Abstract
To examine the effect of lymphocyte specific protein 1 (LSP1) on phagocytic cell motility, stable transfection of LSP1-null U937 cell line with an episomal expression vector carrying the LSP1 complementary DNA created lines expressing varied LSP1 levels. Mock transfectants without LSP1 (U937−) and cell lines with LSP1 levels similar to those of monocytes (U937+) or 4-fold those of monocytes (U937++++) express LSP1 as indicated and express other actin-binding proteins at normal levels before or after monocytic induction (MI) with dibutyryl cyclic adenosine monophosphate. The cell lines were compared for rate of growth and cell division and, after monocytic differentiation, were video-tracked to measure locomotion as distance moved in 2 hours and examined for morphologic changes. Rates of cell division and growth were similar for different U937 cell lines at all LSP1 levels. In contrast, mean rate of locomotion (micrometers moved in 2 hours) was slower in MI–U937++++ (7.78 + 1.11μm, n = 3) and MI-U937− (23.89 + 2.78μm, n = 3) than in MI-U937+ cells (50.77 + 4.11μm, n = 3). Compared with MI-U937−, the locomotive histogram (n = 150 cells) of MI-U937+ or MI-U937++++ cells shows all cells move respectively faster or slower as an entire cell population. In LSP1+ U937 phagocytes, high LSP1 levels inhibit some (locomotion) but not all (cytokinesis) cell motile behaviors and cause the formation of surface projections. In contrast, normal LSP1 levels in U937 phagocytes enhance some (locomotion) but not all (cytokinesis) cellular motile behaviors and have no effect on cell morphology. Therefore, LSP1 level has a unique biphasic effect on cellular locomotion. The data suggest LSP1 is an important regulator of phagocyte locomotion.
In its most general sense, cell motility depends upon dynamic rearrangement of the F-actin–based microfilamentous cytoskeleton and refers to multiple cellular behaviors, including cell division or cytokinesis, locomotion, phagocytosis, chromosome movement, and cell spreading. Dynamic rearrangements of the F-actin–based microfilamentous cytoskeleton are modulated by F- and G-actin interaction with actin-binding and regulatory proteins that control changes in the organization of F-actin filaments and the G- to F-actin equilibrium.
Lymphocyte specific protein 1 (LSP1; also known as WP34, pp52, and leufactin) is a 52-kd F-actin–binding phosphoprotein expressed in all human leukocytes and leukocytic cells lines except the U937 cell line.1-4 Increased amount of LSP1 (4- to 5-fold control) and “hairlike” surface projections are 2 of the characteristics of neutrophils from patients with a unique inherited disorder: neutrophil actin dysfunction with abnormal 47-kd and 89-kd proteins (NAD 47/89).5,6 The rare individuals affected by NAD 47/89 have a congenital defect in neutrophil motility that predisposes them to recurrent infections. To determine if LSP1 affects nonmuscle cell locomotion, Howard et al7 have studied the locomotion of A7 melanoma cells stably transfected to express LSP1. Resultant LSP1 expression inhibited melanoma cell locomotion and re-created the motile and morphologic phenotype characteristic of NAD 47/89 neutrophils in this nonleukocyte cell line. However, the effect of normal and elevated LSP1 levels in phagocytic leukocytes is not known.
Since LSP1 is leukocyte-specific and phagocytes are motile, we used an LSP1-null U937 cell line to examine the effect of normal and high LSP1 level on phagocyte motility and morphology. The U937 cell line is a myelomonocytic cell line that can differentiate into monocytelike cells. Dibutyryl cyclic adenosine monophosphate (db-cAMP), a membrane-permeable analogue of cAMP, induces several functional changes in U937 cells, including increased expression of C5a and fMLP receptors, increased random migration and chemotaxis, increased expression of CR3 and FcRII, and increased nonspecific phagocytosis.8-11 Here we report that LSP1 is not expressed in U937 cells. Stable transfection of U937 cells with pCEP vector carrying the LSP1 complementary DNA creates unique U937 cell lines that express similar levels of several important actin-binding proteins (ABPs) and varied levels of LSP1 before and after monocytic differentiation of the U937 with db-cAMP. In the stably transfected cell lines, LSP1 levels similar to those in monocytes increase rates of cell locomotion but have no effect on cell morphology, while LSP1 levels greater than 4-fold those in monocytes inhibit the differentiated cell locomotion and cause the formation of surface projections as described in A7 melanoma cells. In contrast, varied LSP1 expression, high or low, does not significantly affect the transfected U937 cell division or rate of cell growth. These results show that LSP1 level modulates phagocytic cell motility and differentially affects 2 actomyosin-dependent motile functions in U937 cells. The results suggest LSP1 is an important regulator of phagocytic leukocyte motility.
Materials and methods
Monocyte isolation
Human peripheral blood (35 mL) was drawn into a 60-mL syringe containing 5 mL heparin solution (10 US Pharmacopeiaunits/mL). The whole blood was mixed with 35 mL of RPMI 1640. The mononuclear cells were isolated with the use of histopaque-1077 (Sigma Chemical, St. Louis, MO) and centrifugation. The mononuclear cells were incubated with CD14 microbeads (Miltenyi Biotec, Sunnyvale, CA), and monocytes were isolated by means of magnetic sorting.
Cell culture and transfection
U937 cells (gifts from Thomas A. Rado, University of Alabama, Birmingham, AL) were grown in RPMI 1640 (Fisher Scientific, Pittsburgh, PA) with 10% fetal calf serum (FCS) (HyClone, Logan, UT) and with 100 U/mL penicillin G and 100 μg/mL streptomycin (Fisher Scientific). U937 cells, which are LSP1−, were stably transfected with episomal vector pCEP4 (Invitrogen, San Diego, CA) (pCEP4-LSP1) via electroporation on gene pulsar transfection apparatus (Bio-Rad Laboratories, Hercules, CA). The electroporation was performed as described below. Cell density was 4 × 107/mL in phosphate-buffered saline (PBS) medium; the volume of cells was 900 μL; and DNA concentration was 50 μg/50μL. The cells were incubated with DNA for 30 minutes at room temperature, and the electroporation conditions were the following: cuvette gap was 0.4 cm; voltage was 0.2 kV; field strength was 0.5 kV/cm; capacitor was 960 microfarads; and time constant was 12 to 15 milliseconds. Transfected cells were cultured in medium containing 10% FCS for 2 days, and then 0.2 mg/mL hygromycin B (Boehringer Mannheim, Indianapolis, IN) was added into the culture medium.
Clonal selection in methylcellulose cultures
U937 cell concentration was set at 3 × 104cells/mL. Increasing cell numbers (5 μL, 15 μL, etc) were mixed with 4 mL of methylcellulose mixture, and then placed in 35-mm tissue-culture plates. The cells were cultured for 5 to 10 days. The individual colonies were picked up and resuspended in 0.5 mL RPMI 1640 and 10% FCS without hygromycin B in a 24-well plate. After 1 to 2 days, 0.15 mg/mL of hygromycin B was added to the medium. Methylcellulose mixture consisted of 10 mL of U937-conditioned medium, 8 mL of FCS, 1 mL of glutamine stock solution, 0.5 mL of 100 × penicillin/streptomycin solution, 20 mL of methylcellulose medium (StemCell Technologies, Vancouver, BC, Canada), 11 mL of RPMI 1640, and 50 U/L of 55 mmol/L β-mercaptoethanal (Life Technologies, Grand Island, NY).
Quantitative immunoblot analyses
U937 cells or monocytes were solubilized in sodium dodecyl sulfate (SDS) sample buffer. Protein concentration assays, SDS–polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblots with antihuman LSP1 monoclonal antibodies (mAbs) were performed as previously described.6 Quantitative analysis was performed by densitometry as previously described.4
Cell locomotion assays
Transfected U937 cell lines with variable expression of LSP1 were cultured on glass coverslips and treated with 1 mmol/L db-cAMP for 48 hours. Resulting cells were monocytelike with respect to morphology and histochemistry. Monocytic-differentiated U937 lines are nonspecific esterase-positive (approximately 85%) and generate superoxide as evidenced by reduction of nitroblue tetrazolium (approximately 70%). The cells were put into a Dvorak Stotler chamber, videotaped for 2 hours at 37°C, and tracked to determine the rates of locomotion of 100 cells as a function of LSP1 level as described.12
Cell growth and division assays
Transfected U937 cell lines with variable LSP1 in log-phase growth were inoculated in identical culture medium at the same density: 1 × 105/mL. The cell counts were done every 24 hours from days 1 to 4 to determine the rates of cell growth. Cell division was documented by differential counts of 500 cells at same-day growth, which were attached on the slides by cytospin and stained with Hoescht 33258 (Sigma, St Louis, MO), to determine the number of cells with 1, 2, or 3 nuclei per cell as a percentage of all cells as a function of LSP1 level.
Immunofluorescent staining and microscopy
MI–U937 cells were grown for 2 days on the coverslips, which were treated with poly-D-lysine (Sigma Chemical) for 30 minutes at 37°C before using. The cells on the coverslips were rinsed in PBS and then fixed in 5% formaldehyde (Tousimis, Rockville, MD) in PBS at room temperature for 45 minutes. The cells were washed in PBS and treated with 0.3% Triton X-100 (Sigma Chemical) in PBS for 1 minute. The cells were washed in PBS again and incubated in blocking buffer containing 1% bovine serum albumin and 5% goat serum (Sigma Chemical) in PBS at 37°C for 20 minutes. The cells were incubated with anti-LSP1 mAb (7B3.4) at 37°C for 1 hour. The cells were washed with PBS, incubated in the blocking buffer for 5 minutes, and then incubated with goat antimouse immunoglobulin G mAb conjugated with Oregon Green 488 (Molecular Probes, Eugene, OR) for 30 minutes at 37°C. The cells were washed and incubated with Texas Red-X phalloidin in PBS (1/50 dilution) at 37°C for 30 minutes. The cells were washed, mounted in the mounting media (Vectashield, Burlingame, CA), and sealed with nail polish. The samples were viewed in a Leitz fluorescent microscope (Wetzlar, Germany) equipped with a 100× phase contrast Phaco 3 objective, a 100× PL Fluotar objective and selective filters.
F-actin and LSP1 quantitation and partitioning
MI-U937 cells were washed in PBS, fixed with formalin (3.7%) for 15 minutes, and stained with a cocktail containing lysophosphatidylcholine (100 μg/mL) and NBD-phallacidin (3.3 × 10−7mol/L) (Molecular Probes, Salem, OR) for 10 minutes as previously described.13,14 Fluorescence was quantitated by flow cytometry. Partitioning study of F-actin and LSP1 was performed with antiactin and anti-LSP1 mAbs as previously described.13 14
Results
LSP1 is expressed at varied levels in stably transfected LSP1+ U937 cell lines
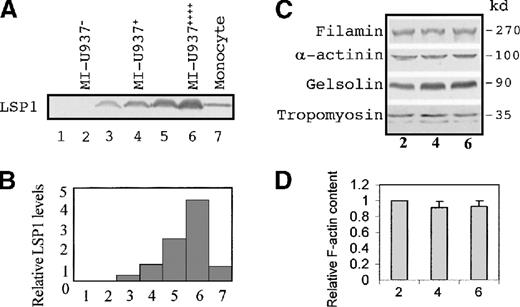
Unlike many actin-binding proteins,15 LSP1 is not expressed in nondifferentiated or differentiated monocytelike U937 cells. However, LSP1-null U937 cells stably transfected with the recombinant pCEP4-LSP1 vector and subcloned express varied levels of LSP1. Immunoblots of transfected cell lines (Figure1A-B) show LSP1 is expressed at varied levels in transfected cell lines (lane 3 and 5) but not in lines stably transfected with vector only (lane 1 and 2). To produce monocytelike motile cells, transfected cell lines were treated with db-cAMP for 2 days8-11 to induce monocyte differentiation. Since the cytomegalovirus immediate/early gene enhancer/promoter in the pCEP vector contains a functional cAMP-responsive element,16 LSP1 expression was doubled following monocytic induction with db-cAMP (lanes 4 and 6). LSP1 levels in the cell lines were compared with the level in peripheral blood monocytes (lane 7). We selected 3 stably transfected U937 cell lines differentiated to monocytelike cells to examine the effect of LSP1 on locomotion. The LSP1-expression levels observed in the cell lines were absent, equal to levels in a monocyte, and 4-fold the monocyte levels (Figure 1B), and the lines were respectively termed MI-U937−, MI-U937+, and MI-U937++++. Therefore, if known actin-binding proteins other than LSP1 are expressed in all 3 lines, the U937 cell lines can serve as useful tools to elucidate the effect of LSP1 on phagocyte motility.
LSP1, but not other actin-binding proteins, is expressed at varied levels in stably transfected, monocyte-differentiated U937 cell lines.
Shown in (A) are immunoblots with anti-LSP1 monoclonal antibody and in (B) the relative LSP1 levels in human monocytes (lane 7) and a variety of stably transfected U937 cell lines (lane 1-6). Quantitative analyses were performed on immunoblot by scanning densitometry. Results are expressed relative to monocytes. Lanes 1, 3, and 5 are U937 cell lines with negative, lower, and higher LSP1 expression, respectively. Lane 1 is the control U937 cell transfected with pCEP4 vector only. The cell lines used in the locomotion study are db-cAMP–treated above cell lines indicated as MI-U937− (lane 2), MI-U937+ (lane 4), and MI-U937++++ (lane 6), respectively. (C) Antifilamin, anti–α-actinin, antigelsolin, and antitropomyosin immunoblots of MI-U937−, MI-U937+, and MI-U937++++ cells. In each lane, 50 μg of total cellular proteins from these cells were loaded. Filamin, gelsolin, α-actinin, and tropomyosin are expressed at equivalent levels in stably transfected monocyte-differentiated U937 cell lines. (D) F-actin quantitative analysis performed on these MI cell lines by flow cytometry of the cells stained with NBD-phallacidin. Results are expressed relative to MI-U937− cells (lane 2) as mean ± SD (n = 4-5).
LSP1, but not other actin-binding proteins, is expressed at varied levels in stably transfected, monocyte-differentiated U937 cell lines.
Shown in (A) are immunoblots with anti-LSP1 monoclonal antibody and in (B) the relative LSP1 levels in human monocytes (lane 7) and a variety of stably transfected U937 cell lines (lane 1-6). Quantitative analyses were performed on immunoblot by scanning densitometry. Results are expressed relative to monocytes. Lanes 1, 3, and 5 are U937 cell lines with negative, lower, and higher LSP1 expression, respectively. Lane 1 is the control U937 cell transfected with pCEP4 vector only. The cell lines used in the locomotion study are db-cAMP–treated above cell lines indicated as MI-U937− (lane 2), MI-U937+ (lane 4), and MI-U937++++ (lane 6), respectively. (C) Antifilamin, anti–α-actinin, antigelsolin, and antitropomyosin immunoblots of MI-U937−, MI-U937+, and MI-U937++++ cells. In each lane, 50 μg of total cellular proteins from these cells were loaded. Filamin, gelsolin, α-actinin, and tropomyosin are expressed at equivalent levels in stably transfected monocyte-differentiated U937 cell lines. (D) F-actin quantitative analysis performed on these MI cell lines by flow cytometry of the cells stained with NBD-phallacidin. Results are expressed relative to MI-U937− cells (lane 2) as mean ± SD (n = 4-5).
LSP1+ and LSP1− U937 cell lines express other actin-binding proteins important for motility
Variations in the level of some actin-binding proteins, specifically ABP280 (filamin) and gelsolin, are known to affect cell motility. Therefore, use of the stably transfected U937 cell line to determine the effect of a single actin-binding protein such as LSP1 on motility requires that lines have similar levels of actin-binding proteins known to affect cell motility and varied LSP1 levels. Quantitative immunoblots for 4 critical actin-binding proteins—filamin, gelsolin, α-actinin, and tropomyosin (Figure 1C)—show that after db-cAMP induction, levels of these actin-binding proteins are similar in the stably transfected U937 cell lines, which vary in LSP1 level. Furthermore, the total actin per cell (data not shown) and the total F-actin levels in cells (Figure 1D) are similar for all 3 U937 cell lines after monocytic induction. Finally, immunoblots of transfected U937 Triton-insoluble cytoskeletons show the percentage of total LSP1 partitioned into the Triton-insoluble fraction from cells with low or high LSP1 level is similar: 15.94 ± 4.21% or 13.68 ± 7.21% (n = 4), respectively. After db-cAMP induction, the percentages of total LSP1 in MI-U937+ or MI-U937++++ Triton-insoluble cytoskeletons decrease to 4.32 ± 2.91% or 3.51 ± 1.66% (n = 4), respectively. The percentages of total cellular actin partitioned into the Triton-insoluble cytoskeleton (also known as Triton-insoluble F-actin) are 21.59 ± 1.58%, 14.81 ± 1.93%, and 10.37 ± 7.14% (n = 4) in null, low, and high LSP1 cells, respectively. After db-cAMP induction, the actin in Triton-insoluble cytoskeletons decreases to 13.89 ± 3.28%, 7.28 ± 3.54%, and 6.12 ± 2.98% (n = 4), respectively. The partitioning of actin and LSP1 is not statistically significantly different in cells with low and high LSP1 levels. Therefore, differences in the motile behaviors and the morphology of the U937 cell lines should reflect the effect of LSP1 and variations in its level alone.
Morphologic effects of LSP1 on MI-U937 cells depend on the level of LSP1 expression
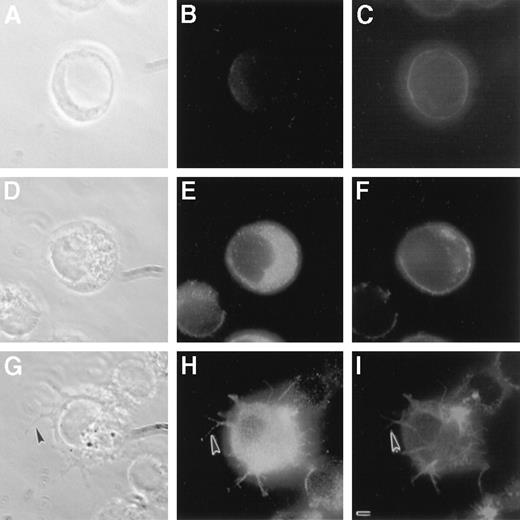
As shown in Figure 2, LSP1 staining is negative in MI-U937−, positive in MI-U937+, and most intense in MI-U937++++. The cellular F-actin distributes primarily to the cell periphery in MI-U937− and MI-U937+. These cells remain round with a smooth surface. In contrast, the MI-U937++++cells displayed surface projections that contain both F-actin and LSP1. Since the expression of F-actin, total actin content, and other critical actin-binding proteins is similar in all cells, the results show that normal LSP1 levels do not alter cellular cytoskeleton or morphology. In contrast, increased level of LSP1 causes formation of surface projections on U937 cells, and the morphologic effect of LSP1 depends on LSP1 level.
Morphologic effects of LSP1 on MI-U937 cells depend on the level of LSP1 expression.
MI-U937− (panels A, B, and C), MI-U937+, (panels D, E, and F), and MI-U937++++(panels G, H, and I) were stained with anti-LSP1 primary antibody with Oregon Green conjugated second antibody and with Texas Red phalloidin for F-actin. Photomicrographs shown are the phase-contrast images (panels A, D, and G), the LSP1 images (panels B, E, and H), and the F-actin images (C, F, and I) of the same cells. Note surface projections with LSP1 and F-actin (arrowhead) in MI-U937++++ cells, but not MI-U937+. The bar is 2 μm.
Morphologic effects of LSP1 on MI-U937 cells depend on the level of LSP1 expression.
MI-U937− (panels A, B, and C), MI-U937+, (panels D, E, and F), and MI-U937++++(panels G, H, and I) were stained with anti-LSP1 primary antibody with Oregon Green conjugated second antibody and with Texas Red phalloidin for F-actin. Photomicrographs shown are the phase-contrast images (panels A, D, and G), the LSP1 images (panels B, E, and H), and the F-actin images (C, F, and I) of the same cells. Note surface projections with LSP1 and F-actin (arrowhead) in MI-U937++++ cells, but not MI-U937+. The bar is 2 μm.
LSP1 modulates the rate of cell locomotion in monocyte-differentiated U937 cells
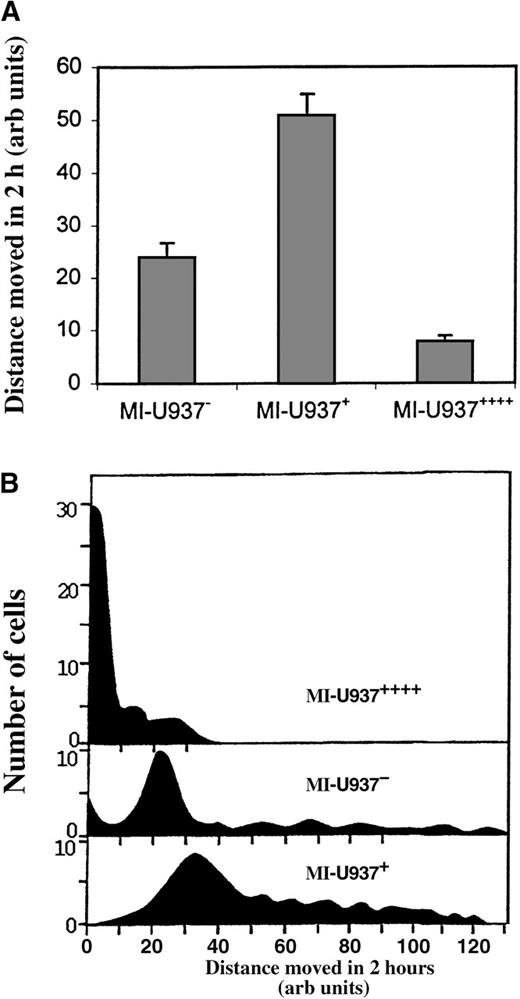
Three previous observations suggest LSP1 is an important regulator of phagocyte locomotion. First, NAD 47/89 neutrophils with elevated levels of LSP1 are immotile.5 Second, phagocytic leukocytes express LSP1.4 Third, LSP1 expression at any level in LSP1-null A7 melanoma cells immobilizes the cell.7 To determine the effect of LSP1 on phagocyte locomotion, we determined the rates of locomotion of monocyte-differentiated U937 cell lines MI-U937−, MI-U937+ and MI-U937++++ by direct single-cell video-tracking in a Dvorak-Stotler chamber as described.12Briefly, cells in the chamber were video-recorded for 2 hours. In each video record, at least 100 cells were tracked and the mean distance in micrometers (μm) traveled during 2 hours was measured. Figure3A shows the mean distance traveled by the LSP1− and LSP1+ cells lines determined in 3 independent experiments with MI-U937−, MI-U937+, and MI-U937++++ cells. To exclude biases due to subcloning, the mean distance moved in 3 affected subclones at each LSP1 level was determined. As shown, the distance traveled by MI-U937+ cells with normal LSP1 levels (50.77 + 4.11 μm, n = 3, or 4.23 × 10−1 μm/min) is 2-fold greater than that of MI-U937− (23.89 + 2.78 μm, n = 3, or 2.01 × 10−1 μm/min), which express no LSP1. This indicates that normal LSP1 levels enhance the rate of locomotion of these phagocytes. Furthermore, a 4-fold increase in LSP1 level in the MI-U937++++ cells markedly decreased the mean distance traveled by these cells (7.78 + 1.11 μm, n = 3, or 0.65 × 10−1 μm/min) when compared with either MI-U937− or MI-U937+. These results show that the effect of LSP1 on phagocyte locomotion is biphasic, enhancing locomotion at normal LSP1 levels and inhibiting locomotion at elevated LSP1 levels. The result suggests LSP1 expression level modulates the rate of phagocyte locomotion.
The mean rate of U937 cell locomotion is a function of LSP1 level.
(A) The mean rates of locomotion (n = 3) for 100 cells analyzed from 3 different experiments in which transfected U937 cells were induced to monocytic differentiation and then MI-U937−, MI-U937+, and MI-U937++++ cells were plated, videotaped, and video-tracked for 2 hours to determine their rates of locomotion. The distribution of rates of locomotion of U937 cells confirms variance of rate of locomotion with LSP1 level. (B) A comparison of the histograms of the rates of locomotion among 150 MI-U937−, MI-U937+, and MI-U937++++ cells.
The mean rate of U937 cell locomotion is a function of LSP1 level.
(A) The mean rates of locomotion (n = 3) for 100 cells analyzed from 3 different experiments in which transfected U937 cells were induced to monocytic differentiation and then MI-U937−, MI-U937+, and MI-U937++++ cells were plated, videotaped, and video-tracked for 2 hours to determine their rates of locomotion. The distribution of rates of locomotion of U937 cells confirms variance of rate of locomotion with LSP1 level. (B) A comparison of the histograms of the rates of locomotion among 150 MI-U937−, MI-U937+, and MI-U937++++ cells.
Histograms confirm rate of locomotion of U937 cells varies with LSP1 level
Since the pCEP vector used in these experiments is episomal, it is possible the observed rates of locomotion (distance traveled in 2 hours) reflect a mixture of 2 cell populations markedly disparate in locomotive behavior rather than a more “normalized” single cell population with respect to locomotion. Existence of disparate cell populations could lead to incorrect conclusions regarding LSP1 effect. To determine if the rates of locomotion were more “normalized,” histograms of the rates of locomotion among 150 cells of each cell line was determined. Figure 3B shows comparison histograms of the rates of locomotion among 150 cells from MI-U937−, MI-U937+, and MI-U937++++ cell lines. As shown in Figure 3B, the distribution of the rates of locomotion among 150 cells with 3 different LSP1 levels is relatively uniform, and differences in the distance traveled in 2 hours represents the behavior of a single average population of cells and not a mixed population of cells widely disparate in locomotive behavior. Therefore the effect of LSP1 level on monocyte-differentiated U937 motility is a homogeneous effect and a uniform characteristic of the cells due to variations in LSP1 level.
LSP1 level does not affect the rate of growth and cell division (cytokinesis) in U937 cells
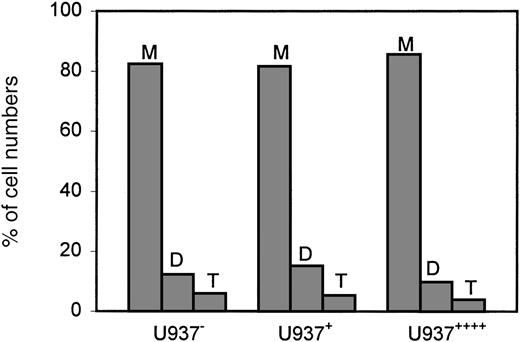
To determine if LSP1 expression affects other motile functions in U937 cells, we determined the effect of LSP1 level on rates of cell growth based on the number of cells produced during a specific time period. Cell growth, as evidenced by increasing numbers of cells, requires actomyosin-dependent cleavage of cells into daughter cells or cytokinesis. The cell lines used varied widely in LSP1 level and were not induced to monocyte differentiation. The LSP1 expression levels observed in the cell lines were absent, equal to levels in a monocyte, and 4-fold greater than a monocyte, and the lines were respectively termed U937−, U937+, and U937++++. These cell lines expressed similar levels of other actin-binding proteins and had similar amounts of total F-actin level and total actin content (data not shown) as described for the MI-U937 cells used to analyze motility. To examine the effect of LSP1 on growth, the U937−, U937+, and U937++++ cells in log-phase growth were placed in culture at the same density on day 0. Daily for the subsequent 4 days, the cell growth rate and cell division were analyzed by daily cell counts and by microscopic review of cells stained with Hoescht stain to determine the number of nuclei per cell. Cytokinesis defects create multinucleate cells and slow growth because nuclear divisions proceed without cleavage of cells into daughter cells. Increased number of nuclei per cell and a decreased rate of growth are expected in the cells defective in this actomyosin-dependent cell function as described for myosin II mutants in Dictyostelium sp17 18Figure 4 shows the rates of growth for these cells. The results indicate that LSP1 level has no effect on the rate of stably transfected cell growth. In Figure5, bar graphs show that the average percentages of cells with 1, 2, and 3 nuclei per cells observed among 500 of the U937−, U937+, and U937++++ cells on day 4 are the same. The results show that LSP1 affects 2 actomyosin-dependent functions differently. While LSP1 modulates cell locomotion, it has no measurable effect on cytokinesis.
LSP1 level does not affect the growth rate of U937 cells.
Shown are the rates of growth for U937− (⧫), U937+ (▪), and U937++++ ([trio])cells. They were in log-phase growth and plated with the same density on day 0. The cell densities were examined on the next 4 days. Results are expressed as the mean ± SD (n = 3).
LSP1 level does not affect the growth rate of U937 cells.
Shown are the rates of growth for U937− (⧫), U937+ (▪), and U937++++ ([trio])cells. They were in log-phase growth and plated with the same density on day 0. The cell densities were examined on the next 4 days. Results are expressed as the mean ± SD (n = 3).
LSP1 does not affect the cell division in U937 cells.
Bar graphs depict the average percentages of cells with 1 (M), 2 (D), and 3 (T) nuclei per cell observed among 500 of the U937−, U937+ and U937++++cells on day 4.
LSP1 does not affect the cell division in U937 cells.
Bar graphs depict the average percentages of cells with 1 (M), 2 (D), and 3 (T) nuclei per cell observed among 500 of the U937−, U937+ and U937++++cells on day 4.
Discussion
Previous reports from 2 different laboratories used genetically engineered cell lines to demonstrate that the level and normal function of at least 3 actin-binding proteins—filamin or ABP280, gelsolin, and myosin II—have dramatic effects on actomyosin-dependent cell functions, including cell shape, locomotion, and cytokinesis. These studies document the power of stably transfected cell lines to elucidate the function of actin-binding proteins in cells. Cunningham et al19 reported that the ABP280-deficient human melanoma cells have impaired locomotion and display circumferential blebbing of the plasma membrane. Expression of ABP280 after transfection restored translocational motility and reduced membrane blebbing. Cunningham et al also showed, in stably transfected NIH3T3 fibroblast cells, gelsolin overexpression to levels 2.25-fold normal enhanced cell locomotion.20 Finally Spudich et al21 22 showed that 2 actomyosin-dependent motile processes in cells—locomotion and cell growth due to lack of cytokinesis or cell division—were lost in genetically engineered Dictyostelium that express myosin II heavy-chain mutants, which cannot assemble in vitro.
The studies reported here investigate the effect of LSP1 on these same 2 actomyosin-dependent cellular functions: locomotion and cytokinesis. The results show LSP1 has a unique effect on locomotion. Specifically, LSP1 expression levels have a biphasic effect on locomotion: normal levels promote locomotion and high levels inhibit locomotion in vitro. In contrast, LSP1 level—high, low, or normal—has no effect on cell growth or cell division (cytokinesis). Our results suggest that LSP1 is not required for cell growth and division and further suggest that the interaction between LSP1 and F-actin cytoskeleton is involved in cell locomotion. Howard et al7 have shown that LSP1 overexpression in A7 melanoma cells causes F-actin–rich, hairlike surface projections to form on cell surfaces and inhibits cell locomotion. This overexpression in a nonmuscle cell recreates the morphologic and motile phenotype of neutrophils in NAD 47/89, a human neutrophil dysfunction associated with recurrent infections. In transfected monocytelike U937 cells reported here, overexpression of LSP1 in MI-U937++++ also causes hairlike projection formation similar to that described on the surface of melanoma cells and decreases the rate of cell locomotion. In contrast to what occurs in MI-U937++++ cells, normal levels of LSP1 expression in MI-U937+ does not result in hairlike projection formation and significantly enhances the rate of cell locomotion. These data indicate LSP1 plays an important role in F-actin–associated motile functions in leukocyte cells. Its impact on locomotion is particularly notable.7 Both the lack of expression of LSP1 and its overexpression result in defective locomotion in the leukocytic cells studied here and possibly in normal cells.
Exactly how LSP1 works is a focus of ongoing studies. LSP1 is an F-actin–bundling protein.7 In fact, preliminary evidence shows it has 4 actin-binding domains: 2 caldesmonlike domains and 2 villinlike domains.23 Theoretically, overexpression of LSP1 could reduce available F-actin in cells by assembling filaments into bundles7 and thereby decrease the motility of the cells. But overexpression is not the case in the normal leukocyte. Exactly how LSP1 affects the locomotion in normal leukocyte still remains to be elucidated. It is likely to be an effector protein activated by phosphorylation. It is reported that LSP1 is a substrate for protein kinase C in human B cells24 and a substrate for mitogen-activated protein kinase–activated protein kinase 2 in human neutrophils.25 Finally, mouse LSP1 is released from the membrane (most likely from F-actin cytoskeleton) when it is phosphorylated in PMA-stimulated T cells.26Like caldesmon or the myristoylated, alanine-rich C kinase substrate (MARCKS) protein, phosphorylation is likely to cause LSP1 to dissociate from microfilaments.27-29 It may affect LSP1's actin-binding affinity and regulate LSP1-actin interaction, or it may be a part of a signal event for cell activation.
Supported by National Institutes of Health Grant 5 RO1 Hl56155. L.H. is a Bradford Dean Dixon fellow.
Reprints:Thomas H. Howard, Department of Pediatrics, Division of Hematology/Oncology, The Children's Hospital of Alabama, 1600 7th Ave South, Birmingham, AL 35233.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 4. LSP1 level does not affect the growth rate of U937 cells. / Shown are the rates of growth for U937− (⧫), U937+ (▪), and U937++++ ([trio])cells. They were in log-phase growth and plated with the same density on day 0. The cell densities were examined on the next 4 days. Results are expressed as the mean ± SD (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.1100/5/m_bloo01533004x.jpeg?Expires=1770753737&Signature=HW5eOErpofJMSMBDcV6F6Gq3DdDLWO~v-JOwe9a5dUUEAw3xq~s4RdRMakdU29RIgDNdXMVew6EJhVCi5ESMSyGo0Qpq3ihBXZLWCJuAxoitq6ghE~UcpXceTMNWMdzrzg8BbCHFTzNeVxaBm2uiSMY~OgT7p~XKxNI7L6xX6K5MEgV3NiSw0Gir5yxsFQM~cYL2BbU3I-775B-t-3h3SVVnx5nRYL828ptQYR3ScSXIY7LEqVMTuPZibtiuvBpGbYTJmFc0XsbogqPbs1kKKIE7p7KoSr-FHo~KFGynC11Edoj~WQuLmhgwAcRFdnAFlEeeQW02rC0QwvLSuCc0MA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal