Abstract
Despite the discovery of thrombopoietin (TPO) and its contribution to megakaryocytopoiesis, the exact mechanisms and sites of platelet production are unknown. It has been shown that mature megakaryocytes (MKs) functionally express the stromal-derived factor 1 (SDF-1) receptor, CXCR4. SDF-1–induced migration of mature MKs through endothelial cell layers results in increased platelet production. Because the migration of polyploid MKs from the bone marrow microenvironment requires remodeling of the perivascular extracellular matrix, it was hypothesized that mature polyploid MKs may express matrix metalloproteinases (MMPs), facilitating their exit into the bone marrow extravascular space. In this report, it is demonstrated that SDF-1 induces the expression and release of gelatinase B (MMP-9) by purified mature polyploid human MKs and an adeno-CXCR4–infected megakaryocytic cell line. Neutralizing antibody to MMP-9, but not MMP-2, blocked SDF-1–induced migration of MKs through reconstituted basement membrane, suggesting that expression of MMP-9 is critical for MK migration. Incubation of mature MKs with a synthetic MMP inhibitor, 5-phenyl-1,10-phenanthrolene, resulted in the inhibition of platelet formation, suggesting that the expression of MMPs is not only critical for megakaryocyte migration but also for subsequent platelet release. Confirming these results, adeno-SDF-1 injection into normal mice resulted in increased platelet counts, a process that could be blocked by a synthetic MMP inhibitor. These results suggest mobilization of MKs involves sequential expression and activation of chemokine receptors such as CXCR4, MMP-9, followed by transendothelial migration. MMP inhibitors may have potential use in the treatment of thrombotic and myeloproliferative disorders.
Introduction
Thrombopoietin (TPO) has been shown to promote megakaryocyte (MK) proliferation and maturation and platelet formation.1-3 Nonetheless, the exact mechanisms and sites of platelet formation are not well defined. The development of MKs can be divided into 2 distinct stages or compartments. The initial stage of MK development involves sequential commitment and proliferation of CD34+ hematopoietic stem cells into proliferating megakaryoblasts, a process referred to as megakaryocytopoiesis. The second phase of MK development comprises a population of morphologically identifiable mature, large polyploid MKs that undergo nuclear endoreplication and ultimately fragment into functional platelets. TPO has been shown to regulate MK proliferation, maturation, and endoreplication and to directly induce platelet formation in vitro.4,5 TPO knockout mice have platelet counts 85% lower than those of normal mice.6 However, despite low platelet counts, these mice do not bleed, and they have morphologically normal MKs and platelets.7 These data suggest that other factors may cooperate with TPO to modulate platelet production. Several studies have shown that mature polyploid MKs are localized to the extracellular space of bone marrow endothelial cells (BMECs).8-11 Electron microscopy studies have shown that these polyploid MKs transverse through BMECs12 and travel to the lungs, where they release platelets within the pulmonary capillaries.13-15
In search of chemokines that may mediate transendothelial migration of MKs, we and others have discovered that mature MKs express the chemokine receptor CXCR4.16-18 Stromal-derived factor 1 (SDF-1), the natural ligand for CXCR4, promotes the transendothelial migration of MKs, enhancing platelet production.16Nevertheless, it is unclear how MKs transmigrate through the basement membrane, which surrounds the BMEC layer. We hypothesized that such movement may involve the production of soluble and membrane-bound proteinases capable of digesting the basement membrane.
Matrix metalloproteinases (MMPs) comprise a family of zinc-binding proteins that degrade proteins within the extracellular matrix.19-21 Of the known MMPs, MMP-2 and -9 degrade collagen IV, one of the main components of the basement membrane, and are thus relevant to MK transendothelial migration. Guinea pig bone marrow MKs and activated platelets have been found to secrete collagenases,22,23 and isolated human platelet releasate has been shown to contain a 72-kd gelatinase (pro-MMP-2).24 The release of pro-MMP-2 correlated with positive in vitro platelet aggregation. With regard to the tissue inhibitors of metalloproteinases (TIMPs), TIMP-1 and -2 have been detected on human MKs and platelets.25,26 To date there are no reports of human MK MMP functional activity, yet MKs have been shown to actively migrate to the bone marrow vascular sinus spaces8-11 and to travel to the lungs, where they release platelets.
In this report, we show for the first time that purified mature polyploid human MKs produce and secrete MMP-9 and that this metalloproteinase is necessary for in vitro MK migration through the basement membrane in response to a chemoattractant stimulus such as SDF-1. MMP enzymatic activity is also required for subsequent platelet formation. Furthermore, administration of a synthetic MMP inhibitor (MMPi) to normal mice blocked SDF-1–induced platelet increase, demonstrating that the enzymatic activity of these proteinases is critical for MK migration out of the bone marrow and subsequent platelet production in vivo. Besides their potential as antimetastatic and antiangiogenic agents,27-31 MMP inhibitors may be useful in the treatment of thrombotic and myeloproliferative disorders.
Materials and methods
All chemicals and reagents were obtained from Sigma Chemical (St Louis, MO), unless stated otherwise.
Adenovirus vectors
AdCXCR4 and AdSDF-1 are Ad5-derived, E1a-deficient, partially E3-deficient vectors with an expression cassette in the E1a region containing the human CXCR4 and human SDF-1 cDNA, respectively, and are driven by the cytomegalovirus major immediate/early promoter/enhancer. The control AdNull is similar in design except that it contains no transgene in the expression cassette. All vectors were amplified and purified as previously described.32 A megakaryocytic leukemic cell line (HEL) was used for the in vitro AdCXCR4 experiments because these cells express low levels of CXCR4 and produce reduced levels of MMPs. For the in vivo assays Balb/c mice received AdSDF-1 and AdNull vectors at 1 × 109plaque-forming units, in a single intravenous injection at the start of the experiment (day 0).
Purification of CD34+ cells from cord blood
CD34+ cells were purified from cord blood samples using the Miltenyi MiniMacs system (Miltenyi Biotec, Auburn, CA), according to the manufacturer's instructions. Briefly, mononuclear cells were separated using a Ficoll-Plaque (Amersham Pharmacia Biotech, Piscataway, NJ) gradient and incubated with a hapten-conjugated anti-CD34+ monoclonal antibody (mAb) in the presence of an Fc receptor blocking reagent. These cells were subsequently incubated with MACS microbeads conjugated to an antihapten antibody and purified using MS+ separation columns (Miltenyi Biotec).
In vitro expansion and purification of CD41a+megakaryocytes from CD34+ cells
CD34+ cells were cultured in serum-free Xvivo-20 cell culture media (BioWhittaker, Walkersville, MD) supplemented with 100 ng/mL rhTPO (Peprotech, Piscataway, NJ), penicillin (100 U/mL), streptomycin (100 μg/mL), and Fungizone (0.25 μg/mL). After 12 days in culture CD41a+ MKs were isolated from the TPO-expanded CD34+ cells. The total cell population was incubated with an anti–CD41a fluorescein isothiocyanate (FITC)–labeled mAb. This incubation was conducted in the presence of 1.4 mmol/L adenosine and 2.74 mmol/L theophylline, known inhibitors of MK activation. These cells were subsequently incubated with MACS anti-FITC microbeads and purified using large-cell separation WHM columns (Miltenyi Biotec). Cells were washed 3 times with Xvivo-20 (BioWhittaker) to ensure complete removal of activation inhibitors.
RNA extraction, cDNA synthesis, and reverse transcription–polymerase chain reaction
Total RNA was extracted from purified MKs with TRI-reagent (Gibco BRL, Grand Island, NY), according to the manufacturer's instructions. cDNA was synthesized using the Ready-to-Go kit (Amersham Pharmacia Biotech) and polymerase chain reaction (PCR) was performed using a PCR thermal cycler (MWG Biotech, High Point, NC). The program used was as follows: an initial cycle of 5 minutes at 94°C, 1 minute at 65°C, and 30 seconds at 72°C. After this initial cycle, the reaction was continued for 35 cycles of 45 seconds at 94°C, 45 seconds at 65°C, and 2 minutes at 72°C, and it concluded with 7 minutes at 72°C. PCR products were run on 1.5% agarose gels with ethidium bromide. The primer sequences for β-actin, MMP-9, TIMP-1, and MMP-2 were as follows: β-actin sense primer, ΤCATGTTTGAGACCTTCAA; β-actin antisense primer, GTCTTTGCGGATGTCCACG; MMP-9 sense primer, ACCGCTATGGTTACACTCGG; MMP-9 antisense primer, GCAGGCAGAGTAGGAGCG; TIMP-1 sense primer, CCAAGTTCGTGGGGACAC; TIMP-1 antisense primer, TGCAGTTTTCCAGCAATGAG, MMP2 sense primer, TCCTTTCACAACCTTCTGTGG; MMP-2 antisense primer, GGGAACCATCACTATGTGGG. Endothelial cell cDNA was used as a positive control for all 4 sets of primers. The specificity of all reverse transcription (RT)–PCR products was confirmed, after extraction from agarose gels, by DNA sequencing (performed at the Cornell University DNA Sequencing Facility).
Analysis of cell culture supernatants by gelatinolytic zymography
Purified CD41a+ MKs and AdCXCR4-infected and AdNull-infected HEL cells were stimulated with and without 200 ng/mL per day rhSDF-1α (R&D Systems, Minneapolis, MN) or 1 U/mL thrombin. Supernatants were collected after 48 hours, and their metalloproteinase activity was measured by gelatinolytic zymography as previously described.33 Briefly, 1 mL cell culture supernatants (from 250 000 viable cells, as determined by trypan blue exclusion) were treated with 50 μL gelatin-agarose beads for 4 to 5 hours at 4°C and processed through SDS-PAGE acrylamide gels containing 1% gelatin. Gels were subsequently incubated in 2.5% Triton X-100 for 1 hour at room temperature, rinsed in distilled water (DW) and placed in low-salt collagenase buffer (50 mmol/L Tris, pH 7.6, 0.2 mol/L NaCl, 5 mmol/L CaCl2, and 0.2% vol/vol Brij-35) at 37°C for 14 to 18 hours. Bands of gelatinolytic activity were visualized after gels were stained with a solution containing 10 mL of a 0.2% Coomassie blue stock and 190 mL destain (DW, methanol and glacial acetic acid, 6:3:1) for 30 minutes and left for 1 hour at room temperature. A pro–MMP-2 standard (Oncogene Research Products, Cambridge, MA) was used as a positive control and to determine the molecular weight of gelatinolytic bands. The Adobe Photoshop 4.0 software application (Adobe Sys Inc, San Jose, CA) and a Umax Astra scanner (Umax Tech Inc, Fremont, CA) were used to scan the gels, and the intensity of the gelatinolytic bands was assessed using NIH Image 1.58 (http://rsb.info.nih.gov/nih-image).
Protein extraction and Western blotting
Purified CD41a+ MKs were stimulated with and without 200 ng/mL per day rhSDF-1α (R&D Systems) or 1 U/mL thrombin. Samples used for gelatinolytic zymography were also analyzed by Western blot to confirm the identity of the MMPs released into the MK supernatant. Briefly, MK supernatant samples were subjected to SDS-PAGE (7.5% gels) under reducing conditions (in the presence of β-mercaptoethanol). Proteins were subsequently blotted onto a nitrocellulose membrane following conventional protocols. Finally, blots were blocked in 1% BSA/PBS–1% Tween-20 for 1 hour at room temperature, followed by incubation with primary and secondary antibodies. Mouse monoclonal anti–MMP-9 antibody (clone 7-11C; Oncogene Research Products) was used at a concentration of 1 μg/mL and secondary antimouse IgG–horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA) was used at a 1:6000 dilution. The ECL chemiluminescence detection system and ECL film (Amersham Pharmacia Biotech) were used to visualize the presence of proteins on the nitrocellulose blots.
Flow cytometry
Cell populations were assessed by flow cytometry using a Coulter Elite flow cytometer (Coulter, Hialeah, FL). The following monoclonal antibodies were used: anit-CD34-phycoerythrin (PE) (clone HPCA2; Becton Dickinson, San Jose, CA), anti–CD41a-FITC (clone HIP8; PharMingen, San Diego, CA), anti–CXCR4-PE (clone 12G5; PharMingen), and IgG isotype control FITC/PE (PharMingen). A minimum of 5 × 104 cells was used for flow cytometry.
Immunohistochemistry
Cytospin preparations of freshly isolated mouse MKs and cultured human MKs were stained for the expression of MMPs following standard protocols. The antibodies used were: anti–MMP-2 mAb (clone 42-5011 for both human and murine samples; Oncogene Research), anti–MMP-9 mAb (clone 6-613 for human samples and clone 7-11C for murine; Oncogene Research), anti–TIMP-1 mAb (clone 7-6C1 for human and murine samples; Oncogene Research), and goat-antimouse-FITC secondary antibody (Kierkegaard & Perry Laboratories, Gaithersburg, MD). Briefly, cell suspensions were spun at 300 rpm for 5 minutes and fixed in 100% methanol for 10 minutes. Slides were washed with PBS 3 times for 5 minutes each and then incubated with 1.5% goat serum for 1 hour at room temperature. Primary antibodies were subsequently applied to the slides at a concentration of 1 μg/mL and incubated for 1 hour at room temperature. Slides were washed twice in PBS for 5 minutes before the application of secondary antibody (20 μg/mL) for 1 hour at room temperature. Finally, the slides were washed with PBS 3 times for 5 minutes each and mounted using SlowFade mounting reagent (Molecular Probes, Eugene, OR). The slides were observed and photographed under a fluorescent light microscope.
Migration assays
A modified version of a previously described transwell migration technique16 was used. Briefly, ex vivo–expanded MK were washed once with serum-free Xvivo-20 and prepared to a final concentration of 106 cells/mL. MK aliquots (100 μL) were added to 8 μm pore transwell inserts, coated with 25 μL growth factor–reduced Matrigel (Becton Dickinson), and placed into the wells of a 24-well plate. The lower compartment contained 600 μL Xvivo-20 serum-free media with or without 100 ng rhSDF-1α (R&D Systems). For the purpose of blocking migration, each condition was prepared in a separate aliquot and incubated with 500 ng/condition of anti–MMP-2 (clone 42-5011; Oncogene Research) or anti–MMP-9 (clone 6-613; Oncogene Research), monoclonal neutralizing antibodies, rhTIMP-1 (300 ng/condition; Oncogene Research), and broad-range proteinase inhibitors such as 2.5 mmol/L EDTA and 100 μmol/L 5-phenyl-1,10-phenanthrolene.34-38 The neutralizing activity of the anti–MMP-2 and anti–MMP-9 antibodies was verified by performing migration assays using leukemic cell lines that express both metalloenzymes and thus require MMP-2 and MMP-9 for SDF-1–induced migration through Matrigel (data not shown). The MK migration was carried out at 37°C and 5% CO2 for 24 hours. Migrated cells were collected from the lower compartment and counted using a hemocytometer, and the percentage of migrated CD41a+ MKs was determined by flow cytometry. Results are shown as a percentage of migrated CD41a+ MKs in response to rhSDF-1α.
Production of platelets by MK in vitro
Day 14 ex vivo–expanded MK were washed once with serum-free Xvivo-20 and prepared to a final concentration of 0.5 × 106 cells/mL, with or without 0.2 mmol/L of a synthetic MMPi, 5-phenyl-1,10-phenanthrolene, supplemented with 100 ng/mL rhTPO and penicillin–streptomycin–Fungizone. After 7 days in culture, the number of CD41a+ platelet particles in culture was determined by flow cytometry.
Animal studies
AdSDF-1 and AdNull vectors were administered to Balb/c mice, treated with or without the MMPi CGS-27 023A (N-hydroxy-2(R)-[4-methoxybenzenesulfonyl]-(3-picoly)amino]-3 methyl-butanamide hydrochloride, a chiral hydroxamic acid derived from D-valine, with a molecular weight of 429.93), provided by Novartis Pharma AG.39 40 The MMPi was administered subcutaneously at 300 μg/mouse twice daily. Control mice were injected with an identical volume of sterile water diluent control. Orbital bleeding was used to determine platelet levels at the start of the experiment, after 3 days, and for the remaining time points (up to day 28). Quantification of peripheral blood platelet levels was performed using a manual determination method (UNOPETTE Test 5854/5855; Becton Dickinson, Rutherford, NJ).
Statistical analysis
Statistical analysis was performed using Microcal Origin 5.0 SR2 (Microcal Software, Northampton, MA). Data are expressed as mean (SEM of 3 to 5 independent experiments). To detect differences between data sets, the Student t test was applied.P < .05 was considered statistically significant.
Results
Mature human MKs express and secrete MMP-9 in vitro
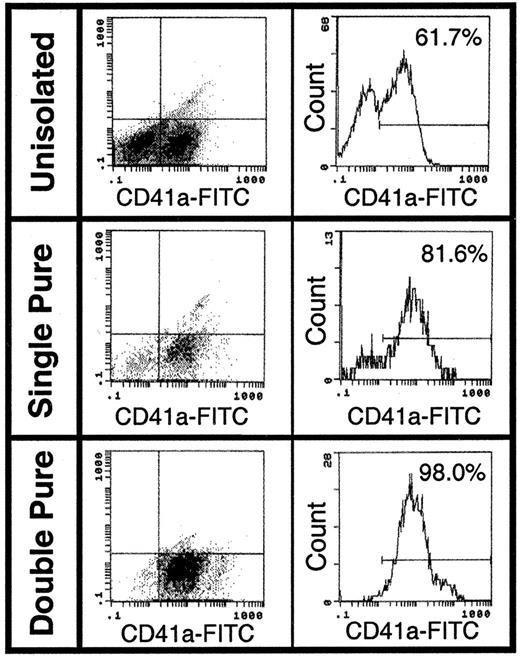
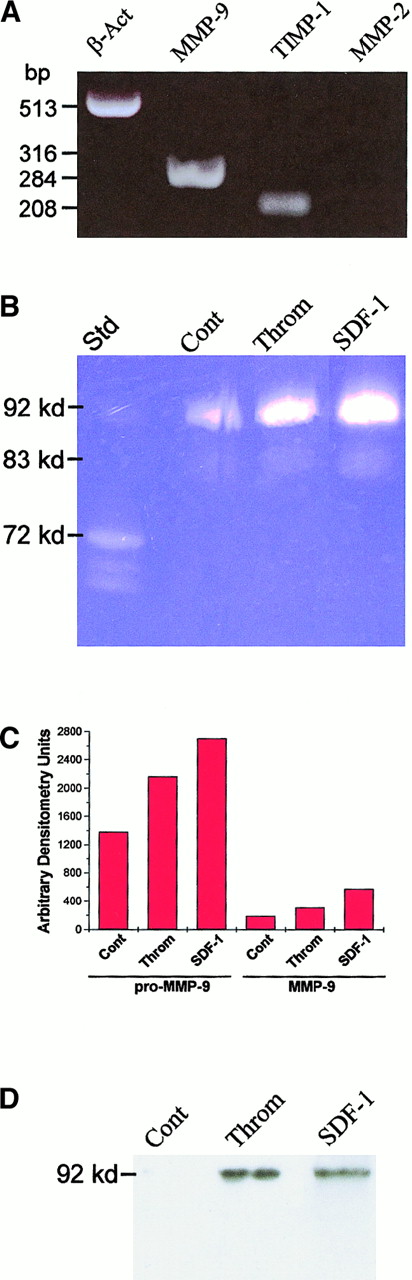
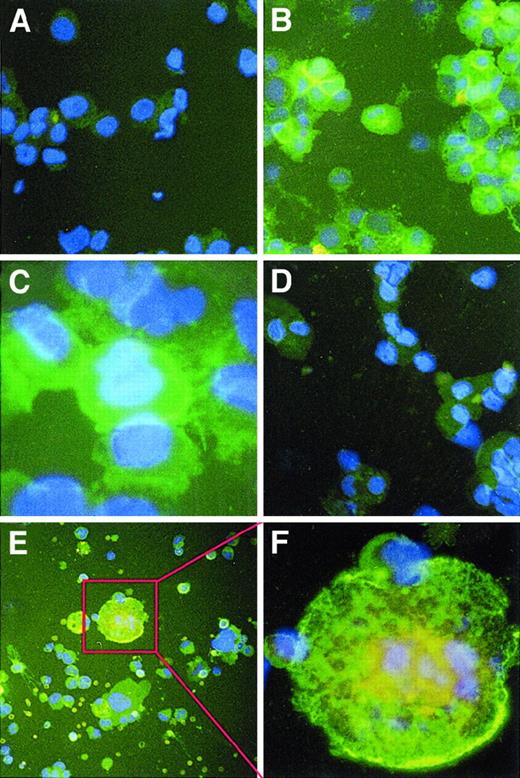
Purified human CD34+ cells were incubated with TPO for 12 to 21 days. CD41a+ MKs were purified from day 14 ex vivo–expanded cells (98% pure population) (Figure1). Purified MKs were analyzed for the expression of MMPs by RT-PCR, immunohistochemistry, zymography, and Western blot analysis. RT-PCR analysis of total RNA extracted from purified MKs showed that these cells expressed MMP-9 and TIMP-1, but not MMP-2, at the RNA level (Figure2A). Immunohistochemistry analysis revealed that mostly mature (polyploid) mouse and human MKs express MMP-9 (Figure 3B,C,E,F) and TIMP-1, but not MMP-2 (Figure 3D). The surface expression of MMP-9 suggested that these cells exported this proteinase to the culture supernatant. This was subsequently confirmed by zymography (Figure 2B) and Western blot analysis (Figure 2D). MK cell culture supernatants had gelatinolytic activity corresponding to human MMP-9 (92 kd), but not to MMP-2 (72 kd MMP-2 standard shown), confirming the results obtained by immunohistochemistry. Zymographic analysis of supernatants from purified MK cultures confirms MKs as the main cell type in those cultures and MMP-9 as the main gelatinase released by these cells. Finally, there was no gelatinolytic activity detected when gels were incubated overnight in buffer containing 1 mmol/L 5-phenyl-1,10-phenanthrolene, confirming the nature of this proteolytic activity.
Purification of human CD41a+ cells.
An enriched population of MKs was isolated after labeling with anti–CD41a-FITC antibody and anti-FITC immunomagnetic microbeads in the presence of 2 inhibitors of MK activation, adenosine and theophylline. The cells were analyzed by flow cytometry after having been passed once (single pure) and twice (double pure) through a magnetic column. This technique resulted in the isolation of a 98% pure CD41a+ MK population.
Purification of human CD41a+ cells.
An enriched population of MKs was isolated after labeling with anti–CD41a-FITC antibody and anti-FITC immunomagnetic microbeads in the presence of 2 inhibitors of MK activation, adenosine and theophylline. The cells were analyzed by flow cytometry after having been passed once (single pure) and twice (double pure) through a magnetic column. This technique resulted in the isolation of a 98% pure CD41a+ MK population.
Expression and secretion of MMPs by purified MKs.
(A) RT-PCR analysis of purified MKs revealed the expression of MMP-9 and TIMP-1, but not of MMP-2. (B) Day 14 ex vivo–expanded MKs (double pure) were cultured with or without rhSDF-1 (200 ng/mL per day) and thrombin (1 U/mL) for 48 hours, and the culture supernatants were analyzed by gelatinolytic zymography. Supernatants from MK cultures have gelatinolytic activity corresponding to human pro–MMP-9 (92 kd) and active MMP-9 (83-88 kd). (C) Quantification of the proteolytic activity detected in MK culture supernatants by densitometry. Data are shown as relative densitometry units. SDF-1 and thrombin stimulation of MK resulted in 95% and 60% increases in pro–MMP-9 and 200% and 65% increases in active MMP-9, respectively. (D) Western blot analysis of MK culture supernatants confirmed the increased expression of pro–MMP-9 on stimulation with SDF-1 and thrombin. Results are representative of 3 separate experiments.
Expression and secretion of MMPs by purified MKs.
(A) RT-PCR analysis of purified MKs revealed the expression of MMP-9 and TIMP-1, but not of MMP-2. (B) Day 14 ex vivo–expanded MKs (double pure) were cultured with or without rhSDF-1 (200 ng/mL per day) and thrombin (1 U/mL) for 48 hours, and the culture supernatants were analyzed by gelatinolytic zymography. Supernatants from MK cultures have gelatinolytic activity corresponding to human pro–MMP-9 (92 kd) and active MMP-9 (83-88 kd). (C) Quantification of the proteolytic activity detected in MK culture supernatants by densitometry. Data are shown as relative densitometry units. SDF-1 and thrombin stimulation of MK resulted in 95% and 60% increases in pro–MMP-9 and 200% and 65% increases in active MMP-9, respectively. (D) Western blot analysis of MK culture supernatants confirmed the increased expression of pro–MMP-9 on stimulation with SDF-1 and thrombin. Results are representative of 3 separate experiments.
Immunohistochemical staining of human and murine megakaryocytes for MMPs.
Cytospin preparations of ex vivo–expanded human and murine MKs were stained with MMP-2 and -9 mouse mAbs and goat-antimouse-FITC antibody, along with DAPI counterstain, and they were observed by fluorescence microscopy at a magnification of 400 × (A,B,D,E) and 1000 × (C,F). (A) An irrelevant IgG control showed little nonspecific staining. There was also low staining for MMP-2 (D). Staining of polyploid human (B,C) and murine (E,F) MKs revealed the presence of MMP-9 on the cell surface. Results are representative of 3 separate experiments.
Immunohistochemical staining of human and murine megakaryocytes for MMPs.
Cytospin preparations of ex vivo–expanded human and murine MKs were stained with MMP-2 and -9 mouse mAbs and goat-antimouse-FITC antibody, along with DAPI counterstain, and they were observed by fluorescence microscopy at a magnification of 400 × (A,B,D,E) and 1000 × (C,F). (A) An irrelevant IgG control showed little nonspecific staining. There was also low staining for MMP-2 (D). Staining of polyploid human (B,C) and murine (E,F) MKs revealed the presence of MMP-9 on the cell surface. Results are representative of 3 separate experiments.
Thrombin and SDF-1 induce MMP-9 secretion by MKs
Treatment of MKs with thrombin or SDF-1 increased pro–MMP-9 (92 kd41) release into the culture supernatant (Figure 2B). Moreover, a lower band of gelatinolytic activity (80-88 kd41) increased in expression after treatment with these cytokines (Figure 2B), corresponding to active MMP-9. Quantification of the gelatinolytic activity by densitometry showed that SDF-1 and thrombin increased pro–MMP-9 release by 95% and 60% and active MMP-9 by 200% and 65%, respectively (Figure 2C). Western blot analysis was performed on the supernatants from SDF-1– and thrombin-stimulated MKs (Figure 2D) to confirm the regulatory effects seen by gelatinolytic zymography. It is worth mentioning that the antibody used to detect MMP-9 recognizes only the pro form of this proteinase. Importantly, Western blot analysis of MK supernatants confirmed the significant increase in MMP-9 levels after thrombin and SDF-1 treatment of mature MKs. In addition, MK cell lysates contained TIMP-1, but not MMP-2, as determined by Western blot analysis (data not shown). These results suggest that the induction of MMP-9 may play a role on MK-induced migration in response to chemokines such as SDF-1.
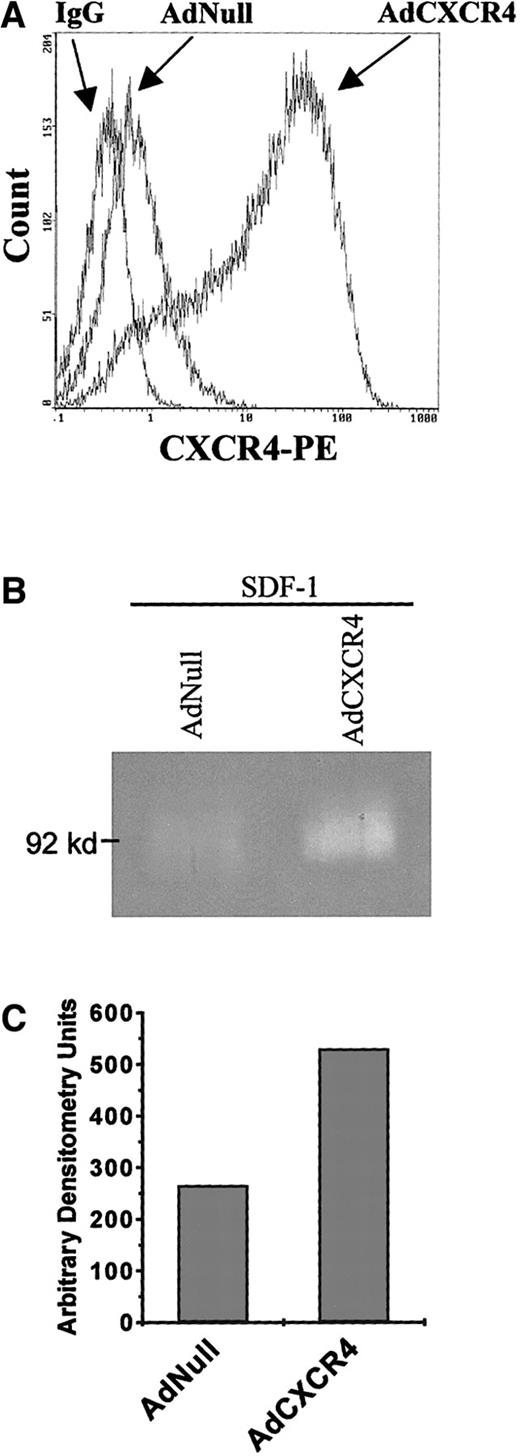
SDF-1 induces MMP production of Adeno-CXCR4 transfected megakaryocytic leukemic cells
Transfection of HEL cells with AdCXCR4 confirmed that SDF-1 signaling, through its receptor, results in increased MMP-9 production. Unstimulated HEL cells release almost undetectable MMP levels (data not shown). SDF-1 treatment of AdNull-infected HEL cells resulted in no detectable increases in gelatinolytic activity. However, SDF-1 treatment of AdCXCR4-infected HEL cells resulted in a 100% increase in pro–MMP-9 levels compared to basal levels (Figure4).
SDF-1 induces MMP-9 expression of AdCXCR4-infected megakaryocytic leukemic cells.
(A) HEL cells, which normally posses low CXCR4 levels, were infected with AdNull and AdCXCR4 adenoviral vectors. After overnight infection, cells were washed 3 times and analyzed for their CXCR4 surface expression. (B) Cells were cultured in the presence of rhSDF-1 (200 ng/mL per day) for 48 hours, and supernatant proteolytic activity was analyzed by gelatinolytic zymography. (C) Gelatinolytic activity was quantified by densitometry. Stimulation of the AdCXCR4-infected cells with SDF-1 resulted in a 100% increase in pro–MMP-9 (92 kd) compared with similarly treated AdNull-infected cells. Results are representative of 3 separate experiments.
SDF-1 induces MMP-9 expression of AdCXCR4-infected megakaryocytic leukemic cells.
(A) HEL cells, which normally posses low CXCR4 levels, were infected with AdNull and AdCXCR4 adenoviral vectors. After overnight infection, cells were washed 3 times and analyzed for their CXCR4 surface expression. (B) Cells were cultured in the presence of rhSDF-1 (200 ng/mL per day) for 48 hours, and supernatant proteolytic activity was analyzed by gelatinolytic zymography. (C) Gelatinolytic activity was quantified by densitometry. Stimulation of the AdCXCR4-infected cells with SDF-1 resulted in a 100% increase in pro–MMP-9 (92 kd) compared with similarly treated AdNull-infected cells. Results are representative of 3 separate experiments.
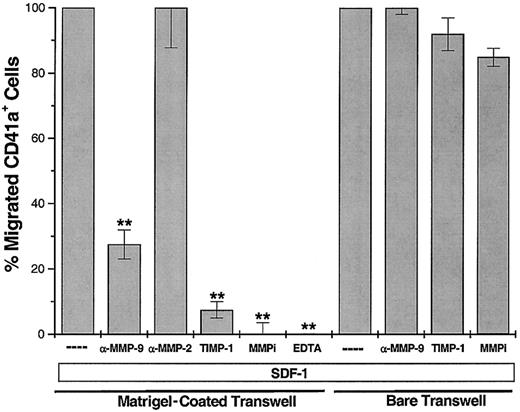
TIMP-1 and immunoneutralizing antibody to MMP-9, but not to MMP-2, blocks SDF-1–induced migration of MK through Matrigel-coated transwells
Others and we have shown that SDF-1, but not other chemokines, can induce the migration of MKs through bare transwells and endothelial monolayers.16-18 To examine the capacity of MKs to migrate through an extracellular matrix similar to endothelial basement membrane, the abluminal surface of 8-μm transwell inserts was coated with a thin layer of growth factor–reduced Matrigel. Similar to our previous results on bare transwells, SDF-1 induced the migration of 20% of the MKs added to the upper chamber through Matrigel-coated transwells. The MMPi 5-phenyl-1,10-phenanthrolene (but not a serine protease inhibitor, phenylmethylsulfonyl fluoride) completely blocked SDF-1–induced migration (Figure 5), suggesting that MKs require MMP activity to migrate through a reconstituted basement membrane. Further, immunoneutralizing antibodies to MMP-9, but not to MMP-2, blocked SDF-1–induced MK migration through Matrigel by 70% (n = 4; P < .005). The MMP-9 mAb used in these studies has been previously shown to specifically block MMP-9 activity.42 Furthermore, TIMP-1 also blocked MK migration through Matrigel-coated transwells, confirming the involvement of MMP-9 in this process. Importantly, MK migration through bare transwells was not inhibited by treatment with the mAb to MMP-9, TIMP-1, or the MMPi 5-phenyl-1,10-phenanthrolene (n = 4; P > .05). These results suggest that the induction of active MMP-9 by SDF-1 may have a role in regulating MK extravasation from the bone marrow, through the endothelial basement membrane, into the sinusoidal space.
Immunoneutralizing antibody for MMP-9 (500 ng/condition) and rhTIMP-1 (300 ng/condition) blocked SDF-1–induced migration of MKs through Matrigel-coated transwells.
This inhibitory effect was not seen with an anti–MMP-2 mAb (500 ng/condition). The anti–MMP-9 mAb and rhTIMP-1 did not block the migration of MK through bare transwells, indicating that MMP-9 is required to break down components of the basement membrane for successful MK migration. Synthetic MMP inhibitors also prevent MK migration. EDTA (1.5 mmol/L) was used as a negative migration control. Results are shown as the percentage of SDF-1–induced migration (SDF-1 alone was considered 100%) and are representative of 4 independent experiments (n = 4; **P < .005 compared to SDF-1 alone).
Immunoneutralizing antibody for MMP-9 (500 ng/condition) and rhTIMP-1 (300 ng/condition) blocked SDF-1–induced migration of MKs through Matrigel-coated transwells.
This inhibitory effect was not seen with an anti–MMP-2 mAb (500 ng/condition). The anti–MMP-9 mAb and rhTIMP-1 did not block the migration of MK through bare transwells, indicating that MMP-9 is required to break down components of the basement membrane for successful MK migration. Synthetic MMP inhibitors also prevent MK migration. EDTA (1.5 mmol/L) was used as a negative migration control. Results are shown as the percentage of SDF-1–induced migration (SDF-1 alone was considered 100%) and are representative of 4 independent experiments (n = 4; **P < .005 compared to SDF-1 alone).
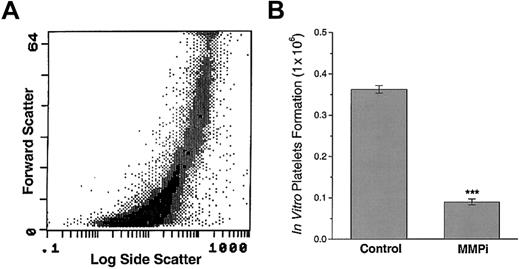
Synthetic MMP inhibitor blocks platelet formation by MKs in vitro
Prolonged (21-day) incubation of MKs with TPO leads to the spontaneous formation of platelet-like particles that have been shown to retain some features of circulating platelets.16Incubation of MKs with the MMPi 5-phenyl-1,10-phenanthrolene for the last 7 days of culture significantly inhibited platelet release (Figure6). Incubation of MKs proliferating in the presence of TPO, with the MMPi, blocked spontaneous MK fragmentation. On the other hand, after 7 days, wells with TPO alone had few viable intact MKs and an abundance of platelet-like particles. MK incubated with TPO alone resulted in a 4-fold increase in the number of platelet-like particles, compared to those treated with the MMPi (n = 4; P < .001). Incubation of MK with recombinant TIMP-1 had only a minor effect on platelet production, suggesting that the process of platelet formation may not be mediated solely by MMP-9 (data not shown). These results show that MMPs are required for the formation of platelets by MKs in vitro.
A synthetic MMPi blocks in vitro MK platelet formation
. (A) In vitro–generated platelets possess forward and log side scatter profile characteristics of in vivo–generated platelets. (B) The addition of a synthetic MMPi to the MK cultures resulted in a 4-fold decrease in the number of in vitro–generated platelets. Results are representative of 3 separate experiments and 4 independent platelet quantifications (n = 4; ***P < .001).
A synthetic MMPi blocks in vitro MK platelet formation
. (A) In vitro–generated platelets possess forward and log side scatter profile characteristics of in vivo–generated platelets. (B) The addition of a synthetic MMPi to the MK cultures resulted in a 4-fold decrease in the number of in vitro–generated platelets. Results are representative of 3 separate experiments and 4 independent platelet quantifications (n = 4; ***P < .001).
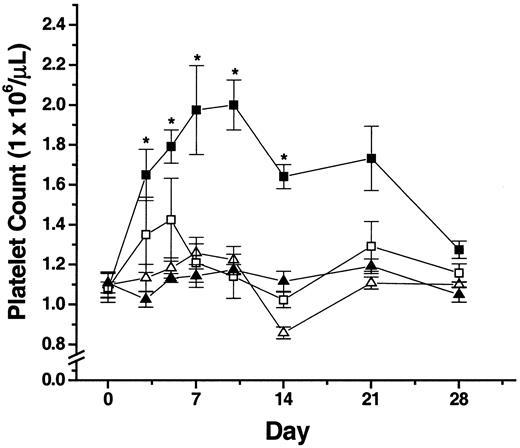
Administration of a synthetic MMP inhibitor to Balb/c mice blocks AdSDF-1–increased platelet levels
The results described above suggest that MMPs (and specifically MMP-9) may play a critical role in facilitating MK migration out of the bone marrow microenvironment into the peripheral circulation and in promoting platelet formation. Therefore, to examine the role of MMPs in regulating platelet production in vivo, AdSDF-1 and the MMPi (CGS-27 023A) were administered to Balb/c mice, and the number of platelets was determined over the course of the experiment.
After 3 days, mice treated with AdSDF-1 alone achieved significantly elevated platelet counts (P < .05), which peaked at days 7 to 10 (Figure 7) and returned to normal by day 28 (Figure 7). However, the administration of MMPi in conjunction with AdSDF-1 (Figure 7) reduced this effect at days 3 to 5 and completely blocked it by day 7 (P < .05).
In vivo administration of a synthetic MMPi blocks AdSDF-1–induced peripheral blood platelet increases.
The MMPi CGS-27023A was administered subcutaneously at 300 μg/mouse twice a day to Balb/c mice. Control mice were injected with an identical volume of control diluent, and platelet counts were determined over the course of the experiment. ▪, AdSDF-1; ■, AdSDF-1 plus MMPi; ▴, AdNull; ▵, AdNull plus MMPi. Results shown are representative of 3 independent experiments (n = 3; *P < .05 compared to diluent control mice).
In vivo administration of a synthetic MMPi blocks AdSDF-1–induced peripheral blood platelet increases.
The MMPi CGS-27023A was administered subcutaneously at 300 μg/mouse twice a day to Balb/c mice. Control mice were injected with an identical volume of control diluent, and platelet counts were determined over the course of the experiment. ▪, AdSDF-1; ■, AdSDF-1 plus MMPi; ▴, AdNull; ▵, AdNull plus MMPi. Results shown are representative of 3 independent experiments (n = 3; *P < .05 compared to diluent control mice).
Discussion
Although TPO has been shown to promote MK proliferation and maturation, the exact mechanisms and sites of platelet formation are not well defined. Studies have shown that MKs transmigrate through BMECs and release platelets within the sinusoidal space or lung capillaries. Others and we have shown that SDF-1 induces the transendothelial migration of MKs.16-18 Because locomotion of large polyploid MKs within the bone marrow microenvironment and subsequent migration requires remodeling of the perivascular extracellular matrix, we hypothesized that mature polyploid MKs may express MMPs, facilitating their exit into the bone marrow extravascular space. In this report, we demonstrate that SDF-1 induces MMP-9 expression by MKs and an adeno-CXCR4–infected megakaryocytic cell line. SDF-1 induces the migration of MKs through Matrigel-coated transwells in an MMP-9–, but not an MMP-2–, dependent manner. In vivo platelet production could be blocked by synthetic inhibitors of MMPs, suggesting these enzymes are required for the process of MK migration and suggesting their subsequent fragmentation into platelets. The process of platelet formation is complex, requiring sequential expression of the SDF-1 receptor CXCR4 by mature MKs, followed by the secretion of MMP-9, which allows for MK migration out of the bone marrow. SDF-1 orchestrates this process by inducing MMP-9 and providing directional cues for MK migration. The collective interaction of MKs with BMECs during the process of transmigration initiates cellular signals, resulting in MK fragmentation and platelet release within the lung capillaries.
MMP-9 has been shown to be primarily produced by cytokine-stimulated endothelial29 and smooth muscle cells,43,44and it plays a key role in the regulation of angiogenesis and the remodeling of connective tissue45 and in disease states associated with acute and chronic inflammation or tumor angiogenesis.29-31 However, recent studies have suggested that MMP-9 not only regulates tissue remodeling but may also play a seminal role in facilitating the migration of hematopoietic cells. MMP-9 is produced by hematopoietic cells, such as eosinophils,46 B-cells,47monocytes,48 and CD34+ cells.49Cytokine activation of CD34+ cells results in the up-regulation of MMPs, including MMP-9. Up-regulation of MMP-9 by various cytokines, including IL-6 and IL-3, facilitates the migration of CD34+ cells through reconstituted basement membranes (Matrigel).49 Moreover, cytokine activation of Epstein-Barr virus B-infected lymphocytes results in increased MMP-9 expression and enhancement of B-cell migration capacity. Furthermore, interferon-β has been shown to inhibit the migration of T cells by inhibiting MMP-9 production.48,50 51
In the current work, we demonstrated that SDF-1 induced MKs to express and secrete MMP-9. Combined SDF-1–induced chemotactic activity and MMP-9 activation results in the promotion of MK migration through vascular basement membrane. So far, among the known chemocytokines, SDF-1 has been shown to be the only chemokine mediating MK migration and activation of MMP-9. Furthermore, SDF-1 also induced MMP-3 release by MKs (data not shown), suggesting that the induction of this proteinase may be the mechanism by which SDF-1 stimulates the production of active MMP-9 by MKs. This mechanism of MMP-9 activation by MMP-3 has been recently shown for other cell types.52Besides their necessity for MK migration, we demonstrate that MMPs are also involved in the process of platelet production by MKs in vitro, in response to TPO.
The MK membrane has unique features distinct from those of platelets. Based on the results obtained by zymography and immunohistochemical staining, it is apparent that MMP-9 is not only secreted by MKs but is localized to the MK membrane. MMP-9 was also localized, though to a much smaller extent, within the MKs, mainly concentrated in demarcation membrane systems. MMP inhibition results in the blockage of platelet production in vitro, suggesting that the localization and activation of MMP-9, and possibly other as yet unrecognized proteinases, may play a key role in facilitating platelet release during MK fragmentation into platelets. It is intriguing that platelets themselves have been shown to contain naturally occurring MMPi such as TIMP-1 and TIMP-2,25 possibly to inhibit further fragmentation once the platelets are released into the peripheral circulation. These observations, in addition to previously published work showing that MMPs are required for platelet aggregation in vitro,53suggests that the inhibitors of these enzymes may be of use for the treatment of thrombophilic disorders.
In support of our in vitro observations, the administration of a synthetic MMPi with high affinity for MMP-9 to normal mice blocked AdSDF-1–induced increases in platelet levels. This MMP inhibitor was chosen over rhTIMP-1 because of the nonspecificity of TIMP-1 and the greater bio-availability of the synthetic MMPi. This MMPi may thus be highly effective at blocking platelet production and MK migration out of the bone marrow and could be useful in the treatment of thrombotic and thrombophilic disorders. Although the MMPi used in these studies has a broad range MMP blocking activity, it blocks preferentially MMP-2, MMP-3, and MMP-9, with IC50 values of 11 nmol/L, 8 nmol/L, and 13 nmol/L, respectively (as determined by Novartis Pharm AG). Of the MMPs investigated, MKs produce MMP-3 and MMP-9 but, unlike other hematopoietic cells, do not appear to produce MMP-2. It would be of the utmost importance to investigate whether a similar effect on MK migration and platelet production is observed after the administration of other available MMP inhibitors. Given that MMP-9, which is essential for MK migration, is also produced by other hematopoietic cells, including monocytes, eosinophils, and lymphocytes, in situations of increased platelet counts, higher levels of MMPi may be necessary to completely block the activity of MMP-9 and to reduce circulating platelet levels. Additionally, it is possible that the synthetic MMP inhibitor might affect ploidy or cytokine release. It is not surprising that factors that inhibit MMP-9 activity, such as interferons,48,50 54-56 have also been shown to decrease platelet production. Unfortunately, unlike MMP inhibitors, such as the one used in the current studies, the administration of interferons is invariably associated with systemic toxicity.
MKs and platelets have the capacity to store and secrete numerous cytokines and angiogenic factors. We have shown that MKs have the capacity to constitutively synthesize and store vascular endothelial growth factor (VEGF), which is the principal specific endothelial mitogenic and survival factor.57 Although MKs and platelets constitutively secrete small quantities of VEGF, activation with thrombin results in the release of large quantities of VEGF.57 In this report, we demonstrate that MMP-9 is not only constitutively secreted by MKs, it is also stored and released on thrombin stimulation. Given that MMP-9 has been shown to promote neo-angiogenesis, we suspect that the release of MMP-9, along with the release of VEGF and numerous other angiogenic regulators, results in locoregional induction of angiogenesis at the site of vascular injury.
In the current report we demonstrate that MMP-9 is required for MK migration out of the bone marrow and for subsequent platelet production in response to a specific chemoattractant stimulus, such as SDF-1. However, to date there have been no reports indicating whether MMP-9–deficient mice have low platelet counts or increased MK bone marrow content.45 However, the degree of redundancy between different members of the MMP family, as demonstrated by their overlapping substrate specificities, suggests that the absence of one proteinase may be compensated by the overexpression or overactivation of another closely related MMP. Another possible explanation is that platelet levels and bone marrow cellularity have simply not been quantified in these mice.
The bone marrow microenvironment has been shown to undergo continual remodeling. There is a balance between extracellular matrix laid down and destroyed. However, in myelofibrosis there is an accumulation of extracellular matrix that may result from defective MMP production by MKs or monocytes. Therefore, it is conceivable that the pathogenesis of myelofibrosis may be due to MK dysfunction, monocyte dysfunction, or both.
We have provided data supporting a new paradigm in hematopoietic cell biology whereby the locomotion of hematopoietic cells may require MMP activity. Therefore, the activation of MMPs may play a key role in the trafficking of hematopoietic cells such as MKs. Production of MMP-9 by MKs is one of the mechanisms by which mature MKs free themselves from the bone marrow matrix and travel to the lungs, where they ultimately release their load of platelets. The mechanisms by which MMP-9 or other as yet unknown MMPs regulate platelet release is the subject of ongoing studies.
Supported by National Heart, Lung, and Blood Institute (NHLBI) grants R01 HL-58707, R01 HL-61849, and Pilot Project P01 HL-59312; the Dorothy Rodbell Foundation for Sarcoma Research; and the Rich Foundation to S.R.; and NHLB grant R01 HL-61401 and the Gar Reichman Fund of the Cancer Research Institute to M.A.S.M.
W.J.L. and S.D. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shahin Rafii, Hematology-Oncology Division, Weill Medical College of Cornell University, Rm C-606, 1300 York Ave, New York, NY 10021; e-mail:srafii@mail.med.cornell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal