Abstract
BAD, the proapoptotic member of the “BH3-only” subfamily of BCL-2 proteins, is inactivated by phosphorylation at serines 112 and 136 and by sequestration in the cytoplasm where it interacts with members of the 14-3-3 family. In BCR/ABL-expressing cells, BAD is constitutively phosphorylated and mainly cytoplasmic, whereas in cells expressing BCR/ABL mutants unable to protect from apoptosis, BAD is nonphosphorylated. We show here that both the wild-type (WT) and the S112A/ S136A double mutant (DM) BAD are more potent inducers of apoptosis in parental than in BCR/ABL-expressing 32D myeloid precursor cells. Stable lines of parental cells expressing DM BAD could not be established and most clones from WT BAD retrovirus-infected parental cells lost BAD expression. On IL-3 withdrawal from parental 32D cells, BAD was rapidly dephosphorylated by the serine-threonine phosphatase 1, and localized in the mitochondria, whereas it remained phosphorylated and did not localize to the mitochondria in the cohort of BCR/ABL-expressing cells escaping apoptosis induced by WT BAD. Moreover, these cells showed high levels of BCL-2 and BCL-XL expression. The cohort of BCR/ABL-expressing cells resistant to apoptosis induced by DM BAD showed only high levels of BCL-2 and BCL-XL. These findings suggest that BCR/ABL-expressing cells are more versatile than normal hematopoietic progenitors in counteracting the apoptotic potential of BAD, and raise the possibility that tumor cells activate multiple antiapoptotic pathways for survival in the face of death-inducing stimuli.
The chimeric oncogene bcr/abl is generated from a chromosomal translocation between chromosomes 9 and 22 (Philadelphia chromosome) which joins sequences from the bcr gene with sequences upstream of c-abl. bcr/abl genes encode 3 proteins: p185, which contains a smaller BCR portion (amino acids 1 to 426), and is associated with acute lymphocytic leukemia (ALL)1; p210, which contains a larger BCR portion (amino acids 1 to 902 or 926), and is found in most cases of chronic myelogenous leukemia (CML)2; and p230, which contains the largest BCR portion among the 3 variants, and is associated with chronic neutrophilic leukemia and with CML.3,4 BCR/ABL proteins are constitutively active tyrosine kinases that can transform fibroblasts and hematopoietic cells in culture,5-7 and induce leukemia in mice.8-10
The BCR/ABL oncoproteins protect cells from apoptosis induced by various stimuli.11-14 In recent years, many molecules involved in either promoting or suppressing apoptosis have been discovered and some of their mechanisms of action have been elucidated. The BCL-2 family of apoptosis regulators is composed of cell death agonists and antagonists. The relative expression levels of inhibitors (BCL-2, BCL-XL) and promoters (BAX, BAD, BCL-XS) determine, in part, how cells will respond to apoptotic stimuli,15,16 but the subcellular localization of various members of the BCL-2 family is also important for controlling apoptotic processes. In healthy cells, the proapoptotic members of the “BH3-only” BCL-2 subfamily BID17,18 and BAD,19,20 which lack a mitochondrial insertion signal, are localized in the cytosol as well as in the mitochondrial membrane. BIM, another “BH3-only” protein, is, instead, sequestered in the cytoskeleton.21 In response to apoptotic stimuli, these proteins translocate to the mitochondrial membrane where they interact with membrane-bound antiapoptotic family members and interfere with their function. Among these proapoptotic members, BAD is the only one regulated by phosphorylation. In the presence of interleukin-3 (IL-3), BAD becomes phosphorylated on serine residues and is sequestered in the cytoplasm by interaction with members of 14-3-3 family, which are motif-specific phosphoserine-binding proteins.22 On IL-3 withdrawal, BAD is unphosphorylated and binds BCL-XL and BCL-2,19,20 thus inhibiting their survival-promoting effects. Akt is one of the kinases responsible for the phosphorylation of BAD at serine 112 and 136.23,24 Mutation of these serine residues (Ser to Ala) converts BAD into a constitutively active inducer of apoptosis.23 BAD can also be regulated at the mitochondrial level.14 In BCR/ABL-expressing cells, BAD is constitutively phosphorylated and mainly cytoplasmic. However, deletion of the BCR domain of BCR/ABL results in the lack of BAD phosphorylation, which can be restored by the overexpression of a dominant-active mutant of Raf-1 targeted to the mitochondria. Moreover, overexpression of Raf-1 in the mitochondria also induces the release of BAD into the cytoplasm.14
Antiapoptotic members of the BCL-2 family inhibit the mitochondrial release of cytochrome c,25 a component of the electron transport chain involved in the activation of procaspase-9, which subsequently cleaves procaspase 3. Once active, caspase-3 can induce DNA fragmentation and other characteristic events of apoptosis.25
The antiapoptotic effects of BCR/ABL might involve the inhibition of upstream preapoptotic mitochondrial events and may also be exerted downstream through the inhibition of the activity of caspase 3. For example, BCR/ABL induces an increase in the levels of Bcl-2,26,27 which may explain its ability to block the mitochondrial release of cytochrome c and the subsequent activation of caspase 3.28
The objective of this study was to extend our investigation on the role of BAD in apoptotic processes in hematopoietic cells by analyzing the effect of wild-type (WT) and mutant BAD proteins on parental and BCR/ABL-expressing 32D myeloid precursor cells. We expected BCR/ABL-expressing cells to be susceptible to overexpression of mutant BAD, which cannot be inactivated by phosphorylation, but to escape the death-inducing effects of WT BAD, thus providing a model to further investigate the antiapoptotic effects of BCR/ABL. We report here that transient expression of WT or mutant BAD is a more potent inducer of apoptosis in parental than in BCR/ABL-expressing 32D cells. On IL-3 starvation of parental 32D cells expressing WT BAD, BAD was rapidly dephosphorylated by the serine-threonine phosphatase 1α (PP-1α) and localized to the mitochondria. In BCR/ABL-expressing cells resistant to apoptosis induced by WT BAD, BAD remained phosphorylated and localized in the cytoplasm. Moreover, resistant clones all showed increased expression of BCL-2 and BCL-XL. BCR/ABL-expressing cells resistant to apoptosis induced by mutant BAD showed elevated levels of BCL-2 and BCL-XL, in excess of those in complex with the mitochondria-localized mutant BAD.
Materials and methods
Plasmids
Hemagglutinin (HA)-tagged WT, S136A, and S112A/S136A double mutant (DM) BAD were cloned into the LXSP retroviral vector as follows: pcDNA3-WTHA-BAD, pcDNA3-S136AHA-BAD, and pcDNA3-DMHA-BAD (kind gift from Dr M. E. Greenberg, Children's Hospital and Department of Neurobiology, Harvard Medical School, Boston, MA) were digested withHindIII/EcoRI, blunt-ended with Klenow, and subcloned into the LXSP vector, linearized by EcoRI digestion, and blunted with Klenow.
WT-BAX LXSP was cloned by digesting pcDNA3-mouse WT-BAX withHindIII, blunt-ending with Klenow, redigesting withXhoI, and subcloning into theHpaI/XhoI-linearized LXSP vector. To obtain GST-BAD, full-length BAD was amplified from BAD-LXSP by polymerase chain reaction (PCR) using the following primers: 5′BAD,GAATTCGAATTC-GGAACCCCAAAGCAG, which contains 2 EcoRI sites (in bold); 3′BAD, GCCGCCAGTGTGATGGATATCTGCA. The amplified fragment that includes an additional EcoRI site at the 3′ end, was then digested with EcoRI and subcloned into theEcoRI-digested pGEX-4T-1 (Pharmacia Biotech, Piscataway, NJ). pHA-MyrAkt-CMV6 was obtained from Dr P. N. Tsichlis (Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA); pSRα p210 BCR/ABL was obtained from Dr A. M. Pendergast (Duke University Medical Center, Durham, NC).
Cell lines
The murine IL-3–dependent 32Dcl3 myeloid precursor cell line29 was maintained at 37°C in IMDM-CM (Iscove's modified Dulbecco medium), supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mmol/L L-glutamine, penicillin/streptomycin (100 μg/mL each), and 15% WEHI-conditioned medium (WEHI-CM) as a source of IL-3.30 Clones of 32D cells expressing p210 BCR/ABL were generated in our laboratory and maintained as described.31 The BCR/ABL-expressing DAGM cell line derived by transfection of the IL-3–dependent parental line was kindly provided by Dr C. Sawyers (University of California-Los Angeles, Los Angeles, CA). The CML-BC K562 cell line was obtained from the American Type Culture Collection (ATCC).
Retrovirus infection and methylcellulose colony assays
The BOSC 23 packaging cell line was cultured and transfected with the empty LXSP vector or the HA-BAD-containing vectors as described.32 At 24 hours after transfection, BOSC 23 cells were cultured for 6 to 8 hours in IMDM-CM, and cocultured with parental or BCR/ABL-expressing 32D cells for 48 hours in the presence of 4 μg/mL polybrene. 32D cells were removed from the plates, washed, and replated in methylcellulose in the presence of puromycin (2.5 μg/mL) and 10% WEHI-CM as source of IL-3, where indicated. Colonies were counted 10 days later. Individual clones picked from methylcellulose plates of BAD-retrovirus–infected cells were transferred to 24-microtiter wells and expanded in the presence of IL-3 to generate cell clones stably expressing wild-type or mutant BAD.
Cell viability and apoptosis assays
Relative numbers of viable cells were estimated by trypan blue exclusion assay. Apoptosis was evaluated by flow cytometry determination of DNA content in propidium iodide-stained nuclei.
Phosphatase inhibitor assays
Cells were cultured for 2 hours in IMDM-CM containing different phosphatase inhibitors: cyclosporin A, 0.5 μmol/L; cantharidin, 30 μmol/L (high) or 250 nmol/L (low); calyculin A, 20 nmol/L (all purchased from Calbiochem Corp, La Jolla, CA). After several washes, cells were cultured for 4 hours in the absence of IL-3. BAD phosphorylation was assessed by Western blotting with a mix of antiphospho BAD (Ser 112 and Ser 136) antibodies.
Preparation of subcellular fractions
Subcellular fractionation was performed as described.14Briefly, cells were lysed in hypotonic buffer (5 mmol/L Tris, pH 7.4, 5 mmol/L KCl, 1.5 mmol/L MgCl2, 0.1 mmol/L EGTA [pH 8.0], 1 mmol/L DTT, 10 μg/mL aprotinin, 10 μg/mL of leupeptin, 10 mmol/L benzamidine, 1 mmol/L PMSF, and 0.2 mmol/L sodium orthovanadate) at 4°C for 30 minutes. After homogenization (20 strokes with a B-pestle Dounce homogenizer), samples were centrifuged (2 000 × g for 5 minutes) to remove the nuclei, and centrifuged again (10 000 × g for 10 minutes) to obtain the heavy membrane (HM) fraction (pellet). The supernatant was centrifuged at 150 000 × g for 1.5 hours to obtain the cytosolic fraction. The HM fractions were solubilized in 1% Triton X-100, 10 mmol/L Tris-HCL (pH 7.4), 150 mmol/L NaCl, 5 mmol/L EDTA, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 mmol/L benzamidine, 1 mmol/L PMSF, and 0.2 mmol/L sodium orthovanadate.
Western blot, immunoprecipitation, and coimmunoprecipation analyses
Western blots were performed using anti-BAD (C-20, K-17: all from Santa Cruz Biotechnology, Inc, Santa Cruz, CA), anti-BCL-2 (Transduction Laboratories, Lexington, KY), anti-BCL-XL (Pharmingen, Torrey Pines, CA), anti-HA (Babco, Richmond, CA), anti-14-3-3β (Santa Cruz Biotechnology), and anti-PP-1 (Santa Cruz Biotechnology) antibodies. Immunoprecipi-tations of BAD and HA-tagged BAD were carried out using anti-BAD (Santa Cruz Biotechnology) and anti-HA antibody (Babco). Immunoprecipitates of PP-1α were performed using anti-PP-1α antibody (kind gift of Dr A. Narnin, Rockefeller University, New York, NY). Coimmunoprecipitations were carried out in 0.2% Triton X-100, 10 mmol/L Tris-HCL (pH 7.4), 150 mmol/L NaCl, 5 mmol/L EDTA, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 mmol/L benzamidine, 1 mmol/L PMSF, and 0.2 mmol/L sodium orthovanadate (immunoprecipitation [IP] buffer). Precleared extracts were incubated with antibody for 2 hours and then with protein G-coated agarose beads for an additional 2 hours. Beads were then washed 5 times in IP buffer and boiled for 2 minutes in sample buffer. Samples were loaded on a 12.5% polyacrylamide-SDS gel for electrophoresis.
Pulse-chase assay
32D cells were cultured for 30 minutes in RPMI-CM (RPMI 1640 without methionine), supplemented with 10% of dialyzed fetal bovine serum and 2 ng/mL of recombinant murine IL-3 (both from Gibco BRL, Grand Island, NY) at 106 cells/mL. Cells were washed and resuspended in RPMI-CM containing 9.25 MBq/mL (250 μCi/mL)of [35S]-methionine (NEN, Life Science Products, Boston, MA) at 5 × 106cells/mL. After 1 hour, cells were washed with methionine-containing IMDM and cultured for 6 hours. At different time points, cells were harvested and lysed in 1% Triton X-100, 10 mmol/L Tris-HCL (pH 7.4), 150 mmol/L NaCl, 2 mmol/L EDTA, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 mmol/L benzamidine, 1 mmol/L PMSF, and 0.2 mmol/L sodium orthovanadate. Precleared extracts were incubated at 4°C for 2 hours with protein G plus (Oncogene Research Products, Boston, MA)-coupled anti-HA antibody. Immunoprecipitated proteins were washed several times in lysis buffer, denatured in gel loading buffer, and resolved by SDS-PAGE. Labeled proteins were visualized by autoradiography.
In vitro dephosphorylation assays
GST-BAD was grown in DH5α bacteria and purified using glutathione sepharose 4B beads (Pharmacia Biotech, NJ). To release GST-BAD, beads were incubated for 1 hour with 200 mmol/L reduced glutathione. 293T cells were transfected with pHA-MyrAKT-CMV6 by calcium phosphate and 24 hours later lysed in 1% Triton X-100, 10 mmol/L Tris-HCL (pH 7.4), 150 mmol/L NaCl, 5 mmol/L EDTA, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 mmol/L benzamidine, 1 mmol/L PMSF, and 0.2 mmol/L sodium orthovanadate. HA-MyrAKT was immunoprecipitated using the anti-HA antibody. Immunoprecipitates were incubated with 1 μg of GST-BAD in the presence of 0.37 MBq (10 μCi) of [32P]-γATP (NEN) for 30 minutes at 30°C. The reaction was stopped by adding 0.1% Triton-X-100, 10 mmol/L Tris-HCL (pH 7.4), 150 mmol/L NaCl, and 5 mmol/L EDTA, and GST-BAD was purified using glutathione sepharose beads (1 hour at 4°C). Phosphorylated GST-BAD was incubated with 2 units of recombinant PP-1α (Calbiochem Corp) in phosphatase buffer (50 mmol/L Tris-HCL, pH 7.5, 5 mmol/L DTT, 200 μmol/L MnCl2, 100 μmol/L EDTA, and 0.02% bovine serum albumin [BSA]) for 30 minutes at 30°C. Reactions were stopped by adding 2 × SDS-sample buffer and run on a 12.5% polyacrylamide gel. The gel was transferred to nitrocellulose and exposed. Levels of GST-BAD were measured with the anti-BAD (C-20) antibody. The PP-1α–dependent release of [32P]-ATP was evaluated by scintillation counting. To assess the potential effect of BCR/ABL expression on PP-1α activity, recombinant PP-1α (Calbiochem) was incubated with anti-ABL immunoprecipitates from parental or BCR/ABL-expressing cells (500 μg cell extracts; anti-c-ABL monoclonal antibody OP20, Calbiochem, CA) in 20 mmol/L PIPES pH 7.0, 20 mmol/L MnCl2 in the presence or absence of 10 μmol/L ATP for 30 minutes at 30°C. Kinase reaction supernatants were collected, diluted 1:10 with phosphatase buffer, and incubated for 30 minutes at 30°C in the presence of the RRApTVA substrate (Promega) to assess PP-1α activity. Released phosphates were detected using a malachite green dye solution.
Northern blot
Total RNA was extracted using Tri-Reagent (Molecular Research Center, Inc, Cincinnati, OH). For Northern blot analysis, RNA (10 μg) was electrophoresed on 1% agarose/6% formaldehyde gels, transferred to nylon membrane (Amersham Pharmacia Biotech, San Francisco, CA), and hybridized to a bcl-2 cDNA probe.33
Results
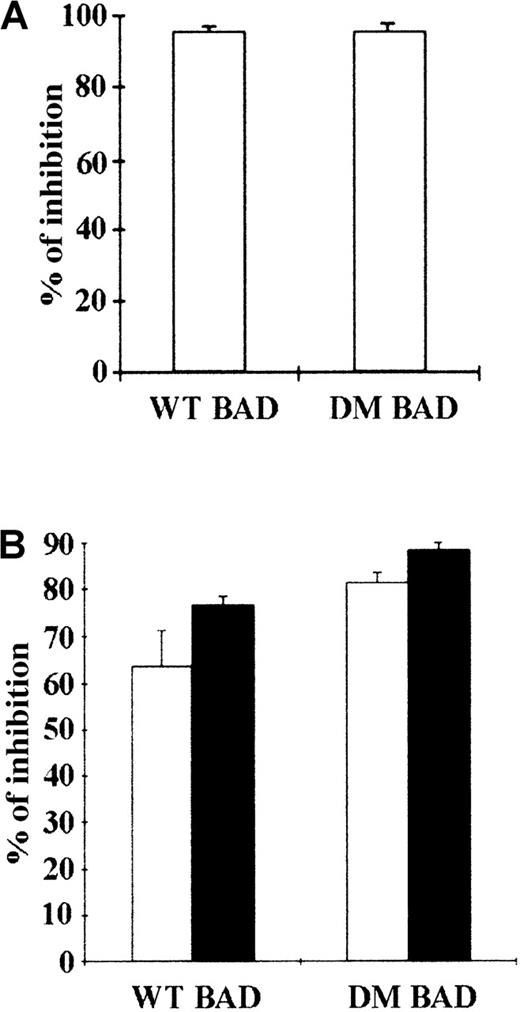
Transient expression of WT and DM BAD induces cell death in parental and BCR/ABL-expressing 32D cells
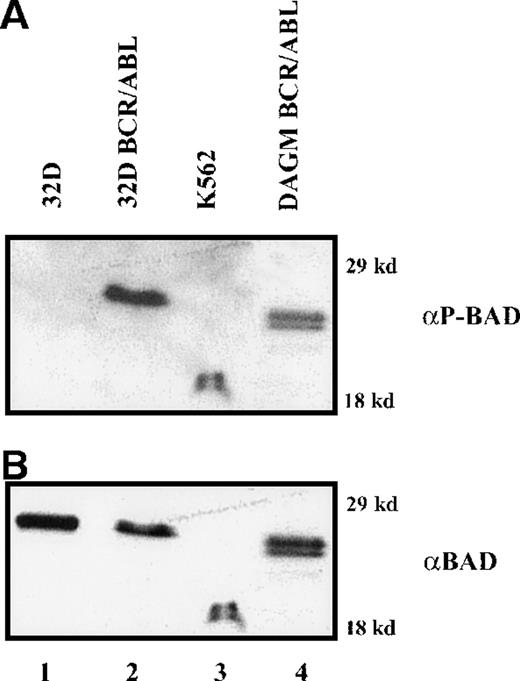
BAD is constitutively phosphorylated in hematopoietic cells expressing WT BCR/ABL (Figure 1), whereas BCR/ABL mutants unable to protect from apoptosis are also defective in promoting BAD phosphorylation (unpublished data).14 This suggests that phosphorylation and inactivation of BAD correlate with the antiapoptotic activity of BCR/ABL. Thus, WT BAD and DM BAD in which serine 112 and 136 were replaced with alanine to prevent the phosphorylation-dependent inactivation were tested for the ability to induce apoptosis in parental and BCR/ABL-expressing 32D cells, as measured by the inhibition of methylcellulose colony formation of cells expressing ectopic BAD. 32D cells were clonogenic only in the presence of IL-3; on infection with a retrovirus carrying either WT or DM BAD, the clonogenic ability of 32D cells was markedly suppressed (approximately 95% decrease) (Figure 2A). Both WT BAD and DM BAD also induced a marked decrease (63.6% and 81%, respectively, in the presence IL-3, and 76.6% and 88%, respectively, in the absence of IL-3), in the clonogenic ability of BCR/ABL-expressing cells (Figure 2B). Similar inhibition of colony formation was also observed on overexpression of the S136A BAD mutant (data not shown).
Phosphorylation of BAD in BCR/ABL-expressing cells.
Western blot shows expression of phosphorylated BAD (A) detected by a mix of antiphosphoserine 112 and 136 BAD antibodies, in IL-3–(32D, 32DBCR/ABL, DAGM BCR/ABL) or serum-(K562) deprived cells (8 and 12 hours, respectively); (B) the same blot was hybridized with an anti-BAD (K17, Santa Cruz, Inc) antibody.
Phosphorylation of BAD in BCR/ABL-expressing cells.
Western blot shows expression of phosphorylated BAD (A) detected by a mix of antiphosphoserine 112 and 136 BAD antibodies, in IL-3–(32D, 32DBCR/ABL, DAGM BCR/ABL) or serum-(K562) deprived cells (8 and 12 hours, respectively); (B) the same blot was hybridized with an anti-BAD (K17, Santa Cruz, Inc) antibody.
Inhibition of colony formation by transient overexpression of WT or DM BAD.
(A) Parental 32D cells were cocultured with LXSP-, WT HA-BAD/LXSP–, or DM HA-BAD/LXSP–transfected BOSC 23 cells. After 48 hours, infected cells were plated in methylcellulose in the presence of IL-3 and puromycin (2.5 μg/mL). Colonies were counted 10 days later. The effect of BAD on the clonogenic capacity of infected cells is expressed as percentage of inhibition of the clonogenic ability of vector-infected cells. (B) BCR/ABL-expressing cells were cocultured with LXSP-, WTHA-BAD/LXSP–, or DMHA-BAD/LXSP–transfected BOSC 23 cells and plated in methylcellulose in the presence of puromycin (2.5 μg/mL). The percentage of inhibition of clonogenic activity (mean + SD) in the presence (□) or absence (▪) of IL-3 is representative of 3 independent experiments.
Inhibition of colony formation by transient overexpression of WT or DM BAD.
(A) Parental 32D cells were cocultured with LXSP-, WT HA-BAD/LXSP–, or DM HA-BAD/LXSP–transfected BOSC 23 cells. After 48 hours, infected cells were plated in methylcellulose in the presence of IL-3 and puromycin (2.5 μg/mL). Colonies were counted 10 days later. The effect of BAD on the clonogenic capacity of infected cells is expressed as percentage of inhibition of the clonogenic ability of vector-infected cells. (B) BCR/ABL-expressing cells were cocultured with LXSP-, WTHA-BAD/LXSP–, or DMHA-BAD/LXSP–transfected BOSC 23 cells and plated in methylcellulose in the presence of puromycin (2.5 μg/mL). The percentage of inhibition of clonogenic activity (mean + SD) in the presence (□) or absence (▪) of IL-3 is representative of 3 independent experiments.
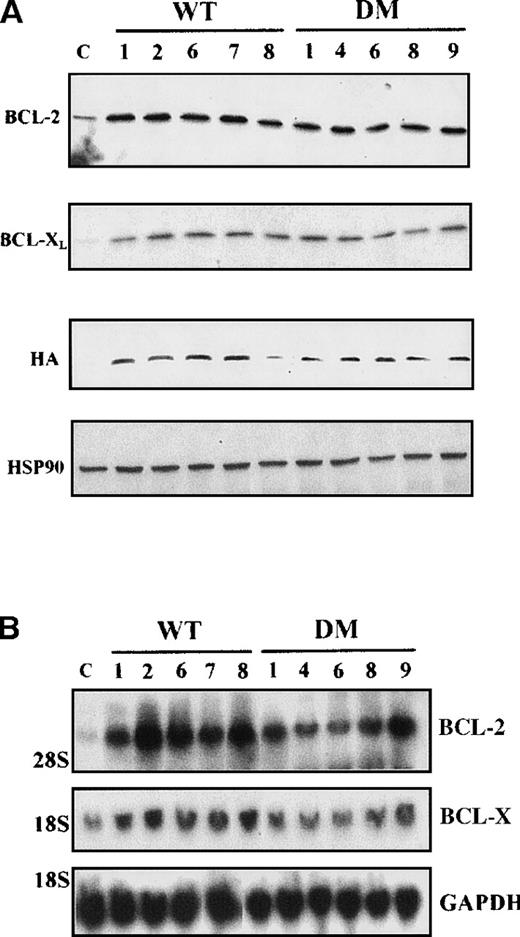
Subcellular localization of WT and DM BAD
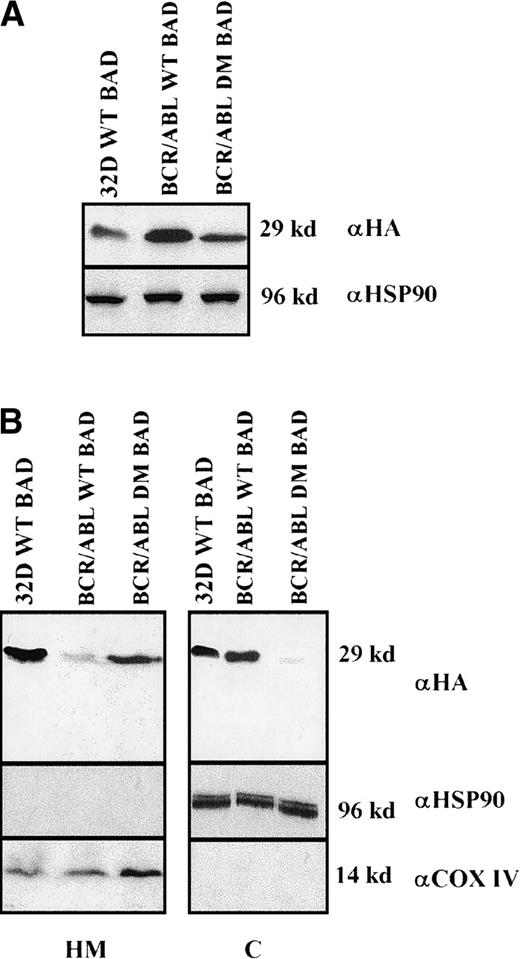
Clones resulting from the infection of wild-type and BCR/ABL-expressing 32D cells with the WT or DM BAD retrovirus were analyzed for the expression of exogenous BAD. After the initial harvest, several clones of infected 32D cells expressed high levels of WT BAD; however, on culturing, most clones lost WT BAD expression. None of the puromycin-resistant clones showed detectable amounts of DM BAD. By contrast, clones from BCR/ABL-expressing cells showed high levels of either WT or DM BAD (Figure 3A). To investigate the subcellular distribution of WT and DM BAD, extracts of parental and BCR/ABL-expressing cells were fractionated and blotted with the anti-HA antibody. WT BAD was equally distributed between the cytoplasm and the mitochondria of parental cells, but was almost exclusively cytoplasmic in BCR/ABL-expressingcells. DM BAD was predominantly associated with mitochondria in BCR/ABL-expressing cells (Figure 3B).
Western blots.
(A) Expression of ectopic HA-BAD in selected clones of parental and BCR/ABL-expressing cells. The Western blot shows expression of WT and DM BAD proteins in representative clones. Levels of HSP90 were detected as control for protein loading. (B) WT and DM BAD expression levels in subcellular fractions of infected parental (32D WT BAD) and BCR/ABL-expressing (BCR/ABL WT and DM BAD) cells. Western blots show HA-BAD levels in fractions enriched for mitochondria (HM), and cytoplasm (C) from infected parental and BCR/ABL-expressing cells. Levels of the mitochondrial marker subunit IV of cytochrome oxidase (COX) and cytoplasmic marker HSP90 were measured as control of equal loading. Results are representative of 3 independent experiments.
Western blots.
(A) Expression of ectopic HA-BAD in selected clones of parental and BCR/ABL-expressing cells. The Western blot shows expression of WT and DM BAD proteins in representative clones. Levels of HSP90 were detected as control for protein loading. (B) WT and DM BAD expression levels in subcellular fractions of infected parental (32D WT BAD) and BCR/ABL-expressing (BCR/ABL WT and DM BAD) cells. Western blots show HA-BAD levels in fractions enriched for mitochondria (HM), and cytoplasm (C) from infected parental and BCR/ABL-expressing cells. Levels of the mitochondrial marker subunit IV of cytochrome oxidase (COX) and cytoplasmic marker HSP90 were measured as control of equal loading. Results are representative of 3 independent experiments.
BAD is constitutively phosphorylated in BCR/ABL-expressing cells
To investigate the mechanisms whereby BCR/ABL counteracts the strong proapoptotic activity of BAD, we first analyzed levels of BAD phosphorylation. Parental and BCR/ABL-expressing cells were metabolically labeled with [32P]-orthophosphate in the absence of growth factors, and exogenous BAD was immunoprecipitated from total extracts using the anti-HA antibody. Phosphorylated WT BAD was detected only in BCR/ABL-expressing cells, whereas DM BAD was not phosphorylated above background (Figure4A). No phosphorylation was detected in 32D/BCR-ABL cells expressing the S136A BAD mutant (data not shown).
HA-BAD in parental and BCR/ABL-expressing cells.
(A) Phosphorylation of HA-BAD in parental and BCR/ABL-expressing cells starved of both IL-3 and serum in [32P]-orthophosphate-containing medium. Immunoprecipitations were carried out using an anti-HA antibody. Vector-infected BCR/ABL-expressing cells were used as negative control. (B) Association of 14-3-3β and HA-BAD in infected parental and BCR/ABL-expressing cells. Infected parental cells were starved of IL-3 and the interaction 14-3-3/BAD was assessed in cytoplasmic extracts 0, 30, and 60 minutes after the removal of IL-3. BCR/ABL-expressing cells infected with WT and DM BAD were starved for 4 hours and then lysed. Total extracts were analyzed for BAD-14-3-3β association. Lane C shows expression of 14-3-3β and HA-BAD in total extracts from BCR/ABL/WT BAD-expressing cells. (C) Levels of phosphorylated BAD after IL-3 removal. Cells were starved of IL-3 for 4 hours, harvested, and lysed at different times (0, 1, 2, 3, and 4 hours). Phosphorylated BAD was detected using a mix of antiphospho BAD antibodies. Levels of HA-BAD were measured as control of equal loading. (D) Stability of WTHA-BAD in starved 32D cells. Levels of [35S]-labeled HA-BAD were assessed at 0, 1, 2, and 4 hours after the simultaneous removal of [35S]-methionine and IL-3. Levels of HA-BAD were measured as control of immunoprecipitation efficiency. The asterisk (in B and D) indicates immunoglobulin light chain. Results are representative of 3 independent experiments.
HA-BAD in parental and BCR/ABL-expressing cells.
(A) Phosphorylation of HA-BAD in parental and BCR/ABL-expressing cells starved of both IL-3 and serum in [32P]-orthophosphate-containing medium. Immunoprecipitations were carried out using an anti-HA antibody. Vector-infected BCR/ABL-expressing cells were used as negative control. (B) Association of 14-3-3β and HA-BAD in infected parental and BCR/ABL-expressing cells. Infected parental cells were starved of IL-3 and the interaction 14-3-3/BAD was assessed in cytoplasmic extracts 0, 30, and 60 minutes after the removal of IL-3. BCR/ABL-expressing cells infected with WT and DM BAD were starved for 4 hours and then lysed. Total extracts were analyzed for BAD-14-3-3β association. Lane C shows expression of 14-3-3β and HA-BAD in total extracts from BCR/ABL/WT BAD-expressing cells. (C) Levels of phosphorylated BAD after IL-3 removal. Cells were starved of IL-3 for 4 hours, harvested, and lysed at different times (0, 1, 2, 3, and 4 hours). Phosphorylated BAD was detected using a mix of antiphospho BAD antibodies. Levels of HA-BAD were measured as control of equal loading. (D) Stability of WTHA-BAD in starved 32D cells. Levels of [35S]-labeled HA-BAD were assessed at 0, 1, 2, and 4 hours after the simultaneous removal of [35S]-methionine and IL-3. Levels of HA-BAD were measured as control of immunoprecipitation efficiency. The asterisk (in B and D) indicates immunoglobulin light chain. Results are representative of 3 independent experiments.
The phosphorylation status of BAD correlates with its ability to interact with 14-3-3β and this interaction is necessary for its sequestration in the cytoplasm.20 Coimmunoprecipitation experiments revealed a weak interaction of WT BAD with 14-3-3β only in the presence of IL-3 and in cytoplasmic extracts of parental 32D cells; at 1 hour after IL-3 withdrawal, 14-3-3β was not detectable in complex with BAD (Figure 4B). In contrast, 14-3-3β strongly and constitutively interacted with WT BAD in BCR/ABL-expressing cells, despite the removal of IL-3. As expected, DM BAD, which is nonphosphorylated and mitochondrial, was not in complex with 14-3-3β (Figure 4B).
Because maintenance of BAD in its phosphorylated form is required to prevent proapoptosis effects, we assessed whether BAD undergoes dephosphorylation on IL-3 withdrawal in parental and BCR/ABL-expressing cells using an antibody mix specific for phosphorylated BAD. In parental cells, BAD became rapidly dephosphorylated after IL-3 removal: At 1 hour, the level of phosphorylation was already decreased by half, and no phosphorylated BAD was detectable at 4 hours (Figure 4C).
By contrast, BAD was phosphorylated at all time points in BCR/ABL-expressing cells and the levels of phosphorylation were much higher than in parental cells (Figure 4C).
Pulse-chase experiments using IL-3–starved WT BAD-expressing parental cells, labeled with [35S]-L-methionine and cultured in the presence of cold L-methionine, showed that newly synthesized HA BAD remained stable during the 4-hour chase period (Figure 4D). Thus, dephosphorylation and lack of complex formation between BAD and 14-3-3β does not appear to reflect a markedly diminished stability (half-life) of BAD, in the absence of IL-3.
BAD is dephosphorylated by PP-1 and PP-1 activity is reduced in BCR/ABL-expressing cells
To assess the potential involvement of known serine phosphatases in the dephosphorylation of BAD detected after IL-3 withdrawal, 32D cells expressing HA-BAD were treated with phosphatase inhibitors (cyclosporin A, or calyculin A, or cantharidin) and examined for levels of phosphorylated BAD by Western blotting with the antibody mix specific for phosphoserine 112 and 136. As shown in Figure5A, BAD remained phosphorylated after treatment with the phosphatase inhibitor calyculin A or cantharidin, but not cyclosporin A. Moreover, BAD was detected in complex with 14-3-3β in cells treated with calyculin A or cantharidin, but not in untreated cells or cells treated with cyclosporin A (Figure 5B). Complex formation between BAD and 14-3-3β was inversely correlated with the mitochondrial localization of BAD in calyculin A-treated and cantharidin-treated cells; BAD was already more abundant in the cytoplasm of cells preincubated for 2 hours with calyculin A or cantharidin (Figure 5C, left panel), and such subcellular localization was clearly predominant after culturing IL-3–deprived cells for 4 additional hours (Figure 5C, right panel). By contrast, BAD was predominantly mitochondrial in untreated cells or in cells treated with cyclosporin A (Figure 5C).
HA-BAD in parental 32D cells.
(A) Phosphorylation of HA-BAD in parental 32D cells treated with phosphatase inhibitors. 32D cells were pretreated either with cyclosporin A (CyA) (0.5 μmol/L), or cantharidin (Cant) (high [cant-high], 30 μmol/L; low[cant-low], 250 nmol/L) or calyculin A (20 nmol/L) for 2 hours and then deprived from IL-3 for 4 hours. At 0 and 4 hours, cells were harvested and lysed. Phosphorylated BAD was detected by using a mix of antiphospho BAD antibodies. Levels of HA-BAD were measured as control of loading. (B) Association between HA-BAD and 14-3-3β. 32D cells were pretreated with phosphatase inhibitors (see above) and then starved from IL-3. The HA-BAD: 14-3-3β interaction was evaluated in cytoplasmic extracts 0 and 4 hours after IL-3 withdrawal. Levels of immunoprecipitated HA-BAD were measured as control of loading. (C) BAD expression levels in subcellular fractions of parental cells treated with phosphatase inhibitors. Western blots show exogenous BAD levels in fractions enriched for cytoplasm (C) and mitochondria (HM) from untreated and phosphatase inhibitor-treated 32D cells at 0 and 4 hours after IL-3 withdrawal. Levels of mitochondrial marker subunit IV of cytochrome oxidase (COX) and cytoplasmic marker HSP90 were measured as control of equal loading and purity of subcellular fractions. Representative of 3 different experiments.
HA-BAD in parental 32D cells.
(A) Phosphorylation of HA-BAD in parental 32D cells treated with phosphatase inhibitors. 32D cells were pretreated either with cyclosporin A (CyA) (0.5 μmol/L), or cantharidin (Cant) (high [cant-high], 30 μmol/L; low[cant-low], 250 nmol/L) or calyculin A (20 nmol/L) for 2 hours and then deprived from IL-3 for 4 hours. At 0 and 4 hours, cells were harvested and lysed. Phosphorylated BAD was detected by using a mix of antiphospho BAD antibodies. Levels of HA-BAD were measured as control of loading. (B) Association between HA-BAD and 14-3-3β. 32D cells were pretreated with phosphatase inhibitors (see above) and then starved from IL-3. The HA-BAD: 14-3-3β interaction was evaluated in cytoplasmic extracts 0 and 4 hours after IL-3 withdrawal. Levels of immunoprecipitated HA-BAD were measured as control of loading. (C) BAD expression levels in subcellular fractions of parental cells treated with phosphatase inhibitors. Western blots show exogenous BAD levels in fractions enriched for cytoplasm (C) and mitochondria (HM) from untreated and phosphatase inhibitor-treated 32D cells at 0 and 4 hours after IL-3 withdrawal. Levels of mitochondrial marker subunit IV of cytochrome oxidase (COX) and cytoplasmic marker HSP90 were measured as control of equal loading and purity of subcellular fractions. Representative of 3 different experiments.
In cells treated with a low concentration (250 nmol/L) of cantharidin, which is inhibitory for PP2α, but not for PP-1, BAD was dephosphorylated (Figure 5A, lane Cant-Low), and not in complex with 14-3-3β (data not shown), suggesting that PP-1 is involved in the dephosphorylation of BAD after IL-3 withdrawal.
Consistent with the potential role of PP-1α in dephosphorylating BAD, PP-1α was detected in complex with BAD in exponentially growing parental and BCR/ABL-expressing 32D cells and in cells deprived of IL-3 for 2 hours (Figure 6A, left). In control experiments, PP-2A was not in complex with HA-tagged BAD in BCR/ABL-expressing 32D cells (Figure 6A, middle). Moreover, the interaction of PP-1α and BAD appears to be specific because PP-1α did not interact with BAX (Figure 6A, right). To determine whether PP-1α dephosphorylates BAD, bacterially purified GST-BAD was phosphorylated by Akt using anti-Akt immunoprecipitates from 293T cells transiently transfected with constitutively active Akt and incubated with recombinant PP-1α. In 2 separate experiments, the fraction of32P-labeled GST-BAD was markedly reduced (more than 80%) after treatment with PP-1α (Figure 6B). To assess whether the activity of PP-1α might be down-modulated in BCR/ABL-expressing cells, anti–PP-1α immunoprecipitates from parental and BCR/ABL-expressing cells were used in phosphatase assays with phosphorylated GST-BAD or a synthetic phosphopeptide as substrates. However, using 2 different anti–PP-1α antibodies, we were unable to demonstrate measurable phosphatase activity in the immunoprecipitate (data not shown). Thus, on the assumption that BCR/ABL might directly or indirectly inhibit PP-1α activity, recombinant PP-1α was incubated with anti-ABL immunoprecipitates from parental or BCR/ABL-expressing cells and assessed for the ability to dephosphorylate a synthetic phosphopeptide. In 3 different experiments, PP-1α activity was inhibited by incubation with anti-ABL immunoprecipitates from BCR/ABL-expressing cells (50% to 80% inhibition compared with immunoprecipitates from parental cells) (Figure 6C). The inhibitory effect was only observed when PP-1α was incubated with anti-ABL immunoprecipitates in kinase buffer supplemented with 10 μmol/L ATP, suggesting that BCR/ABL-dependent PP-1α phosphorylation is involved in the inhibition of enzyme activity.
PP-1α and BAD in parental and BCR/ABL-expressing cells.
(A) Association of PP-1α and BAD in parental and BCR/ABL-expressing 32D cells. The association of HA-BAD and PP-1α was assessed in cytoplasm extracts of exponentially growing and IL-3–starved (2 hours) parental and BCR/ABL-expressing cells (left). As control, the interaction of BAD with PP-2α (middle, lane 2) and that of BAX with PP-1α (right, lanes 1 and 2) were also assessed in BAD- and BAX- expressing cells. Levels of HA-BAD (left and middle panels), and PP-1α (right) were measured as immunoprecipitation controls (left panel, lane 2 of middle panel, lanes 1 and 2 of right panel); levels of PP-2α (lane 1, middle) and BAX (lane 3, right) were measured in whole cell lysate as Western blot controls. Arrows indicate PP-1α HA-BAD, and PP-2α proteins. Asterisks indicate immunoglobulin chains. (B) PP-1α–dependent dephosphorylation of [32P]-labeled GST-BAD. Constitutively active HA-MyrAkt was immunoprecipitated from transiently transfected 293T cells and incubated with 1 μg of GST-BAD and 0.37 MBq (10 μCi) [32P]-γATP for 30 minutes at 30°C. Phosphorylated GST-BAD was then incubated with glutathione sepharose beads for 1 hour at 4°C. Phosphatase reactions were carried out in the presence of 2 units of recombinant PP-1α for 30 minutes at 30°C, and stopped by adding 2 × SDS-sample buffer. Levels of BAD were measured as control of loading. Two independent experiments are shown. (C) Inhibition of PP-1α activity by anti-BCR/ABL immunoprecipitates. Histograms show the percentage of inhibition (mean + SD) of PP-1α activity by anti-ABL immunoprecipitates from BCR/ABL-expressing cells, compared with parental cells, in the presence or in the absence of 10 μmol/L ATP. PP-1α activity was assessed using a malachite green dye solution to detect phosphates released from the synthetic substrate. Samples were also assessed for PP-1α levels by Western blotting with anti-PP-1α antibody.
PP-1α and BAD in parental and BCR/ABL-expressing cells.
(A) Association of PP-1α and BAD in parental and BCR/ABL-expressing 32D cells. The association of HA-BAD and PP-1α was assessed in cytoplasm extracts of exponentially growing and IL-3–starved (2 hours) parental and BCR/ABL-expressing cells (left). As control, the interaction of BAD with PP-2α (middle, lane 2) and that of BAX with PP-1α (right, lanes 1 and 2) were also assessed in BAD- and BAX- expressing cells. Levels of HA-BAD (left and middle panels), and PP-1α (right) were measured as immunoprecipitation controls (left panel, lane 2 of middle panel, lanes 1 and 2 of right panel); levels of PP-2α (lane 1, middle) and BAX (lane 3, right) were measured in whole cell lysate as Western blot controls. Arrows indicate PP-1α HA-BAD, and PP-2α proteins. Asterisks indicate immunoglobulin chains. (B) PP-1α–dependent dephosphorylation of [32P]-labeled GST-BAD. Constitutively active HA-MyrAkt was immunoprecipitated from transiently transfected 293T cells and incubated with 1 μg of GST-BAD and 0.37 MBq (10 μCi) [32P]-γATP for 30 minutes at 30°C. Phosphorylated GST-BAD was then incubated with glutathione sepharose beads for 1 hour at 4°C. Phosphatase reactions were carried out in the presence of 2 units of recombinant PP-1α for 30 minutes at 30°C, and stopped by adding 2 × SDS-sample buffer. Levels of BAD were measured as control of loading. Two independent experiments are shown. (C) Inhibition of PP-1α activity by anti-BCR/ABL immunoprecipitates. Histograms show the percentage of inhibition (mean + SD) of PP-1α activity by anti-ABL immunoprecipitates from BCR/ABL-expressing cells, compared with parental cells, in the presence or in the absence of 10 μmol/L ATP. PP-1α activity was assessed using a malachite green dye solution to detect phosphates released from the synthetic substrate. Samples were also assessed for PP-1α levels by Western blotting with anti-PP-1α antibody.
Of note, the ability of calyculin A and cantharidin to prevent the dephosphorylation of BAD induced by IL-3 withdrawal correlated with a reduced susceptibility of treated cells to undergo apoptosis during the first 24 hours of IL-3 deprivation (Figure7). However, at 48 hours, there was no difference in the viability of untreated and phosphatase-inhibitor–treated cells (not shown).
Treatment of 32D cell with phosphatase inhibitors.
Viability of parental (A) and WT BAD-expressing (B) 32D cells treated with phosphatase inhibitors. Numbers of viable cells from triplicate wells were determined for each treatment at the indicated times after the removal of IL-3 by trypan blue exclusion. Representative of 3 independent experiments. Similar results were obtained in representative samples when apoptosis was evaluated by DNA content analysis in propidium iodide-stained nuclei. C, control; CyA, cyclosporin A; Cant, cantharidin; and Caly, calyculin.
Treatment of 32D cell with phosphatase inhibitors.
Viability of parental (A) and WT BAD-expressing (B) 32D cells treated with phosphatase inhibitors. Numbers of viable cells from triplicate wells were determined for each treatment at the indicated times after the removal of IL-3 by trypan blue exclusion. Representative of 3 independent experiments. Similar results were obtained in representative samples when apoptosis was evaluated by DNA content analysis in propidium iodide-stained nuclei. C, control; CyA, cyclosporin A; Cant, cantharidin; and Caly, calyculin.
BCL-2 and BCL-XL levels are increased in WT and DM BAD-expressing cells
In WT BAD BCR/ABL-expressing cells, BAD inactivation is probably due to sequestration into the cytoplasm by phosphorylation and interaction with 14-3-3, and to its relative resistance to PP-1α dephosphorylation. On the other hand, DM BAD is exclusively mitochondrial and potentially proapoptotic. To investigate the mechanisms by which the proapoptotic activity of DM BAD is neutralized in BCR/ABL-expressing cells at the mitochondrial level, we analyzed the levels of expression and the localization of BCL-2 and BCL-XL, the natural targets of BAD.
In 5 random clones of BCR/ABL-expressing cells transduced with either WT or DM BAD, levels of BCL-2 and BCL-X were greatly enhanced, compared with vector-infected cells (Figure 8A), and mostly restricted to mitochondria-enriched fractions (data not shown). To determine whether the observed differences in protein levels reflected differences in messenger RNA (mRNA) levels, total RNA from IL-3–starved clones of WT and DM-infected BCR/ABL-expressing cells was analyzed by Northern blot for BCL-2 and BCL-XL expression. Compared with vector-infected cells, all clones tested showed enhancedbcl-2 mRNA levels, but no consistent differences inbcl-x mRNA levels (Figure 8B).
Expression levels of proteins and mRNA.
(A) Expression levels of BCL-2 and BCL-XLproteins in total extracts from clones of WT BAD- and DM BAD-infected BCR/ABL-expressing cells. Cell extracts were blotted with anti-BCL-2, anti-BCL-X, and anti-HA antibodies. Levels of HSP90 were analyzed as control of equal loading. Lane C indicates extracts from vector-infected BCR/ABL-expressing cells. (B) Analysis of bcl-2and bcl-x mRNA levels in clones of WT BAD- and DM BAD-infected BCR/ABL-expressing cells. Northern blots were hybridized with probes for bcl-2 and bcl-x. GAPDH mRNA levels (bottom panel) were also measured as control of loading.
Expression levels of proteins and mRNA.
(A) Expression levels of BCL-2 and BCL-XLproteins in total extracts from clones of WT BAD- and DM BAD-infected BCR/ABL-expressing cells. Cell extracts were blotted with anti-BCL-2, anti-BCL-X, and anti-HA antibodies. Levels of HSP90 were analyzed as control of equal loading. Lane C indicates extracts from vector-infected BCR/ABL-expressing cells. (B) Analysis of bcl-2and bcl-x mRNA levels in clones of WT BAD- and DM BAD-infected BCR/ABL-expressing cells. Northern blots were hybridized with probes for bcl-2 and bcl-x. GAPDH mRNA levels (bottom panel) were also measured as control of loading.
BAD interacts with BCL-2 and BCL-XL in BCR/ABL-expressing 32D cells
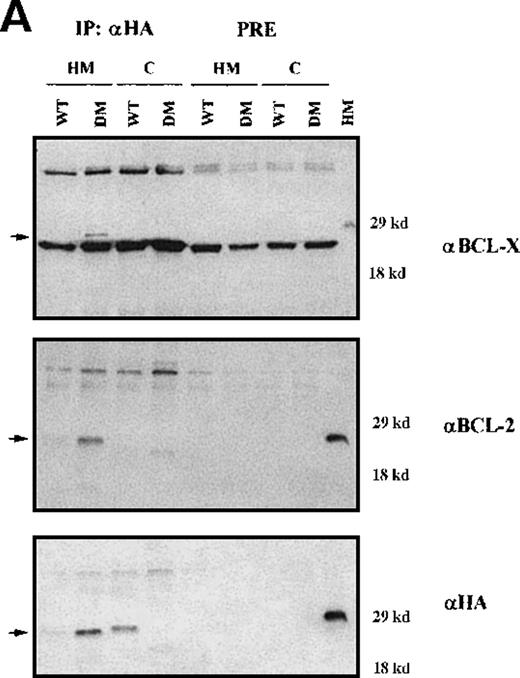
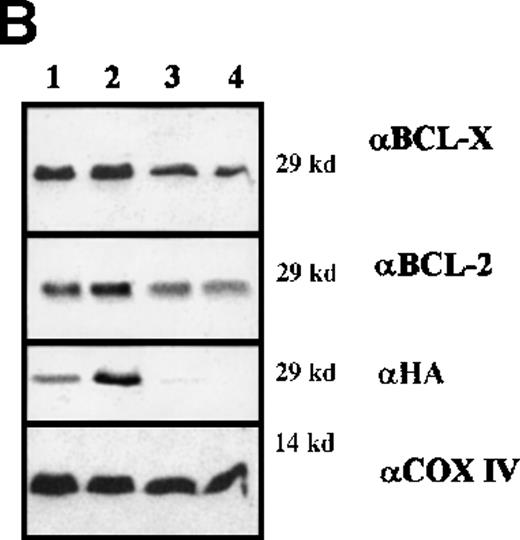
To assess the interaction(s) of BAD, cytosolic and mitochondrial extracts of IL-3–starved BCR/ABL-expressing cells infected with WT or DM BAD were immunoprecipitated using the anti-HA antibody and blotted with anti-BCL-2 or BCL-XL antibodies (Figure9A). DM BAD did interact with both BCL-2 and BCL-XL in mitochondrial extracts, where it resides. Conversely, very little WT BAD, which is mainly cytoplasmic, was found in association with mitochondrial BCL-2 and BCL-XL.
Immunoprecipitation and immunodepletion for cytoplasmic and mitochondrial extracts.
(A) Coimmunoprecipitation of BAD:BCL-2 and BAD:BCL-XL in BCR/ABL-expressing cells. HA-BAD immunoprecipitates (IP) from cytoplasmic and mitochondrial extracts of WT BAD- and DM BAD-infected BCR/ABL-expressing cells were blotted with anti–BCL-XL, anti-BCL-2, and anti-HA antibodies. Preclearings were used as controls. Lane HM shows BCL-XL,BCL-2, and BAD expression in mitochondrial extracts from BCR/ABL/WT BAD-expressing cells. In the upper panel, samples were electrophoresed in the presence of β-mercaptoethanol as reducing agent. (B) BCL-2 and BCL-XL levels in HA-BAD-immunodepleted mitochondrial extracts. Western blot shows levels of BCL-2 and BCL-XL in mitochondrial extracts of WTHA-BAD– (lanes 1 and 3) or DMHA-BAD– (lanes 2 and 4) expressing cells before (lanes 1 and 2) or after (lanes 3 and 4) immunodepletion with the anti-HA antibody. Levels of HA-BAD and COX IV were measured as control of immunodepletion efficiency and of equal loading, respectively.
Immunoprecipitation and immunodepletion for cytoplasmic and mitochondrial extracts.
(A) Coimmunoprecipitation of BAD:BCL-2 and BAD:BCL-XL in BCR/ABL-expressing cells. HA-BAD immunoprecipitates (IP) from cytoplasmic and mitochondrial extracts of WT BAD- and DM BAD-infected BCR/ABL-expressing cells were blotted with anti–BCL-XL, anti-BCL-2, and anti-HA antibodies. Preclearings were used as controls. Lane HM shows BCL-XL,BCL-2, and BAD expression in mitochondrial extracts from BCR/ABL/WT BAD-expressing cells. In the upper panel, samples were electrophoresed in the presence of β-mercaptoethanol as reducing agent. (B) BCL-2 and BCL-XL levels in HA-BAD-immunodepleted mitochondrial extracts. Western blot shows levels of BCL-2 and BCL-XL in mitochondrial extracts of WTHA-BAD– (lanes 1 and 3) or DMHA-BAD– (lanes 2 and 4) expressing cells before (lanes 1 and 2) or after (lanes 3 and 4) immunodepletion with the anti-HA antibody. Levels of HA-BAD and COX IV were measured as control of immunodepletion efficiency and of equal loading, respectively.
After immunodepletion with the anti-HA antibody, mitochondrial extracts were blotted with the anti–BCL-XL or the anti–BCL-2 antibody. Both proteins were readily detectable in the immunodepleted extracts (Figure 9B), suggesting that an excess of BCL-2 and BCL-XL not in complex with BAD remains available to promote cell survival.
Discussion
Serine phosphorylation of proapoptotic BAD and its sequestration in the cytoplasm by interaction with 14-3-3β leads to its inactivation.Inactivation of BAD is important in BCR/ABL-dependent protection from apoptosis because: (1) BCR/ABL mutants that fail to induce BAD phosphorylation are also unable to protect transfected cells from apoptosis14 (data not shown); and (2) overexpression of constitutively active Akt or mitochondria-targeted c-Raf-1, two molecules that can phosphorylate and inactivate BAD, restores the antiapoptotic and leukemogenic potential of BCR/ABL mutants.14 31
This study was undertaken to address 2 main questions: (1) Is BAD inactivation important for the antiapoptotic effects of BCR/ABL, and (2) are BCR/ABL-expressing cells a useful model in investigating mechanisms, whereby tumor cells escape apoptosis triggered by death-inducer effectors? To address these issues, parental and BCR/ABL 32D cells expressing WT or a DM BAD were assessed for colony formation and the ability to counteract ectopic expression of death-inducer BAD.
Colony formation of parental 32D cells was markedly suppressed (approximately 95% inhibition) by either WT or DM BAD. Moreover, clones expressing DM BAD could not be rescued, and WT BAD expression was rapidly lost from most clones selected after infection with the WT BAD retrovirus, implying that parental cells can only tolerate low levels of BAD.
Either WT or DM BAD induced a marked decrease in the clonogenic capacity (67% and 75%, respectively) of BCR/ABL-expressing cells. In the absence of IL-3, this effect was even more pronounced (81% and 88%, respectively). However, stable clones obtained after infection of BCR/ABL-expressing cells showed readily detectable levels of WT or DM BAD and were IL-3–independent for survival and proliferation. Thus, parental cells can rarely overcome the death-inducing effect of BAD, whereas BCR/ABL-expressing cells are more resilient in tolerating WT BAD and the more potent DM derivative. The ability of a cohort of BCR/ABL-expressing cells to escape the death-inducing effect of BAD appears to depend on a variety of mechanisms that can be recognized by comparing the effects on BCL-2 family apoptosis regulators in the clone of parental 32D cells able to tolerate ectopic BAD expression.
In WT BAD-infected parental cells cultured in IL-3, BAD is equally distributed between cytoplasm and mitochondria. By contrast, in BCR/ABL-expressing cells, WT BAD is almost exclusively cytoplasmic. This may depend on the ability of BCR/ABL to be more potent than IL-3 as an activator of the kinase(s) that phosphorylate BAD and to suppress the activity of the phosphatase(s) that dephosphorylates BAD. Indeed, Akt and mitochondrial Raf-1 are both constitutively activated by BCR/ABL,14 31 and on IL-3 deprivation, WT BAD was rapidly dephosphorylated in parental, but not in BCR/ABL-expressing 32D cells (Figure 4, panel C), even if treated with PI-3k inhibitors (not shown).
BAD is sequestered into the cytoplasm through the interaction with members of the 14-3-3 family, and the binding is dependent on the serine phosphorylation of BAD.20 In parental cells, the interaction of BAD with 14-3-3 was detected only by immunoprecipitating HA BAD from cytosolic extracts. Furthermore, this interaction was very labile and, after 30 minutes of growth factor deprivation, nearly undetectable. By contrast, BAD and 14-3-3 remained in complex in BCR/ABL-expressing cells. Two mechanisms can be proposed to explain the IL-3 deprivation-induced changes in BAD phosphorylation and 14-3-3/BAD association in parental cells. BAD might have a very rapid turnover after IL-3 withdrawal, so that in the absence of BAD kinase activities, newly synthesized BAD remains unphosphorylated. Alternatively, BAD might be a target of a specific serine phosphatase activated on IL-3 removal or whose function simply remains unchecked in the absence of BAD kinase activities. Pulse-chase analysis revealed constant levels of newly synthesized BAD during the first 4 hours of starvation, a time point at which BAD phosphorylation was markedly diminished (Figure 4C). These findings support the hypothesis that dephosphorylation of BAD is an active process that involves a specific serine phosphatase. Indeed, PP-1α was able to dephosphorylate BAD in vitro after its phosphorylation by Akt. This finding is relevant for the regulation of the activity of BAD in living cells because: (1) BAD remained phosphorylated in cells treated with PP-1α inhibitors before growth factor withdrawal; (2) cells treated with these inhibitors (but not with cyclosporin A), were more resistant to apoptosis induced by IL-3 deprivation; and (3) PP-1α was detected in complex with BAD in parental and BCR/ABL-expressing. Moreover, BCR/ABL appears to inhibit PP-1α activity, directly or indirectly. The inhibition of PP-1α activity by anti-BCR/ABL immunoprecipitates is not inconsistent with the detection of the PP-1α–BAD complex in BCR/ABL-expressing cells because such complex was also detected in parental cells in which PP-1α activity was inhibited by treatment with calyculin A and cantharidin (not shown). Consistent with the inhibition of PP-1α activity, tyrosine phosphorylated PP-1α was detected in complex with BCR/ABL, and recombinant PP-1α was phosphorylated on incubation with anti-ABL immunoprecipitates from BCR/ABL-expressing cells (data not shown). Incidentally, previous studies support a role for v-Abl and v-Src as inhibitors of PP-1α activity.35,36 Thus, although the primary determinant for the maintenance of phosphorylated and inactivated BAD in BCR/ABL-expressing cells might be the constitutive activity of BAD kinases, it is also likely that the inhibition of PP-1α activity is a contributing factor. The finding of PP-1α involvement in BAD dephosphorylation expands the observation of a recent study37 showing calcineurin-dependent BAD dephosphorylation in cells sensitive to apoptosis induced by agents that elevate cytosolic Ca++. In our model of apoptosis, the calcineurin inhibitor cyclosporin A did not prevent BAD dephosphorylation induced by IL-3 withdrawal, suggesting that the activity of BAD can be regulated by different mechanisms in different cell types. The role of serine phosphatases in apoptosis is controversial. Some reports suggest that protein phosphatases can act as inducers of apoptosis,37-41 whereas other studies imply an antiapoptotic role for some phosphatases.42 In our studies, inhibition of BAD dephosphorylation by phosphatase inhibitors was associated with a delay in the apoptosis induced by IL-3 deprivation; however, 48 hours after IL-3 withdrawal, cells were all dead probably because of the potency of the apoptosis inducer and of the pleiotropic effects of the phosphatase inhibitors.38
In summary, in parental cells, BAD is rapidly dephosphorylated by PP-1α and relocates to the mitochondria after IL-3 removal; by contrast, in the cohort of BCR/ABL-expressing cells that escape the death-inducing effects of WT BAD, BAD remains phosphorylated and does not relocate to the mitochondria. But how can BCR/ABL-expressing cells escape the apoptotic effects of the mitochondria-localized DM BAD? Although the cytoplasmic localization of WT BAD impedes its interaction with BCL-2 and BCL-XL, DM BAD strongly interacts with both BCL-2 and BCL-XL, indicating that it is fully competent in binding its natural targets. Compared with empty vector-infected cells, BCL-2 and BCL-XL protein levels were, however, enhanced in mitochondrial extracts of each clone of WT and DM BAD-infected BCR/ABL-expressing cells, suggesting that BCR/ABL neutralizes the proapoptotic activity of BAD by inducing an increase in the levels of BCL-2 and BCL-XL.
The increased expression of bcl-2 mRNA in all clones tested is consistent with the participation of transcriptional mechanisms in the enhanced expression of BCL-2. The bcl-x mRNA levels were not augmented in all the clones, suggesting that the primary mechanism controlling BCL-XL levels might be posttranscriptional even though transcriptional mechanisms cannot be excluded. Previous reports have demonstrated that BCR/ABL can induce the expression of BCL-214,33 and that BCL-XL is augmented in murine hematopoietic malignancies.43 The robust expression of BCL-2 and BCL-XL in the BCR/ABL-expressing cells resistant to ectopic expression of WT or DM BAD likely reflects a process of selection whereby the clones with highest BCL-2/BCL-XL expression have a survival advantage.
As indicated by the coimmunoprecipitation and the immunodepletion experiments, expression of BCL-2 and BCL-XL was in excess of that interacting with the mitochondria-localized DM BAD, suggesting that the levels of BCL-2 and BCL-XL are sufficient for promoting cell survival.
In conclusion, hematopoietic cells transformed by the BCR/ABL oncoprotein, although highly sensitive to ectopic expression of the death inducer BAD, can utilize several strategies to escape apoptosis. These findings raise the possibility that other tumor cells may effectively utilize similar or different antiapoptotic mechanisms to avoid eradication by therapeutic strategies designed to promote apoptosis.44
Acknowledgments
We thank Dr M. E. Greenberg for providing WT and mutant BAD cDNAs, and D. Perrotti for helpful discussion and advice.
Supported in part by NIH and ACS grants to B.C; P.S. was supported in part by a doctoral fellowship from the University of Modena, Medical School, Italy; and F.C. was supported in part by a doctoral fellowship from the University of Catania Medical School, Italy.
Reprints:Bruno Calabretta, Department of Microbiology and Immunology, Kimmel Cancer Center, Thomas Jefferson University, Bluemle Life Sciences Building, Room 630, 233 S 10th St, Philadelphia, PA, 19107; e-mail: b_calabretta@lac.jci.tju.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 4. HA-BAD in parental and BCR/ABL-expressing cells. / (A) Phosphorylation of HA-BAD in parental and BCR/ABL-expressing cells starved of both IL-3 and serum in [32P]-orthophosphate-containing medium. Immunoprecipitations were carried out using an anti-HA antibody. Vector-infected BCR/ABL-expressing cells were used as negative control. (B) Association of 14-3-3β and HA-BAD in infected parental and BCR/ABL-expressing cells. Infected parental cells were starved of IL-3 and the interaction 14-3-3/BAD was assessed in cytoplasmic extracts 0, 30, and 60 minutes after the removal of IL-3. BCR/ABL-expressing cells infected with WT and DM BAD were starved for 4 hours and then lysed. Total extracts were analyzed for BAD-14-3-3β association. Lane C shows expression of 14-3-3β and HA-BAD in total extracts from BCR/ABL/WT BAD-expressing cells. (C) Levels of phosphorylated BAD after IL-3 removal. Cells were starved of IL-3 for 4 hours, harvested, and lysed at different times (0, 1, 2, 3, and 4 hours). Phosphorylated BAD was detected using a mix of antiphospho BAD antibodies. Levels of HA-BAD were measured as control of equal loading. (D) Stability of WTHA-BAD in starved 32D cells. Levels of [35S]-labeled HA-BAD were assessed at 0, 1, 2, and 4 hours after the simultaneous removal of [35S]-methionine and IL-3. Levels of HA-BAD were measured as control of immunoprecipitation efficiency. The asterisk (in B and D) indicates immunoglobulin light chain. Results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.676/5/m_bloo01425004aw.jpeg?Expires=1767796309&Signature=MJMUpYzh8udBOFjI1qUSEaSt1heDP0Lj~whpl9G0T29ci9mrhBdiOoMMMS-BbAYA6o~mAt2RdhFsc4-eTfkhoDUIYnuY0jX23aLZWtkigda5KVOepfws7RKnEBSXVH8CV1ht0SnU1CxTnoxtQuN5hVXo-bojPcjMpJEtuN1W6DGFytpQjPLZ6bepncV~vcfQmyibzz-CMr16PDxNqK6GBUtWr-os8V~MczWmTEcSwBZmlgClU4hCH5KpdkkfDjZ4QflcrCrvHxXkR69PdDYnvqBfH-6TBZCsPk-8Uz5vIsyaFr03zlRU7yc-ma~N-ydxfn9n4MD3wROCmfgAOLEqkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. HA-BAD in parental and BCR/ABL-expressing cells. / (A) Phosphorylation of HA-BAD in parental and BCR/ABL-expressing cells starved of both IL-3 and serum in [32P]-orthophosphate-containing medium. Immunoprecipitations were carried out using an anti-HA antibody. Vector-infected BCR/ABL-expressing cells were used as negative control. (B) Association of 14-3-3β and HA-BAD in infected parental and BCR/ABL-expressing cells. Infected parental cells were starved of IL-3 and the interaction 14-3-3/BAD was assessed in cytoplasmic extracts 0, 30, and 60 minutes after the removal of IL-3. BCR/ABL-expressing cells infected with WT and DM BAD were starved for 4 hours and then lysed. Total extracts were analyzed for BAD-14-3-3β association. Lane C shows expression of 14-3-3β and HA-BAD in total extracts from BCR/ABL/WT BAD-expressing cells. (C) Levels of phosphorylated BAD after IL-3 removal. Cells were starved of IL-3 for 4 hours, harvested, and lysed at different times (0, 1, 2, 3, and 4 hours). Phosphorylated BAD was detected using a mix of antiphospho BAD antibodies. Levels of HA-BAD were measured as control of equal loading. (D) Stability of WTHA-BAD in starved 32D cells. Levels of [35S]-labeled HA-BAD were assessed at 0, 1, 2, and 4 hours after the simultaneous removal of [35S]-methionine and IL-3. Levels of HA-BAD were measured as control of immunoprecipitation efficiency. The asterisk (in B and D) indicates immunoglobulin light chain. Results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.676/5/m_bloo01425004bw.jpeg?Expires=1767796309&Signature=YbaiTKOTDK5XuuQEh0zR-HowUZXywr55AnMDApu1r29p~vC~4HTEAelG6NoCgNqjxNLpP1kAygIDdObSYFBKtFQKMpQDpMp8eeCqvgDaBpk2Hi~iYnBJkwSc8XvhuGH9G5qzel9-VmFK7LD0aspTGBzJ3ZdgQwagwA6kOHC2IP6mhlcwAS7VTJm-ZNzlES9JNV0FLVqx9XUpkoPAvrx16QzUNDPSs3uwOmLCCksBbO2xceXG6S7Kb1ksopQhzVaps5HyagRmmlkbeCOSscz1sRWEfP6cCJb-PT72yLcPFzKJfnHFoJivry~-3ztwSUjlmzIg1So6A9eDAWOfONyakw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. HA-BAD in parental and BCR/ABL-expressing cells. / (A) Phosphorylation of HA-BAD in parental and BCR/ABL-expressing cells starved of both IL-3 and serum in [32P]-orthophosphate-containing medium. Immunoprecipitations were carried out using an anti-HA antibody. Vector-infected BCR/ABL-expressing cells were used as negative control. (B) Association of 14-3-3β and HA-BAD in infected parental and BCR/ABL-expressing cells. Infected parental cells were starved of IL-3 and the interaction 14-3-3/BAD was assessed in cytoplasmic extracts 0, 30, and 60 minutes after the removal of IL-3. BCR/ABL-expressing cells infected with WT and DM BAD were starved for 4 hours and then lysed. Total extracts were analyzed for BAD-14-3-3β association. Lane C shows expression of 14-3-3β and HA-BAD in total extracts from BCR/ABL/WT BAD-expressing cells. (C) Levels of phosphorylated BAD after IL-3 removal. Cells were starved of IL-3 for 4 hours, harvested, and lysed at different times (0, 1, 2, 3, and 4 hours). Phosphorylated BAD was detected using a mix of antiphospho BAD antibodies. Levels of HA-BAD were measured as control of equal loading. (D) Stability of WTHA-BAD in starved 32D cells. Levels of [35S]-labeled HA-BAD were assessed at 0, 1, 2, and 4 hours after the simultaneous removal of [35S]-methionine and IL-3. Levels of HA-BAD were measured as control of immunoprecipitation efficiency. The asterisk (in B and D) indicates immunoglobulin light chain. Results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.676/5/m_bloo01425004cw.jpeg?Expires=1767796309&Signature=EIK7GJKeh9bzl7tzwH5YTSqLOwP0SYtsj1KFbHIS5sdcGRdZ-J4amoTiHqEbEw2U2E~Ll5NvGC59DOpDu8qT10J6YixrT3GC-z9BUW3kEntxIAX2Wz8tvO0fxxV7V0iLa6iOJajeB17gqnaFYyHWfWzLvTd1xCO2lvwQIhMO3viCYmnGtdzt~e3dQAZG5cXCMf-8tVcxUF79-o3rszKTJrmrzIb5sFElWf4VG37lXRGGQsGttKrTF5zQiYV6IMsvOSfe6PrpVvCfQG7OrozdlAOy3bCVc3BncCmam4HeT0pWT0uTaimK8sxXIeXzmTAuaQLlgbJMb0g6xl15rIwzLw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. HA-BAD in parental and BCR/ABL-expressing cells. / (A) Phosphorylation of HA-BAD in parental and BCR/ABL-expressing cells starved of both IL-3 and serum in [32P]-orthophosphate-containing medium. Immunoprecipitations were carried out using an anti-HA antibody. Vector-infected BCR/ABL-expressing cells were used as negative control. (B) Association of 14-3-3β and HA-BAD in infected parental and BCR/ABL-expressing cells. Infected parental cells were starved of IL-3 and the interaction 14-3-3/BAD was assessed in cytoplasmic extracts 0, 30, and 60 minutes after the removal of IL-3. BCR/ABL-expressing cells infected with WT and DM BAD were starved for 4 hours and then lysed. Total extracts were analyzed for BAD-14-3-3β association. Lane C shows expression of 14-3-3β and HA-BAD in total extracts from BCR/ABL/WT BAD-expressing cells. (C) Levels of phosphorylated BAD after IL-3 removal. Cells were starved of IL-3 for 4 hours, harvested, and lysed at different times (0, 1, 2, 3, and 4 hours). Phosphorylated BAD was detected using a mix of antiphospho BAD antibodies. Levels of HA-BAD were measured as control of equal loading. (D) Stability of WTHA-BAD in starved 32D cells. Levels of [35S]-labeled HA-BAD were assessed at 0, 1, 2, and 4 hours after the simultaneous removal of [35S]-methionine and IL-3. Levels of HA-BAD were measured as control of immunoprecipitation efficiency. The asterisk (in B and D) indicates immunoglobulin light chain. Results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.676/5/m_bloo01425004dw.jpeg?Expires=1767796309&Signature=prf8PIhnlXhJZ2-00jha3ob4fc5nRXoNXxamXfDuJuf8DeXbGET4~oFGT2txK5XzBiB5U4yrzAVDJxG7dYxRyyG5cqoJPNE0Zc2Yk-XTcQQ67IfNt3EJb1NCG-v38ex-ZmHCA7v2B2MUcCAhDWGKxxJDkBOfmiPvL~-klJhT6MPWlP3GkpOFIbdYJqamDDOGa3Wi22WY92T4feGNfKwHtBKrryVjpFTSRUZd5xyFEXGKPyUSjyBadeUGNSHqVQkjbdG2ygPCCb9oLnEefLfuqSYzMsvH-n7tHL7lSEmW1hqV1P~c0iBTTjsryxiIczUrIBaNogUNaFLOv9x7qQqikQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. HA-BAD in parental 32D cells. / (A) Phosphorylation of HA-BAD in parental 32D cells treated with phosphatase inhibitors. 32D cells were pretreated either with cyclosporin A (CyA) (0.5 μmol/L), or cantharidin (Cant) (high [cant-high], 30 μmol/L; low[cant-low], 250 nmol/L) or calyculin A (20 nmol/L) for 2 hours and then deprived from IL-3 for 4 hours. At 0 and 4 hours, cells were harvested and lysed. Phosphorylated BAD was detected by using a mix of antiphospho BAD antibodies. Levels of HA-BAD were measured as control of loading. (B) Association between HA-BAD and 14-3-3β. 32D cells were pretreated with phosphatase inhibitors (see above) and then starved from IL-3. The HA-BAD: 14-3-3β interaction was evaluated in cytoplasmic extracts 0 and 4 hours after IL-3 withdrawal. Levels of immunoprecipitated HA-BAD were measured as control of loading. (C) BAD expression levels in subcellular fractions of parental cells treated with phosphatase inhibitors. Western blots show exogenous BAD levels in fractions enriched for cytoplasm (C) and mitochondria (HM) from untreated and phosphatase inhibitor-treated 32D cells at 0 and 4 hours after IL-3 withdrawal. Levels of mitochondrial marker subunit IV of cytochrome oxidase (COX) and cytoplasmic marker HSP90 were measured as control of equal loading and purity of subcellular fractions. Representative of 3 different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.676/5/m_bloo01425005aw.jpeg?Expires=1767796309&Signature=gr6UlaGEXv0fa66BXIc88~R-MGNyoF4oOwHVBhTNBicx8qqNhf2yQLe~UGpmuw72H3ufMEQLXhyBWtBfnIlcJ6E~uf6XcoEI~gqQQYqVegYV0hqCHVesDZQrvYxbFM8-g9KTXMLddo4Xzk8SS-8R5iYkUv7Oeil4~WYk2DFwFu6N2Ah9EUm3xL1uRULv1NmPXyp6Q-4ytZmG3qEeqcHvZvIF2IE7EhtKBThH~vbBlDxpU2Nd-mlJjHupLj9NYq2JoWqR4dZPRQWRQckdpQ3RNKdGD7TPHLxafUu5ux-v14NYFmz8HlRSYTR4YeDoYpCOAwyIt-dyuKuH11i8F1K7vQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. HA-BAD in parental 32D cells. / (A) Phosphorylation of HA-BAD in parental 32D cells treated with phosphatase inhibitors. 32D cells were pretreated either with cyclosporin A (CyA) (0.5 μmol/L), or cantharidin (Cant) (high [cant-high], 30 μmol/L; low[cant-low], 250 nmol/L) or calyculin A (20 nmol/L) for 2 hours and then deprived from IL-3 for 4 hours. At 0 and 4 hours, cells were harvested and lysed. Phosphorylated BAD was detected by using a mix of antiphospho BAD antibodies. Levels of HA-BAD were measured as control of loading. (B) Association between HA-BAD and 14-3-3β. 32D cells were pretreated with phosphatase inhibitors (see above) and then starved from IL-3. The HA-BAD: 14-3-3β interaction was evaluated in cytoplasmic extracts 0 and 4 hours after IL-3 withdrawal. Levels of immunoprecipitated HA-BAD were measured as control of loading. (C) BAD expression levels in subcellular fractions of parental cells treated with phosphatase inhibitors. Western blots show exogenous BAD levels in fractions enriched for cytoplasm (C) and mitochondria (HM) from untreated and phosphatase inhibitor-treated 32D cells at 0 and 4 hours after IL-3 withdrawal. Levels of mitochondrial marker subunit IV of cytochrome oxidase (COX) and cytoplasmic marker HSP90 were measured as control of equal loading and purity of subcellular fractions. Representative of 3 different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.676/5/m_bloo01425005bw.jpeg?Expires=1767796309&Signature=edQK~j2Ua0KtYavjikNAfTjHegD~asM0r-Ljw4Ob76M5GFFFUff8zU1ZuEwGMyyRKYVvJc4ebW-tw1HF10R6jtpOgStifBNj7CfvNK5DFF2PI3QyG9GM3gUayIt8sfHezgUNev8OAzDe4e3eNdWcWUO6sCwjnJV3j1syP-byZegHIlsqbGPlQIH-Z5JrVKLxkzHY9x~XyVtmsM2IBrEjSFY0nBHsYkUIXYQRXrUqAVGMtKLaTCQ40LoGG0lpv2Y9VH7VGDB47qhYjwhHR7Rq7KxqNXJ6JLj0yO2pkMDdFaNetZQHG0qROrsBxGLzPLS~u344lDBdRP-R~wU8FOuB~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. HA-BAD in parental 32D cells. / (A) Phosphorylation of HA-BAD in parental 32D cells treated with phosphatase inhibitors. 32D cells were pretreated either with cyclosporin A (CyA) (0.5 μmol/L), or cantharidin (Cant) (high [cant-high], 30 μmol/L; low[cant-low], 250 nmol/L) or calyculin A (20 nmol/L) for 2 hours and then deprived from IL-3 for 4 hours. At 0 and 4 hours, cells were harvested and lysed. Phosphorylated BAD was detected by using a mix of antiphospho BAD antibodies. Levels of HA-BAD were measured as control of loading. (B) Association between HA-BAD and 14-3-3β. 32D cells were pretreated with phosphatase inhibitors (see above) and then starved from IL-3. The HA-BAD: 14-3-3β interaction was evaluated in cytoplasmic extracts 0 and 4 hours after IL-3 withdrawal. Levels of immunoprecipitated HA-BAD were measured as control of loading. (C) BAD expression levels in subcellular fractions of parental cells treated with phosphatase inhibitors. Western blots show exogenous BAD levels in fractions enriched for cytoplasm (C) and mitochondria (HM) from untreated and phosphatase inhibitor-treated 32D cells at 0 and 4 hours after IL-3 withdrawal. Levels of mitochondrial marker subunit IV of cytochrome oxidase (COX) and cytoplasmic marker HSP90 were measured as control of equal loading and purity of subcellular fractions. Representative of 3 different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.676/5/m_bloo01425005cw.jpeg?Expires=1767796309&Signature=xEXjJnl5P-3nAMbNsLrPcE~4HpeZS~2dL1XrJI8BgFAy6EqejBmI43OMbkSWUyOvQRRVcSAG2iQOqznVrF7krktuoet~VKJDLHSz~lIhbfc7hpAEQl2KavE5TjwHc1rWBxJCQP1F2WKNK3PDTL6-2WES4vQLgT2QOvz8Z1B0YY68uDHFvECUr1iiPvufG~WXNXY-le-W5YVpXqqTxy7L3yGU8TSRse84mupbggGhc3FqWa1Y-E~KOg~uIPnPC51vPCNakVHeYYcNwKoSgC~w73puLW~16iJwtc37Ki965T0EIeLtJQTk5AsSRRhspCYqBgvcvFBHoaUrPxV710Qq2A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. PP-1α and BAD in parental and BCR/ABL-expressing cells. / (A) Association of PP-1α and BAD in parental and BCR/ABL-expressing 32D cells. The association of HA-BAD and PP-1α was assessed in cytoplasm extracts of exponentially growing and IL-3–starved (2 hours) parental and BCR/ABL-expressing cells (left). As control, the interaction of BAD with PP-2α (middle, lane 2) and that of BAX with PP-1α (right, lanes 1 and 2) were also assessed in BAD- and BAX- expressing cells. Levels of HA-BAD (left and middle panels), and PP-1α (right) were measured as immunoprecipitation controls (left panel, lane 2 of middle panel, lanes 1 and 2 of right panel); levels of PP-2α (lane 1, middle) and BAX (lane 3, right) were measured in whole cell lysate as Western blot controls. Arrows indicate PP-1α HA-BAD, and PP-2α proteins. Asterisks indicate immunoglobulin chains. (B) PP-1α–dependent dephosphorylation of [32P]-labeled GST-BAD. Constitutively active HA-MyrAkt was immunoprecipitated from transiently transfected 293T cells and incubated with 1 μg of GST-BAD and 0.37 MBq (10 μCi) [32P]-γATP for 30 minutes at 30°C. Phosphorylated GST-BAD was then incubated with glutathione sepharose beads for 1 hour at 4°C. Phosphatase reactions were carried out in the presence of 2 units of recombinant PP-1α for 30 minutes at 30°C, and stopped by adding 2 × SDS-sample buffer. Levels of BAD were measured as control of loading. Two independent experiments are shown. (C) Inhibition of PP-1α activity by anti-BCR/ABL immunoprecipitates. Histograms show the percentage of inhibition (mean + SD) of PP-1α activity by anti-ABL immunoprecipitates from BCR/ABL-expressing cells, compared with parental cells, in the presence or in the absence of 10 μmol/L ATP. PP-1α activity was assessed using a malachite green dye solution to detect phosphates released from the synthetic substrate. Samples were also assessed for PP-1α levels by Western blotting with anti-PP-1α antibody.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.676/5/m_bloo01425006w.jpeg?Expires=1767796309&Signature=m61Lm-D1keoiKw4~7yaSk~1-RINibsoZmzK1MjZ8gVPlJyP5s8LC2Akzi~9NGYmd2JP3YkER4pmtjcpxNhnj~osN3ok1UIv~b~Inb4uUdOFyimWC-8RzSvHM3I0W4cvZjyD3EheifACnKQHpNniKZjp5jtC-ZUBTILKMCVkF8daE0d8ZwW8Jmcvqx4HL9YMX27LrwbV4cb-rRUwsJauGL8rEsSt9W8kixSifQQtw955rr~Xw3Ia9GBbevLWQv-s8Bu3RbBgxuHO9ah87hDY-Fa-cz~039EUXjOOSgudPLaZ5CGxMzCaHIHlu9dUdjGTEMz7O1N4arubSYchexPip7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal