rapid recovery phase that followed high-dose cytarabine therapy, and the cells were stored overnight at 4°C until the results of PCR analysis were available. If the cells were still PCR positive, the harvested cells were incubated with anti-CD19 monoclonal antibody and subjected to in vitro negative selection using a Miltenyi Super MACS cell-sorting system.21 The overall purging procedure, including (if required) ex vivo purging, was considered successful when it was possible to harvest a minimum of 8 × 106PCR-negative CD34+ cells/kg, ie, the total amount required for reinfusions 2 and 3.
Monitoring, harvesting, and processing of hematopoietic progenitor cells
Hematopoietic progenitor cells in peripheral blood and leukapheresis components were assessed by counting total CD34+cells by direct immunofluorescence flow cytometry with the phycoerythrin-conjugated HPCA-2 CD34 antibody (Becton Dickinson, San Jose, CA) as previously described.21
Leukaphereses were performed during growth-factor expanded mobilization of progenitor cells occurring after administration of either high-dose cyclophosphamide, high-dose cytarabine, or both. On the first recovery day after chemotherapy that the CD34+ cell count reached at least 0.02 × 109/L, the patients received 1 dose of rituximab; 24 hours later, they underwent leukapheresis procedures until the target number of CD34+cells was collected. Mononuclear cells were collected with use of a novel automated leukapheresis system (Auto PBSC Spectra; COBE, Lakewood, CO), as previously described.22
Molecular monitoring of MRD was accomplished by assessing DNA samples from peripheral blood, bone marrow, and leukapheresis products with use of either nested PCR amplification of either the bcl-2/IgH or bcl-1/IgH translocation or semi-nested amplification of clonal rearrangement of IgH genes, essentially as described by Corradini et al9 and Andersen et al.23 The limit of detection of MRD was reproducible at the level of 10−6 for the bcl-2/IgH translocation and at 10−5 for the other 2 rearrangements. The progenitor cells to be autografted were processed, cryopreserved, thawed, and reinfused as described previously.24
Response to treatment and statistical analysis
Complete response to treatment was defined according to international working group recommendations, which entail complete disappearance of all detectable clinical and radiographic evidence of disease, regression of lymph nodes and any enlarged organ to normal size, and clearance of lymphoma involvement in the bone marrow on morphologic analysis.25
The characteristics of the patients and their response to treatment were compared with the Fisher exact test. The significance of differences among the different groups in myelosuppression duration, yield of CD34+ cells, number of leukaphereses performed, and transfusions required was calculated with the nonparametric Mann-Whitney U test or t test, as appropriate.
Results
Patient characteristics
The characteristics of the 25 patients enrolled into the study are listed in Table 1. All 15 patients with follicular or marginal-zone lymphoma had previously received 1 line of polychemotherapy (either cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone [CHOP]; cyclophosphamide, vincristine, and prednisone; or fludarabine, mitoxantrone, and dexamethasone), with or without local-regional radiotherapy. At the time of entry into the study, these patients had progression of disease requiring treatment that occurred either after an initial partial response (12 patients) or a complete response that lasted less than 1 year (3 patients). The bcl-2/IgH rearrangement was detected in 10 of the 14 patients with follicular lymphoma. All 15 patients in this subgroup had PCR-detectable disease in the bone marrow; in 10, histologic studies showed marrow involvement.
The diagnosis of MCL was confirmed in all 10 enrolled cases by expression of CD5 and lack of expression of CD23 on the tumor cells. No patient with MCL had previously received chemotherapy or radiotherapy. The bcl-1/IgH rearrangement was detected in 6 patients and absence of the bcl-2/IgH rearrangement in all 10. All MCL patients had involvement of multiple nodal sites and histologic evidence of bone marrow involvement, which was extensive in 7. The latter patients had also circulating lymphoma cells in the peripheral blood, confirmed by immunophenotyping (ie, CD20+, CD5+, and CD23− cells), in amounts ranging from 0.04 × 109/L to more than 1.0 × 109/L (in 4 patients).
Table 1 shows that the patient characteristics that might have influenced the purging (ie, disease extent and bone marrow and peripheral blood involvement by lymphoma cells) were equally represented in the 2 groups. The major differences between the groups were a higher number of men, MCL diagnoses, and molecular diseases (IgH rearrangements) in the R-HDS group. All these differences most likely reflect a single factor, ie, a chance overrepresentation of mantle cell histologic features in the group that received rituximab. In fact, MCL occurs predominantly in men,26 and because of considerable heterogeneity in the site of the underlying molecular rearrangement, the bcl-1/IgH translocation can be amplified by PCR in less than half of MCLs.23
Mobilization and harvesting of CD34+ cells after cyclophosphamide and cytarabine
Table 2 shows the peak values of CD34+ cells circulating in the peripheral blood during the growth-factor–supported recovery phase, with and without rituximab infusion. No significant differences were observed among the 4 groups analyzed, indicating that rituximab infusion had no adverse effect on mobilization and that previous cyclophosphamide treatment did not impair progenitor mobilization after cytarabine administration, which was done soon afterward. This surprising lack of negative influence27 was confirmed by statistical analysis with a paired test of peak values in the same patient. Likewise, no significant differences were observed among the 4 groups in the day of the first apheresis, the number of aphereses, and the total number of CD34+ cells harvested (Table 2).
Results of the aphereses
| Group/patient no. . | Post-cyclophosphamide harvests . | Post-cytabarine harvests . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis . | Probe . | No. of aphereses (day of 1st apheresis) . | CD34+ (×106/kg) . | Results of PCR analysis . | No. of aphereses (day of 1st apheresis) . | CD34+ (×105/kg) . | Results of PCR analysis . | CD34+ (×106/kg) after ex vivo purging . | PCR analysis after ex vivo purging . | |
| HDS (n = 10) | ||||||||||
| 1 | MCL | bcl1 | 2 (13) | 24.3 | + | 2 (12) | 24.9 | − | ||
| 2 | MCL | bcl1 | 1 (13) | 28.8 | + | 2 (14) | 29.9 | + | 8.6 | − |
| 3 | Foll | bcl2 | 1 (12) | 2.4 | + | 2 (12) | 45 | + | 12.2 | − |
| 4 | Foll | bcl2 | 1 (13) | 3 | + | 2 (12) | 12.9 | + | 7.6 | − |
| 5 | Foll | bcl2 | 1 (16) | 5.7 | − | 2 (14) | 20.4 | − | ||
| 6 | Foll | bcl2 | 1 (13) | 8.3 | + | 3 (13) | 11.8 | − | ||
| 7 | Foll | bcl2 | 1 (12) | 17 | + | 1 (12) | 17.8 | + | 5.3 | + |
| 8 | Foll | bcl2 | 1 (13) | 14.5 | + | 2 (13) | 33.6 | + | 9.2 | − |
| 9 | MCL | bcl1 | 1 (12) | 16.1 | + | 1 (13) | 37 | − | ||
| 10 | Foll | IgH | ND | 1 (17) | 3.2 | + | ||||
| R-HDS (n = 15) | ||||||||||
| 11 | Foll | bcl2 | 1 (13) | 15 | + | 1 (13) | 65.2 | − | ||
| 12 | Foll | IgH | 1 (17) | 14.8 | − | ND | ||||
| 13 | Foll | bcl2 | 1 (17) | 2.3 | + | 1 (14) | 17.5 | − | ||
| 14 | MCL | IgH | 2 (15) | 13 | − | ND | ||||
| 15 | Foll | bcl2 | 1 (15) | 33.2 | − | ND | ||||
| 16 | Foll | IgH | 1 (15) | 21.6 | − | ND | ||||
| 17 | Foll | bcl2 | 1 (33) | 4.2 | + | 2 (12) | 23.6 | − | ||
| 18 | MCL | bcl1 | 1 (15) | 17.6 | + | 2 (12) | 26.6 | − | ||
| 19 | MCL | bcl1 | 1 (10) | 24.4 | + | 1 (10) | 32.4 | − | ||
| 20 | MALT | IgH | 1 (17) | 2.6 | + | 1 (13) | 14 | + | ND | |
| 21 | MCL | IgH | 1 (14) | 12 | + | 1 (13) | 63.6 | − | ||
| 22 | MCL | IgH | 1 (14) | 8 | + | 2 (13) | 15 | − | ||
| 23 | MCL | IgH | 1 (17) | 7 | + | 1 (13) | 28 | − | ||
| 24 | MCL | bcl1 | 1 (13) | 5.8 | + | 2 (12) | 16 | − | ||
| 25 | Foll | IgH | 2 (15) | 7.8 | − | 1 (11) | 6.3 | − | ||
| Group/patient no. . | Post-cyclophosphamide harvests . | Post-cytabarine harvests . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis . | Probe . | No. of aphereses (day of 1st apheresis) . | CD34+ (×106/kg) . | Results of PCR analysis . | No. of aphereses (day of 1st apheresis) . | CD34+ (×105/kg) . | Results of PCR analysis . | CD34+ (×106/kg) after ex vivo purging . | PCR analysis after ex vivo purging . | |
| HDS (n = 10) | ||||||||||
| 1 | MCL | bcl1 | 2 (13) | 24.3 | + | 2 (12) | 24.9 | − | ||
| 2 | MCL | bcl1 | 1 (13) | 28.8 | + | 2 (14) | 29.9 | + | 8.6 | − |
| 3 | Foll | bcl2 | 1 (12) | 2.4 | + | 2 (12) | 45 | + | 12.2 | − |
| 4 | Foll | bcl2 | 1 (13) | 3 | + | 2 (12) | 12.9 | + | 7.6 | − |
| 5 | Foll | bcl2 | 1 (16) | 5.7 | − | 2 (14) | 20.4 | − | ||
| 6 | Foll | bcl2 | 1 (13) | 8.3 | + | 3 (13) | 11.8 | − | ||
| 7 | Foll | bcl2 | 1 (12) | 17 | + | 1 (12) | 17.8 | + | 5.3 | + |
| 8 | Foll | bcl2 | 1 (13) | 14.5 | + | 2 (13) | 33.6 | + | 9.2 | − |
| 9 | MCL | bcl1 | 1 (12) | 16.1 | + | 1 (13) | 37 | − | ||
| 10 | Foll | IgH | ND | 1 (17) | 3.2 | + | ||||
| R-HDS (n = 15) | ||||||||||
| 11 | Foll | bcl2 | 1 (13) | 15 | + | 1 (13) | 65.2 | − | ||
| 12 | Foll | IgH | 1 (17) | 14.8 | − | ND | ||||
| 13 | Foll | bcl2 | 1 (17) | 2.3 | + | 1 (14) | 17.5 | − | ||
| 14 | MCL | IgH | 2 (15) | 13 | − | ND | ||||
| 15 | Foll | bcl2 | 1 (15) | 33.2 | − | ND | ||||
| 16 | Foll | IgH | 1 (15) | 21.6 | − | ND | ||||
| 17 | Foll | bcl2 | 1 (33) | 4.2 | + | 2 (12) | 23.6 | − | ||
| 18 | MCL | bcl1 | 1 (15) | 17.6 | + | 2 (12) | 26.6 | − | ||
| 19 | MCL | bcl1 | 1 (10) | 24.4 | + | 1 (10) | 32.4 | − | ||
| 20 | MALT | IgH | 1 (17) | 2.6 | + | 1 (13) | 14 | + | ND | |
| 21 | MCL | IgH | 1 (14) | 12 | + | 1 (13) | 63.6 | − | ||
| 22 | MCL | IgH | 1 (14) | 8 | + | 2 (13) | 15 | − | ||
| 23 | MCL | IgH | 1 (17) | 7 | + | 1 (13) | 28 | − | ||
| 24 | MCL | bcl1 | 1 (13) | 5.8 | + | 2 (12) | 16 | − | ||
| 25 | Foll | IgH | 2 (15) | 7.8 | − | 1 (11) | 6.3 | − | ||
PCR indicates polymerase chain reaction; HDS, high-dose sequential therapy; MCL, mantle cell lymphoma; Foll, follicular lymphoma; ND, not done because of failure of mobilization after high-dose cyclophosphamide; R-HDS, rituximab and high-dose sequential therapy; and MALT, marginal-zone lymphoma (mucosa-associated lymphoid tissue type).
PCR analysis of bone marrow, peripheral blood, and leukapheresis products
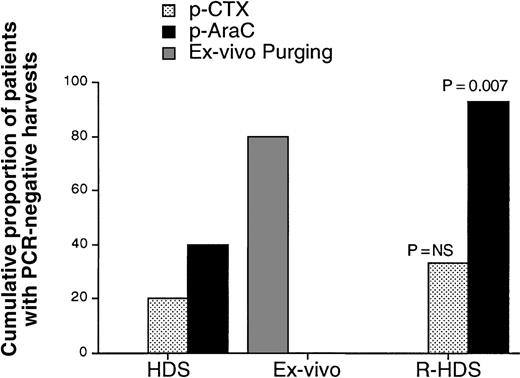
Major and significant differences were observed in the ability to harvest PCR-negative CD34+ cells. As shown in Table 2 and Figure 2, after administration of cyclophosphamide, PCR-negative products were harvested in 2 patients (20%) who did not receive rituximab but in 5 patients (33%) who did. On the other hand, after administration of cytarabine, the proportion of harvests free of lymphoma cells increased to 40% in the HDS group and to 93% in the R-HDS group (P = .007).
Cumulative proportion of patients from whom PCR-negative harvests were obtained after either cyclophosphamide (p-CTX), cytarabine (p-AraC), or cytarabine plus ex vivo purging (ex vivo purging).
The latter group includes only patients in the high-dose sequential therapy arm.
Cumulative proportion of patients from whom PCR-negative harvests were obtained after either cyclophosphamide (p-CTX), cytarabine (p-AraC), or cytarabine plus ex vivo purging (ex vivo purging).
The latter group includes only patients in the high-dose sequential therapy arm.
Ex vivo purging was planned for all 7 patients in whom in vivo purging had failed (6 patients in the HDS group and 1 in the R-HDS group), but it was actually performed in 5. The other 2 patients did not undergo ex vivo purging because of lack of an adequate number of CD34+cells (patient 10) and mechanical failure of the cryopreservation device (patient 20).
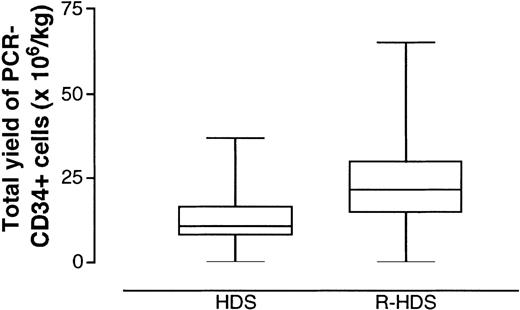
Thus, the rate of success in harvesting PCR-negative products remained higher in the R-HDS group, although not significantly so, even after ex vivo purging (93% versus 80%; Figure 2). This qualitative advantage was strengthened by quantitative differences in the overall yield of CD34+/PCR-negative cells between the 2 groups (Figure3). In fact, when we used an intent-to-treat analysis to compare the ability of the 2 different procedures to yield lymphoma-free progenitors, the R-HDS regimen was clearly found to be superior (mean yield of CD34+/PCR-negative cells per kg, 26 versus 13;P = .029).
Total yield of PCR-negative and CD34+ cells harvested from the peripheral blood.
Each patient underwent the minimum number of procedures sufficient to complete the therapeutic program (Figure 1). Boxes extend from the 25th to the 75th percentile, with the horizontal line indicating the median. Whiskers extend from the largest to smallest values.
Total yield of PCR-negative and CD34+ cells harvested from the peripheral blood.
Each patient underwent the minimum number of procedures sufficient to complete the therapeutic program (Figure 1). Boxes extend from the 25th to the 75th percentile, with the horizontal line indicating the median. Whiskers extend from the largest to smallest values.
The in vivo purging efficacy of the combination of high-dose chemotherapy and rituximab was further emphasized by a subgroup analysis of patients with MCL, a disease well known to be recalcitrant to both in vivo9 and in vitro purging.23 In fact, in vivo purging was successful in all 7 MCL patients in the R-HDS group. Of note, 4 of these 7 patients (patients 18, 19, 21, and 22) had initial massive bone marrow involvement and leukemic findings in the peripheral blood (basal CD5+/CD23−mononuclear cells counts of 0.18, 1.7, 3.8, and 6.9 × 109/L , respectively).
Informative data were derived from simultaneous PCR analyses of bone marrow, peripheral blood, and leukapheresed cells done both after administration of cyclophosphamide and after administration of cytarabine. If the bone marrow was PCR positive, leukapheresis products were invariably PCR positive in the HDS group (12 of 12 cases). Interestingly, in the R-HDS group, 4 of 13 leukaphereses done in patients with PCR-positive bone marrow were PCR negative. A lack of absolute concordance between the molecular status of bone marrow and leukapheresis products also emerged from analysis of patients with PCR-negative bone marrow. In fact, of 24 total leukaphereses performed in patients with PCR-negative bone marrow, 4 showed a PCR-positive signal (3 in the HDS group and 1 in the R-HDS group). Similarly low was the concordance between peripheral blood and leukapheresis products. In fact, although all 18 patients with PCR-positive blood had PCR-positive aphereses, the reverse was not true: 8 of 18 harvests from patients with PCR-negative peripheral blood were contaminated with lymphoma cells. Thus, the molecular status of neither bone marrow nor peripheral blood could be used as a convenient surrogate test for predicting tumor-free leukaphereses and prospectively guiding harvesting procedures.
Hematopoietic recovery after autografting
Rituximab treatment had no adverse effect on the capacity of in vivo purged cells to support a rapid and complete hematopoietic reconstitution after the final myeloablative course (mitoxantrone and melphalan). In fact, no significant difference was observed between the HDS and R-HDS groups in the mean number of days spent with less than 0.1 ×109 neutrophils/L (5.5 versus 6.2 days) and less than 0.5 ×109 neutrophils/L (7.4 versus 7.7 days), whereas a borderline or significantly favorable effect was observed for platelet recovery. The patients who received autografts of cells exposed in vivo to rituximab had a mean of 7 days with a platelet count below 25 × 109/L (versus 13.6 days in the HDS patients; P = .59) and required significantly fewer single-donor platelet transfusions (mean, 5 versus 10; P = .05). They also required fewer transfusions of red blood cells (mean, 3.6 versus 9;P = .03). Both beneficial effects most likely reflected the higher dose of CD34+ cells autografted in the R-HDS patients (Figure 3).
Overall treatment toxicity and tumor response
There were 2 treatment-related deaths, 1 in each group. Both occurred after the second autotransplantation. One patient with a history of cardiac arrhythmia died suddenly on day 14 after mitoxantrone and melphalan administration after an otherwise uneventful recovery, and 1 patient positive for antibody to hepatitis B core antigen (HBcAb) and negative for HBsAg died of fulminant hepatitis B on day 180 after administration of mitoxantrone and melphalan.
The 15 patients enrolled in the study received a total of 88 infusions of rituximab (375 mg/m2). Only 2 patients (2.3%) had adverse events with infusion. Both events—development of a rash in 1 patient and dyspnea in the other—occurred during the first infusion only and were rated as being of grade 1 severity.
Of the 10 evaluable patients who received HDS, 1 patient died of fulminant hepatitis B, 2 additional patients did not achieve complete remission and died of disease progression, and 1 patient never attained molecular eradication of the disease in the bone marrow. After a median follow-up duration of 10 months, 7 patients in the HDS group were free of both clinical and molecular disease. Of the 14 R-HDS patients evaluable for response (1 died as a result of toxicity too early for an accurate assessment), all achieved a complete clinical and molecular response and all were relapse-free after a median follow-up duration of 14 months. Of note, all 9 evaluable patients with MCL achieved a complete and durable response.
Discussion
Contamination of bone marrow collections by tumor cells can contribute to relapse. This fact, proved formally by gene-marking studies,3-5 represents the strongest argument for using measures to eliminate malignant cells from the graft (purging). In fact, although it will be difficult to proof definitively that purging has a role in improving disease-free survival, it is hard to justify immediately following the delivery of a myeloablative therapy course aimed at eradicating disease with an intravenous infusion of tumor cells capable of causing relapse.
The ex vivo techniques for purging, while usually effective in reducing by several logs the amount of contaminating tumor cells, have severe limitations. In fact, these techniques are labor intensive, they may delay bone marrow recovery as a consequence of substantial loss and variable damage of stem and committed progenitor cells, and they increase costs. Furthermore, elimination of accessory and lymphoid cells by techniques for selecting stem cells might adversely influence immune reconstitution or antitumor effects. A variety of strategies have been proposed as alternatives to ex vivo purging. Collectively referred to as “in vivo purging,” these methods are aimed at reducing or eliminating contaminating cells by means of treatments delivered to or procedures carried out in patients. The techniques include harvesting of peripheral blood instead of marrow cells (a so-far largely unproved method) and administration of cytotoxic drugs9,28-30 or monoclonal antibodies before the harvest.12,31 However, in many cases, successful in vivo purging has only been inferred from indirect clinical data,29-31 whereas in others, residual neoplastic cells were detected by immunophenotypic or cytogenetic methods with relatively low sensitivity and specificity. Indeed, when Boquéand coworkers32 used reverse transcriptase-PCR to analyze hematopoietic progenitors harvested from patients with chronic myeloid leukemia according to the program developed by Carella et al,28 all Philadelphia chromosome–negative bags were positive for the bcr/abl translocation.
Proof that an intensive in vivo chemotherapy treatment can foster the subsequent collection of lymphoma-free progenitors was provided by Corradini et al,9 who harvested PCR-negative peripheral blood products from 1 of 9 patients (12%) with mantle cell lymphoma and 8 of 19 (42%) with follicular lymphoma. Furthermore, toxicologic studies in cynomolgus monkeys10 showed that infusion of the monoclonal antibody rituximab caused a rapid and reproducible clearance of CD20+ cells from the blood of treated animals. These 2 observations provided the rationale for the current study. In fact, only subsequently12 was it reported that a combination of rituximab and CHOP chemotherapy converted bcl-2/IgH–positive marrow to PCR negativity in a subset of patients with low-grade B-cell lymphoma, a finding that provides additional independent support for our in vivo purging strategy.
The present study demonstrated that in vivo purging can be accomplished successfully in most CD20+ mantle cell and follicular lymphomas (93%) and that successful purging resulted from both chemotherapy and immunotherapy. The role of chemotherapy was confirmed by the facts that chemotherapy alone was able to provide PCR-negative harvests in 40% of cases (Figure 2) and that the proportion of PCR-negative products was invariably higher when harvesting was carried out after the second course of chemotherapy (ie, high-dose cytarabine) (Figure 2 and Table 2). These results are in agreement with those reported by Corradini et al.9
The important role of rituximab was demonstrated by the fact that PCR-negative harvests were obtained from 93% of the patients who had received 4 rituximab infusions but only 40% of the controls (P = .007; Figure 2). A possible bias in our assessment of the in vivo purging role of rituximab was the higher proportion of patients in the R-HDS group whose MRD was examined by PCR amplification of the less sensitive IgH rearrangement. This unbalance reflects the presence of a higher number of patients in the R-HDS group than in the HDS (8 [53%] versus 3 [33%]) with histotypes for which a bcl-2/IgH probe was not applicable (7 cases of MCL and 1 case of mucosa-associated lymphoid tissue lymphoma). The in vivo purging role of rituximab in this small population could not be determined by subset analysis but is strongly suggested by comparison with results obtained in studies using the same technique adopted here to detect MRD in bone marrow23 and peripheral blood harvests9,33 from patients with MCL. In fact, all 7 patients in the R-HDS group had PCR-negative harvests. For comparison, in the study by Corradini et al,9 high-dose chemotherapy alone was effective in 1 of 9 cases, whereas immunologic ex vivo purging failed to eradicate PCR-detectable disease in 15 of the 17 MCL patients reported on by Andersen et al23 and all 3 MCL patients treated by Tarella et al.33
The addition of ex vivo purging converted to PCR negativity the harvests from 5 of the 6 HDS patients in whom in vivo purging had failed (Table 2). This increased the total success rate in the HDS group to 80%, close to the 93% rate in the R-HDS arm (P not significant). However, the total yield of CD34+ cells was significantly lower in the HDS group (P = .029; Figure 3), a finding that could explain the delayed marrow recovery observed in those patients. In fact, recovery after transplantation, especially platelet recovery, is directly correlated with CD34+ cell dose.8
Of note, although administration of high-dose cytarabine occurred close to administration of high-dose cyclophosphamide, it did not influence the extent of CD34+ cell mobilization (Table 2). This effect was surprising and in contrast with our previous finding that short intervals between high-dose drug courses severely impair progenitor mobilization.27 However, a different sequence of drugs was used here (ie, cyclophosphamide followed by cytarabine compared with etoposide followed by cyclophosphamide in our previous study27), and recovery after cytarabine was hastened by reinfusion of progenitor cells.
Finally, in assessing overall efficacy, it is tempting to speculate that the very high complete response rate observed in the R-HDS group might reflect an important therapeutic role of rituximab given during maximal expansion and activation of the granulocyte-macrophage system by concomitant treatment with growth factors.34
Acknowledgments
We thank Dr Marco Bregni, Dr Salvatore Siena, and the staff at the various INT Units for expert patient care; Marco Milanesi and Paolo Longoni for technical assistance; and Dr Luca Baldini, Dr Attilio Gabbas, Dr Giovanni Martinelli, Dr Enrico Pogliani, and Dr Carlo Tondini for referring patients with MCL.
Supported in part by AIRC and by Ministero della Sanitàgrant ICS 030.1/RF96.278.
M.M. and M.D.N. contributed equally to this work.
Reprints:Alessandro M. Gianni, Cattedra di Oncologia Medica, Istituto Nazionale Tumori, Via Venezian 1, 20133 Milan, Italy; e-mail:alessandro.gianni@unimi.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal