Abstract
Interleukin-6 (IL-6) is reported to be central to the pathogenesis of myeloma, inducing proliferation and inhibiting apoptosis in neoplastic plasma cells. Therefore, abrogating IL-6 signaling is of therapeutic interest, particularly with the development of humanized anti–IL-6 receptor (IL-6R) antibodies. The use of such antibodies clinically requires an understanding of IL-6R expression on neoplastic cells, particularly in the cycling fraction. IL-6R expression levels were determined on plasma cells from patients with myeloma (n = 93) and with monoclonal gammopathy of undetermined significance (MGUS) or plasmacytoma (n = 66) and compared with the levels found on normal plasma cells (n = 11). In addition, 4-color flow cytometry was used to assess the differential expression by stage of differentiation and cell cycle status of the neoplastic plasma cells. IL-6R alpha chain (CD126) was not detectable in normal plasma cells, but was expressed in approximately 90% of patients with myeloma. In all groups, the expression levels showed a normal distribution. In patients with MGUS or plasmacytoma, neoplastic plasma cells expressed significantly higher levels of CD126 compared with phenotypically normal plasma cells from the same marrow. VLA-5− “immature” plasma cells showed the highest levels of CD126 expression, but “mature” VLA-5+ myeloma plasma cells also overexpressed CD126 when compared with normal subjects. This study demonstrates that CD126 expression is restricted to neoplastic plasma cells, with little or no detectable expression by normal cells. Stromal cells in the bone marrow microenvironment do not induce the overexpression because neoplastic cells express higher levels of CD126 than normal plasma cells from the same bone marrow in individuals with MGUS.
Introduction
Interleukin-6 (IL-6) was initially identified as a factor that promoted differentiation of B-lymphocytes into class-switched immunoglobulin-secreting plasma cells, without inducing proliferation.1 For normal circulating plasma cell precursors, the molecule is a critical survival factor.2,3In contrast to its effects on normal B-cells, IL-6 induces proliferation of myeloma cell lines and freshly isolated myeloma plasma cells, but does not induce immunoglobulin secretion.4,5Kawano et al6 have demonstrated that myeloma cells at an early stage of differentiation are more proliferative in response to IL-6. This “immature” fraction can be identified because it lacks VLA-5 (CD49e) expression, has plasmablastic morphology, and produces low levels of immunoglobulin.6 In addition, IL-6 can inhibit the apoptotic effects of anti-fas antibodies or dexamethasone on myeloma cell lines, but not of agents that induce DNA damage such as doxorubicin or etoposide.7,8 IL-6 can function in both an autocrine and paracrine fashion,9 but most evidence indicates that its primary mode of action in vivo is paracrine in nature.10,11 Transgenic mice that overexpress IL-6 have an excess of plasma cells and polyclonal immunoglobulins, but do not develop overt myeloma.12 13
The central role of IL-6 in the pathogenesis of myeloma has led to a number of therapeutic strategies to abrogate IL-6 signaling.14 Clinical studies with murine anti–IL-6 antibodies showed evidence of disease regression in some patients, but these were not maintained, probably because of the development of a human anti-mouse reaction.15 Phase 1 studies of chimeric anti–IL-6 in patients refractory to second-line therapy showed effective blocking of IL-6 signals, but again no clinical response by standard criteria.16 A possible reason for the lack of clinical response is the inability to neutralize serum IL-6 completely, as therapy appears most effective in patients with low serum IL-6 levels.17 Serum IL-6 receptor (IL-6R) levels are increased to a much lesser extent than IL-6 levels in patients with myeloma,18 and immunotherapeutic strategies are currently focused on the receptor for IL-6 because it is more effective to target the receptor than the IL-6 molecule.19
The IL-6 receptor is a complex of 2 molecules, CD126 and gp130 (CD130).20 The alpha-chain CD126 is a glycoprotein that contains the ligand-binding site.21 Although this molecule is sufficient for low-affinity binding, high-affinity binding and signal transduction require the presence of the CD130 molecule.20 CD130 is also the common signal transduction unit for IL-11, oncostatin M, ciliary neurotropic factor, and leukemia inhibitory factor.22 The soluble form of CD126 that is generated by both normal and myeloma cells23 can bind IL-6 and mediate signal transduction through membrane-bound CD130.20 Thus, successful anti-CD126 immunotherapy must inhibit the binding of IL-6 to both soluble and membrane CD126. A number of studies have assessed the levels of serum CD126 in myeloma and monoclonal gammopathy of undetermined significance (MGUS).24 25 However, the use of therapeutic strategies in the clinical environment also demands a full understanding of the pattern of expression of the membrane IL-6R.
Although receptor transcripts can be detected in the majority of myeloma plasma cells,26 early flow cytometric studies were unable to detect surface expression.27 However, recent studies using more sensitive phycoerythrin (PE)-conjugated antibodies, which are unaffected by endogenous IL-6, have been able to demonstrate CD126 and CD130 expression by plasma cells from most patients with myeloma and MGUS.28
The aim of this study was to determine whether it is possible to identify differences in IL-6R expression between normal and neoplastic plasma cells that might explain their differential responses to IL-6. A central part of this approach was to compare expression between normal and neoplastic plasma cells from individual patients with MGUS, as well as to assess the differential expression in precursor and cycling plasma cell fractions.
Patients, materials, and methods
Patients
Bone marrow aspirate specimens were analyzed from 93 patients with multiple myeloma at diagnosis (median age, 66 years; range, 45-92 years), 54 patients with MGUS (median age, 70 years; range, 45-90 years), 12 patients with solitary plasmacytoma (SP) (median age, 67 years; range, 58-89 years), and 11 normal controls (3 allogeneic donors and 8 patients undergoing cardiothoracic surgery with no history of hematologic malignancy). Approval for these studies was obtained from the institutional review board. Informed consent was provided according to the Declaration of Helsinki.
Sample preparation and fluorescence-activated cell sorter (FACS) analysis
Leukocytes were prepared by incubation with a 10-fold excess of ammonium chloride (8.6 g/L in distilled H2O) for 5 minutes and washed twice in FACSFlow (BD Biosciences, Oxford, United Kingdom) containing 0.3% bovine serum albumin (BSA; Sigma-Aldrich, Dorset, United Kingdom). A total of 1 × 106 leukocytes were stained with 10-μL volumes of each pretitered antibody per test for 20 minutes at 4°C, washed twice, and acquired using a BD Biosciences FACSort with CELLQuest v3.1 software. At least 50 000 cells were analyzed in each test. In all cases, a minimum of 500 plasma cells were analyzed.
Plasma cells were identified using a sequential gating strategy, shown in Figure 1. An initial region (R1) was set around cells expressing a high level of CD38 and CD138, and then a second region (R2) was set on the light scatter of gated CD38+CD138+ cells. A third region (R3) was set around the cells satisfying both R1 and R2 for CD38 and CD45 expression. The final plasma cell gate used was a combination of R2 and R3 (CD38, CD45, and scatter characteristics). CD138 was not used for the final plasma cell gate because the level of expression is not sufficient to separate plasma cells from other leukocytes, particularly in the peripheral blood.29
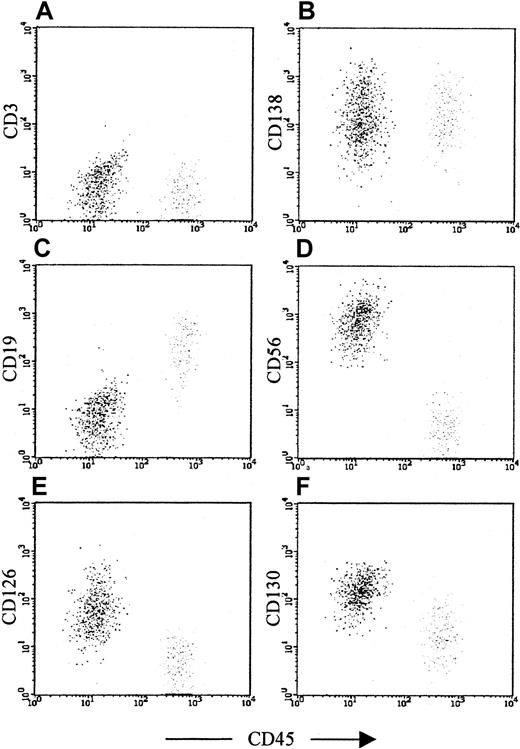
Sequential gating strategy for identification of plasma cells.
The gating strategy is demonstrated in a patient with MGUS, with approximately even numbers of normal and myeloma plasma cells. (A) Plot showing all cells, with CD138 (Syndecan-1, B-B4) expression against CD38 expression. High CD138 and CD38 expression (region 1) usually provides the best separation of bone marrow plasma cells from other leukocytes, but also contains a significant proportion of cells binding antibodies nonspecifically (the diagonal line of dots). Contaminating cells can be removed in most cases using a region set on forward- and side-scatter characteristics. (B) Scatter characteristics of cells identified in region 1 (R1). Plasma cell scatter characteristics vary from patient to patient, in keeping with their morphologic heterogeneity. However, by excluding cells with very low or very high forward- and light-scatter characteristics (apoptotic cells or debris and doublets or electronic noise, respectively), it is possible in most cases to exclude the majority of contaminating events. (C) Plot showing all cells, with CD38 against CD45 expression. The neoplastic plasma cells are clearly visible as a distinct population in the upper left area of the plot; however, the normal plasma cells in the upper right are not a discrete population and overlap with other leukocytes as well as nonspecific binding events. The contaminating leukocyte population is, in this case, normal B-cell progenitor cells, which are often more numerous in MGUS patients than plasma cells. Activated T cells expressing the same level of CD38 as plasma cells are also common in myeloma. (D) The same plot as (C), but the only cells displayed are those satisfying the scatter region (R2), indicating that 2 clear populations are present with no obvious contamination. The rationale for using CD38 versus CD45, as opposed to CD138 versus CD38, as the primary phenotypic gate has been reported previously,29but is, briefly, because of the following: (1) an immunophenotypic gate set on dual positivity is more likely to contain contamination than a gate set on the basis of one positive and one negative marker because a cell that binds one antibody nonspecifically also binds others, and hence is more likely to contaminate a dual-positive region; and (2) CD138 is expressed at a significantly lower level by circulating plasma cells than by paired marrow counterparts, whereas CD38/CD45 expression does not vary and therefore provides a more consistent approach for all samples. Internal controls to ensure that plasma cells have been accurately identified are shown in Figure 2.
Sequential gating strategy for identification of plasma cells.
The gating strategy is demonstrated in a patient with MGUS, with approximately even numbers of normal and myeloma plasma cells. (A) Plot showing all cells, with CD138 (Syndecan-1, B-B4) expression against CD38 expression. High CD138 and CD38 expression (region 1) usually provides the best separation of bone marrow plasma cells from other leukocytes, but also contains a significant proportion of cells binding antibodies nonspecifically (the diagonal line of dots). Contaminating cells can be removed in most cases using a region set on forward- and side-scatter characteristics. (B) Scatter characteristics of cells identified in region 1 (R1). Plasma cell scatter characteristics vary from patient to patient, in keeping with their morphologic heterogeneity. However, by excluding cells with very low or very high forward- and light-scatter characteristics (apoptotic cells or debris and doublets or electronic noise, respectively), it is possible in most cases to exclude the majority of contaminating events. (C) Plot showing all cells, with CD38 against CD45 expression. The neoplastic plasma cells are clearly visible as a distinct population in the upper left area of the plot; however, the normal plasma cells in the upper right are not a discrete population and overlap with other leukocytes as well as nonspecific binding events. The contaminating leukocyte population is, in this case, normal B-cell progenitor cells, which are often more numerous in MGUS patients than plasma cells. Activated T cells expressing the same level of CD38 as plasma cells are also common in myeloma. (D) The same plot as (C), but the only cells displayed are those satisfying the scatter region (R2), indicating that 2 clear populations are present with no obvious contamination. The rationale for using CD38 versus CD45, as opposed to CD138 versus CD38, as the primary phenotypic gate has been reported previously,29but is, briefly, because of the following: (1) an immunophenotypic gate set on dual positivity is more likely to contain contamination than a gate set on the basis of one positive and one negative marker because a cell that binds one antibody nonspecifically also binds others, and hence is more likely to contaminate a dual-positive region; and (2) CD138 is expressed at a significantly lower level by circulating plasma cells than by paired marrow counterparts, whereas CD38/CD45 expression does not vary and therefore provides a more consistent approach for all samples. Internal controls to ensure that plasma cells have been accurately identified are shown in Figure 2.
The expression of CD138 (positive control) and CD3 (negative control) was assessed to ensure that the gated cells were plasma cells with minimal contamination. The expression of CD19-PE and CD56-PE was then used to distinguish between normal and neoplastic plasma cells. The former are consistently CD19+CD56−, whereas the latter are CD19− or CD19+CD56+.29-31 This is demonstrated in Figure 2, which shows representative plots from an MGUS patient with both normal and neoplastic plasma cells.
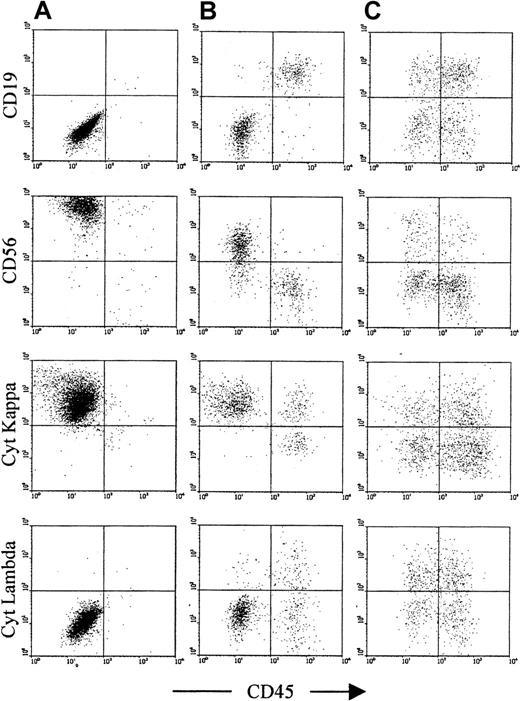
Assessment of antigen expression by normal and neoplastic plasma cells.
The plots show cells that satisfy both R2 and R3 from Figure 1 and demonstrate the controls used to determine that the gated cells are plasma cells. In addition, they show the antibodies used to discriminate normal (right side of each plot) from neoplastic plasma cells (left side of each plot) and to assess the expression of CD126 and CD130. In this patient with MGUS, the neoplastic plasma cells and the normal plasma cells have distinct CD45 expression. This is the case in approximately half of patients with MGUS. However, in all cases, the CD19 and CD56 expression is sufficient to discriminate normal from neoplastic cells.30 31 (A) and (B) show the negative and positive controls, respectively; both normal and neoplastic cells are CD3− and CD138+. The former identifies whether contaminating T cells or nonspecific binding events have been included in the plasma cell gate. The latter determines whether any CD138− nonplasma cells have been included in the plasma cell gate, particularly B-cell progenitors, which are CD38++CD19+CD56− (as normal plasma cells), but lack CD138 expression. (C) and (D) show CD19 and CD56, respectively. Normal plasma cells are consistently CD19+CD56− and appear on the right side of the plots, as they generally show higher CD45 expression than neoplastic cells. Myeloma plasma cells are CD19− with variable CD56 expression in approximately 95% of patients, and are CD19+CD56++ in a further 4% of patients, and can thus be discriminated from normal plasma cells in more than 99% of patients. In MGUS, multiple populations (ie, CD19+CD56+, CD19−CD56−, and CD19−CD56+) are often detected, although in this case a single neoplastic population (CD19−CD56+) is present. (E) and (F) show the CD126 and CD130 (IL-6 receptor alpha-chain and gp130, respectively) expression on the gated plasma cells, demonstrating that expression is restricted to the phenotypically neoplastic population.
Assessment of antigen expression by normal and neoplastic plasma cells.
The plots show cells that satisfy both R2 and R3 from Figure 1 and demonstrate the controls used to determine that the gated cells are plasma cells. In addition, they show the antibodies used to discriminate normal (right side of each plot) from neoplastic plasma cells (left side of each plot) and to assess the expression of CD126 and CD130. In this patient with MGUS, the neoplastic plasma cells and the normal plasma cells have distinct CD45 expression. This is the case in approximately half of patients with MGUS. However, in all cases, the CD19 and CD56 expression is sufficient to discriminate normal from neoplastic cells.30 31 (A) and (B) show the negative and positive controls, respectively; both normal and neoplastic cells are CD3− and CD138+. The former identifies whether contaminating T cells or nonspecific binding events have been included in the plasma cell gate. The latter determines whether any CD138− nonplasma cells have been included in the plasma cell gate, particularly B-cell progenitors, which are CD38++CD19+CD56− (as normal plasma cells), but lack CD138 expression. (C) and (D) show CD19 and CD56, respectively. Normal plasma cells are consistently CD19+CD56− and appear on the right side of the plots, as they generally show higher CD45 expression than neoplastic cells. Myeloma plasma cells are CD19− with variable CD56 expression in approximately 95% of patients, and are CD19+CD56++ in a further 4% of patients, and can thus be discriminated from normal plasma cells in more than 99% of patients. In MGUS, multiple populations (ie, CD19+CD56+, CD19−CD56−, and CD19−CD56+) are often detected, although in this case a single neoplastic population (CD19−CD56+) is present. (E) and (F) show the CD126 and CD130 (IL-6 receptor alpha-chain and gp130, respectively) expression on the gated plasma cells, demonstrating that expression is restricted to the phenotypically neoplastic population.
For analysis of cytoplasmic light-chain expression, leukocytes were incubated with antibodies to surface antigens as described above. The cells were then washed twice with FACSFlow containing 0.3% BSA, incubated with 100 μL of FACSLyse (BD Biosciences) for 10 minutes, and washed twice with FACSFlow containing 5% BSA. For permeabilization, the cells were incubated with 100 μL of FACSFlow containing 5% BSA and 0.05% NP-40 (Sigma-Aldrich) for 5 minutes and washed twice with FACSFlow containing 5% BSA. The cells were then incubated with 10 μL antibodies to intracellular antigens for 20 minutes, washed twice with FACSFlow containing 0.3% BSA, and acquired and analyzed as above. Representative plots are shown in Figure3.
Confirmation of light-chain restriction in samples with an excess of phenotypically neoplastic plasma cells.
This figure shows representative plots from 3 patients. (A) A patient with myeloma at presentation, with only neoplastic phenotype (CD19−CD56+) plasma cells detectable. (B) A MGUS patient with more than 80% of CD45− plasma cells having a neoplastic phenotype (CD19−CD56+). (C) A MGUS patient with a small population of phenotypically neoplastic plasma cells (in this case, approximately 20% of total plasma cells). For both patients (A) and (B), there is clear cytoplasmic light-chain restriction in the CD45− plasma cell population. For patient B, the CD45+ plasma cell population is polyclonal. For patient (C), the neoplastic clone is at a low level, and both CD45+ and CD45− are polyclonal, although there is a slight lambda preponderance within the CD45−population. MGUS patients were considered suitable for comparison of neoplastic and normal plasma cell CD126 expression if their phenotypic profile was similar to that of patient (B), whereas MGUS patients with a phenotypic profile similar to that of patient (C) were excluded from further analysis.
Confirmation of light-chain restriction in samples with an excess of phenotypically neoplastic plasma cells.
This figure shows representative plots from 3 patients. (A) A patient with myeloma at presentation, with only neoplastic phenotype (CD19−CD56+) plasma cells detectable. (B) A MGUS patient with more than 80% of CD45− plasma cells having a neoplastic phenotype (CD19−CD56+). (C) A MGUS patient with a small population of phenotypically neoplastic plasma cells (in this case, approximately 20% of total plasma cells). For both patients (A) and (B), there is clear cytoplasmic light-chain restriction in the CD45− plasma cell population. For patient B, the CD45+ plasma cell population is polyclonal. For patient (C), the neoplastic clone is at a low level, and both CD45+ and CD45− are polyclonal, although there is a slight lambda preponderance within the CD45−population. MGUS patients were considered suitable for comparison of neoplastic and normal plasma cell CD126 expression if their phenotypic profile was similar to that of patient (B), whereas MGUS patients with a phenotypic profile similar to that of patient (C) were excluded from further analysis.
For 4-color analysis of VLA-5 and Ki-67 expression, the same gating strategy and antibodies were used, but CD38-APC and CD45-PE/Cy5 were used to identify plasma cells. Assessment of VLA-5 expression was performed using fluorescein isothiocyanate (FITC)-conjugated anti–VLA-5, with no other changes to the methodology. For assessment of nuclear Ki-67 expression, we labeled the cells with PE, PE/Cy5, and APC conjugates as above and then incubated them overnight in 1% paraformaldehyde/FACSFlow (Sigma-Aldrich). The cells were then washed twice and incubated with 0.5% Triton X-100/FACSFlow (Sigma-Aldrich/BD Biosciences) for 10 minutes, washed once, and incubated with Ki-67–FITC, then washed twice and analyzed as described above.
Because there was significant overlap between negative control and test histograms for anti-CD126 fluorescence, standard methods of analysis (ie, percentage positive and mean fluorescence intensity) were considered unsuitable. Therefore, Kolmogorov-Smirnov (K-S) analysis was used to assess the level of expression. CD126 and CD130 expression on plasma cells (gated as described above) was compared with control expression by K-S analysis with CELLQuest software. Briefly, the cumulative test and control distributions were compared, and the largest difference (D) between the 2 was calculated. This method has a number of advantages over other analyses (see Discussion) and is the standard method of comparing the difference between 2 overlapping histograms. To determine the reproducibility of the assay, we analyzed cell lines K620, U266b, JJN3, and JIM-1 in triplicate and produced 9 D values for each antigen on each cell line. The coefficient of variation for these values was consistently less than 5%.
Cell culture
Leukocytes were prepared from freshly aspirated (within 24 hours) bone marrow cells by density gradient centrifugation (Lymphoprep; Nycomed Amersham, Bucks, United Kingdom). Plasma cells were then magnetically purified with CD138 microbeads (Miltenyi Biotec, Surrey, United Kingdom). A total of 5 × 104 cells were incubated for 24 hours in 200 μL RPMI 1640 containing 5% fetal calf serum; 10 μg/mL dexamethasone; 0.1 μg/mL IL-6 (Sigma-Aldrich); and humanized anti–IL-6R (MRA, derived fron anti-CD126 clone PM-1, kindly provided by Chugai Pharma Europe, London, United Kingdom) at a concentration of 100, 10, 1, or 0.1 μg/mL. A control well, lacking both MRA and IL-6, was also prepared. All tests were performed in triplicate. After incubation, the cells were washed and incubated with 10 μL each CD38-PE/Cy5, 7-AAD (Sigma-Aldrich 1:20), and CD45-FITC. The cells were washed again, and 10 μL of 1:100 FITC CaliBRITE beads (BD Biosciences) was added to each well. The cells were acquired as described above. Plasma cells were identified by CD38 versus CD45 expression, and the proportion of viable (7-AAD−) plasma cells to beads was calculated for each well. Test values (mean ± SE) were then reported as a percentage of the mean of the dexamethasone-only control.
Antibodies
CD3 (OKT3)-PE, CD19 (FMC7)-PE, CD38 (OKT8)-PE/Cy5/APC, and CD45 (4B2)-FITC/PE/Cy5 were prepared from hybridoma supernatant and conjugated and titered in-house. MRA (humanized anti-CD126) was conjugated and titered in-house. Other antibodies, which were used according to the manufacturers' recommendations, were VLA-5–FITC (SAM-1; Serotec), CD138-PE (B-B4; Serotec), CD56-PE (MY-31; Becton Dickinson), Ki-67–FITC (MIB-1; Coulter), CD126-PE (M91; Immunotech), and CD130-PE (AM64; Pharmingen).
Results
Myeloma plasma cells have significantly higher levels of CD126 expression than normal plasma cells, which express negligible levels of CD126
Bone marrow plasma cells from patients with myeloma at presentation (n = 93) and from normal controls (n = 11) were assessed for expression of CD126. For normal plasma cells, CD126 fluorescence intensity was virtually identical to the control (median D = 0; range, 0-0.2). CD126 expression was significantly increased in myeloma patients (median D = 0.45; range, 0-0.99; Mann-WhitneyU test, P < .0001) (Figure4).
Neoplastic plasma cells express significantly higher levels of CD126 and CD130 than normal plasma cells.
Box and whisker plots showing the minimum; 25th, 50th, 75th percentiles; and maximum expression of (A) CD126 (IL-6 receptor alpha-chain) and (B) CD130 (IL-6 receptor beta-chain) by normal and neoplastic plasma cells. Values shown are the K-S D values for the separation of test (either CD126- or CD130-PE fluorescence) and control (CD3-PE fluorescence) cumulative frequencies. P values shown are for the difference in expression levels of the patient group and normal plasma cells in both cases (Mann-Whitney Utest).
Neoplastic plasma cells express significantly higher levels of CD126 and CD130 than normal plasma cells.
Box and whisker plots showing the minimum; 25th, 50th, 75th percentiles; and maximum expression of (A) CD126 (IL-6 receptor alpha-chain) and (B) CD130 (IL-6 receptor beta-chain) by normal and neoplastic plasma cells. Values shown are the K-S D values for the separation of test (either CD126- or CD130-PE fluorescence) and control (CD3-PE fluorescence) cumulative frequencies. P values shown are for the difference in expression levels of the patient group and normal plasma cells in both cases (Mann-Whitney Utest).
In both myeloma patients and normal individuals, the level of CD126 expression in the group as a whole showed a normal distribution (Anderson-Darling, P < .01). To determine a reference for the level of CD126 expression, we assessed the K-S D value for the U266b cell line. This cell line has been shown to express approximately 27 000 binding sites.20 Of the myeloma patients analyzed, 10% showed expression above this level, 13% expressed CD126 at control levels, and the remainder had an intermediate level of expression.
To determine whether the different levels of surface CD126 result in functional differences, we compared the ability of exogenous IL-6 to rescue CD126+ and CD126− plasma cells from dexamethasone-induced apoptosis. For CD126+ plasma cells, IL-6 was able to inhibit apoptosis. Figure5 demonstrates the dose-dependent increases in apoptosis for CD126+ plasma cells cultured with MRA in the presence of dexamethasone and IL-6. However, MRA was ineffective against plasma cells that lacked CD126 expression, and the proportion of viable plasma cells after culture was not significantly different from control.
IL-6 “rescue” from dexamethasone-induced apoptosis: dose-dependent inhibition in CD126+ cases with MRA
. The graph shows the number of viable plasma cells remaining after culture with dexamethasone, IL-6, and varying concentrations of MRA (humanized anti-CD126) for a CD126+ and a CD126− patient. Plasma cell numbers are expressed as a proportion of the control (dexamethasone only).
IL-6 “rescue” from dexamethasone-induced apoptosis: dose-dependent inhibition in CD126+ cases with MRA
. The graph shows the number of viable plasma cells remaining after culture with dexamethasone, IL-6, and varying concentrations of MRA (humanized anti-CD126) for a CD126+ and a CD126− patient. Plasma cell numbers are expressed as a proportion of the control (dexamethasone only).
VLA-5− plasma cells express the highest levels of CD126, but all stages of differentiation show overexpression in comparison with normal plasma cells
Previous studies have shown that lack of expression of VLA-5 identifies proliferative immature myeloma cells that are IL-6 responsive.6 We therefore assessed the level of CD126 expression in both the immature VLA-5− fraction and the cycling fraction, identified by Ki-67 expression. This analysis was performed in 10 patients with myeloma at presentation. The majority of plasma cells lacked VLA-5 expression (median 88%; range, 44% to 98%). Coexpression studies indicated that the Ki-67+ cells were restricted to the VLA-5− fraction, with more than 95% of the Ki-67+ plasma cells lacking VLA-5 expression in all cases. The Ki-67+ fraction represented a median of 4% (range, 0%-39%) of plasma cells, which is consistent with previous studies.32
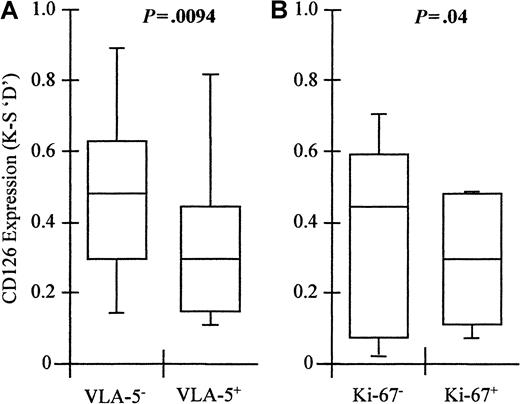
The VLA-5− “immature” fraction of myeloma plasma cells expressed significantly higher levels of CD126 (median D = 0.52; range, 0.13-0.94) than VLA-5+ “mature” cells (median D = 0.33; range, 0.12-0.87; n = 10; Wilcoxon matched-pairs signed ranks, P = .0094) (Figure6). However, the level of expression in the VLA-5+ “mature” fraction was still significantly increased over normal levels (Mann-Whitney U test,P = .016). Ki-67+ plasma cells had significantly lower levels of CD126 on their surface (median D = 0.35; range, 0.06-0.55) than those in G0 (median D = 0.46; range, 0.02-0.69; n = 10; Wilcoxon matched-pairs signed ranks, P = .02) (Figure 6). Thus, the level of C126 expression is independent of the proportion of plasma cells in cycle. The highest levels of CD126 expression are found in the “immature” fraction, but the “mature” fraction still expresses higher levels than normal plasma cells. Therefore, the differences in CD126 expression observed between normal and neoplastic cells do not reflect differences in stage of differentiation or cell cycle status.
VLA-5− myeloma plasma cells express significantly higher levels of CD126 than VLA-5+ plasma cells.
However, cycling (Ki-67+) plasma cells are VLA-5− but express lower levels of CD126 than noncycling plasma cells. Box and whisker plots showing the minimum; 25th, 50th, and 75th percentiles; and maximum expression of CD126 (IL-6 receptor alpha-chain) by (A) VLA-5+ and VLA-5− myeloma plasma cells and (B) Ki-67− and Ki-67+ myeloma plasma cells. Values shown are the K-S D values for the separation of test (CD126-PE fluorescence) and control (CD3-PE fluorescence) cumulative frequencies. P values shown are for the difference in expression levels between VLA-5+ versus VLA-5−, and Ki-67+ versus Ki-67−plasma cells, respectively (Mann-Whitney U test).
VLA-5− myeloma plasma cells express significantly higher levels of CD126 than VLA-5+ plasma cells.
However, cycling (Ki-67+) plasma cells are VLA-5− but express lower levels of CD126 than noncycling plasma cells. Box and whisker plots showing the minimum; 25th, 50th, and 75th percentiles; and maximum expression of CD126 (IL-6 receptor alpha-chain) by (A) VLA-5+ and VLA-5− myeloma plasma cells and (B) Ki-67− and Ki-67+ myeloma plasma cells. Values shown are the K-S D values for the separation of test (CD126-PE fluorescence) and control (CD3-PE fluorescence) cumulative frequencies. P values shown are for the difference in expression levels between VLA-5+ versus VLA-5−, and Ki-67+ versus Ki-67−plasma cells, respectively (Mann-Whitney U test).
There is no correlation between disease stage or serum β2m level and CD126 expression
The proportion of plasma cells in cycle is one of the most powerful prognostic factors in myeloma.33 34 Because there appeared to be no correlation between Ki-67 expression and CD126 expression, we decided to compare expression levels with other prognostic factors, namely stage and β2m level. Patients with stage I or II disease showed CD126 expression (median D = 0.62; range, 0.0-0.90) similar to that of patients with stage III disease (median D = 0.46; range, 0.0-0.95; Mann-Whitney U test,P > .05). Furthermore, patients with high levels (more than 4.2 mg/L) of serum β2m did not show a higher level of CD126 expression (median D = 0.48; range, 0.03-0.90) compared with patients with low β2m levels (median D = 0.40; range, 0.0-0.69; Mann-WhitneyU test, P > .05). Thus, CD126 expression does not correlate with any major prognostic factor.
In MGUS and solitary plasmacytoma, CD126 is expressed on neoplastic, but not normal, plasma cells
We and others have previously shown that patients with MGUS and solitary plasmacytoma have 2 populations of bone marrow plasma cells, one with a normal phenotype and another with a neoplastic phenotype.29 35 We therefore used this flow cytometric approach to compare the level of expression of CD126 on normal and malignant plasma cells derived from the same patient. Plasma cells were discriminated by their CD45 expression; they were considered normal if more than 80% of the cells were CD19+CD56−, and neoplastic if more than 80% of the cells were CD19−or CD19+CD56+. Only cases with more than 100 events in each population were included. The gating strategy is demonstrated in Figures 1 and 2. Figure 3 shows representative plots of cytoplasmic light-chain restriction and immunophenotype in patients with myeloma and MGUS.
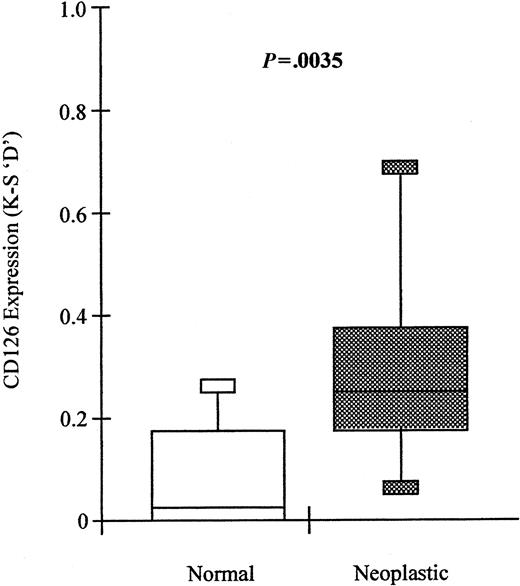
In 19 of 39 cases, it was possible to identify these 2 populations and to directly compare the level of CD126 expression on normal and neoplastic cells from the same individual. Plasma cells with a neoplastic phenotype expressed significantly higher levels of CD126 (median D = 0.25; range, 0-0.69) than their normal counterparts (median D = 0.02; range, 0-0.28; Wilcoxon matched-pairs signed ranks,P = .0035) (Figure 7). Furthermore, expression of CD126 by the normal-phenotype plasma cells in patients with MGUS or plasmacytoma was not significantly different from that of plasma cells from normal donors (Mann-Whitney Utest, P = .4031). However, expression of CD126 by the neoplastic plasma cells from MGUS was significantly lower than that of plasma cells from myeloma (Mann-Whitney U test,P = .0048).
Neoplastic plasma cells express significantly higher levels of CD126 than normal plasma cells derived from the same bone marrow in patients with MGUS.
Box and whisker plots showing the minimum; 25th, 50th, and 75th percentiles; and maximum expression of CD126 (IL-6 receptor alpha-chain) by phenotypically neoplastic and normal plasma cells identified using the sequential gating strategy shown in Figures 1 and2. Values shown are the K-S D values for the separation of test (CD126-PE fluorescence) and control (CD3-PE fluorescence) cumulative frequencies. The P value shown is for the difference in expression levels between paired phenotypically normal and neoplastic plasma cells, respectively (Wilcoxon signed-rank test).
Neoplastic plasma cells express significantly higher levels of CD126 than normal plasma cells derived from the same bone marrow in patients with MGUS.
Box and whisker plots showing the minimum; 25th, 50th, and 75th percentiles; and maximum expression of CD126 (IL-6 receptor alpha-chain) by phenotypically neoplastic and normal plasma cells identified using the sequential gating strategy shown in Figures 1 and2. Values shown are the K-S D values for the separation of test (CD126-PE fluorescence) and control (CD3-PE fluorescence) cumulative frequencies. The P value shown is for the difference in expression levels between paired phenotypically normal and neoplastic plasma cells, respectively (Wilcoxon signed-rank test).
Normal plasma cells express CD130, but neoplastic plasma cells express significantly higher levels
Bone marrow plasma cells from patients with myeloma at presentation (n = 46), with MGUS or solitary plasmacytoma (n = 26), and from normal controls (n = 8) were also assessed for expression of the CD130 molecule. The results are shown in Figure 4. In contrast to the CD126 molecule, there was clear expression of CD130 by normal plasma cells. K-S analysis indicated that the normal level of expression was a median D value of 0.68 (range, 0.58-0.79; 95% confidence upper limit = 0.71). Plasma cells from MGUS or SP patients showed significantly higher levels (median D = 0.78; range, 0.51-0.99; Mann-Whitney U test, P = .023; 77% of patients above the normal range). Plasma cells from patients with myeloma also showed a higher level of CD130 expression than both normal and MGUS plasma cells (median D = 0.92; range, 0.41-0.99; Mann-WhitneyU test, P = .0013 for normal andP = .0286 for MGUS; 83% of patients above the normal range). Although there was no correlation between CD126 and CD130 expression, CD130 was always expressed by cells with detectable levels of CD126 expression.
Discussion
IL-6 is essential for differentiation of normal B cells into plasma cells1 and has also been demonstrated to be an essential survival factor for circulating plasma cell precursors.2 CD126 is detectable on the surface of circulating precursors from patients with reactive plasmacytoses.3 However, despite having a highly sensitive assay for detection of the receptor, we can find little or no evidence of expression on normal plasma cells. This strongly suggests that the CD126 chain is down-regulated during terminal differentiation of normal B-cells. In contrast, plasma cells from more than 80% of patients with myeloma, MGUS, or plasmacytoma show IL-6R expression above control levels. These data clearly demonstrate that the normal down-regulation of CD126 does not occur in neoplastic plasma cells.
The proportion of myeloma patients expressing CD126 is consistent with previous studies that have detected CD126 mRNA in approximately 70% of patients with myeloma26 and surface receptor expression in 60% of patients.28 We have analyzed CD126 expression using the D value generated by K-S analysis, which has previously been shown to correlate directly with the number of receptor molecules determined by a radioligand-binding assay in a similar setting.36 K-S analysis is highly sensitive to small shifts in expression, and this may explain why the results of this study are more compatible with reverse transcriptase–polymerase chain reaction analysis than previous flow cytometry studies. Patients with MGUS or plasmacytoma, who have a mixture of normal and neoplastic plasma cells detectable by flow cytometry, provided an internal control population for this study. The difference in CD126 expression seen between normal and neoplastic plasma cells in MGUS patients is similar to the difference in expression seen in plasma cells from normal individuals and those from patients with myeloma.
The overexpression of CD126 in neoplastic cells therefore provides a possible mechanism for the differential responses of neoplastic and normal plasma cells to IL-6. However, it is less clear whether the major effect in vivo is proliferative or antiapoptotic. Kawano et al6,37 have demonstrated that the VLA-5−subset of plasma cells is morphologically less differentiated, shows increased tritiated-thymidine uptake in response to IL-6, and expresses cyclin D1 mRNA. In contrast, the VLA-5+ subset does not respond to IL-6 and lacks cyclin D1 expression.6 37 It is possible that the higher levels of CD126 in myeloma are the result of an increased proportion of VLA-5− cells in this patient group. This study demonstrates that the VLA-5− fraction contains virtually all the cells in cycle and that the VLA-5− fraction expresses significantly higher levels of CD126. However, the VLA-5+ cells also express CD126 at a significantly higher level than normal plasma cells. Thus, CD126 overexpression is inherent to neoplastic cells and is not due to maturation arrest at a stage of differentiation that would normally express CD126.
Ki-67+ cells have significantly lower CD126 expression than their noncycling counterparts, and in one patient with 30% Ki-67+ plasma cells, there was no detectable CD126 expression. The proliferative capacity of the myeloma cells clearly does not correlate with CD126 expression. This is not consistent with studies that showed increased proliferation of neoplastic cells in response to IL-6.5,37,38 It is possible that CD126 expression is transient and occurs only before entry into the cell cycle. However, studies suggesting a proliferative role for IL-6 are mostly based on tritiated-thymidine incorporation, in which decreased apoptosis could produce similar results to increased proliferation. The effects of IL-6 have been analyzed in vivo, and myeloma patients having infusion of IL-6 showed no significant alteration in plasma cell labeling index.39 We did not find increased CD126 expression in patients with more aggressive stage III disease, and CD126 expression did not correlate with high serum β2m levels. The expression of CD126 by virtually all neoplastic cells and the lack of correlation with cell cycle status or tumor bulk suggest that the effect of IL-6 on myeloma in vivo is antiapoptotic. This is further supported by the finding that patients with higher proportions of VLA-5− plasma cells, ie, with higher levels of CD126 expression, respond less well to chemotherapy.40
A key finding of this study is the differential expression of CD126 in phenotypically normal and neoplastic plasma cells within the same individuals with MGUS and solitary plasmacytoma. This demonstrates that the level of CD126 expression is not a function of the marrow environment, but of the neoplastic plasma cells. Several studies have suggested that the marrow microenvironment plays a key role in the pathogenesis of myeloma. Lokhorst et al41 have shown that stromal layers depleted of plasma cells from myeloma patients produce higher levels of IL-6 than those from normal donors. Furthermore, the level of IL-6 produced correlates with disease stage in myeloma patients.41 In addition, the recent but controversial findings of Rettig et al suggested that infection of stromal cells by HHV-8,42 which produces a functional homologue of IL-6,43 may be central to the pathogenesis of myeloma. The finding that up-regulation of CD126 expression is present on neoplastic but not normal plasma cells from the same marrow aspirate indicates that aberrant expression is inherent to the neoplastic clone. Thus, stromal overproduction of IL-6 may inhibit apoptosis or promote growth of the neoplastic clone, but the primary abnormality is within the plasma cells, as their normal counterparts retain CD126 expression identical to that from normal individuals.
In summary, the results suggest that a key feature of myeloma plasma cells compared with normal plasma cells is a failure to down-regulate CD126. This overexpression is unlikely to be the result of an arrest in maturation at a stage in which this receptor would normally be expressed because even the most “mature” myeloma plasma cells overexpress CD126. The overexpression is also not induced by stromal cells because neoplastic plasma cells from patients with MGUS express higher levels of CD126 than normal plasma cells from the same marrow. Increased CD126 expression is therefore inherent to the neoplastic cells, and, in combination with other studies, our results suggest that the major role of IL-6 in vivo is antiapoptotic.
The significance of these results in the context of CD126 immunotherapy is 2-fold. First, the difference in expression between normal and neoplastic plasma cells supports the use of anti-CD126 antibodies for treatment of myeloma. However, this will require measurement of surface CD126 expression levels in each patient because those who lack CD126 expression are unlikely to benefit. Further studies are required to determine the relation between membrane and soluble CD126. Second, the therapeutic effects of CD126 antagonism may be either to induce apoptosis in malignant cells or to sensitize the cells to the apoptotic effects of other chemotherapeutic agents.
Supported in part by research funding from Chugai Pharma Europe, Yorkshire Cancer Research, and the Leukaemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gareth Morgan, Department of Haematology, University of Leeds, Leeds General Infirmary, Great George St, Leeds, West Yorkshire, lS1 3EX, United Kingdom; e-mail:garethm@pathology.leeds.ac.uk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal