Abstract
In this study, it is shown that short-term exposure of normal human marrow CD34+CD38− cells to low concentrations of tumor necrosis factor (TNF) in the presence of 100 ng/mL Flt3 ligand and Steel factor and 20 ng/mL interleukin-3 (IL-3), IL-6, and granulocyte colony-stimulating factor, in either bulk or single-cell serum-free cultures, markedly reduces their ability subsequently to generate colony-forming cells (CFCs) in 6-week stromal cell–containing long-term cultures without affecting their viability, mitogenic response, or short-term ability to produce CFCs. A similar differential effect on the functional attributes of CD34+CD38− cells was seen when C2- or C6-ceramide, but not dihydro-C2-ceramide (an inactive analog of ceramide), was substituted for TNF. The addition of D-erythro-MAPP (a specific inhibitor of intracellular ceramide degradation) enhanced the ability of TNF to selectively eliminate long-term culture–initiating cell (LTC-IC) activity. These findings indicate that TNF can directly modulate the ability of CD34+CD38− cells to maintain their LTC-IC function at doses below those required to initiate apoptosis, cell cycle arrest, or both, and they suggest that this may be mediated by the TNF-induced generation of intracellular ceramide. Identification of a signaling intermediate that can influence primitive hematopoietic cell fate decisions offers a new approach to the investigation of signaling mechanisms in normal stem cell populations and to how these may be altered in leukemic cells.
Introduction
Normal adult human bone marrow contains a small population of transplantable totipotent hematopoietic stem cells, each with the capacity to generate a large and diverse population of mature progeny over prolonged periods of time in vivo1-3. These stem cells are closely related to cells that can sustain the production of hematopoietic cells in cultures provided with certain types of fibroblasts for at least 5 weeks2,4,5—hence the designation of the original input cells as long-term culture–initiating cells (LTC-ICs).6 In mice and humans, LTC-ICs and transplantable stem cells can be physically separated from most colony-forming cells (CFCs) that proliferate and differentiate in semisolid media in response to appropriate growth factor stimulation.7-9 Interestingly, the distinction between repopulating stem cells, LTC-ICs, and CFCs is not fully explained by differences in the particular growth factors to which these different cell types can respond because a number of these, either alone or in combination, can stimulate all 3 types of cells.2,10-14One key difference lies in the inability of primitive hematopoietic cells to proliferate in semisolid as opposed to liquid media.11 15-17
Tumor necrosis factor (TNF) has been shown to elicit a variety of responses, inhibitory and stimulatory, on primitive human and murine hematopoietic progenitor cells, depending on the cell population exposed and the other growth factors present.18-22 Some of these effects may be indirectly mediated owing to the ability of TNF to induce the production and release of other cytokines.20,22-24 However, direct inhibitory and stimulatory effects on primitive hematopoietic cells have also been demonstrated.21,25,26 Production of intracellular ceramide is a well-described consequence of TNF stimulation,27,28and the apoptosis-inducing effect of TNF on many cells, including hematopoietic cells, is mimicked by exposure to C2-ceramide, a diffusible analog of ceramide that enters cells passively.29 30
In previous studies, we showed that TNF in the presence of Flt3 ligand (FL), Steel factor (SF), interleukin-3 (IL-3), IL-6, and granulocyte colony-stimulating factor (G-CSF) can, at low concentrations, markedly decrease the output of cells with LTC-IC activity. This was seen in short-term cultures of normal human CD34+CD38−marrow cells under conditions that did not appear to affect the production of CFCs.12 These observations raised the possibility that TNF, like suboptimal concentrations of FL, SF, IL-3, IL-6, and G-CSF or excessive concentrations of IL-3,31 may activate intracellular events that strongly promote the differentiation of primitive human CD34+CD38− hematopoietic cells without influencing their viability or proliferative activity. The studies described here were designed to investigate this hypothesis and to determine whether increased levels of intracellular ceramide might be involved in mediating the effects observed.
Materials and methods
Cells
Heparinized aspirate cells were obtained with informed consent from healthy donors of marrow for allogeneic bone marrow transplantation at our center or were from cadaveric sources (Northwest Tissue Center, Seattle, WA). Low-density (less than 1.077 g/cm3) cells were stored overnight at 4°C in Iscove medium containing 50% fetal calf serum (FCS; StemCell Technologies, Vancouver, BC, Canada) for fractionation by fluorescence-activated cell sorting (FACS, see below) or were frozen in 90% FCS and 10% dimethyl sulfoxide and stored at −135°C before further use. Highly purified (more than 99% pure) CD34+CD38− populations were isolated from fresh or thawed cell samples by FACS and collected in bulk in tubes or as single cells in the individual wells of a 96-well microtiter plate using the single-cell deposition unit of the FACS, as previously described.15 31 Briefly, cells to be sorted were washed once in phosphate-buffered saline (StemCell) and once in Hanks HEPES-buffered salt solution. In some cases, they were then resuspended in Hanks HEPES-buffered salt solution containing 0.1 μg/mL fluorescein isothiocyanate (FITC)–conjugated Annexin V (Bio-Whittaker, La Jolla, CA) in addition to 2 μg/mL propidium iodide (PI; Sigma Chemical Co, St Louis, MO) to improve the yield of viable cells. Annexin V− PI− cells were isolated using a FACStar Plus (Becton Dickinson, San Jose, CA) equipped with a 5-W argon laser and a helium-neon laser before they were stained with antibodies specific for CD34 (8G12-Cy5) and CD38 (Leu17-phycoerythrin; Becton Dickinson). After one wash in Hanks HEPES-buffered salt solution containing 2% FCS (HF) and another in HF containing 2 μg/mL PI (Sigma), the CD34+CD38− subset of Annexin V− PI− cells was isolated. In other cases, the first staining with Annexin V was omitted, and PI−CD34+CD38− cells were isolated in a single step. Alternatively, the cells were first incubated with a 5–growth factor cocktail (see below) for 2 days, then restained with Annexin V–FITC to allow newly appearing Annexin V+ (apoptotic) cells to be removed. In all cases, cells were kept at 4°C throughout the labeling procedures, and positive cells were selected as those exhibiting a level of fluorescence that excluded 99% of cells incubated with control antibodies of the same isotype and labeled with the same fluorochrome.
Serum-free liquid cultures of primary cells
Sorted cells were cultured in suspension in 96-well plates at 1 or 100 to 200 cells/well in 100-μL volumes (or in 24-well plates at 500 cells/well in 500 μL volumes) of serum-free Iscove medium supplemented with 10−4 mol/L 2-mercaptoethanol, 10 mg/mL bovine serum albumin plus 10 μg/mL human insulin and 200 μg/mL human transferrin (BIT; StemCell), 40 μg/mL low-density lipoproteins (Sigma), and, as indicated, 100 ng/mL FL (Immunex, Seattle, WA), 100 ng/mL SF (Terry Fox Laboratory), 20 ng/mL IL-3 (Novartis, Basel, Switzerland), 20 ng/mL G-CSF (StemCell), 20 ng/mL IL-6 (Cangene, Mississauga, ON, Canada), and TNF (R&D System, Minneapolis, MN) with or without D-erythro-MAPP (Kamiya Biomedical Co, Seattle, WA), C2-ceramide (Sigma), C6-ceramide (Calbiochem, La Jolla, CA), or dihydro-C2-ceramide (Calbiochem). Cells were incubated undisturbed at 37°C in a humidified atmosphere of 5% CO2 in air before they were harvested and assayed at the times indicated in each experiment.
CFC assays
CFC numbers were determined by plating 1.1-mL aliquots of washed cells suspended in Iscove methylcellulose medium (MethoCult H4330; StemCell) supplemented with 3 U/mL human erythropoietin (StemCell), 50 ng/mL SF, and 20 ng/mL each of IL-6, GM-CSF (Novartis), G-CSF, and IL-3 in 35-mm Petri dishes. Erythroid, granulocyte-macrophage, and mixed-lineage colonies were enumerated in situ after 16 days of incubation at 37°C using standard scoring criteria to identify pure and mixed erythroid and granulocyte-macrophage colonies (from BFU-E, CFU-GM, and CFU-GEMM). Total CFC counts refer to the sum of all of these.
LTC-IC assays
Cells to be tested were co-cultivated, as described previously,9 with irradiated (8000 cGy) mouse fibroblast feeders (3 × 105 cells/35-mm dish) engineered to produce 10 ng/mL human IL-3, 130 ng/mL human G-CSF, and 10 ng/mL human SF, with subsequent weekly replacement of half the medium (human long-term culture medium, HCC-5100, StemCell; supplemented just before use with 10−6 mol/L freshly dissolved hydrocortisone sodium hemisuccinate, Sigma). Cultures were then maintained at 37°C, usually for 6 weeks, at the end of which both the nonadherent and the adherent cells were harvested, combined, washed, and assayed for their CFC content. These 6-week CFC output values provided a relative measure of the number of LTC-ICs in the original input suspension and could thus be used directly to compare the effects of different treatments on LTC-IC activity. Although limiting-dilution LTC-IC assays were not performed to allow formal distinction between the effects on LTC-IC frequency and the effects on their CFC-producing activity, previous experiments have shown that the latter parameter is not affected when CD34+CD38− cells are cultured under the serum-free conditions described here.9 31 In one set of experiments, some LTCs were harvested at the end of 3 weeks of incubation, and their CFC contents were similarly determined.
Statistics
Differences between progenitor numbers in different treatment groups were assessed using the Student t test.
Results
Dose- and time-dependence of TNF-induced suppression of LTC-ICs in short-term cultures of human marrow CD34+CD38−cells stimulated with FL, SF, IL-3, IL-6, and G-CSF
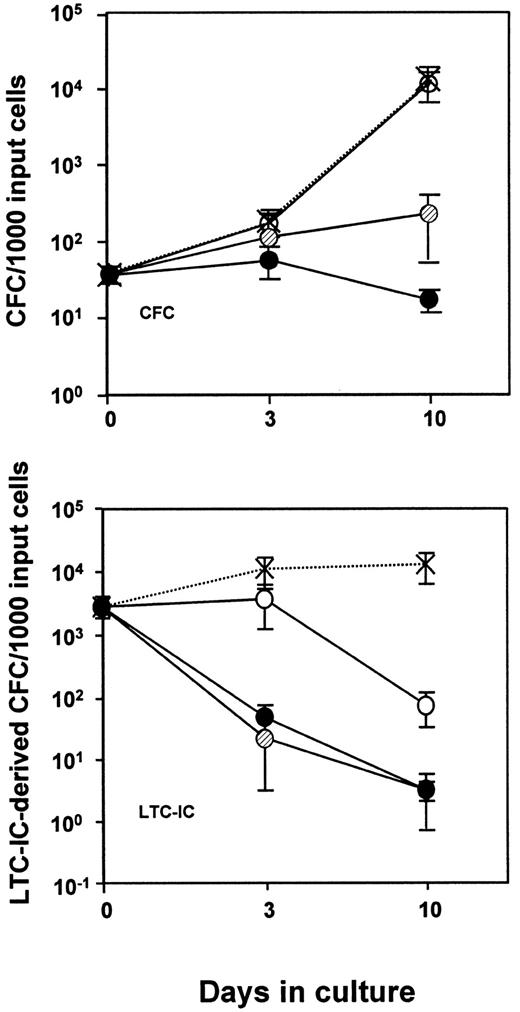
In a first series of experiments, 1000 to 2000 PI−/CD34+CD38− cells isolated from normal adult human marrow were placed in culture at 500 to 1000 cells/mL in serum-free medium supplemented with FL (100 ng/mL), SF (100 ng/mL), IL-3 (20 ng/mL), IL-6 (20 ng/mL), and G-CSF (20 ng/mL) and varying concentrations of TNF. After 3, 10, and in some cases 20 days, the LTC-IC, CFC, and total cell numbers were evaluated. Under these conditions, TNF inhibited the total cell expansion (data not shown) and the expansion of CFCs and LTC-ICs in a time- and dose-dependent fashion (Figure 1). However, the loss of LTC-IC activity was the most TNF-sensitive response. Thus, exposure of CD34+CD38− cells for 10 days to a concentration of TNF as low as 0.1 ng/mL caused a rapid and marked reduction in LTC-IC activity (more than 10-fold below input values;P < .05) in the absence of any effect on CFC production. The addition of 0.1 ng/mL TNF also had little effect on the total number of cells generated in these cultures (less than 2-fold reduction; P > .05; data not shown). Moreover, the selective effect of TNF on LTC-IC activity was not abrogated if the addition of the TNF was delayed for 2 days, as indicated by the similar results obtained in 2 such experiments, one of which one is shown in Table 1. On the other hand, a similar but an even more rapid effect (within 24 hours) could be demonstrated when the input cells were exposed to higher concentrations of TNF (20 to 100 ng/mL, data not shown, but see also Figure 3, discussed below).
Time-course of changes in CFC and 6-week LTC-IC (expressed as LTC-IC–derived CFC) numbers in suspension cultures of CD34+CD38− human marrow cells incubated in serum-free medium containing 100 ng/mL FL and SF and 20 ng/mL IL-3, IL-6, and G-CSF with varying amounts of TNF.
0 (X), 0.1 ng/mL (○), 1 ng/mL (○), and 10 ng/mL (●) of TNF added. Values shown are the mean ±SEM of data from 4 to 11 experiments, each with a different marrow source. At each time point, the progeny of 200 (n = 2), 500 (n = 3), or 1000 (n = 6) CD34+CD38− cells were analyzed. Input CFC and total LTC-IC–derived CFC values (per 1000 CD34+CD38− cells) were 37 ± 10 and 2500 ± 960, respectively.
Time-course of changes in CFC and 6-week LTC-IC (expressed as LTC-IC–derived CFC) numbers in suspension cultures of CD34+CD38− human marrow cells incubated in serum-free medium containing 100 ng/mL FL and SF and 20 ng/mL IL-3, IL-6, and G-CSF with varying amounts of TNF.
0 (X), 0.1 ng/mL (○), 1 ng/mL (○), and 10 ng/mL (●) of TNF added. Values shown are the mean ±SEM of data from 4 to 11 experiments, each with a different marrow source. At each time point, the progeny of 200 (n = 2), 500 (n = 3), or 1000 (n = 6) CD34+CD38− cells were analyzed. Input CFC and total LTC-IC–derived CFC values (per 1000 CD34+CD38− cells) were 37 ± 10 and 2500 ± 960, respectively.
Differential effect of tumor necrosis factor on different progenitor activities measured in assays of cells from 7-day cultures of CD34+CD38− human marrow cells
| Progenitor type assayed . | TNF addition (on day 2) . | Cells washed before assay . | CFC/1000 input cells . | ||
|---|---|---|---|---|---|
| Input . | Day 2 . | Day 7 . | |||
| CFCs | − | − | 45 | 140 | 3500 |
| − | + | 3400 | |||
| + | − | 7000 | |||
| + | + | 7900 | |||
| LTC-IC (6-wk) | − | − | 20 000 | 35 000 | 85 000 |
| − | + | 48 000 | |||
| + | − | 3700 | |||
| + | + | 1300 | |||
| Progenitor type assayed . | TNF addition (on day 2) . | Cells washed before assay . | CFC/1000 input cells . | ||
|---|---|---|---|---|---|
| Input . | Day 2 . | Day 7 . | |||
| CFCs | − | − | 45 | 140 | 3500 |
| − | + | 3400 | |||
| + | − | 7000 | |||
| + | + | 7900 | |||
| LTC-IC (6-wk) | − | − | 20 000 | 35 000 | 85 000 |
| − | + | 48 000 | |||
| + | − | 3700 | |||
| + | + | 1300 | |||
TNF indicates tumor necrosis factor; CFC, colony-forming cell; LTC-IC, long-term culture-initiating cell; FACS, fluorescence-activated cell sorting; FL, Flt3 ligand; SF, Steel factor; IL, interleukin; G-CSF, granulocyte colony-stimulating factor.
FACS-purified suspensions of Annexin V− PI−CD34+CD38− cells were isolated from the light-density fraction of normal human marrow samples, and 1200 of these cells per treatment group (ie, 6 × 100 μL cultures each containing 200 cells) were incubated for 7 days in serum-free medium supplemented with FL and SF (at 100 ng/mL each) and IL-3, IL-6, and G-CSF (at 20 ng/mL each). At the end of the first 2 days, the entire culture was restained with FITC-Annexin V, and any new Annexin V+ cells (3% in this case) were removed by resorting the cells. The Annexin V− fraction was then resuspended in fresh medium containing the same 5 growth factors, with or without 0.1 ng/mL TNF. At the end of another 5 days at 37°C, the cells in each type of culture were harvested, pooled, and washed once (or not, as shown), and aliquots were assayed for CFCs and LTC-ICs. The numbers of directly detected CFCs and of CFCs produced by 6-week LTC-ICs in the serum-free suspension cultures at the end of the 5-day exposure to TNF are shown in comparison with the corresponding input values (or the postreselection values measured after the first 2 days in the absence of TNF), as indicated. Results are from 1 of 2 such experiments; similar findings were obtained in each.
To determine whether the effects of TNF pretreatment on LTC-IC activity might influence CFC production within the first 3 weeks in culture, additional experiments were performed in which the LTCs were harvested at this earlier time point. In addition, in these experiments, some cells were incubated with TNF for only part of the pretreatment phase in suspension culture with FL, SF, IL-3, IL-6, and G-CSF and were then cultured for another 2 or 4 days in the same medium but without TNF (removed by washing). The purpose of this protocol was to determine whether the effect of exposing LTC-ICs to TNF for a given period could be reversed by further incubation in the TNF-free medium with high concentrations of stimulatory cytokines before assessing residual LTC-IC activity. The results of these experiments (performed in triplicate, ie, with CD34+CD38− cells isolated from 3 different normal bone marrows) are shown in Table2. It can be seen that, in spite of the expected minimal effects of TNF (0.1 ng/mL) pretreatment on directly assayed CFCs (groups 1 and 4 vs groups 3 and 6, respectively, in Table2; P > .05), the CFC content of 3-week-old LTCs initiated with cells cultured in the presence of TNF was significantly lower than the CFC content of 3-week-old LTCs initiated with cells cultured in the absence of TNF (groups 1 and 4 vs groups 3 and 6, respectively, in Table 2; P < .05). Moreover, there was no evidence that the loss of CFC-generating potential in LTCs could be reversed by further culture in TNF-free but growth factor–supplemented medium before plating the cells in LTC (group 3 vs group 5 in Table 2). Indeed, the data indicate that the TNF effect is fixed fairly rapidly—exposure for 3 versus 5 days (group 2 vs group 3 in Table 2) or for 5 versus 9 days (group 5 vs group 6 in Table 2) gave similar decreases in 3-week LTC-IC activity, with little effect on directly assayed CFCs in the same experiments.
Differential effect of tumor necrosis factor on the generation of cells detectable directly as colony-forming cells or as cells able to generate colony-forming cells in 3-week-old long-term cultures
| Progenitor type assayed . | Treatment group . | TNF addition (on day 2) . | Duration of TNF exposure (days) . | Total duration of expansion culture ± TNF (days) . | CFC/1000 input cells . |
|---|---|---|---|---|---|
| CFCs | 1 | − | 0 | 7 | 6200 ± 1400 |
| 2 | + | 3 | 7 | 3200 ± 600 | |
| 3 | + | 5 | 7 | 4100 ± 1200 | |
| 4 | − | 0 | 11 | 27 100 ± 2600 | |
| 5 | + | 5 | 11 | 17 100 ± 3300 | |
| 6 | + | 9 | 11 | 18 800 ± 3100 | |
| LTC-ICs (3 wk) | 1 | − | 0 | 7 | 55 000 ± 1600 |
| 2 | + | 3 | 7 | 16 600 ± 3200 | |
| 3 | + | 5 | 7 | 17 500 ± 4400 | |
| 4 | − | 0 | 11 | 91 500 ± 11 000 | |
| 5 | + | 5 | 11 | 9900 ± 6500 | |
| 6 | + | 9 | 11 | 4000 ± 2400 |
| Progenitor type assayed . | Treatment group . | TNF addition (on day 2) . | Duration of TNF exposure (days) . | Total duration of expansion culture ± TNF (days) . | CFC/1000 input cells . |
|---|---|---|---|---|---|
| CFCs | 1 | − | 0 | 7 | 6200 ± 1400 |
| 2 | + | 3 | 7 | 3200 ± 600 | |
| 3 | + | 5 | 7 | 4100 ± 1200 | |
| 4 | − | 0 | 11 | 27 100 ± 2600 | |
| 5 | + | 5 | 11 | 17 100 ± 3300 | |
| 6 | + | 9 | 11 | 18 800 ± 3100 | |
| LTC-ICs (3 wk) | 1 | − | 0 | 7 | 55 000 ± 1600 |
| 2 | + | 3 | 7 | 16 600 ± 3200 | |
| 3 | + | 5 | 7 | 17 500 ± 4400 | |
| 4 | − | 0 | 11 | 91 500 ± 11 000 | |
| 5 | + | 5 | 11 | 9900 ± 6500 | |
| 6 | + | 9 | 11 | 4000 ± 2400 |
TNF indicates tumor necrosis factor; CFC, colony-forming cell; LTC, long-term culture; LTC-IC, long-term culture-initiating cell; FACS, fluorescence-activated cell sorting; FL, Flt3 ligand; SF, Steel factor; IL, interleukin; G-CSF, granulocyte colony-stimulating factor.
FACS-purified Annexin V− PI−CD34+CD38− cells were isolated from the light-density fraction of 3 normal human marrow samples, and 1500 of these cells per treatment group (ie, 3 × 500 μL cultures, each containing 500 cells) were incubated for either 7 or 11 days in serum-free medium supplemented with FL and SF (at 100 ng/mL each) and IL-3, IL-6, and G-CSF (at 20 ng/mL each). At the end of the first 2 days, 0.1 ng/mL TNF was added as indicated. Three or 5 days later (for the 7 day and 11 day groups, respectively), the cells in each culture were washed, resuspended in fresh medium with growth factors ± TNF at 0.1 ng/mL as indicated, and returned to the incubator. At the end of the 7 or 11 days in suspension culture, the cells were harvested and washed, and aliquots were assayed for CFC before and after further culture under LTC conditions for 3 weeks. The results of these assays are shown as the mean ± SEM for the pooled data from the 3 experiments performed. Input CFCs and 3-week LTC-IC–derived CFC numbers (per 1000 initial CD34+CD38− cells) were 45 ± 4 and 2200 ± 1600, respectively.
TNF-induced loss of LTC-IC function does not result from a cytotoxic effect on a subset of CD34+CD38−cells
Another series of experiments was undertaken to examine whether the differential TNF-induced loss of LTC-IC activity (but not CFC activity) might reflect modulation of the functional repertoire of the responding CD34+CD38− cells rather than selective killing of those that initially possessed LTC-IC activity. In these experiments, CD34+CD38− cells were cultured in serum-free medium plus FL, SF, IL-3, IL-6, and G-CSF plus 0.1 ng/mL TNF as before but as isolated single cells as well as in bulk cultures initiated with 500 cells each. Direct visualization of the single-cell cultures indicated no effect of this TNF dose on the viability of the input CD34+CD38− cells (proportion of wells with 1 or more refractile cell/well) or the ability of the input cells to divide (proportion of wells with 2 or more refractile cells/well), when the cultures were assessed after 3 or 10 days of incubation (Table 3).
Lack of effect of low-dose tumor necrosis factor on the viability and clonal growth of individual CD34+CD38− human marrow cells assessed in single-cell cultures
| Period of culture (days) . | TNF addition . | Proportion (%) of wells containing different numbers of cells . | ||
|---|---|---|---|---|
| 0 cells . | 1 cell . | 2 cells . | ||
| 3 | − | 4.3 ± 1.1 | 82 ± 5 | 14 ± 5 |
| + | 7.8 ± 1.7 | 81 ± 3 | 12 ± 4 | |
| 10 | − | 27 ± 8 | 34 ± 15 | 40 ± 20 |
| + | 32 ± 7 | 32 ± 13 | 36 ± 18 | |
| Period of culture (days) . | TNF addition . | Proportion (%) of wells containing different numbers of cells . | ||
|---|---|---|---|---|
| 0 cells . | 1 cell . | 2 cells . | ||
| 3 | − | 4.3 ± 1.1 | 82 ± 5 | 14 ± 5 |
| + | 7.8 ± 1.7 | 81 ± 3 | 12 ± 4 | |
| 10 | − | 27 ± 8 | 34 ± 15 | 40 ± 20 |
| + | 32 ± 7 | 32 ± 13 | 36 ± 18 | |
TNF indicates tumor necrosis factor; FACS, fluorescence-activated cell sorting; FL, Flt3 ligand; SF, Steel factor; IL, interleukin; G-CSF, granulocyte colony-stimulating factor.
Annexin V− PI−CD34+CD38− cells were isolated from low-density normal human marrow samples and deposited directly using the FACS into the round-bottomed wells of 96-well plates preloaded with serum-free medium containing FL and SF (at 100 ng/mL) and IL-3, IL-6, and G-CSF (at 20 ng/mL), with or without 0.1 ng/mL TNF, as indicated. Cultures were assessed by visual examination at the times shown, and the proportions of wells containing 0, 1, or more than 1 viable (refractile) cell per well were identified. Results shown are the mean ± SEM of data obtained in 5 independent experiments (with different marrow samples), in each of which at least 96 single-cell cultures were evaluated.
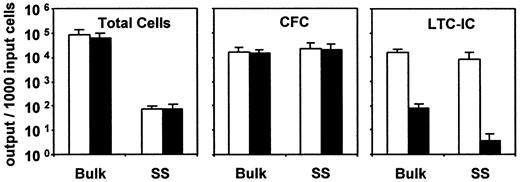
A comparison of the number of LTC-ICs, CFCs, and total cells measured in the single-cell and parallel-bulk cultures at the end of the 10-day period of incubation is presented in Figure2. As can be seen, the presence of TNF had little effect (P > .05) on either CFC or total cell expansion, regardless of whether 1 or 500 cells were used to initiate the cultures. However, the negative effect of TNF on LTC-IC yields (P < .05) was more pronounced in the single-cell cultures. These results establish the ability of TNF to act directly on LTC-ICs to eliminate their function without concomitantly affecting their ability to survive and divide.
Effect of added TNF (0.1 ng/mL) on the changes measured in total viable cells, CFCs, and 6-week LTC-ICs (expressed as LTC-IC–derived CFCs) after 10 days in 5 experiments, in each of which parallel cultures were initiated with 500 (bulk) versus at least 96 single CD34+CD38− human marrow cells (SS) (same experiments as described in Table 3).
Cells from the single-cell cultures were pooled at the end of the 10-day incubation before assay for their CFC and LTC-IC contents. Results shown are the mean ±SEM. ■, control data; ▪, data for the TNF-treated cultures. Input CFCs and total LTC-IC–derived CFC values (per 1000 initial CD34+CD38− cells) in these experiments were 22 ± 7 and 3500 ± 1900, respectively.
Effect of added TNF (0.1 ng/mL) on the changes measured in total viable cells, CFCs, and 6-week LTC-ICs (expressed as LTC-IC–derived CFCs) after 10 days in 5 experiments, in each of which parallel cultures were initiated with 500 (bulk) versus at least 96 single CD34+CD38− human marrow cells (SS) (same experiments as described in Table 3).
Cells from the single-cell cultures were pooled at the end of the 10-day incubation before assay for their CFC and LTC-IC contents. Results shown are the mean ±SEM. ■, control data; ▪, data for the TNF-treated cultures. Input CFCs and total LTC-IC–derived CFC values (per 1000 initial CD34+CD38− cells) in these experiments were 22 ± 7 and 3500 ± 1900, respectively.
Evidence that the TNF-induced loss of LTC-IC function involves the sphingolipid pathway
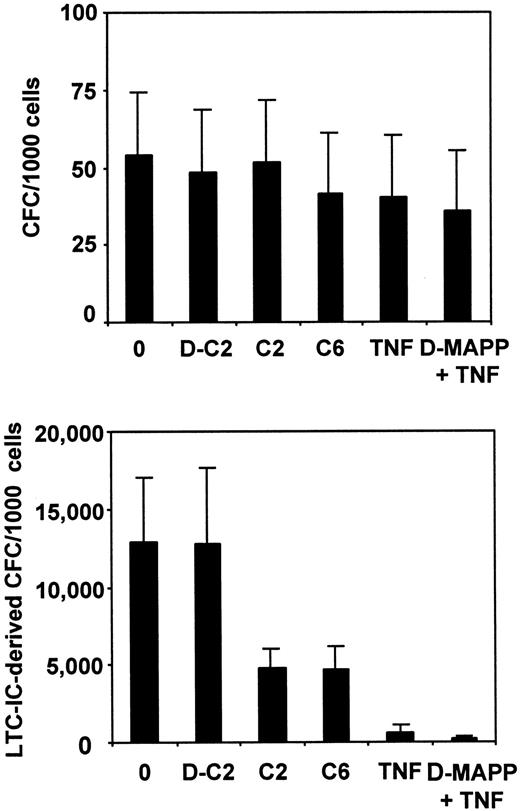
To investigate whether TNF might affect the cellular attributes required for LTC-IC function as a result of activation of the ceramide pathway, human marrow CD34+CD38− cells were cultured for 24 hours in serum-free media supplemented with the same 5–growth factor cocktail as before, in the presence (or not) of 20 ng/mL TNF with or without D-erythro-MAPP (an inhibitor of the alkaline ceramidase that breaks down ceramide to sphingosine), 30 μmol/L C2-ceramide, C6-ceramide (another active analog of ceramide whose structure is more closely related to the natural ceramide than C2-ceramide29), or dihydro-C2-ceramide, an inactive ceramide analog.32 After 24 hours, the cultures were assayed for CFCs and LTC-ICs. As shown in Figure 3, there was a similarly marked and selective decrease in LTC-IC activity in cultures to which TNF, C2-ceramide, or C6-ceramide had been added (P < .05), and the effect of TNF was slightly enhanced when D-erythro-MAPP was present. In contrast, exposure of the cells to dihydro-C2-ceramide had no significant (P > .05) effect on LTC-IC (or CFC) numbers by comparison with control cultures containing the standard 5–growth factor cocktail only.
Comparison of the effects of various reagents that can modulate the sphingomyelinase pathway on the maintenance of CFC and LTC-IC activity in 24-hour serum-free cultures of CD34+CD38− human marrow cells.
Values shown are the mean ± SEM of data from 3 independent experiments (different marrow samples). All cultures contained 100 ng/mL FL and SF, 20 ng/mL IL-3, IL-6, and G-CSF, and 30 μmol/L concentrations of various ceramide analogs: dihydro-C2-ceramide (D-C2), C2-ceramide (C2), C6-ceramide (C6), or 5 μmol/L D-erythro-MAPP (D-MAPP), with or without 20 ng/mL TNF or diluent (0), as indicated. Input CFCs and 6-week LTC-IC–derived CFC values (per 1000 initial CD34+CD38− cells) were 45 ± 10 and 12 400 ± 1200, respectively.
Comparison of the effects of various reagents that can modulate the sphingomyelinase pathway on the maintenance of CFC and LTC-IC activity in 24-hour serum-free cultures of CD34+CD38− human marrow cells.
Values shown are the mean ± SEM of data from 3 independent experiments (different marrow samples). All cultures contained 100 ng/mL FL and SF, 20 ng/mL IL-3, IL-6, and G-CSF, and 30 μmol/L concentrations of various ceramide analogs: dihydro-C2-ceramide (D-C2), C2-ceramide (C2), C6-ceramide (C6), or 5 μmol/L D-erythro-MAPP (D-MAPP), with or without 20 ng/mL TNF or diluent (0), as indicated. Input CFCs and 6-week LTC-IC–derived CFC values (per 1000 initial CD34+CD38− cells) were 45 ± 10 and 12 400 ± 1200, respectively.
Of interest, it should be noted that in all the overnight cultures, including the control cultures, the number of CFCs detectable was twice the input value, even though CD34+CD38− cells from adult marrow do not begin to divide until the third day of incubation under these conditions (Petzer et al15 and Table2). This shows that the 5–growth factor cocktail can “prime” CD34+CD38− cells in liquid culture to overcome their inability to proliferate in semisolid medium, even in the absence of cell division. A similar effect of mitogenic growth factors on primitive Lin− CD34−CD38− human marrow cells has also been demonstrated.17 Although the mechanism responsible has not yet been investigated, we speculate that an effect on the organization of the cellular cytoskeleton may be involved.
Discussion
In this study, we have demonstrated an ability of nontoxic concentrations of TNF to promote the loss of certain functional properties of primitive (CD34+CD38−) human hematopoietic cells without altering their viability or capacity to become detectable as CFCs either before or after they proliferate in response to FL, SF, IL-3, IL-6, and G-CSF. This 5–growth factor cocktail was chosen because it has been shown to optimize the expansion of both LTC-IC and CFC populations from the input population studied here.12,31 Experiments with single cells showed that the response to TNF is directly mediated and can occur in the absence of effects on cell survival or proliferation. The increased LTC-IC activity obtained when CD34+CD38− cells are stimulated by FL in combination with SF, IL-3, IL-6, and G-CSF is due to the response of a substantial proportion (more than 30%) of the initial viable population.15 Therefore, any substantial loss of these cells, such as by apoptosis, should have been readily detectable by direct visual examination of single-cell cultures. The lack of such an observation argues strongly in favor of an alternative explanation—that, under the conditions of TNF exposure used here, CD34+CD38− cells are induced to alter certain biologic functions associated with their original undifferentiated state, including those that allow their detection as LTC-ICs. Convincing examples of a cytokine-determined alteration in primitive hematopoietic cell differentiation status, independent of effects on survival or mitogenesis, are rare and are largely restricted to studies with murine cells.33-35 However, we have recently reported similar findings in cultures of human marrow CD34+CD38− cells exposed either to suboptimal concentrations of FL, SF, IL-3, IL-6, and G-CSF or to excess concentrations of IL-3 (in the presence of low levels of FL and SF).31 Evidence of extrinsic determination of hematopoietic cell differentiation decisions has also been obtained in various transformed or immortalized hematopoietic cell lines of mouse and human origin.36-39
The current observations are of particular interest because they suggest the involvement of an intracellular signaling pathway that is activated by TNF and appears able to affect hematopoietic stem cell fate decisions. This is further supported by the observation that a closely related subpopulation of cells, the Thy-1+ subset of CD34+ cells in human cord blood, contains transcripts for both TNF receptor species (p55 and p75) and can be stimulated directly by TNF to enhance their proliferative response in combination with FL, SF, G-CSF, and GM-CSF.21 However, in that study, effects on LTC-IC activity were not analyzed.
Previous investigations have identified a number of downstream targets of ceramide, including a proline-directed serine–threonine protein kinase (ceramide-activated protein kinase),40 the proto-oncogene Vav,41 a cytosolic ceramide-activated protein phosphatase,42 and protein kinase C-ζ.43 Some of these are also known to be regulated by interactions with 1,2-diacylglycerol (DG). Interestingly, the outcome of raising the intracellular level of ceramide in T cells appears to be subject to modulation according to whether the cells are costimulated by receptors that activate intracellular DG.44 More recently, evidence of ceramide-induced phosphorylation of the cyclic adenosine monophosphate response element binding protein (and a related transcription factor, ATF-1) by p38 mitogen-activated protein kinase has been reported.45 Moreover, the inhibition of this pathway had no effect on the induction of apoptosis by ceramide or TNF. This is consistent with the hypothesis that these agents may elicit different biologic responses by the activation of different intracellular intermediates. Accordingly, the cellular context in which TNF receptor activation occurs is likely to be an important determinant of the final biologic outcome it elicits in a given target cell. The ability to control over time the in vitro cytokine environment of highly purified populations of primitive hematopoietic cells should facilitate the future identification of events downstream of sphingomyelin degradation that may precipitate the accelerated differentiation of these cells and allow the potential relevance of the current findings to leukemic stem cell responses to be investigated.
Acknowledgments
We thank the members of the Clinical Stem Cell Assay Service of the British Columbia Cancer Agency for assistance in accessing and initial processing of the human marrow samples, the staff of the Flow Cytometry Laboratory for assistance in cell sorting, and T. Palmater for manuscript preparation. We also thank Dr P. Lansdorp (Terry Fox Laboratory) and Amgen, Cangene, Immunex, Novartis, and StemCell for providing reagents.
Supported by grants from Novartis and the National Cancer Institute of Canada (NCIC), with funds from the Terry Fox Run. C.J.E. is a Terry Fox Cancer Research Scientist of the NCIC.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Connie J. Eaves, Terry Fox Laboratory, British Columbia Cancer Agency, 601 West 10th Ave, Vancouver, BC V5Z 1L3, Canada; e-mail: connie@terryfox.ubc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal