Abstract
The migration of leukocytes into tissues is regulated by chemokines and other chemotactic factors that act on receptors that signal through Gi proteins. It seems likely that the colonization of tissues during dissemination of hematopoietic tumor cells is similarly regulated. In fact, dissemination of a T-cell hybridoma, a model for T lymphoma, was blocked when Gi proteins were inactivated by the S1 catalytic subunit of pertussis toxin that had been transfected into those cells. Pertussis toxin S1 blocked dissemination of MDAY-D2 murine myeloid leukemia cells to the liver and spleen, as in T-cell hybridoma cells, but it did not prevent bone marrow colonization. In contrast, overexpression of a function-defective mutant of the Gq/11 protein blocked dissemination to the bone marrow and also prevented Gq/11 dissemination to the liver and spleen. This indicates that the influx of these myeloid cells into all tissues requires the Gq/11 protein in addition to the Gi protein in the liver and spleen.
The influx of leukocytes into inflamed tissues is induced by chemoattractants including small peptides called chemokines, such as interleukin 8 (IL-8) and monocyte chemoattractant protein–1 (MCP-1).1 The synthesis of these factors is induced by proinflammatory cytokines. In addition, certain chemokines are constitutively expressed in hematopoietic and lymphoid tissues in which they regulate the migration and recirculation of various types of blood cells. The secondary lymphoid tissue chemokine (SLC) acts on the chemokine receptor CCR7, and the B-cell–attracting chemokine–1 (BCA-1) acts on the CXCR5 receptor. Both receptors control lymphocyte trafficking in lymph nodes.2-4 Furthermore, stromal cell–derived factor-1 (SDF-1) and its receptor CXCR4 are required for homing of progenitor cells to the bone marrow.5,6 Also, chemokines are constitutively produced in nonhematopoietic tissues. For instance, SDF-1, BCA-1, and the liver and activation-regulated chemokine (LARC) are expressed in the liver.4,7 8 These chemokines are probably involved in the constitutive migration of lymphoid and myeloid cells into and through these tissues.
Chemotaxis triggered by chemokines is blocked by the pertussis toxin,9 which inactivates members of the Gi subclass of G proteins.10 In fact, Gi-coupled receptors are generally able to induce chemotaxis, in contrast to similar heptahelical receptors that are not coupled to Gi proteins.11-13 Accordingly, migration of T cells in the thymus, lymph nodes, and many other tissues is blocked by the pertussis toxin.14-16 In addition, however, chemokine receptors couple to several other G proteins17,18 including Gq and G11 (Gq/11), 2 highly homologous proteins that are both expressed in most cells and tissues. With few exceptions, their functions are redundant.19 Gq/11 appears not to be required for chemotaxis,20 but it may be involved in other effects. For instance, chemokines induce the activation of integrins such as leukocyte function–associated antigen–1 (LFA-1) and α4β1. This induces adhesion of cells to the integrin ligands intercellular adhesion molecule–1 (ICAM-1), the vascular cell adhesion molecule–1 (VCAM-1), and fibronectin, which is necessary for migration through the endothelium and in general for invasion into cell monolayers.21This migration depends on activation of the small guanosine 5′-triphosphatase (GTPase) RhoA,22 which can be activated by Gq protein signals but apparently not by Gi protein signals.23
It is reasonable to assume that migration of disseminating malignant hematopoietic cells and normal counterpart cells are regulated by similar mechanisms. In fact, we found that malignant T-cell hybridomas, which are made from cytotoxic T lymphocyte (CTL) clones, disseminated to multiple tissues.24 This probably reflects the constitutive recirculation of memory T cells through these tissues.25 The dissemination of T-cell hybridoma cells that had been transfected with the pertussis toxin S1 catalytic subunit was completely blocked,26 27 suggesting the involvement of chemokines that are constitutively expressed in these tissues. Here we show that pertussis toxin S1 only partly blocked the dissemination of a myeloid leukemia cell line with characteristics of a bone marrow neutrophil progenitor. The Gi proteins were required for dissemination to the liver and spleen but not to the bone marrow. Instead, we obtained evidence for involvement of the Gq/11 proteins in the invasion of all tissues.
Materials and methods
Cells and culture conditions
Generation and transduction of DNA constructs
The complementary DNA (cDNA) encoding the pertussis toxin catalytic subunit S1 was removed from the pcDNAhyg (hygromycin) vector26 and cloned into the retroviral vector pMFG-IRES-geo. This vector contains an intraribosomal entry site (IRES) 3′ of the inserted cDNA. This is followed by the cDNA encoding the geo protein, which is a fusion of the neomycin resistance and β-galactosidase (lacZ) proteins. As a result, both S1 and geo proteins are translated from 1 bicistronic messenger RNA (mRNA).30 From the neomycin-resistant transduced cells, clones can be selected with high β-galactosidase activity, which correlates with the high expression of S1.31 The vector was transfected by calcium phosphate precipitation into the virus-packaging cell line BOSC23.32 As a control, we used the empty vector, from which the IRES was deleted. The G208A mutant of G11,33kindly provided by Dr C. D. Tsoukas (San Diego State University, San Diego, CA), was cloned into pLZRS-IRES-Hygro-EGFP. This was made by introducing an IRES, a cDNA encoding a fusion protein of the hygromycin-resistance protein (Hygro), and the enhanced green fluorescent protein (EGFP) into the LZRS vector.34 This vector was transfected by calcium phosphate precipitation into Phoenix cells.35
MDAY-D2 cells (2 × 105) were transduced with the virus vectors by cocultivation or by incubation with the BOSC23 or Phoenix supernatants. After 24 hours, MDAY-D2 cells were transferred to fresh medium, and after another 24 hours, they were plated in 96-well plates (Costar, Cambridge, MA) at 2000-10 000 cells per well in 1 mg/mL G418 (Life Technologies, Rockville, MD) or 1 mg/mL hygromycin B (Calbiochem, La Jolla, CA). After 1-2 weeks, clones of the S1-transduced cells were stained for β-galactosidase. If necessary, the cells were subcloned or sorted by fluorescence activated cell sorter (FACS) (FACScan; Becton Dickinson, San Jose, CA) to select clones with homogeneous high and stable β-galactosidase levels. As controls, we generated cells expressing only the geo protein in the same vector, but without the IRES. GFP fluorescence of the G208A-G11–transduced cell population was measured on a FACScan, and the population with the highest expression levels (approximately 30%) was sorted. Single cell sorting from this population yielded several high expressor clones. We selected those clones with stable and homogeneous GFP levels for further analysis. G208A-G11 was similarly transduced into 1 of the S1-transduced clones, and a bulk population with a GFP expression profile similar to that of the population expressing only G208A-G11 was selected.
Antibodies and flow cytometry
The following products were used: hybridomas producing the rat antimouse α4 monoclonal antibody (mAb) PS/2 and αL mAb M17/4 (American Type Culture Collection [ACCT], Rockville, MD); rat antimouse α5 mAb MFR5, hamster antimouse β3 mAb 2C9.G2, and hamster antirat β1 mAb Ha2/5 (PharMingen, San Diego, CA); rat antihuman α6 mAb GoH3 (gift of Dr A. Sonnenberg, The Netherlands Cancer Institute, Amsterdam, The Netherlands); rat antimouse αM mAb 5C6 (Dr C. A. Figdor, University of Nÿmegen, The Netherlands); rat antimouse β2 mAb GAME36; rat antihuman VCAM-1 mAb 4B9 (Becton Dickinson); and rabbit antifibronectin polyclonal antibody (pAb) (Dako, Glostrup, Denmark). For flow cytometry, antibody incubations and washing steps were performed on ice in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA), 0.02% NaN3, 1 mmol/L magnesium (Mg++), and 1 mmol/L calcium (Ca++). Secondary antibodies used were fluorescence isothiocyanate–labeled (FITC-labeled) mouse antirat immunoglobulin (Ig) and rabbit antirat antibodies (Nordic, Tilburg, The Netherlands) and goat antirat and antimouse phycoerythrin-labeled (PE-labeled) F(ab′)2 fragments (Jackson Immune Research Laboratories, West Grove, PA). Fluorescence was measured using a FACScan. The controls were cells incubated with secondary antibody only.
Immunoblotting
SDS-PAGE–separated (sodium dodecyl sulfate–polyacrylamide gel electrophoresis–separated) cell lysates were blotted to nitrocellulose, which was then blocked with 3% BSA and 0.4% Tween-20. The membranes were incubated for 1 hour with the mouse 151C1 mAb against S137 or the rabbit antihuman G11 pAb QL38 at 20°C, followed by incubation with sheep antimouse or donkey antirabbit horseradish peroxidase–coupled Ig, respectively (Amersham Life Sciences, Little Chalfont, England). Stained proteins were visualized by enhanced chemiluminescence (ECL kit, Amersham).
ADP-ribosylation assay
The cells were homogenized with a Teflon glass homogenizer in 20 mmol/L Tris HCl (tris[hydroxymethyl] aminomethane hydrogen chloride) (pH 7.5) containing 1 mmol/L ethylenediamine tetraacetic acid) (EDTA), 1 mmol/L dithiothreitol (DTT), 20 μg/mL soybean trypsin inhibitor (Sigma, St Louis, MO), 2 μg/mL aprotinin, and 0.5 mmol/L Pefabloc (Boehringer Mannheim, Mannheim, Germany). After centrifugation at 200g for 10 minutes, the membranes were collected from the supernatant by centrifugation at 160 000g for 30 minutes and suspended in 20 mmol/L Tris HCl (pH 7.5) supplemented with 1 mmol/L EDTA and 1 mmol/L DTT. The protein concentration was determined by BCA protein assay (Pierce, Rockford, IL). To determine whether the Gi proteins had been ADP-ribosylated (adenosine 5′-diphosphate–ribosylated) by the endogenously produced S1 protein, 20-μg membranes of the S1 transfectants were treated with pertussis toxin (List Biology Laboratory, Campbell, CA) for 60 minutes at 30°C. The reaction was performed in 50 μL 0.1 mol/L Tris HCl (pH 8.0) supplemented with 10 mmol/L thymidine, 1 mmol/L adenosine 5′-triphosphate (ATP), 0.1 mmol/L GTP, 2.5 mmol/L magnesium dichloride (MgCl2), 1 mmol/L EDTA, 10 mmol/L DTT, 10 μmol/L nicotinamide adenine dinucleotide (NAD), and 0.037 MBq (1 μCi) 32P-NAD (NEN, Dreieich, Germany). Reactions were stopped by the addition of up to 20% trichloroacetic acid. After 15 minutes on ice, the samples were centrifuged at maximum speed in a microfuge, and the pellets were washed twice with 200 μL ethyl ether. The samples were dissolved in Laemmli sample buffer containing 0.1 mol/L DTT and subjected to SDS-PAGE (10% gel). After drying, the gels were exposed to Kodak X-AR films (Eastman Kodak, Rochester, NY).
Northern blot analysis
Total RNA was extracted from murine neutrophils and MDAY-D2 parental and transfectant cells (Ultraspec RNA isolation system; BIOTECX, Houston, TX). The RNA was size-fractionated on a 1.5% agarose gel containing 2.2 mol/L formaldehyde, transferred to a Nytran 13 N membrane (Schleicher and Schuell, Dassel, Germany), hybridized with the full-length 32P-dATP–labeled cDNA probe of CXCR2, and exposed to X-ray film.
Dissemination and histology
Cells (2 × 104) were mixed in 200 μL PBS supplemented with 1 mmol/L Ca++ and 1 mmol/L Mg++ and injected into the lateral tail veins of 6- to 8-week-old syngeneic DBA/2 mice. The animals were autopsied when moribund or after a fixed time period. Metastasis formation was determined both macroscopically and microscopically. For microscopic examination, the tissues were fixed in ethanol–acetic acid–formol saline fixative and embedded in paraffin; 5-μm sections were mounted onto slides and stained with hematoxylin and eosin according to standard procedures. For classification of MDAY-D2 cells, a smear preparation was stained with May-Grünwald-Giemsa. To test tumorigenicity, 2 × 104 cells were injected intraperitoneally. After 3 weeks, the peritoneal cavity was flushed with PBS, and the cells were counted.
Results
Characterization of MDAY-D2 cells as myeloid leukemia cells
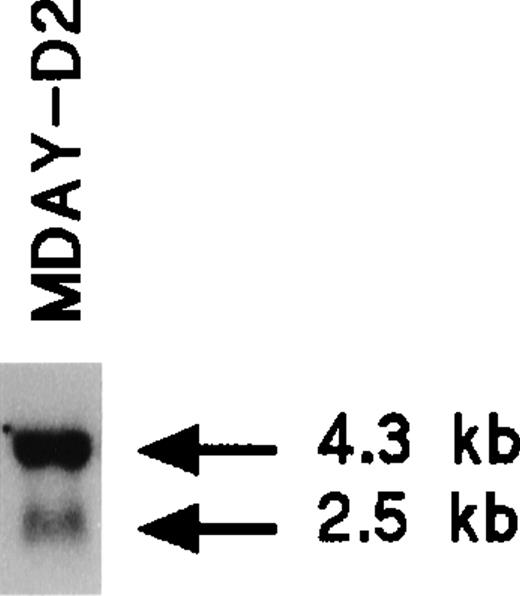
The MDAY-D2 hematopoietic tumor that arose in DBA/2 mice28 disseminated extensively to the liver, spleen, and bone marrow.39 The phenotype of the cells has not yet been further defined. We observed that MDAY-D2 cells express the chemokine receptor CXCR2 (IL8RB). Northern blot analysis demonstrated a major 4.3-kb mRNA species and a minor 2.5-kb species (Figure1), which are similar to those species described for myeloid precursors isolated from mouse bone marrow.40 Table 1 shows the integrins expressed by MDAY-D2 cells, which include Mac-1 (αMβ2; CD11b/CD18). Furthermore, the cells express Fc receptors.41 Both surface proteins are myeloid markers. Finally, cytological staining revealed small granules (see Figure 4E below), which are typical for early neutrophil development. Thus, MDAY-D2 cells have characteristics of bone marrow myeloid precursors and can therefore be classified as myeloid leukemia cells.
Surface levels of integrin subunits on MDAY-D2 cells
| Integrin subunit . | Mean fluorescence . |
|---|---|
| Control antirat IgG | 2.3 |
| α4 (CD49d) | 19.1 |
| α5 (CD49e) | 25.5 |
| α6 (CD49f) | 37.9 |
| αL (CD11a) | 9.0 |
| αM (CD11b) | 7.0 |
| β2 (CD18) | 16.0 |
| Control antihamster IgG | 2.4 |
| β3 (CD61) | 5.6 |
| Control antihamster IgM | 2.3 |
| β1 (CD29) | 40.7 |
| Integrin subunit . | Mean fluorescence . |
|---|---|
| Control antirat IgG | 2.3 |
| α4 (CD49d) | 19.1 |
| α5 (CD49e) | 25.5 |
| α6 (CD49f) | 37.9 |
| αL (CD11a) | 9.0 |
| αM (CD11b) | 7.0 |
| β2 (CD18) | 16.0 |
| Control antihamster IgG | 2.4 |
| β3 (CD61) | 5.6 |
| Control antihamster IgM | 2.3 |
| β1 (CD29) | 40.7 |
Surface levels, as measured by FACS. Fluorescence in arbitrary units. Controls are cells incubated with only the appropriate secondary antibodies.
Inactivation of Gi proteins by pertussis toxin S1 expression in MDAY-D2 cells
To investigate a role for pertussis toxin–sensitive Gi proteins in dissemination, we generated MDAY-D2 cells expressing the pertussis toxin catalytic subunit S1. This was achieved by retroviral transduction of a bicistronic retroviral vector31 that contained 2 cDNAs separated by an IRES. The first encoded S1, and the second encoded a fusion protein of β-galactosidase and the neomycin-resistant protein. Because expression of the 2 cDNAs correlated,31 selection for the high and stable lacZ expression resulted in stable clones with S1 levels sufficient to inactivate all Gi proteins.
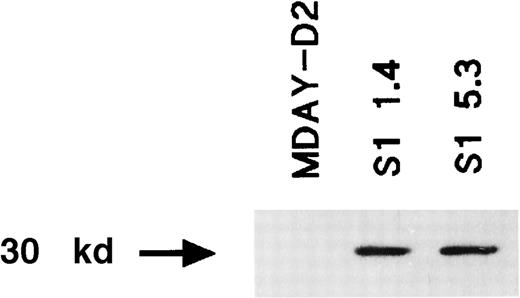
Many independent clones were obtained, and 2 of these (S1 1.4 and S1 5.3) were studied in more detail. For a control, we transduced the empty vector and selected clones with similar lacZ levels. Western blot analysis demonstrated that the S1 protein was present (Figure2). To demonstrate that the S1 ADP-ribosyltransferase was active, we assessed pertussis toxin–induced ADP-ribosylation of Gi proteins in isolated cell membranes. Labeling should not occur if the Gi proteins have already been ADP-ribosylated by the endogenously expressed S1 enzyme. Figure3 shows that treatment of the MDAY-D2 membranes in the presence of 32P-NAD resulted in a high level of 32P incorporation into the 40-kd Gi proteins. In contrast, no incorporation of label was observed in membranes of the MDAY-D2 S1 transfectants, indicating that all Gi proteins were ADP-ribosylated by the endogenously expressed S1 protein and, therefore, that all Gi protein activity was blocked in the S1-transduced MDAY-D2 cells.
Expression of the S1 catalytic subunit of pertussis toxin in the MDAY-D2 transfectants S1 1.4 and S1 5.3 but not in untransfected MDAY-D2 cells.
The expression was detected by Western blot analysis using an anti-S1 mAb.
Expression of the S1 catalytic subunit of pertussis toxin in the MDAY-D2 transfectants S1 1.4 and S1 5.3 but not in untransfected MDAY-D2 cells.
The expression was detected by Western blot analysis using an anti-S1 mAb.
ADP-ribosylation of Gi proteins by pertussis toxin in the plasma membranes of MDAY-D2 cells but not in the MDAY-D2 transfectants S1 1.4 and S1 5.3.
The figure shows that in these transfectants, all Gi proteins had already been ADP-ribosylated by the transfected S1 catalytic subunit of pertussis toxin (PT).
ADP-ribosylation of Gi proteins by pertussis toxin in the plasma membranes of MDAY-D2 cells but not in the MDAY-D2 transfectants S1 1.4 and S1 5.3.
The figure shows that in these transfectants, all Gi proteins had already been ADP-ribosylated by the transfected S1 catalytic subunit of pertussis toxin (PT).
Dissemination of S1 transfectants
Upon injection of 2 × 104 cells into the tail vein of syngeneic mice, MDAY-D2 cells invaded different tissues, predominantly the liver, spleen, and bone marrow, and after 13-20 days, most of the mice became moribund, as described previously.39 Table 2 shows the results of 2 independent experiments. In the first, the dissemination pattern of the empty vector control transfectants, with the same β-galactosidase activity as the S1 transfectants, was similar to that of the parental cells. This shows that retrovirus transduction and β-galactosidase expression does not lead to immune rejection of the cells. In contrast, the dissemination of S1 transfectants to the liver and spleen was completely or (in few mice) almost completely blocked, as shown macroscopically by β-galactosidase staining in Figure4A-D. Remarkably, however, the dissemination of the S1 transfectants to the bone marrow was not affected and did not differ from the parental cells.
Metastases of MDAY-D2 and S1 transfectants from 2 experiments
| Location of metastases . | Transfectants . | |||||||
|---|---|---|---|---|---|---|---|---|
| MDAY-D2 . | Control vector . | MDAY-D2 S1 1.4 . | MDAY-D2 S1 5.3 . | |||||
| Subjects . | Tumor extent . | Subjects . | Tumor extent . | Subjects . | Tumor extent . | Subjects . | Tumor extent . | |
| Experiment 1 | ||||||||
| Liver | 10/10 | ++++ | 10/10 | ++++ | 0/10 | − | 0/10 | − |
| Spleen | 10/10 | ++++ | 10/10 | ++++ | 3/10 | +/− | 4/10 | +/− |
| Bone marrow | 10/10 | ++++ | 10/10 | ++++ | 10/10 | ++++ | 10/10 | ++++ |
| Experiment 2 | ||||||||
| Liver | 10/10 | ++++ | − | − | 0/5 | − | 2/10 | +/− |
| Spleen | 10/10 | ++++ | − | − | 5/10 | +/− | 3/10 | +/− |
| Bone marrow | 10/10 | ++++ | − | − | 8/10 | ++++ | 8/10 | ++++ |
| Location of metastases . | Transfectants . | |||||||
|---|---|---|---|---|---|---|---|---|
| MDAY-D2 . | Control vector . | MDAY-D2 S1 1.4 . | MDAY-D2 S1 5.3 . | |||||
| Subjects . | Tumor extent . | Subjects . | Tumor extent . | Subjects . | Tumor extent . | Subjects . | Tumor extent . | |
| Experiment 1 | ||||||||
| Liver | 10/10 | ++++ | 10/10 | ++++ | 0/10 | − | 0/10 | − |
| Spleen | 10/10 | ++++ | 10/10 | ++++ | 3/10 | +/− | 4/10 | +/− |
| Bone marrow | 10/10 | ++++ | 10/10 | ++++ | 10/10 | ++++ | 10/10 | ++++ |
| Experiment 2 | ||||||||
| Liver | 10/10 | ++++ | − | − | 0/5 | − | 2/10 | +/− |
| Spleen | 10/10 | ++++ | − | − | 5/10 | +/− | 3/10 | +/− |
| Bone marrow | 10/10 | ++++ | − | − | 8/10 | ++++ | 8/10 | ++++ |
In both experiments, 2 × 104 cells were injected intravenously, and the mice were killed when moribund. The number of subjects indicated is given as the number of mice that had metastases per the number of mice injected for each indicated organ. The tumor extent indicates the extent of metastases formation: +/− indicates a few isolated tumor cells; +, some foci; ++, some foci and some diffusely spread tumor cells; +++, more foci and more diffusely spread tumor cells; ++++, many foci and many diffusely spread tumor cells. The data were obtained by macroscopic and microscopic analyses. Cells transfected with a control vector were used only for experiment 1.
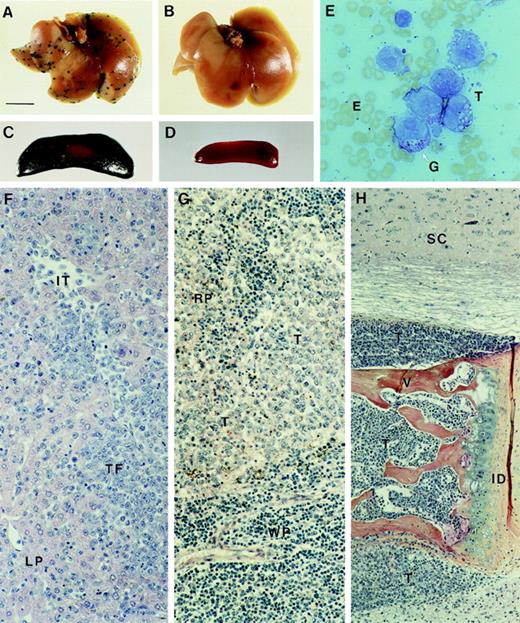
Dissemination of MDAY-D2 cells and S1 transfectants to liver, spleen, and bone marrow.
Whole (A) liver and (C) spleen from mice killed 17 days after tail vein injection of control MDAY-D2 transfectants (empty vector). Multiple foci are visible in the liver, and the spleen is greatly enlarged and consists mainly of β-galactosidase–positive MDAY-D2 cells. Whole (B) liver and (D) spleen from mice injected with the S1 1.4 transfectant and killed after 17 days. No tumor cells can be detected. (E) A smear preparation of MDAY-D2 cells, stained with May-Grünwald Giemsa. Note the presence of granules (G). Histology of tissues in mice injected with control (empty vector) transfectants: (F) Liver. In the panel, LP indicates liver parenchyma; IT, intravascular tumor cells; and TF, tumor focus. (G) Spleen. RP indicates red pulp; WP, white pulp; and T, tumor cells. (H) Bone marrow. In mice injected with S1 transfected cells, the bone marrow is similarly affected, whereas the liver and spleen are tumor-free. SC indicates spinal cord; V, vertebral body; ID, intervertebral disc; and T, tumor cells. Note tumor infiltration into the spinal cord (top) and muscle (below).
Dissemination of MDAY-D2 cells and S1 transfectants to liver, spleen, and bone marrow.
Whole (A) liver and (C) spleen from mice killed 17 days after tail vein injection of control MDAY-D2 transfectants (empty vector). Multiple foci are visible in the liver, and the spleen is greatly enlarged and consists mainly of β-galactosidase–positive MDAY-D2 cells. Whole (B) liver and (D) spleen from mice injected with the S1 1.4 transfectant and killed after 17 days. No tumor cells can be detected. (E) A smear preparation of MDAY-D2 cells, stained with May-Grünwald Giemsa. Note the presence of granules (G). Histology of tissues in mice injected with control (empty vector) transfectants: (F) Liver. In the panel, LP indicates liver parenchyma; IT, intravascular tumor cells; and TF, tumor focus. (G) Spleen. RP indicates red pulp; WP, white pulp; and T, tumor cells. (H) Bone marrow. In mice injected with S1 transfected cells, the bone marrow is similarly affected, whereas the liver and spleen are tumor-free. SC indicates spinal cord; V, vertebral body; ID, intervertebral disc; and T, tumor cells. Note tumor infiltration into the spinal cord (top) and muscle (below).
In a second experiment, in which similar results were obtained, histological analysis was also performed (Table 2). This confirmed the lack of tumor foci in the livers of mice injected with the S1 transfectants. In most mice the spleen was also tumor-free, but in some, a few isolated tumor cells or small foci were observed in the red pulp area. Thus, in contrast to the parental cells (Figure 4F,G), S1 transfectants were unable to invade the liver and migrate between the hepatocytes, and extravasation into the red pulp area of the spleen was blocked or strongly reduced. In contrast, dissemination of S1 transfectants to the bone marrow was not affected and was comparable to the parental cells. The MDAY-D2 cells completely replaced the bone marrow where they blocked normal hematopoiesis. Eventually, the cells expanded outside the bone marrow, and they oppressed the spinal cord (Figure 4H). Spinal cord oppression and the outgrowth of tumor cells from the bone marrow into the skeletal muscle are probably responsible for the observed hind leg paralysis.
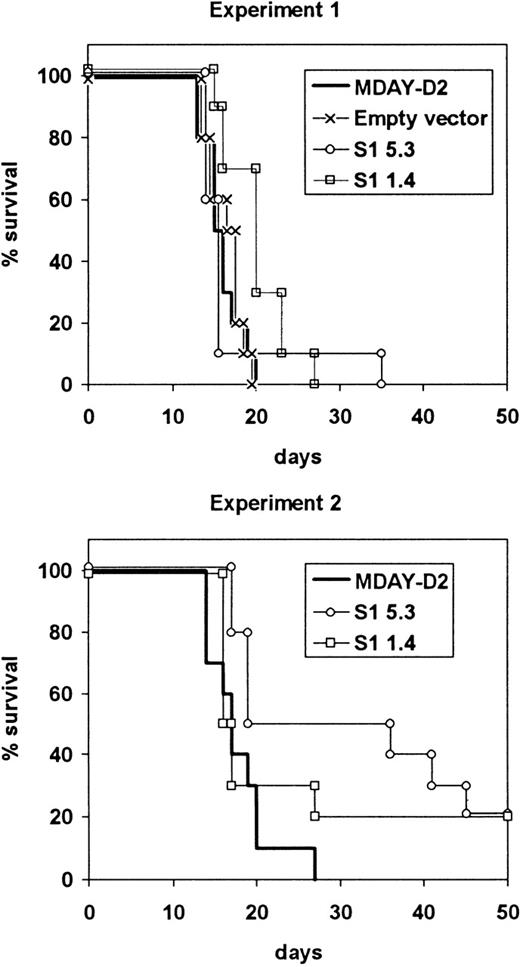
The average survival time of mice injected with the S1 transfectants was slightly longer compared to the parental or control cells, but most of the mice had to be killed within the same 13-20 day period (Figure5). In the case of S1 transfectants, this was necessary because of hind leg paralysis, although the mice were clearly not as ill as those injected with parental cells. In both experiments, some mice survived up to approximately 40 days, and in the second experiment, 2 of 10 mice injected with each of the S1 transfectants survived until they were killed after 100 days, and no dissemination was observed. The growth of the cells in the bone marrow showed that the S1 transfectants were tumorigenic, and this was confirmed by intraperitoneal injection of the cells. The average doubling time in the peritoneal cavity was between 36 and 38 hours for both the parental cells and the S1 transfectants.
Survival of mice injected with S1 transfectants.
Mice were injected with either the empty vector control transfectants or one of the 2 transfectant clones S1 1.4 and S1 5.3 expressing the S1 catalytic subunit of pertussis toxin. The mice were killed when moribund. The results from 2 independent experiments are shown.
Survival of mice injected with S1 transfectants.
Mice were injected with either the empty vector control transfectants or one of the 2 transfectant clones S1 1.4 and S1 5.3 expressing the S1 catalytic subunit of pertussis toxin. The mice were killed when moribund. The results from 2 independent experiments are shown.
Expression of the G208A mutant of G11
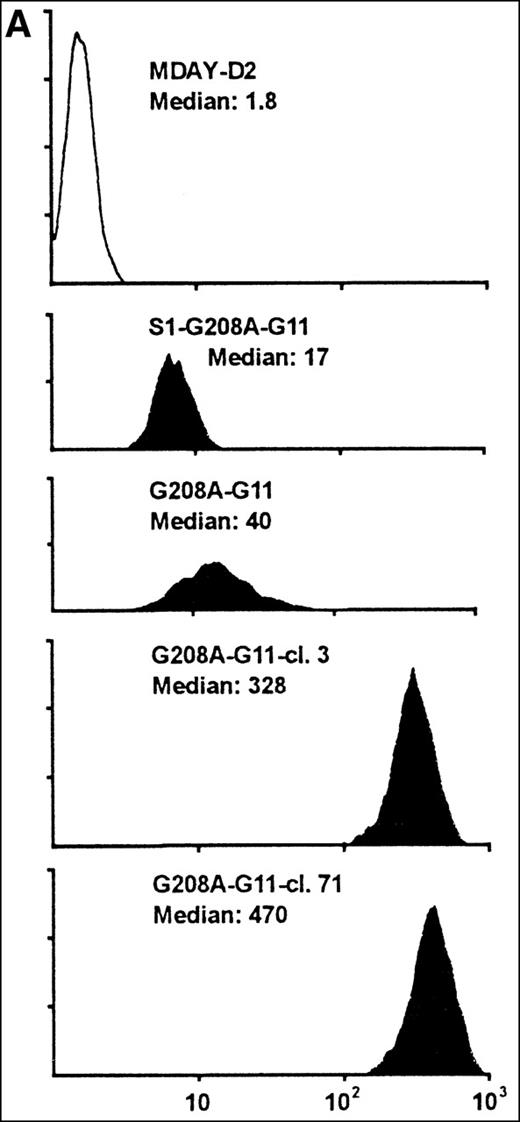
Assuming that the MDAY-D2 cells require signals to activate integrins for invasion into the bone marrow, we wondered whether pertussis toxin–insensitive G proteins might be involved. To study a possible role of Gq/11 proteins, we overexpressed a function-defective G208A mutant of G11, which has been reported to suppress the function of the 2 highly homologous G proteins, Gq and G11.33 This mutant was transduced with the retroviral vector LZRS,34which yields high virus titers resulting in high transduction efficiency. The vector used contained an IRES followed by Hyg-EGFP. This allowed the selection of transfectants by hygromycin resistance as well as GFP-based sorting by FACS of cells that express the bicistronic mRNA at high levels. At first, we used an unsorted bulk population with moderate GFP levels (G208A-G11) rather than clones (Figure6A). This has the advantage that the results are not influenced by clonal variation. In addition, we transduced the G11 construct into the S1-expressing clone S1 5.3 and obtained a population (S1-G208A-G11) with similar, although on an average somewhat lower, GFP expression levels (Figure 6A).
MDAY-D2 cells transfected with the G208A mutant of the G11 protein, coexpressing EGFP.
G208A-G11: Bulk population of transfected parental cells. S1-G208A-G11: Cells transfected with both the S1 catalytic subunit of pertussis toxin and the G11 mutant (S1-G208A-G11). Clones 3 and 71 of the G208A-G11 transfectants were selected for their high GFP expression. (A) FACS profiles of GFP expression. The median fluorescence in arbitrary units is indicated. Untransfected MDAY-D2 cells serve as negative controls. (B) Expression of G11 in these populations and clones, as detected by Western blot analysis with a pAb.
MDAY-D2 cells transfected with the G208A mutant of the G11 protein, coexpressing EGFP.
G208A-G11: Bulk population of transfected parental cells. S1-G208A-G11: Cells transfected with both the S1 catalytic subunit of pertussis toxin and the G11 mutant (S1-G208A-G11). Clones 3 and 71 of the G208A-G11 transfectants were selected for their high GFP expression. (A) FACS profiles of GFP expression. The median fluorescence in arbitrary units is indicated. Untransfected MDAY-D2 cells serve as negative controls. (B) Expression of G11 in these populations and clones, as detected by Western blot analysis with a pAb.
Initially, it appeared that bone marrow dissemination was suppressed by the combination of pertussis toxin S1 and G208A-G11, but not by the G11 mutant alone (see below). However, the G208A-G11 protein levels differed between the 2 populations and were higher in the cells that also expressed S1 (Figure 6B). This was quite unexpected because the GFP level was actually lower in the S1 plus G208A-G11 transfectant population (Figure 6A). To obtain cells with higher expression of the mutant, several clones were selected from the G208A-G11 population by single cell sorting of the few cells with quite high GFP levels. The clones differed in homogeneity of expression (ie, in the variation between individual cells) and also in stability. Some clones rapidly lost expression over time, but other clones retained the same expression level. Clones 3 and 71, which have a stable andhomogeneous GFP concentration that is 10-fold higher than in the original population (Figure 6A), were chosen for further analysis. The G208A-G11 protein levels detected by Western blot analysis were substantially increased, and they were also increased when compared to the S1 plus G208A-G11 population (Figure 6B). The antiserum used stained 2 bands of approximately 41 and 43 kd. It is noteworthy that in the high expressor clones, mainly the 43-kd band was seen. The endogenous Gq/11 proteins were not visible (Figure 6B) and were in fact only seen after prolonged exposure. This shows that the mutant is highly overexpressed compared to the endogenous proteins.
Effect of G208A-G11 on dissemination
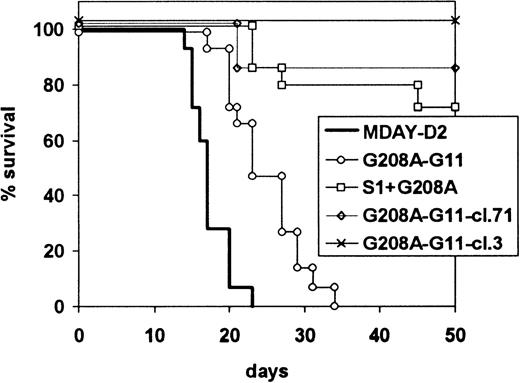
The dissemination capacities of the mutant G11 transfectants were first assessed for the 2 bulk populations with and without S1. In the first experiment, all mice were killed after 15 days. The results are shown in Table 3. Expression of the dominant-negative G11 reduced dissemination to all sites. Bone marrow colonization was reduced in extent and seen only in 7 of 10 mice. However, invasion of the liver and spleen was also less extensive. This was observed in 5 of 10 mice and 6 of 10 mice, respectively, and the extent of tumor growth was much reduced. The dissemination capacity of the population that also contained S1 but, as described above, expressed higher levels of G208A-G11 was even more reduced. The occasional spleen tumors that we had seen with cells expressing only S1 were no longer observed, and in only 1 of 10 mice, bone marrow colonization was seen. These results were confirmed in 2 independent subsequent experiments, in which the mice were killed when moribund rather than after a fixed time period. The combined results of the 2 experiments are shown in Figure 7. Mice injected with the G208A-G11 transfectants became ill 10 days later than mice injected with the parental cells. The population expressing both S1 and G11-G208A had even lower dissemination capacity: only 4 of 15 mice showed hind leg paralysis after an extended time period.
Metastasis of MDAY-D2 S1 and dominant-negative G11 transfectants
| Location of metastases . | Transfectants . | |||||||
|---|---|---|---|---|---|---|---|---|
| MDAY-D2 . | MDAY-D2 S1 5.3 . | MDAY-D2 Gq-DN . | MDAY-D2 S1 + Gq-DN . | |||||
| Subjects . | Tumor extent . | Subjects . | Tumor extent . | Subjects . | Tumor extent . | Subjects . | Tumor extent . | |
| Liver | 10/10 | ++++ | 0/10 | − | 6/10 | ++ | 0/10 | − |
| Spleen | 10/10 | ++++ | 10/10 | +/− | 5/10 | ++ | 0/10 | − |
| Bone marrow | 10/10 | ++++ | 10/10 | ++++ | 7/10 | +++ | 1/10 | +++ |
| Location of metastases . | Transfectants . | |||||||
|---|---|---|---|---|---|---|---|---|
| MDAY-D2 . | MDAY-D2 S1 5.3 . | MDAY-D2 Gq-DN . | MDAY-D2 S1 + Gq-DN . | |||||
| Subjects . | Tumor extent . | Subjects . | Tumor extent . | Subjects . | Tumor extent . | Subjects . | Tumor extent . | |
| Liver | 10/10 | ++++ | 0/10 | − | 6/10 | ++ | 0/10 | − |
| Spleen | 10/10 | ++++ | 10/10 | +/− | 5/10 | ++ | 0/10 | − |
| Bone marrow | 10/10 | ++++ | 10/10 | ++++ | 7/10 | +++ | 1/10 | +++ |
Cells (2 × 104) were injected intravenously, and the mice were killed at day 15. The number of subjects indicated is given as the number of mice that had metastases per the number of mice injected for each indicated organ. The extent of metastases formation: +/− indicates a few isolated tumor cells; +, some foci; ++, some foci and some diffusely spread tumor cells; +++, more foci and more diffusely spread tumor cells; ++++, many foci and many diffusely spread tumor cells. Data were obtained by macroscopic and microscopic analyses.
Survival of mice injected with G208A-G11 transfectants.
Mice were injected with MDAY-D2 of cells transfected with the G208A mutant of the G11 protein (G208A-G11), coexpressing EGFP, both the S1 catalytic subunit of pertussis toxin and the G11 mutant (S1-G208A-G11), or clones 3 or 71 selected from the G208A-G11 transfectants for high GFP expression. The mice were killed when moribund. The combined results of 2 independent experiments are shown.
Survival of mice injected with G208A-G11 transfectants.
Mice were injected with MDAY-D2 of cells transfected with the G208A mutant of the G11 protein (G208A-G11), coexpressing EGFP, both the S1 catalytic subunit of pertussis toxin and the G11 mutant (S1-G208A-G11), or clones 3 or 71 selected from the G208A-G11 transfectants for high GFP expression. The mice were killed when moribund. The combined results of 2 independent experiments are shown.
The difference between the 2 populations could be due either to the presence of S1 or the higher levels of G208A-G11. To study this, we injected the clones expressing only G208A-G11, but at higher levels than the S1-G208A-G11 population, as described above (Figure 6). The results, also included in Figure 7, show that G208A-G11 alone is able to block dissemination. Of the mice injected with these cells, 13 of 14 mice survived without developing a tumor, and after 21 days, only 1 of 14 developed hind leg paralysis. This indicates that Gq/11 is involved in dissemination not only to the bone marrow but also to the liver and spleen. In combination, our results indicate that both Gi and Gq/11 protein signals are involved in invasion of the liver and spleen.
Discussion
We have shown here that MDAY-D2 myeloid leukemia cells require Gi signals to colonize the liver and spleen, as we have previously shown for T-cell hybridoma cells,26,27 but they do not require Gi signals for dissemination to the bone marrow. In the liver and spleen, Gi signals are apparently activated by factors in these tissues, most probably chemokines, and it seems likely that their role in dissemination is to induce invasion of the cells into these tissues. SDF-1, BCA-1, and LARC are chemokines that are constitutively expressed in the liver.4,7,8 SDF-1 was a major candidate27 for activating T-cell hybridomas, and in fact, we have recently obtained evidence that SDF-1 is involved in the dissemination of these cells (Zeelenberg et al, unpublished data). However, MDAY-D2 cells do not express the appropriate receptors for the 3 chemokines, or the expression is only at low levels, and the cells show no chemotactic response toward SDF-1 (Soede et al, unpublished data). SDF-1 is therefore not likely to be involved in bone marrow colonization by MDAY-D2 cells. This possibility is suggested by the reported role of SDF-1 in engraftment of the bone marrow by hematopoietic progenitors as well as in the invasion of chronic lymphocytic leukemia B cells into monolayers of bone marrow stromal cells.5,6 42 MDAY-D2 cells do express CXCR2 (Figure 1), but chemokines acting on this receptor are not known to be present in the liver, and the CXCR2 ligand CINC (cytokine-induced neutrophil chemoattractant) does not induce chemotaxis of these cells (Soede et al, unpublished data). Thus, at present there is no obvious candidate for the chemokine involved in the liver and spleen colonization of MDAY-D2 cells.
Whereas the pertussis toxin inhibited dissemination to the spleen and liver, it had no effect on colonization of the bone marrow. This is in line with a recent report43 on the engraftment of hematopoietic stem cells to the bone marrow, which was also independent of Gi protein signals. Since engraftment of human stem cells is dependent on SDF-1 and CXCR4, at least in mice,6 this indicates that CXCR4 triggers not only Gi proteins but also activates other pathways that are more relevant for bone marrow colonization. In fact, several chemokine receptors are coupled to more than 1 G protein; this includes CXCR4, which is coupled to both Gi and Gq/11 proteins.17,44 To investigate a possible role of Gq/11, we overexpressed the function-defective G208A mutant of G11 and found that this blocked dissemination of MDAY-D2 myeloid leukemia cells to the bone marrow, liver, and spleen. The G208A mutation is analogous to that in dominant-negative Gαi and Gαs mutants45-47and prevents dissociation of the α subunit from the βγ subunits. Evidence that the G208A mutant impairs Gq/11 signaling in a dominant-negative fashion has been presented.33 Our results thus indicate that MDAY-D2 cells also require Gq/11 signals to disseminate to the liver, spleen, and bone marrow. However, as with liver invasion, we have no obvious candidate for a chemokine that would regulate bone marrow colonization because SDF-1 has no effect on these cells.
Chemotaxis induced by chemokines does not appear to require Gq/11.11,13,20 In line with this, we have not seen an effect of G208A-G11 on SDF-1–induced chemotaxis in preliminary experiments with T-cell hybridoma cells expressing this G11 mutant. However, chemokines have other relevant effects as well. Transendothelial migration depends on integrins such as LFA-1 (αLβ2) and α4β1, which are both expressed on MDAY-D2 cells (Table 1). Similarly, as in most hematopoietic cells, these integrins are not active because the cells do not bind spontaneously to integrin ligands.48 Chemokine signals activate integrins and thus promote adhesion to cell monolayers. This involves activation of the small GTPase RhoA.22 The role of RhoA appears to be specific for G protein–induced adhesion because it is not required for integrin activation by the phorbol ester PMA.49,50 RhoA is activated by constitutively active mutants of G12/13 and Gq/11 but not of Gi.23Activation of Gq/11 by chemokine receptors may thus be required to induce adhesion to the endothelium and to the underlying extracellular matrix. In line with this notion, platelet aggregation involving the integrin αIIbβ3, triggered via heptahelical receptors for ADP and thrombin, was shown to require Gq/11 signals in addition to Gi signals.51 52
Although a role for Gq/11 in the induction of adhesion thus seems likely, we have so far not obtained convincing evidence for this hypothesis. MDAY-D2 cells express several integrins (Table 1), including LFA-1 and α4β1, which we have shown previously to be involved in dissemination of T-cell hybridoma and ESb T-lymphoma cells, respectively. The wide-spread dissemination of the T-cell hybridoma cells, including that to the liver and spleen, was completely dependent on LFA-1, as demonstrated with LFA-1–deficient mutant cells.53 Instead, ESb cells required α4β1 for colonization of the liver and spleen, as shown using β1-deficient double knockout mutant cells.54 The 2 integrins are expressed by MDAY-D2 cells, and thus, both may be involved. Also αVβ3, present on MDAY-D2 cells, has been implicated in liver metastasis of a lymphoma cell line,55 although this integrin could not substitute for α4β1 in ESb cells.54Dissemination to the bone marrow is likely to involve α4β1 and α5β1, which mediate interaction of myeloid cells with monolayers of bone marrow stromal cells.56 Also, homing of myeloid precursors to the bone marrow depends on α4β1,57 and transfection of α4β1 into Chinese hamster ovary cells was sufficient to confer the capacity to metastasize to the bone marrow.58 However, adhesion induced by chemokines, as measured in standard adhesion assays, is often very transient and weak, and only a small percentage of the cells bind.59 We have tested the induction of adhesion by the CXCR2 ligand CINC. The cells did not adhere to the LFA-1 and Mac-1 ligand ICAM-1, but adhesion to the α4β1 and α5β1 ligand fibronectin was sometimes observed. However, this adhesion was weak and very poorly reproducible, and it was impossible to assess an effect of the G208A-G11 mutant. The phorbol ester PMA did induce adhesion to fibronectin, but as expected, this was not affected by either S1 or the G208A-G11 mutant.
For dissemination in the bone marrow, Gq/11 appears to be required, whereas Gi is not. If Gq/11 mediates the proadhesive effects of chemokines, this may be the only requirement in this tissue. However, the migration signal may also be provided by factors that do not act on G protein–coupled receptors. For instance, hepatocyte growth factor/scatter factor (HGF/SF), which acts on the receptor tyrosine kinase c-met, is produced by bone marrow stromal cells and is chemotactic for myeloid cells.60 Signals by such factors may allow the cells to overcome the lack of Gi signals.
In conclusion, our results indicate that Gq/11 plays an important role in the dissemination of myeloid cells. Whereas the requirement for Gi proteins in liver and spleen colonization is not surprising given the established role of chemokines and the involvement of Gi proteins in chemokine-induced chemotaxis, the role of Gq/11 is novel and unexpected. The fact that bone marrow colonization occurs normally in the absence of Gi protein activity, whereas Gq/11 appears to be essential, underscores the importance of the latter. It remains to be established in which essential process the Gq/11 proteins are involved.
Acknowledgments
We thank Ton Schrauwers for excellent technical assistance in animal experiments, and Ingrid Zeelenberg for help with some of these experiments. We are grateful to Dr C. D. Tsoukas (University of San Diego, San Diego, CA) for the G208A mutant G11 cDNA; Dr G. P. Nolan (Stanford University, Stanford, CA) for the LZRS vector and Phoenix cells; Drs G. A. M. Michiels and J. G. Collard (Netherlands Cancer Institute, Amsterdam, The Netherlands) for modifications of the LZRS vector; and Dr S. Hermouet (Université de Nantes, Nantes, France) for the anti-Gq antiserum.
Supported by grant NKI 95-969 from the Dutch Cancer Society, Amsterdam, The Netherlands.
Submitted January 7, 2000; accepted March 6, 2000.
Reprints:E. Roos, Division of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; e-mail: eroos@nki.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal