Abstract
Chemokine receptors mediate the migration of lymphocytes through the binding of soluble ligands, and their expression is differentially regulated in lymphocyte subsets. The pattern of chemokine receptor expression in T-cell non-Hodgkin lymphoma has not been previously studied. Using a panel of mouse monoclonal antibodies, we studied the immunohistochemical expression of the Th1-associated chemokine receptor CXCR3 in 141 patients with T-cell lymphoma, and we studied the receptors CCR4 and CCR5 and some of their ligands in a subset of these tumors. Expression of CXCR3 was typical of the smaller T cells in angioimmunoblastic lymphoma (15 of 18 patients), angiocentric lymphoma (3 of 3 patients), histiocyte-rich tumors (4 of 5 patients), and unspecified T-cell lymphomas (17 of 39 patients). CXCR3 expression was seen in only 1 of 15 patients with anaplastic lymphoma kinase (ALK)-positive anaplastic large-cell lymphoma. In contrast, all ALK-positive tumors showed diffuse reactivity for the Th2-associated receptor CCR4 (5 of 5 patients). CCR4 expression was also a consistent feature of the large-cell transformation of mycosis fungoides. CCR5 expression showed no consistent association with any T-cell tumor type. The chemokines Mig (CXCR3 ligand), TARC (CCR4 ligand), and MCP-2 (CCR5 ligand) were detected in intratumoral blood vessels and histiocytes. Mig was also coexpressed by a subset of CXCR3-positive tumor cells in 6 of 20 lymphomas. MCP-2 was highly expressed in stromal cells in 3 patients with nodal involvement by cutaneous T-cell lymphoma. As with normal T-cell subsets, we demonstrated that there is frequent differential expression of chemokine receptors in T-cell tumors, which may explain, in part, the distinctive patterns of spread in different tumor subtypes.
Chemokines are soluble proteins that regulate leukocyte migration and activation through binding to transmembrane receptors differentially expressed on lymphocyte subsets.1 It has recently been demonstrated that the expression pattern of chemokine receptors in normal T-cell subsets correlates with the pattern of cytokine secretion in these cells. Specifically, the expression of the receptors CCR3, CCR4, and CCR8 is associated with Th2-polarized T cells expressing the cytokines, IL-4 and IL-5.2-4 In contrast, CXCR3 expression is highest in cells with a predominant Th1 pattern of cytokine secretion.2,3,5 CCR5 is constitutively expressed in circulating memory T cells and is differentially regulated on T-cell activation in response to different cytokines.6-9
To date, the classification of T-cell lymphoma has been largely based on morphologic or clinical criteria.10 For example, mycosis fungoides (MF) and intestinal T-cell lymphoma are defined based on sites of involvement and can have a wide range of histologic appearances. Other tumors, such as angioimmunoblastic lymphoma, are largely defined by morphologic criteria. Some lymphoma types are defined by specific phenotypic criteria, such as the presence of HTLV-I in adult T-cell leukemia–lymphoma and of CD30 expression (± ALK) in anaplastic large-cell lymphoma. We report that several of the T-cell–associated chemokine receptors are differentially expressed in distinct subtypes of T-cell lymphoma and are correlated with the expression of other T-cell surface markers.
Materials and methods
All T-cell tumors diagnosed at Brigham and Women's Hospital (Boston, MA) in the past 10 years were included if material was available; additional cases were drawn from Boston Medical Center (Boston, MA), Washington University Hospital (St Louis, MO), and Children's Hospital (Boston, MA). Diagnosis was based on the revised European–American lymphoma (REAL) classification after examination of routinely stained histologic sections and a panel of immunohistochemical markers, including the B-cell marker CD20 (L26) and the T-cell–associated markers CD3, CD45RO, and CD43 (Leu22).10 If fresh tissue was available, additional immunostains for the T-cell markers CD2, CD4, CD5, and CD8 were performed as previously described.11 Our specific criteria for the diagnosis of anaplastic large-cell lymphoma and angioimmunoblastic lymphoma have been previously published.12 13 Cases with a T-cell genotype and prominent vascular infiltration were regarded as angiocentric T-cell lymphoma. Predominant tumor cell size and presence of abundant admixed histiocytes (ie, Lennert lymphoma) were recorded for cases in the REAL category of peripheral T-cell lymphoma, unspecified.
Immunoperoxidase studies were performed on formalin-fixed, paraffin-embedded material (for CXCR3, CD30, CD134/OX40) after microwave antigen retrieval and on cryostat sections using antibodies directed against the chemokine receptors CCR4 (1G1),14 CCR5 (3A9),5,15 CXCR3 (1C6),5,16 and the chemokines TARC(2D8),14 Mig (4G10), and MCP-2 (2D5),17 and the activation markers CD134/OX40 (ACT35; Pharmingen, San Diego, CA) and CD30 (BerH2; DAKO, Carpinteria, CA). All antibodies directed against chemokine receptors were tested for specificity by the ability to stain to L1.2 stable transfectants expressing the target chemokine receptor but not L1.2 transfectants expressing other chemokine receptors.
Statistical analyses were performed on certain pairwise comparisons using either Student t test or Fisher exact test methods, depending on analyzed sample size, using the StatDirect package (Camcode, Ashwell, UK).
Results
Chemokine receptor expression
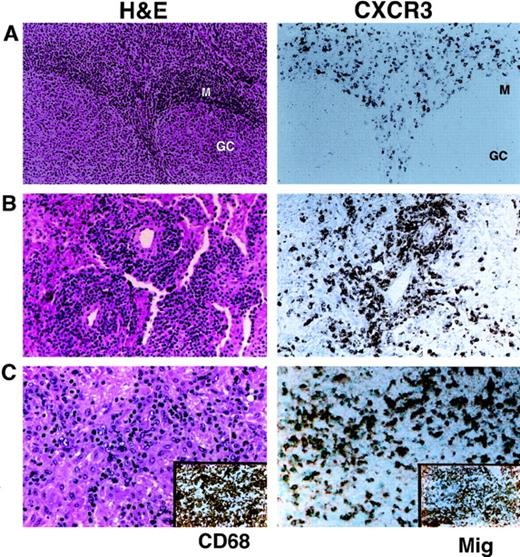
CXCR3 has been previously demonstrated to be expressed in most circulating peripheral T cells. In normal lymph node and tonsil, numerous CXCR3-positive interfollicular T cells were noted by immunohistochemistry, comprising 20% to 50% of the total cellularity in these regions (Figure 1A). B cells, in the follicles and mantle zone, were negative for CXCR3. We examined the expression pattern of CXCR3 in paraffin and cryostat sections of 141 T-cell lymphomas of all histologic types. Sixty-six patients (47%) showed CXCR3 positivity in more than 5% of tumor cells, with 45 patients showing diffuse reactivity in most tumor cells (Table1). Diffuse CXCR3 expression was seen in the smaller T cells from 15 of 18 patients with angioimmunoblastic lymphoma, in 3 of 3 patients with angiocentric T cell lymphoma, and in 4 of 5 patients with histiocyte-rich T-cell lymphoma (Figure 1B,C).
Expression of the chemokine receptor CXCR3.
(A) Normal tonsil shows staining of approximately 50% of T cells in the interfollicular areas for CXCR3. B cells, in follicles and mantle zones, are negative. (B) Angiocentric T-cell lymphoma of the lung shows diffuse positivity for CXCR3 in the angioinvasive component. (C) Histiocyte-rich T-cell (lymphoepithelioid) lymphoma shows a mixture of predominantly small tumor lymphocytes and numerous CD68-positive epithelioid histiocytes (inset, left). Tumor cells are positive for CXCR3. Histiocytes show strong expression of Mig (inset, right).
Expression of the chemokine receptor CXCR3.
(A) Normal tonsil shows staining of approximately 50% of T cells in the interfollicular areas for CXCR3. B cells, in follicles and mantle zones, are negative. (B) Angiocentric T-cell lymphoma of the lung shows diffuse positivity for CXCR3 in the angioinvasive component. (C) Histiocyte-rich T-cell (lymphoepithelioid) lymphoma shows a mixture of predominantly small tumor lymphocytes and numerous CD68-positive epithelioid histiocytes (inset, left). Tumor cells are positive for CXCR3. Histiocytes show strong expression of Mig (inset, right).
Expression pattern of T-cell–associated chemokine receptors and their ligands in T-cell lymphoma
| Tumor types . | CXCR3 . | CCR4 . | CCR5 . | CCR4/CXCR3 coexpression . | Mig† . | TARC‡ . | MCP-21-153 . |
|---|---|---|---|---|---|---|---|
| Anaplastic large-cell lymphoma | |||||||
| ALK-positive | 1/15 | 5/5 | 3/4 | ||||
| ALK-negative | 8/21 | 3/6 | 1/6 | 2/6 | 1/5 | 0/3 | 0/5 |
| Primary cutaneous | 2/5 | 0/1 | |||||
| Angioimmunoblastic lymphoma | 15/18 | 0/2 | — | ||||
| T-cell lymphoma, unspecified | |||||||
| Large-cell morphology | 6/21 | 3/13 | 4/11 | 0/13 | 2/12 | 0/8 | 0/12 |
| Mixed-cell morphology | 10/13 | 1/2 | 0/2 | 3/3 | 0/4 | 0/4 | |
| Small-cell morphology/CLL | 1/5 | — | — | ||||
| Histiocyte-rich (Lennert) | 4/5 | 0/1 | 0/1 | 0/1 | 0/1 | ||
| Angiocentric T-cell lymphoma | 3/3 | 0/1 | 1/1 | ||||
| Mycosis fungoides | 7/9 | 1/7 | 0/3 | ||||
| Mycosis fungoides in transformation | 5/8* | 5/5* | — | 2/5 | 1/4 | 0/4 | 0/4 |
| Enteropathy-associated | 0/2 | — | — | ||||
| Adult T-cell lymphoma/leukemia | 0/2 | — | — | ||||
| T-lymphoblastic lymphoma | 1/5 | 0/2 | 1/3 | ||||
| Other T-cell tumors1-155 | 3/9 | 1/3 | 1/1 | 0/1 | 0/1 |
| Tumor types . | CXCR3 . | CCR4 . | CCR5 . | CCR4/CXCR3 coexpression . | Mig† . | TARC‡ . | MCP-21-153 . |
|---|---|---|---|---|---|---|---|
| Anaplastic large-cell lymphoma | |||||||
| ALK-positive | 1/15 | 5/5 | 3/4 | ||||
| ALK-negative | 8/21 | 3/6 | 1/6 | 2/6 | 1/5 | 0/3 | 0/5 |
| Primary cutaneous | 2/5 | 0/1 | |||||
| Angioimmunoblastic lymphoma | 15/18 | 0/2 | — | ||||
| T-cell lymphoma, unspecified | |||||||
| Large-cell morphology | 6/21 | 3/13 | 4/11 | 0/13 | 2/12 | 0/8 | 0/12 |
| Mixed-cell morphology | 10/13 | 1/2 | 0/2 | 3/3 | 0/4 | 0/4 | |
| Small-cell morphology/CLL | 1/5 | — | — | ||||
| Histiocyte-rich (Lennert) | 4/5 | 0/1 | 0/1 | 0/1 | 0/1 | ||
| Angiocentric T-cell lymphoma | 3/3 | 0/1 | 1/1 | ||||
| Mycosis fungoides | 7/9 | 1/7 | 0/3 | ||||
| Mycosis fungoides in transformation | 5/8* | 5/5* | — | 2/5 | 1/4 | 0/4 | 0/4 |
| Enteropathy-associated | 0/2 | — | — | ||||
| Adult T-cell lymphoma/leukemia | 0/2 | — | — | ||||
| T-lymphoblastic lymphoma | 1/5 | 0/2 | 1/3 | ||||
| Other T-cell tumors1-155 | 3/9 | 1/3 | 1/1 | 0/1 | 0/1 |
Differential staining of the larger tumor cells for CCR4 and the smaller tumor cells for CXCR3 was seen in 2 patients.
Strong Mig immunostaining of histiocytes seen; blood vessels show variable Mig immunoreactivity.
Strong TARC immunoreactivity seen in the muscularis/adventitia of (larger) blood vessels, with variably weak staining of histiocytes and dendritic cells.
MCP-2 immunoreactivity was seen in blood vessels and nodal sinus lining cells and strongly seen in histiocytes and mononuclear cells of uncertain lineage in nodal involvement by mycosis fungoides in 3 of 4 patients.
Includes CD8-positive tumors (5) and difficult to classify cases.
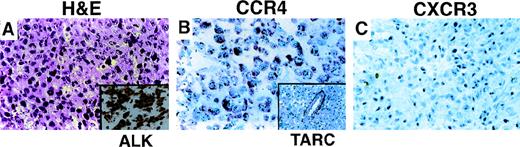
We also examined patients with CD30-positive anaplastic large-cell lymphoma (ALCL) of T-cell or null lineage previously characterized by immunostaining for the ALK kinase. Nuclear ALK1 immunohistochemical expression is a marker of the t(2;5) chromosomal translocation seen in a subset of patients with ALCL. ALK-positive ALCL was negative for CXCR3 in 14 of 15 patients (Figure 2C). Among noncutaneous ALK-negative ALCL tumors, CXCR3 expression was more variable, with 8 of 21 (38%) showing diffuse positivity. CXCR3 was diffusely positive in 2 of 5 patients with primary cutaneous ALCL, all of whom were ALK negative.
Chemokine receptor expression in ALCL.
(A) Tumor shows a diffuse infiltrate of anaplastic cells with cytoplasmic and nuclear reactivity for ALK (inset). CD30 was uniformly positive (not shown). (B) Immunostain for CCR4 shows membrane and granular cytoplasmic reactivity in all tumor cells. Admixed small lymphocytes are negative. Immunostain for the CCR4 ligand TARC shows staining of intratumoral blood vessels; tumor cells are negative (inset). (C) Immunostain for CXCR3 is negative in tumor cells.
Chemokine receptor expression in ALCL.
(A) Tumor shows a diffuse infiltrate of anaplastic cells with cytoplasmic and nuclear reactivity for ALK (inset). CD30 was uniformly positive (not shown). (B) Immunostain for CCR4 shows membrane and granular cytoplasmic reactivity in all tumor cells. Admixed small lymphocytes are negative. Immunostain for the CCR4 ligand TARC shows staining of intratumoral blood vessels; tumor cells are negative (inset). (C) Immunostain for CXCR3 is negative in tumor cells.
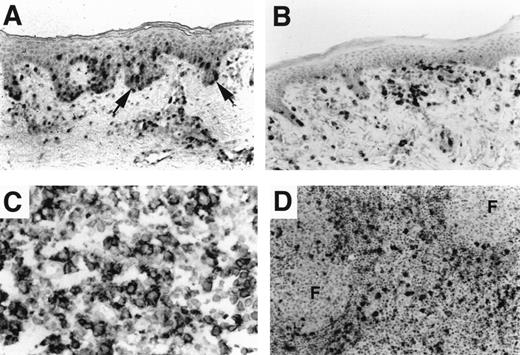
Cutaneous T-cell lymphoma of the MF type showed strong CXCR3 expression in 7 of 9 patients. CXCR3-positive cells included most of the epidermotropic tumor cells and most lymphocytes infiltrating the superficial dermis (Figure 3A). Two of 3 lymph node biopsies from patients with MF showed numerous CXCR3-positive tumors in the sinuses and interfollicular areas, including morphologically atypical cells with cerebriform nuclei. In MF that underwent large-cell transformation, CXCR3 reactivity occurred only in small lymphocytes in the lymph nodes of 5 of 8 patients; the remaining patients were entirely negative for CXCR3 (data not shown). Thus, there was a highly significant association between CXCR3 expression in low-grade MF but not in large-cell transformation of MF (P = .002).
Chemokine receptor expression in cutaneous lymphoma.
(A) Patch-stage MF with an epidermotropic and superficial dermal infiltrate showing CXCR3 reactivity. CCR4 immunostain was negative (not shown). (B) Cutaneous lymphoma with slack skin presentation shows reactivity of the large epidermotropic tumor cells for CCR4. CXCR3 immunostain was negative (not shown). (C) Nodal large-cell transformation of MF. Large tumor cells show diffuse reactivity for CCR4; CXCR3 was positive only in admixed small lymphocytes (not shown). (D) Increased MCP-2 in stromal cells in lymph node involvement by MF. Interfollicular macrophages and nontumor mononuclear cells show MCP-2 expression in 4 of 4 patients with nodal involvement by low-grade MF. Increased MCP-2 was not seen in nodal involvement by transformed MF (not shown).
Chemokine receptor expression in cutaneous lymphoma.
(A) Patch-stage MF with an epidermotropic and superficial dermal infiltrate showing CXCR3 reactivity. CCR4 immunostain was negative (not shown). (B) Cutaneous lymphoma with slack skin presentation shows reactivity of the large epidermotropic tumor cells for CCR4. CXCR3 immunostain was negative (not shown). (C) Nodal large-cell transformation of MF. Large tumor cells show diffuse reactivity for CCR4; CXCR3 was positive only in admixed small lymphocytes (not shown). (D) Increased MCP-2 in stromal cells in lymph node involvement by MF. Interfollicular macrophages and nontumor mononuclear cells show MCP-2 expression in 4 of 4 patients with nodal involvement by low-grade MF. Increased MCP-2 was not seen in nodal involvement by transformed MF (not shown).
We compared CXCR3 expression with immunohistochemical expression of 2 other T-cell–associated chemokine receptors, CCR4 and CCR5, in 48 T-cell tumors for which we had frozen, unfixed material available. In non-neoplastic lymph nodes and tonsil, CCR4 was expressed in the endothelium of most blood vessels and in a small fraction of interfollicular T cells comprising approximately 1% of the overall cellularity (data not shown). By immunohistochemistry, CCR5 was predominantly expressed in endothelium and histiocytes and weakly expressed in a small number of lymphocytes (data not shown). In contrast, a subset of T-cell tumors showed diffuse expression of CCR4 (19 of 48, 40%), CCR5 (10 of 31, 32%), or both (Table 1).
The most striking finding was in ALK-positive ALCL, in which all 5 tumors tested showed diffuse positivity for CCR4 (Figure 2B). Tumor cells typically showed membrane and granular cytoplasmic staining patterns that resembled those seen in immunostains for granular cytotoxic proteins such as TIA-1. CCR4 was positive in 3 of 6 ALK-negative, noncutaneous anaplastic tumors. CCR5 expression was seen in 3 of 4 ALK-positive patients and in 1 of 6 ALK-negative anaplastic tumors. Strong CCR4 positivity was present in 4 of 5 patients with nodal large-cell transformation of MF (Figure 3C), with the remaining patient showing focal CCR4 reactivity. In contrast, only 2 patients with epidermotropic cutaneous lymphoma showed positive staining of tumor cells for CCR4. Both cases were unusual; 1 patient had the “slack-skin” variant of MF and a largely epidermotropic infiltrate of large, irregular lymphocytes (Figure 3B), and the second patient had an unclassifiable CD8-positive tumor (data not shown).
Chemokine expression in T-cell tumors
We also examined the immunohistochemical expression of high-affinity ligands for these chemokine receptors, including Mig, which binds to CXCR3, TARC, which binds to CCR4, and MCP-2, which binds to CCR5. Variable staining for all 3 chemokines was seen in blood vessels and admixed histiocytes (Table 1). TARC showed the strongest staining of the muscular/adventitial layer of blood vessels and scattered stromal cells in lymph node and connective tissue of the dermis (Figure 2B inset and data not shown).
Mig immunostaining was more variable and was abundantly expressed in macrophages of histiocyte-rich T-cell tumors (Figure 1C inset). Mig expression was also seen in variable numbers of tumor cells in 6 of 21 patients with T-cell lymphoma, mostly those in the peripheral T-cell lymphoma, unspecified category. All patients with Mig staining of tumor cells also showed tumor expression of its receptor, CXCR3. MCP-2 immunostaining was not seen in normal lymphocytes or in any T-cell tumors tested. However, we noted increased MCP-2 expression in various stromal cells in 3 of 4 patients with nodal involvement by MF (Figure3D). In only 1 of these patients were increased numbers of CCR5-expressing tumor cells present (data not shown).
Correlation with expression of other surface activation markers
Using the same set of tumors, we previously reported that some members of the tumor necrosis factor (TNF) receptor gene family, namely CD30 and CD134/OX40, are differentially expressed in T-cell lymphoma.13 Expression of these markers of activated T cells correlates with histologic tumor type (Table 1). For instance, ALK-positive ALCL is uniformly CD30-positive but almost always negative for CD134/OX40. In contrast, angioimmunoblastic lymphoma nearly always shows clusters of OX40-positive cells but not CD30 expression in tumor cells.
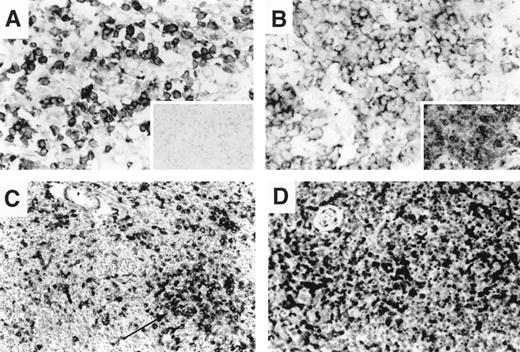
We compared our previous results for CD30 and CD134/OX40 with those of the chemokine receptors. Coexpression of CD30 and CCR4 was present in 8 of 11 patients with ALCL. Tumor cells in the large-cell transformation of MF were also positive for both CCR4 and CD30 in 4 of 5 patients. However, 4 CCR4-positive non-anaplastic PTCLs were uniformly negative for CD30 (Figure 4A inset). Considering all tumor types, CD30 and CCR4 were coexpressed in most tumor cells in 13 of 44 patients (30%), and neither was expressed in 19 of 44 patients (43%). Thus, there was a significant association between CD30 and CCR4 coexpression in T-cell tumors (P = .04).
Variability of chemokine receptor and TNF receptor expression in nodal T-cell lymphoma.
(A) Nodal peripheral T-cell lymphoma with a predominant large-cell morphology showing diffuse membrane and cytoplasmic reactivity for CCR4 in most tumor cells. CD30 immunostain is negative (inset). (B) Nodal peripheral T-cell lymphoma with a predominant large-cell morphology showing diffuse reactivity for CXCR3; CD134/OX40 immunostain was also diffusely positive (inset). (C, D) Angioimmunoblastic T-cell lymphoma in lymph node. CD134/OX40 immunostain highlights clusters of OX40-positive larger tumor cells (C). CXCR3 immunostain is diffusely positive in small- to intermediate-sized lymphocytes from the same biopsy (D). Large, clear cells are variably positive.
Variability of chemokine receptor and TNF receptor expression in nodal T-cell lymphoma.
(A) Nodal peripheral T-cell lymphoma with a predominant large-cell morphology showing diffuse membrane and cytoplasmic reactivity for CCR4 in most tumor cells. CD30 immunostain is negative (inset). (B) Nodal peripheral T-cell lymphoma with a predominant large-cell morphology showing diffuse reactivity for CXCR3; CD134/OX40 immunostain was also diffusely positive (inset). (C, D) Angioimmunoblastic T-cell lymphoma in lymph node. CD134/OX40 immunostain highlights clusters of OX40-positive larger tumor cells (C). CXCR3 immunostain is diffusely positive in small- to intermediate-sized lymphocytes from the same biopsy (D). Large, clear cells are variably positive.
In contrast, CD134/OX40 and CXCR3 were coexpressed in 14 of 18 patients with angioimmunoblastic lymphoma (AIL), 4 of 5 patients with histiocyte-rich T-cell tumors, and 3 of 3 patients with angiocentric T-cell lymphoma. Considering all tumor types, there was more than 50% expression of CXCR3 and CD134/OX40 in tumor cells in 37 of 122 (30%) patients and no significant expression of these markers in 54 of 122 (44%) patients. Thus, there was a highly significant association between the expression of these 2 markers overall in T-cell non-Hodgkin lymphoma (P < .0001). However, certain tumor types showed great variations in the extent of expression of these markers. In AIL, CD134/OX40 and CXCR3 appeared to be expressed in nonidentical populations of lymphocytes within the tumor, based on a comparison of serial sections (Figure 4C,D). OX40 was consistently expressed in the neoplastic clusters of larger cells with clear cytoplasm characteristic of AIL (Figure 4C). In contrast, CXCR3 was more uniformly expressed in the small- and intermediate-sized lymphocytes, possibly representing reactive infiltrating T cells (Figure 4D).
In ALK-positive ALCL, only 1 patient each expressed CD134 and CXCR3. In ALK-negative ALCL, there was no significant correlation between CD134/OX40 and CXCR3, with 5 patients each expressing neither marker, CXCR3 alone, or CD134 alone, and 6 patients expressing both. Similarly, among the nonanaplastic large cell tumors, there was no correlation noted between CXCR3 expression and that of CD134/OX40 (Figure 4B). These results likely reflect the morphologic and immunophenotypic heterogeneity of these categories.
Discussion
We present the immunohistochemical expression pattern of 3 T-cell–associated chemokine receptors, CXCR3, CCR4, and CCR5, in a large series of T-cell lymphoma of all types. CXCR3 is expressed in several tumor subtypes, including most cases of AIL, histiocyte-rich T-cell tumors, and angiocentric T-cell lymphoma. In these tumors, the detection of CXCR3 is highly correlated with the coexpression of the TNF receptor CD134/OX40. In contrast, ALK-positive ALCL shows immunoreactivity for CCR4 and is negative for CXCR3 in most patients. Lymphomas with a naive or immature phenotype, namely lymphoblastic lymphoma and T-CLL, were predominantly negative for both CXCR3 and CCR4.
The expression patterns of ALK-negative anaplastic tumors and peripheral T-cell lymphoma of unspecified type are highly variable with regard to chemokine receptor expression, and there is no obvious correlation with CD30 or CD134/OX40 expression. These findings provide additional evidence that these categories are morphologically and immunophenotypically heterogeneous and do not correlate with a distinct T-cell phenotype.
In T-cell cultures and cell lines, expression of the chemokine receptor CCR4 has been correlated with the Th2 phenotype.3,8,9,18 Our finding of the preferential expression of CCR4 in CD30-positive anaplastic tumors is intriguing given the frequent association of CD30 expression in normal T cells with a Th2-like phenotype. Increased numbers of CD30-positive cells and elevated levels of circulating soluble CD30 have been reported in prototypic “Th2-type” inflammatory states.19-21 This observation is hypothesized to be related to the induction of CD30 expression in T cells after production of the Th2 cytokine IL-4.22,23 The pattern of cytokine expression in ALCL has not been well-studied. However, 1 small study showed increased expression of Th2 cytokines in cutaneous ALCL by transcriptional analysis.24 By demonstrating CCR4 expression and lack of CXCR3, our study provides evidence for the phenotypic similarity of ALK-positive ALCL to a Th2-like T-cell population.
Conversely, CXCR3 expression has been associated with cell lines and primary cultures polarized toward Th1 cytokine expression.3,5,8,9,18 Our finding that AIL and histiocyte-rich T-cell lymphoma highly express CXCR3 and CD134/OX40 supports a Th1-like phenotype for these tumors. In this regard, increased levels of the Th1 cytokine interferon-gamma (produced by tumor-associated macrophages) have been previously reported in a small series of Lennert lymphoma.25 We also noted abundant Mig production by admixed histiocytes, suggesting an important role for this chemokine interaction with CXCR3 in tumor dissemination or growth.
Angioimmunoblastic lymphoma is a complex tumor characterized by morphologic, immunophenotypic, and genotypic instability and a high rate of histologic progression.12,26,27 Historically, early stages of this tumor (often labeled AILD) have been regarded as pre-neoplastic or even reactive because occasional patients experience nonprogression or even regression without treatment. Given this complexity, it is likely that the phenotype of the tumor cell population and cytokine expression pattern fluctuates over the course of the disease. Indeed, increased levels of IL-2, IL-4, TNF-α and TNF-β, soluble CD30, and interferon-γ have all been variably reported in AIL/AILD.25,28 29 Our findings of distinct populations of CD134-positive and CXCR3-positive populations of cells within AIL is further evidence of tumor heterogeneity and shows that these 2 receptors can be differentially regulated.
Each chemokine receptor typically binds a number of related chemokines with varying affinity. This complexity makes identification of functionally relevant ligand-receptor interactions difficult. As an initial step toward correlating receptor expression with chemokine sources, we performed immunostaining for a high-affinity ligand for each of the studied receptors. In normal and tumor lymphoid tissues, Mig, TARC, and MCP-2 were all primarily localized to blood vessels and admixed histiocytes. We did not note discernible differences in chemokine expression in the stromal component of different T-cell tumor subtypes in the small number of patients examined. Given the detection sensitivity of immunohistochemistry, it is possible that some chemokines are expressed at lower levels in other nodal cell types.
Correlation of chemokine expression with tumor localization is further complicated by the ability of 1 chemokine to bind multiple receptors. For instance, the CCR5 ligands RANTES, MIP-1α, and MCP-2 can also bind CCR1 or CCR2.17,30 Therefore, identification of an altered pattern of chemokine expression does not immediately suggest a target receptor. This point is well illustrated by our finding of increased MCP-2 expression in stromal cells in patients with nodal involvement by MF. CCR5, however, was not widely expressed in these tumors, suggesting functional interactions of MCP2 with the other binding partners, perhaps CCR2b present on T cells and histiocytes.31
We noted expression of the chemokine Mig in a subset of tumor cells in some lymphomas that also expressed the receptor CXCR3. We previously noted this as a consistent finding in CXCR3-positive B-cell CLL.16 Whether endogenously produced Mig functions to deliver an autocrine signal in these tumors or, rather, to desensitize tumor cells to exogenous stimulation through CXCR3 remains to be explored.
Overall, our results confirm the distinctive nature of most recognized subtypes of T-cell lymphoma that is correlated with expression patterns of both chemokine receptors and TNF receptors. For instance, the expression of CD30 and CCR4, but not CD134/OX40 or CXCR3, is the typical phenotype of ALK-positive ALCL.13 Similarly, the expression of CD134 in large cells and CXCR3 in smaller cells, but not CD30, appears to be characteristic of AIL. In ALK-negative ALCL and the unspecified category of PTCL, we have not yet identified distinct patterns of chemokine receptor or TNF receptor expression, suggesting that these are heterogeneous entities. Studies with additional T-cell markers will be required before functional similarities between tumors within this undifferentiated category are recognized.
Acknowledgments
We thank LeukoSite Incorporated (Cambridge, MA) for providing chemokine receptor antibodies, Dr G. Pinkus for contributing cases, and J. Ebel for preparing the manuscript.
Reprints:David Dorfman, Brigham and Women's Hospital, Department of Pathology, 75 Francis St, Boston, MA 02115; e-mail:dmdorfman@bics.bwh.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal