Abstract
Recent observations have underscored the biologic relevance of intratumoral angiogenesis and its potential impact on prognosis. Increased bone marrow angiogenesis has been demonstrated in a variety of hematologic disorders, including multiple myeloma. The extent and prognostic significance of bone marrow angiogenesis in 114 patients with myelofibrosis with myeloid metaplasia (MMM) was investigated. A control group of 44 patients without bone marrow disease, 15 patients with polycythemia vera, and 17 patients with essential thrombocythemia was also studied. Bone marrow microvessel density was assessed by a semiquantitative method, visual microvessel grading, and 2 separate quantitative methods, visual count and computerized image analysis. Angiogenesis estimation by all 3 methods was highly comparable. On visual microvessel grading, a grade 3 or 4 increase in bone marrow angiogenesis was demonstrated in 70% of patients with MMM, 33% of patients with polycythemia vera, 12% of patients with essential thrombocythemia, and 0% of normal controls. In a multivariate analysis, increased angiogenesis in MMM correlated significantly with increased spleen size and was found to be a significant and independent risk factor for overall survival. Increases in marrow angiogenesis correlated with hypercellularity and megakaryocyte clumping. In contrast, these 2 features were inversely proportional to reticulin fibrosis, whereas increases in marrow angiogenesis were independent of reticulin fibrosis. These preliminary findings suggest that neo-angiogenesis is an integral component of the bone marrow stromal reaction in MMM and may provide useful prognostic information and a rationale for the therapeutic investigation of anti-angiogenic agents.
Introduction
Angiogenesis, or the formation of new blood vessels, may be integral to solid tumor growth and metastasis.1 Quantification and analysis of the degree of intratumoral angiogenesis may provide prognostic information for patients with certain solid tumors.2-7 In hematologic malignancies, the bone marrow is a primary site of disease activity and a readily accessible tissue for the investigation of angiogenesis. Under normal conditions, the human bone marrow is supplied by a small number of blood vessels. The concentration of these vessels, or bone marrow microvessel density (MVD), has been shown to be increased in various hematologic disorders, including acute lymphoid8 or myeloid9 leukemia, myelodysplastic syndrome,10chronic myeloid leukemia,11 and plasma cell proliferative disorders.12 Furthermore, increased bone marrow angiogenesis has been correlated with unfavorable prognosis in multiple myeloma.13 14
Myelofibrosis with myeloid metaplasia (MMM) is one of the Philadelphia-negative chronic myeloid disorders and is characterized by clonal megakaryocytic hyperplasia and secondary bone marrow fibrosis.15 Clinically, patients with MMM manifest progressive anemia and hepatosplenomegaly that may be associated with substantial constitutional symptoms. Life expectancy is severely compromised, and causes of death include progressive cachexia and transformation of the disease process to acute myeloid leukemia.16 Several clinical parameters, including hemoglobin concentration, have been used to develop prognostic models that help facilitate treatment decisions.17,18 At present, treatment is primarily palliative and includes the use of androgen preparations to alleviate anemia19 and chemotherapy, surgical resection,20 or radiation therapy21to manage symptomatic splenomegaly. Most recently, both allogeneic22 and autologous23 hematopoietic stem cell transplantations have been shown to benefit some patients.
The current pathogenetic hypothesis in MMM is that clonal proliferation of megakaryocytes or monocytes (or both) is accompanied by an abnormal cytokine release that mediates a detrimental bone marrow stromal reaction.24 Implicated cytokines include transforming growth factor-beta (TGF-β), basic fibroblast growth factor (bFGF), and platelet-derived growth factor (PDGF). The resultant secondary processes include polyclonal proliferation of fibroblasts and osteoblasts, which is associated with collagen fibrosis and new bone formation, respectively.25 In addition, however, recent evidence suggests that the aberrant bone marrow microenvironment in MMM may also host excess angiogenic cytokines and that megakaryocyte expression of vascular endothelial growth factor may be increased.10,26 Similarly, previous morphometric studies have suggested increased bone marrow vascularity in MMM.27Accordingly, we performed a quantitative analysis of bone marrow MVD in a retrospective cohort of 114 patients with MMM and investigated the prognostic relevance of increased angiogenesis.
Patients and methods
Patients
After approval by the institutional review board, the study patients were identified through a search of a comprehensive database of medical diagnoses and procedures. The medical records of all patients with MMM who were seen at our institution from 1991 through 1995 and for whom bone marrow biopsies were available for analysis were reviewed. Patient selection was dictated by the availability of bone marrow sections for estimation of MVD. Therefore, the study cohort included patients whose disease was newly diagnosed and patients whose disease course was further along.
For the purposes of this study, the designation MMM was used to include patients with agnogenic myeloid metaplasia, post-polycythemic myeloid metaplasia, and post-thrombocythemic myeloid metaplasia. All study patients had bone marrow fibrosis, atypical megakaryocytic hyperplasia, and peripheral blood leukoerythroblastosis and dacryocytosis.28 Patients with the Philadelphia chromosome (or its molecular equivalent), dyserythropoiesis (myelodysplastic syndrome with myelofibrosis), or acute myelofibrosis were excluded. Clinical and laboratory information was obtained at diagnosis, at the time of bone marrow studies, and during the clinical course, including last follow-up. The bone marrow MVD estimations in patients with MMM were compared with those of normal bone marrow specimens and with specimens from patients with essential thrombocythemia or polycythemia vera.
Bone marrow: general review
Bone marrow biopsy slides were prepared from paraffin-embedded blocks. Marrows were stained with hematoxylin and eosin in standard fashion. All marrows were reviewed by one of the authors (C.A.H.) to ensure the accuracy of the patient's MMM diagnosis. In a uniform fashion, each marrow was evaluated for cellularity. In addition, the marrows were subsequently graded for the presence of reticulin fibrosis (1-4), osteosclerosis (0-2), and presence and degree of megakaryocyte clumping (1-3). Increases in MVD were estimated from gross inspection of these stained specimens.
Use of CD34 as the endothelial antigen of choice
During the initial stages of the current project, we evaluated the staining performances of commercial antibodies to formalin-resistant endothelial cell antigens, including CD31, CD34, and factor VIII, in 15 patients with MMM. The results demonstrated substantial cross-reactivity of both CD31 and factor VIII with the increased megakaryocyte pool that was expected in this disease. In addition, CD31 staining was comparatively weak and was also present in a broader group of myeloid precursors, compared with CD34 staining. Although the antibody to CD34 stained myeloid progenitors as well, the number of cells stained was sufficiently small as not to interfere with our analysis. Regardless, we were careful in documenting vessel specificity of the CD34-stained stroma that was considered for analysis. In general, CD34 has been found to be a useful antigen for assessing intratumor angiogenesis in various solid tumors.29,30 In one instance, staining for CD34 was directly compared with that for both CD31 and the von Willebrand factor.31 As in our initial observations, staining intensity for CD31 was found to be inferior to that of the other 2 antigens. Similar findings were also reported in a more recent study of the myelodysplastic syndrome in which both CD31 and CD34 were used to target bone marrow vessels.10
Bone marrow microvessel staining
Bone marrow biopsy slides were prepared from paraffin-embedded blocks. Bone marrow microvessels were visualized by immunohistochemical staining for CD34 with the use of a labeled streptavidin-biotin peroxidase method. After deparaffinization, slides were steam pretreated in 0.01 mol/L EDTA buffer, pH 8, in a Black and Decker Handy Steamer Plus (Black and Decker, Towson, MD) for 30 minutes. After rinsing in cool water, slides were immunostained by a Ventana ES automated stainer (Ventana Systems, Tucson, AZ) using buffers and detection reagents supplied by the manufacturer. The primary antibody (Clone HPCA-1; Becton Dickinson, San Jose, CA) was incubated with tissue sections for 24 minutes in a 1:50 dilution. The AEC (aminoethyl carbazole) detection kit (Ventana Systems) was used for antigen visualization; sections were counterstained with a light hematoxylin, and a coverslip of Kaiseri's glycerol jelly (Mayo Medical Laboratories, Rochester, MN) was applied.
Measurement of microvessel density
Three separate methods were used to estimate MVD. In the first method, visual microvessel grading, the study slides were visually scanned at 100×, 200×, and 400× magnification and semiquantitatively graded for the extent of CD34 staining (Figure1). To ensure the accuracy of the grading method, each stained sample was reviewed by 2 of the authors, in a blinded fashion. Morphologic analysis was performed carefully to ensure vessel specificity of the CD 34-stained stroma considered for analysis. The second method, visual count, involved actual counting of microvessels according to previously described methods.2In performing this visual count, each of the study slides was first scanned at 100× magnification, and 3 areas with abundant microvessels were chosen and defined as “hot spots.” The number of microvessels in each of these hot spots was then determined at 400× magnification. The final MVD number (microvessels per high-power [400×] field) was assigned by taking the average of the 3 separate visual counts. During the counting process, large vessels and vessels in the periosteum or bone and open sinusoids were excluded. Areas of staining with no discrete breaks were counted as single vessels, and the presence of a lumen was not required.
Visual microvessel grades of bone marrow angiogenesis in patients with myelofibrosis with myeloid metaplasia.
MVG 1, normal or slightly increased MVD; MVG 2, microvessels are easy to find and are definitely increased from normal; MVG 3, abundant microvessels; MVG 4, markedly increased MVD.
Visual microvessel grades of bone marrow angiogenesis in patients with myelofibrosis with myeloid metaplasia.
MVG 1, normal or slightly increased MVD; MVG 2, microvessels are easy to find and are definitely increased from normal; MVG 3, abundant microvessels; MVG 4, markedly increased MVD.
In the third and final method, bone marrow MVD was estimated by using computerized image analysis.32 The 3 hot spots used for the visual count were quantified by computer-based image analysis. A PC-compatible computer running the image analysis software (Optimas 6.0 for Windows 95; Optimas, Seattle, WA) was used for analysis of digitally captured images. With computerized pixel counting, microvessel surface area was determined and expressed as the percentage of a bone marrow hot spot occupied by CD34 staining. An optimized microvessel surface area was then determined by eliminating the area occupied by fat and expressing the result as a percentage of cellular area occupied by CD34 staining.
Statistical analysis
Overall survival was defined as the interval from diagnosis to death or last contact. Survival from the date of bone marrow MVD study was also analyzed. An event was defined as a death from any cause, unless otherwise indicated. The MVD estimation from each of the aforementioned methods was separately studied for possible correlations with various clinical, histologic, or laboratory variables obtained at the time of the bone marrow study. Various univariate techniques were applied, including the Fisher exact test for categorical variables and the Kruskal-Wallis and Wilcoxon rank-sum tests for continuous variables. Results were subsequently evaluated by multivariate analysis. Cox proportional hazards regression analysis was used to assess the prognostic relevance of several clinical parameters, including degree of bone marrow angiogenesis, on survival from the time of diagnosis. All data were analyzed by using SAS software (SAS, Cary, NC).
Results
One hundred fourteen patients with MMM, 15 patients with polycythemia vera, 17 patients with essential thrombocythemia, and 44 normal controls were studied. MVD was clearly increased compared with normal controls (Table 1) (P < .01). Results from the 3 different methods of estimating angiogenesis showed a significant positive correlation. In addition, there was excellent interobserver reproducibility of the qualitative grading system, with more than 95% agreement between blinded reviewers. Specifically, there was a 1-value discrepancy (microvessel grade) in 8 patients and a 2-value discrepancy in 3. In those patients with a discrepancy, consensus was reached after re-review. With the visual microvessel grading method, approximately 70% of the patients with MMM had a substantial increase (grade 3 or 4) in bone marrow MVD compared with 33% of those with polycythemia vera and 12% of those with essential thrombocythemia (Table 1). Furthermore, none of the patients with either polycythemia vera or essential thrombocythemia displayed grade 4 bone marrow angiogenesis, whereas 32.5% of the patients with MMM did. In general, almost all the patients with MMM had some degree of increase in microvessels. Increased vascularity was usually appreciable even in the specimens stained with hematoxylin and eosin (P < .01).
Bone marrow microvessel density estimation in 114 patients with myelofibrosis with myeloid metaplasia compared with a control group of 44 patients without bone marrow disease (normal controls), 15 patients with polycythemia vera, and 17 patients with essential thrombocythemia
| MVD evaluation methods . | MMM (n = 114 pts) . | Normal controls (n = 44 pts) . | Polycythemia vera (n = 15 pts) . | Essential thrombo-cythemia (n = 17 pts) . |
|---|---|---|---|---|
| Visual MVD grade | ||||
| 1 (normal) (%) | 2 (1.8) | 34 (77.3) | 4 (26.7) | 4 (23.5) |
| 2 (%) | 32 (28) | 10 (22.7) | 6 (40) | 11 (64.7) |
| 3 (%) | 43 (37.7) | 0 (0) | 5 (33) | 2 (12) |
| 4 (%) | 37 (32.5) | 0 (0) | 0 (0) | 0 (0) |
| Visual count, median (range) (vessels/high-power field) | 44.3 (7.8-112.9) | |||
| Image analysis, median (range) (% area occupied by vessels) | 7.3 (1.2-21.2) |
| MVD evaluation methods . | MMM (n = 114 pts) . | Normal controls (n = 44 pts) . | Polycythemia vera (n = 15 pts) . | Essential thrombo-cythemia (n = 17 pts) . |
|---|---|---|---|---|
| Visual MVD grade | ||||
| 1 (normal) (%) | 2 (1.8) | 34 (77.3) | 4 (26.7) | 4 (23.5) |
| 2 (%) | 32 (28) | 10 (22.7) | 6 (40) | 11 (64.7) |
| 3 (%) | 43 (37.7) | 0 (0) | 5 (33) | 2 (12) |
| 4 (%) | 37 (32.5) | 0 (0) | 0 (0) | 0 (0) |
| Visual count, median (range) (vessels/high-power field) | 44.3 (7.8-112.9) | |||
| Image analysis, median (range) (% area occupied by vessels) | 7.3 (1.2-21.2) |
Clinical and laboratory characteristics of the 114 patients with MMM, obtained at the time of the bone marrow MVD study, are outlined in Table 2. These characteristics were investigated for possible correlations with the extent of bone marrow MVD, as measured by each of the 2 different visual methods of assessing angiogenesis (Table 3). By univariate analysis, both methods revealed a significant correlation between increased MVD and increased spleen size.
Clinical and laboratory characteristics of 114 patients with myelofibrosis with myeloid metaplasia at the time of bone marrow study for angiogenesis
| Clinical parameters . | n . | Values . |
|---|---|---|
| Age | 114 | Median, 65.9 y (range, 29.7-92 y) |
| Sex | ||
| Males | 62 | 54.4% |
| Females | 52 | 45.6% |
| Type of MMM | ||
| AMM | 86 | 75.4% |
| PPMM | 18 | 15.8% |
| PTMM | 10 | 8.8% |
| Palpable spleen size below the left costal margin (in patients with intact spleen) | 109 | Median, 7 cm (range, 0-32 cm) |
| Peripheral blood count | 114 | Median (range) |
| Hemoglobin | 10.3 g/dL (5.7-17.2) | |
| White blood cell count | 9.1 × 109/L (1.1-125.7) | |
| Platelet count | 175 × 109/L (3-1580) | |
| Circulating blasts | 0.5% (0-24.5) | |
| Red cell transfusion dependency | ||
| Yes | 38 | 33.3% |
| No | 76 | 66.7% |
| Dupriez score | ||
| 0 | 46 | 40.4% |
| 1 | 48 | 42.1% |
| 2 | 20 | 17.5% |
| Presence of ascites | ||
| Yes | 13 | 11.7% |
| No | 101 | 88.3% |
| Bone marrow cellularity | 114 | Median, 70% (range, 5-100%) |
| Reticulin fibrosis grade | 99 | |
| ≤1 | 9 | 9% |
| 2 | 25 | 25% |
| 3 | 50 | 51% |
| 4 | 15 | 15% |
| Megakaryocyte clumping | 114 | |
| ≤ + 1 | 30 | 26.3% |
| + 2 | 41 | 36.0% |
| + 3 | 43 | 37.7% |
| Osteosclerosis grading | 114 | |
| 0 (Absent) | 65 | 57% |
| 1 | 32 | 28% |
| 2 | 17 | 15% |
| Bone marrow cytogenetic studies | 85 | |
| Normal | 34 | 41.5% |
| Trisomy 8 | 10 | 11.7% |
| 13q- | 10 | 11.7% |
| 20q- | 8 | 9.5% |
| Other abnormalities | 23 | 25.6% |
| Clinical parameters . | n . | Values . |
|---|---|---|
| Age | 114 | Median, 65.9 y (range, 29.7-92 y) |
| Sex | ||
| Males | 62 | 54.4% |
| Females | 52 | 45.6% |
| Type of MMM | ||
| AMM | 86 | 75.4% |
| PPMM | 18 | 15.8% |
| PTMM | 10 | 8.8% |
| Palpable spleen size below the left costal margin (in patients with intact spleen) | 109 | Median, 7 cm (range, 0-32 cm) |
| Peripheral blood count | 114 | Median (range) |
| Hemoglobin | 10.3 g/dL (5.7-17.2) | |
| White blood cell count | 9.1 × 109/L (1.1-125.7) | |
| Platelet count | 175 × 109/L (3-1580) | |
| Circulating blasts | 0.5% (0-24.5) | |
| Red cell transfusion dependency | ||
| Yes | 38 | 33.3% |
| No | 76 | 66.7% |
| Dupriez score | ||
| 0 | 46 | 40.4% |
| 1 | 48 | 42.1% |
| 2 | 20 | 17.5% |
| Presence of ascites | ||
| Yes | 13 | 11.7% |
| No | 101 | 88.3% |
| Bone marrow cellularity | 114 | Median, 70% (range, 5-100%) |
| Reticulin fibrosis grade | 99 | |
| ≤1 | 9 | 9% |
| 2 | 25 | 25% |
| 3 | 50 | 51% |
| 4 | 15 | 15% |
| Megakaryocyte clumping | 114 | |
| ≤ + 1 | 30 | 26.3% |
| + 2 | 41 | 36.0% |
| + 3 | 43 | 37.7% |
| Osteosclerosis grading | 114 | |
| 0 (Absent) | 65 | 57% |
| 1 | 32 | 28% |
| 2 | 17 | 15% |
| Bone marrow cytogenetic studies | 85 | |
| Normal | 34 | 41.5% |
| Trisomy 8 | 10 | 11.7% |
| 13q- | 10 | 11.7% |
| 20q- | 8 | 9.5% |
| Other abnormalities | 23 | 25.6% |
AMM, agnogenic myeloid metaplasia; PPMM, postpolycythemic myeloid metaplasia; PTMM, post-thrombocythemic myeloid metaplasia.
Correlation of bone marrow microvessel study, measured in two different visual methods, with various patient characteristics in 114 patients with myelofibrosis with myeloid metaplasia
| Patient characteristics . | P . | |||||
|---|---|---|---|---|---|---|
| MVG . | VC . | CELL . | RF . | OS . | MC . | |
| Sex | .43 | .58 | .41 | .59 | .17 | .91 |
| Age | .28 | .18 | .96 | .06 | < .01 | .99 |
| Subsequent progression to splenectomy | .08 | .02 | .13 | .17 | .02 | .14 |
| Subsequent blastic transformation | .14 | .84 | .07 | .47 | .63 | .66 |
| Cytogenetic profile (normal vs abnormal) | .43 | .37 | .75 | .45 | .38 | .97 |
| Visual microvessel grading (MVG) | < .01 | .32 | .09 | < .01 | ||
| Visual microvessel counting (VC) | < .01 | .36 | .14 | .05 | ||
| Bone marrow cellularity (CELL) | .46 | .52 | .19 | < .01 | < .01 | |
| Degree of reticulin fibrosis (RF) | .97 | .06 | .19 | < .01 | .11 | |
| Osteosclerosis (OS) | .09 | .14 | < .01 | < .01 | < .01 | |
| Megakaryocyte clumping (MC) | .01 | .05 | < .01 | < .01 | < .01 | |
| Dupriez score | .91 | .29 | .47 | .12 | < .01 | .11 |
| Hemoglobin level | .16 | .23 | .05 | .02 | .06 | .05 |
| White blood cell count | .59 | .39 | .07 | .89 | .32 | .86 |
| Platelet count | .87 | .28 | .46 | .03 | .50 | .02 |
| Circulating blast percentage | .77 | .15 | < .01 | .27 | .26 | .02 |
| Need for red cell transfusion | .48 | .80 | .14 | .05 | .07 | .13 |
| Spleen size | .04 | < .01 | .59 | .03 | .41 | .07 |
| Liver size | .22 | .17 | .06 | .32 | .02 | .11 |
| History of weight loss | .78 | .21 | .05 | .26 | .31 | .1 |
| Patient characteristics . | P . | |||||
|---|---|---|---|---|---|---|
| MVG . | VC . | CELL . | RF . | OS . | MC . | |
| Sex | .43 | .58 | .41 | .59 | .17 | .91 |
| Age | .28 | .18 | .96 | .06 | < .01 | .99 |
| Subsequent progression to splenectomy | .08 | .02 | .13 | .17 | .02 | .14 |
| Subsequent blastic transformation | .14 | .84 | .07 | .47 | .63 | .66 |
| Cytogenetic profile (normal vs abnormal) | .43 | .37 | .75 | .45 | .38 | .97 |
| Visual microvessel grading (MVG) | < .01 | .32 | .09 | < .01 | ||
| Visual microvessel counting (VC) | < .01 | .36 | .14 | .05 | ||
| Bone marrow cellularity (CELL) | .46 | .52 | .19 | < .01 | < .01 | |
| Degree of reticulin fibrosis (RF) | .97 | .06 | .19 | < .01 | .11 | |
| Osteosclerosis (OS) | .09 | .14 | < .01 | < .01 | < .01 | |
| Megakaryocyte clumping (MC) | .01 | .05 | < .01 | < .01 | < .01 | |
| Dupriez score | .91 | .29 | .47 | .12 | < .01 | .11 |
| Hemoglobin level | .16 | .23 | .05 | .02 | .06 | .05 |
| White blood cell count | .59 | .39 | .07 | .89 | .32 | .86 |
| Platelet count | .87 | .28 | .46 | .03 | .50 | .02 |
| Circulating blast percentage | .77 | .15 | < .01 | .27 | .26 | .02 |
| Need for red cell transfusion | .48 | .80 | .14 | .05 | .07 | .13 |
| Spleen size | .04 | < .01 | .59 | .03 | .41 | .07 |
| Liver size | .22 | .17 | .06 | .32 | .02 | .11 |
| History of weight loss | .78 | .21 | .05 | .26 | .31 | .1 |
P values calculated by univariate analysis.
Values in boldface indicate that in a multivariate analysis the significant correlation between bone marrow microvessel density and subsequent need for splenectomy was accounted for by spleen size.
Various histologic features were quantified from the hematoxylin and eosin preparations, including cellularity, megakaryocyte clumping, reticulin fibrosis, and osteosclerosis. These features were then compared with each another, with bone marrow angiogenesis, and with clinical features (Table 3). Increases in marrow angiogenesis correlated with increases in cellularity (P = .01) and megakaryocyte clumping (P = .01) and were independent of reticulin fibrosis (P = .32) and osteosclerosis (P = .09). In contrast, MVD in the control marrows was independent of either age (P = .66) or cellularity (P = .92). In patients with MMM, increases in cellularity correlated with increases in megakaryocyte clumping (P < .01) and were inversely proportional to osteosclerosis (P < .01). Clinically, hypocellularity was associated with anemia (P = .05), circulating blasts (P < .01), hepatomegaly (P = .06), and weight loss (P = .05). Megakaryocyte clumping was correlated with hypercellularity and increased angiogenesis but was inversely proportional to osteosclerosis (P < .01), thrombocytopenia (P = .04), and weight loss (P = .05). Osteosclerosis and reticulin fibrosis were well correlated (P < .01) and were reflective of hypocellularity (P < .01), anemia (P = .02), thrombocytopenia (P = .03), higher Dupriez score (P < .01), red cell transfusion dependence (P = .05), and hepatomegaly (P = .02).
Bone marrow studies of angiogenesis were performed at a median of 2 months (range, 0.2-175 months) from the initial diagnosis of MMM. The median follow-up duration from the time of the marrow study was 31.5 months (range, 0-187 months). During this period, 29 patients (25.4%) required splenectomy and 12 patients (10.5%) experienced leukemic transformation. Increased MVD predicted subsequent splenectomy but not leukemic transformation (Table 3). However, in a multivariate analysis, the significant correlation between MVD and the subsequent need for splenectomy was accounted for by spleen size (Table 3).
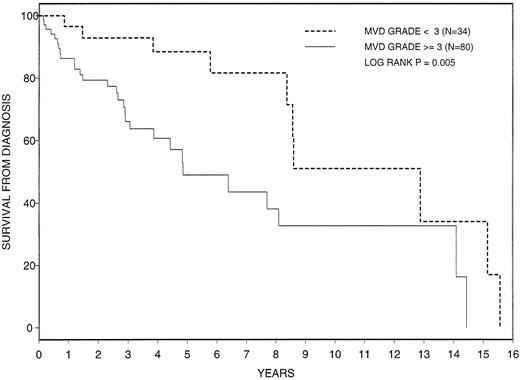
The prognostic relevance of increased angiogenesis to survival was investigated by using the MVD values obtained from microvessel visual grading (Figure 1). Median overall survival from diagnosis in all 114 patients with MMM was 101 months (range, 1.8-187.7 months). Survival was significantly shorter in patients with a grade 3 or 4 increase in angiogenesis (median survival, 155 months [range, 10.4-187.7 months] vs 58.6 months [range, 1.8-174.2 months]; P = .005) (Figure 2). Although not significant, survival from the time of the bone marrow MVD study was also shorter in patients with a grade 3 or 4 marrow angiogenesis (median survival, 69.7 months [range, 3.9-80.6 months] vs 32.1 months [range, 1-79.2 months]; P = .11) (Figure3). In addition to angiogenesis, various other disease parameters and histologic features, measured at the time of initial diagnosis, were tested for their prognostic relevance (Table4). Univariate and multivariate analyses identified increased angiogenesis, advanced age, and increased proportion of circulating blasts as risk factors for overall survival.
Cumulative survival from time of diagnosis in 114 patients with myelofibrosis with myeloid metaplasia classified by extent of bone marrow angiogenesis. MVD = microvessel density.
Cumulative survival from time of diagnosis in 114 patients with myelofibrosis with myeloid metaplasia classified by extent of bone marrow angiogenesis. MVD = microvessel density.
Cumulative survival from time of bone marrow study of angiogenesis in 114 patients with myelofibrosis with myeloid metaplasia, classified by extent of bone marrow angiogenesis. MVD = microvessel density.
Cumulative survival from time of bone marrow study of angiogenesis in 114 patients with myelofibrosis with myeloid metaplasia, classified by extent of bone marrow angiogenesis. MVD = microvessel density.
Prognostic evaluation of various clinical and laboratory parameters in 114 patients with myelofibrosis with myeloid metaplasia
| Parameter . | Effect on survival from time of diagnosis (P) . |
|---|---|
| Bone marrow angiogenesis | < .01 |
| Sex | .20 |
| Age | < .01 |
| Bone marrow cytogenetics (normal vs abnormal) | .27 |
| Bone marrow cellularity | .45 |
| Degree of reticulin fibrosis | .50 |
| Degree of osteosclerosis | .25 |
| Megakaryocyte clumping | .95 |
| Dupriez score | .88 |
| Hemoglobin level | .13 |
| White blood cell count | .61 |
| Platelet count | .84 |
| Circulating blast percentage | .02 |
| Red cell transfusion dependency | .41 |
| Spleen size | .16 |
| Liver size | .24 |
| History of weight loss | .76 |
| Parameter . | Effect on survival from time of diagnosis (P) . |
|---|---|
| Bone marrow angiogenesis | < .01 |
| Sex | .20 |
| Age | < .01 |
| Bone marrow cytogenetics (normal vs abnormal) | .27 |
| Bone marrow cellularity | .45 |
| Degree of reticulin fibrosis | .50 |
| Degree of osteosclerosis | .25 |
| Megakaryocyte clumping | .95 |
| Dupriez score | .88 |
| Hemoglobin level | .13 |
| White blood cell count | .61 |
| Platelet count | .84 |
| Circulating blast percentage | .02 |
| Red cell transfusion dependency | .41 |
| Spleen size | .16 |
| Liver size | .24 |
| History of weight loss | .76 |
Values in boldface indicate parameters that were identified by both univariate and multivariate analyses as risk factors for overall survival.
Discussion
The current study clearly demonstrated a substantial increase of marrow vascularity in most patients with MMM compared with normal controls. In addition, the extent of the abnormality was more pronounced in patients with MMM than in those with either polycythemia vera or essential thrombocythemia. Another recent report10suggests a higher degree of vascular proliferation in chronic myeloproliferative diseases than in other myeloid disorders, including myelodysplastic syndrome and acute myeloid leukemia. Therefore, substantial increased angiogenesis, along with collagen fibrosis and osteosclerosis, may be an integral component of the bone marrow stromal reaction in MMM. In regard to the method of evaluating angiogenesis in MMM, both the semiquantitative and the quantitative methods we used were relatively accurate and showed satisfactory correlation. However, because of an associated architectural disorder of the bone marrow stroma in MMM, the semiquantitative method (visual microvessel grading) may be more suitable for minimizing inaccuracies that may arise from uneven distribution of the lesions.
The observation of increased marrow vascularity in MMM is consistent with our current understanding of the pathogenesis of this disease. The proliferation of an aberrant clone, most likely of megakaryocytic or of monocytic origin (or of both), is believed to be the underlying cause for the induction of an abnormal cytokine milieu that is critical to the kinetic and synthetic stimulation of polyclonal fibroblasts (causing collagen fibrosis) and osteoblasts (causing osteosclerosis).33-36 The 2 primary cytokines that have been implicated in this pathogenetic process are TGF-β37,38 and bFGF.39 These same cytokines, however, are also potent regulators of angiogenesis.40-42 TGF-β has been shown to promote endothelial cell migration, enhance stromal cell production of vascular endothelial growth factor (VEGF), and possibly inhibit the generation of anti-angiogenic molecules.40-45 On the other hand, bFGF may either directly stimulate endothelial cell proliferation or facilitate VEGF–endothelial cell interaction through the modulation of endothelial cell integrin or VEGF-receptor expression.46-48
VEGF is the most potent endothelial cell mitogen whose mechanism of action may involve the stimulation of tyrosine kinase receptors (flt-1/KDR).49 Increased constitutive expression and secretion of VEGF has been demonstrated in human megakaryocytes, and such VEGF expression may be inducible by either a paracrine or an autocrine mechanism.50,51 Prominent megakaryocyte VEGF expression has also been shown in the chronic myeloid disorders.10 Furthermore, a recent study26 has demonstrated increased serum levels of VEGF in most patients with MMM, and the concentration of the cytokine was significantly higher in platelet-rich than in platelet-poor plasma. These observations suggest that the cytokine-mediated bone marrow stromal reaction in MMM includes angiogenesis and that clonally expanded megakaryocytes and related cells are also capable of secreting angiogenic cytokines.
An increase in either intratumoral angiogenesis or serum levels of VEGF has been associated with poor prognosis in a variety of solid malignancies.3,52-55 The possible prognostic relevance of these measurements in hematologic malignancies has so far been appreciated in acute myeloid leukemia,56,57 multiple myeloma,13,14 and non-Hodgkin lymphoma.58 In the current study, we show that the degree of increased angiogenesis in patients with MMM may have an independent prognostic value and could serve as an additional variable in clinical prognostic models. In most studies of patients with MMM, anemia, advanced age, and increased percentage of circulating blasts have been independently correlated with poor survival.15 In the current study, univariate analysis revealed that increased angiogenesis, along with advanced age and increased percentage of circulating blasts, was a highly significant risk factor for survival. Furthermore, all 3 variables maintained their prognostic relevance in a subsequent multivariate analysis. Although a correlation existed among angiogenesis, cellularity, and megakaryocyte clumping, the different relationship these features exhibit with osteosclerosis, reticulin fibrosis, and survival suggests that angiogenesis may have an independent and biologically more important role in disease progression. Neither the extent of bone marrow collagen fibrosis nor the degree of osteosclerosis has been shown to have consistent prognostic value in MMM.17,59 60 It is emphasized, however, that the currently observed prognostic value of bone marrow angiogenesis in MMM should be confirmed by well-designed prospective studies.
Increased marrow angiogenesis in MMM was also associated with marked splenomegaly and a subsequent need for splenectomy (a surrogate for symptomatic splenomegaly). Because splenomegaly in MMM is primarily a result of extramedullary hematopoiesis,61 it is possible that increased marrow vascularity either facilitates splenic extramedullary hematopoiesis or shares a common pathogenetic cytokine that is responsible for both pathologic processes. The former possibility is consistent with the view that splenic extramedullary hematopoiesis arises from the migration of bone marrow hematologic precursor cells that enter the systemic circulation through the marrow sinusoids and are filtered in the spleen, where they undergo further progenitor cell expansion.62,63 Such a process may further be enhanced by the presence of increased intravascular hematopoiesis in MMM.64 There is limited information regarding the potential for VEGF, TGF-β, or bFGF to cause extramedullary hematopoiesis. Nonetheless, the observed connection between angiogenesis and extramedullary hematopoiesis suggests that one of the expected treatment benefits from the use of an anti-angiogenic agent in MMM may be a decrease in hepatosplenomegaly.
The preliminary findings in the current study suggest that microvessel proliferation is a major component of the mixed stromal reaction in MMM and that it may be a marker of disease activity and progressive extramedullary hematopoiesis. Possible prognostic significance was demonstrated and supports the investigation of anti-angiogenic agents in the treatment of patients with MMM. Accordingly, we have initiated a phase 2 study using thalidomide, a drug with anti-angiogenic properties.65 Thalidomide has been successfully used in patients with multiple myeloma,66 and it may also have therapeutic activity in patients with chronic myeloproliferative diseases, including some with MMM.67 However, it is unknown whether the therapeutic benefit of thalidomide is related to its anti-angiogenic, immunomodulatory, or cytokine regulatory properties. Nevertheless, it is reasonable to consider the investigative use of more potent anti-angiogenic agents in patients with MMM.
Supported in part by National Cancer Institute grant CA85818. S.V.R. is a Leukemia and Lymphoma Society Translational Research Awardee.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ayalew Tefferi, Division of Hematology and Internal Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal