Abstract
The effect of traumatic lumbar puncture at the time of initial diagnostic workup on treatment outcome in children with newly diagnosed acute lymphoblastic leukemia (ALL) was investigated. The findings of the first 2 lumbar punctures performed on 546 patients with newly diagnosed ALL treated on 2 consecutive front-line studies (1984-1991) at St Jude Children's Research Hospital were retrospectively reviewed. Lumbar punctures were performed at the time of diagnosis and again for the instillation of first intrathecal chemotherapy. The event-free survival (EFS) experience for patients with 1 cerebrospinal fluid (CSF) sample contaminated with blast cells was worse than that for patients with no contaminated CSF samples (P = .026); that of patients with 2 consecutive contaminated CSF samples was particularly poor (5-year EFS = 46 ± 9%). In a Cox multiple regression analysis, the strongest prognostic indicator was 2 consecutive contaminated CSF samples, with a hazard ratio of 2.39 (95% confidence interval, 1.36-4.20). These data indicate that contamination of CSF with circulating leukemic blast cells during diagnostic lumbar puncture can adversely affect the treatment outcome of children with ALL and is an indication to intensify intrathecal therapy.
Introduction
The presence of overt central nervous system (CNS) disease at the time of diagnosis, as defined by cerebrospinal fluid (CSF) criteria or the presence of cranial nerve palsies, negatively affects the event-free survival (EFS) of children with acute lymphoblastic leukemia (ALL).1-4 The effect of a small number of leukemic blasts in the CSF at diagnosis on EFS is controversial. Investigators from the Children's Cancer Group have demonstrated that this finding is of no prognostic significance in patients with intermediate-risk ALL in the context of their systemic and CNS-directed therapy.5,6 In contrast, we and the investigators from the Pediatric Oncology Group have shown that the presence of blast cells in the CSF, even if small in number, resulted in a high risk of relapse, requiring more intensive intrathecal therapy.7 8 The literature currently contains no information to guide physicians in assigning risk classifications (hence treatment) for patients who experience a traumatic diagnostic lumbar puncture, nor does it elucidate the effect of such a procedure on EFS rates. Our working hypothesis was that the iatrogenic introduction of circulating blast cells into the subarachnoid space by a traumatic lumbar puncture (TLP) would adversely affect the EFS. In the present study, we sought to determine whether TLP at the time of diagnosis affected the treatment outcome for patients with newly diagnosed ALL.
Materials and methods
CNS status
From 1984 to 1991, 546 patients with newly diagnosed ALL were enrolled in 2 consecutive Total Therapy studies [XI (n = 358) and XII (n = 188)] at St Jude Children's Research Hospital.9 10 All patients underwent lumbar puncture at the time of diagnostic bone marrow aspiration and again on the first day of induction therapy, generally within 24 to 48 hours after diagnostic procedures when intrathecal therapy was to be instilled. In both studies, patients' CNS status was defined as CNS leukemia if the initial CSF sample contained 5 leukocytes or more per microliter and blast cells. If the initial lumbar puncture was traumatic, CNS status was based on the findings of the second puncture provided that it was atraumatic. If both procedures were traumatic, the patient was assumed to have no leukemic cells in the cerebrospinal fluid (for the purposes of treatment assignment).
For the current analysis, the CNS status of all patients was retrospectively reclassified into 1 of the following groups: CNS 1 (puncture not traumatic; < 10 red blood cells per microliter and no identifiable leukemic blast cells after cytocentrifugation); CNS 2 (puncture not traumatic; < 5 white blood cells [WBCs] per microliter with leukemic blast cells after cytocentrifugation); CNS 3 (puncture not traumatic; ≥ 5 WBCs per μL with leukemic blast cells after cytocentrifugation), TLP− (puncture traumatic [ ≥ 10 red blood cells per microliter] with no leukemic blast cells after cytocentrifugation), or TLP+ (TLP with leukemic blast cells after cytocentrifugation). The CNS status of patients for whom 2 consecutive TLPs contained leukemic blast cells was classified as TLP++.
Supportive care
All patients had a complete blood count and coagulation profile at the time of admission to the hospital. There was no consistent institutional policy regarding platelet transfusion prior to procedures. Generally, patients received platelet transfusions if there were signs of overt bleeding. Because the presence of coagulopathy at diagnosis has little if any consequence in childhood ALL, no therapeutic intervention was initiated for this finding.11
The procedures were performed by a variety of clinicians, including the attending physicians, fellows, nurse practitioners, and pediatric residents generally without sedation in this treatment era.
Treatment
The treatment regimens for both protocols have been described previously.9,10 In brief, remission induction therapy was identical for both protocols, consisting of 6 drugs (prednisone, vincristine, daunorubicin, l-asparaginase, teniposide, and cytarabine). In study XI,9 all patients received 2 high doses of methotrexate as consolidation therapy. On completion of consolidation therapy, patients were assigned to receive 1 of 3 continuation therapy regimens. Continuation treatment for patients with higher-risk disease consisted of 4 drug pairs given in either rotational or sequential schedule and in those with lower-risk disease 4 rotational drug pairs or antimetabolites with pulses of prednisone and vincristine. All patients in study XII10 received antimetabolite-based therapy with alternating pulses of high-dose methotrexate and teniposide plus cytarabine, given as 10 pulses during the first of 2.5 years of continuation treatment.
In both protocols, all patients received triple intrathecal treatment with methotrexate, hydrocortisone, and cytarabine. It was administered 3 times (days 2, 22, and 43) during remission induction therapy for all patients except those with CNS leukemia (ie, CNS 3 status), who were given 2 additional treatments on days 8 and 15. Intrathecal treatment was given every 8 weeks in study XI and every 6 weeks in study XII until 1 year after remission induction, when cranial irradiation plus 5 intrathecal treatments were given to patients with high-risk disease (18 Gy) and to those with CNS leukemia at the time of diagnosis (24 Gy). No intrathecal therapy was given after 1 year of continuation therapy. Because CNS 2 status and TLP+ were not recognized at that time as adverse features, patients with either finding were not given additional intrathecal therapy. The Institutional Review Board approved the treatment protocols, and signed informed consent was obtained from the patients, their parents, or their guardians, as appropriate.
Study design and statistical analysis
Event-free survival was measured from the date of patient enrollment to the date of the first treatment failure of any kind (relapse, second malignancy, or death) or the date of the last follow-up. Patients who did not achieve a complete response (CR) by day 43 were assigned an EFS value of zero. Duration of CNS remission was measured from the date of initial complete remission to the date of isolated or combined CNS relapse for patients who had such a relapse, or to the last follow-up date for those whose disease remained in CR. All non-CNS relapses, second malignancies, and deaths in CR were considered competing risks for developing a CNS relapse and were analyzed accordingly. Similarly, duration of hematologic remission was measured from the date of initial complete remission to the date of isolated hematological relapse for patients who had such a relapse, or to the last follow-up date for those whose disease remained in CR. All other types of relapse, second malignancies, and deaths in CR were considered competing risks for developing an isolated hematologic relapse and were analyzed accordingly.
Fisher exact test and the exact chi-square test were used to test for associations between 2 categorical variables. Distributions of EFS were estimated by the method of Kaplan and Meier,12 with standard error (SE) calculated as suggested by Peto et al.13,14 The Mantel-Haenszel statistic15 was used to compare distributions of EFS, stratified by study and by National Cancer Institute (NCI)/Rome criteria. Estimates of cumulative incidence of isolated or combined CNS relapse, as well as estimates of isolated hematological relapse, were calculated by the methods of Kalbfleisch and Prentice16 and were compared by the methods of Gray.17 All estimates of outcome are reported as ± 1 SE. All analyses of outcome were stratified by study number and by NCI/Rome risk criteria,18 which are defined as follows for patients with B-lineage ALL: standard risk (WBC at diagnosis < 50 × 109/L and age at diagnosis ≥ 1 and < 10 years) and high risk (WBC at diagnosis ≥ 50 × 109/L or age at diagnosis ≥ 10 years). For the purposes of this study, patients with T-lineage ALL and infants younger than 1 year of age were also considered high risk. The Wald statistic from a Cox proportional hazards regression model19 was used to evaluate the significance of having 2 consecutive traumatic taps with blasts with respect to EFS while controlling for other known adverse risk features. All P values reported are two-sided, and all analyses were conducted with the use of SAS release 6.12 or StatXact version 4.0.
Results
The number of patients reclassified into each of the CNS status groups was as follows: CNS 1 (n = 336), CNS 2 (n = 80), CNS 3 (n = 16), TLP− (n = 54), and TLP+(n = 60). With the possible exception of 1 patient, there was no indication of preexisting CNS or subarachnoid hemorrhage in any of the patients, as evidenced by the gross description or microscopic examination of the cerebrospinal fluid and the procedure note. Although a diagnostic traumatic tap, with or without blast cells, was not associated either with age at the time of diagnosis (P = .96) or with presenting WBC count (P = .12), a diagnostic TLP with blast cells (TLP+) was associated with a higher presenting WBC count (P < .001; Table 1).
Frequency of diagnostic traumatic lumbar puncture positive for blasts according to patients' age and white blood cell count at the time of diagnosis
| Factor . | Category . | No. of patients (%) . | P value* . | P value† . | ||
|---|---|---|---|---|---|---|
| Nontraumatic . | TLP− . | TLP+ . | ||||
| Age (y) | < 1 | 15 (79) | 0 (0) | 4 (21) | .30 | .96 |
| ≥ 1 to < 10 | 306 (80) | 36 (9) | 43 (11) | |||
| ≥ 10 | 111 (78) | 18 (13) | 13 (9) | |||
| WBC (× 109/L) | ≤ 50 | 329 (80) | 48 (12) | 32 (8) | < .001 | .12 |
| > 50 to ≤ 100 | 39 (83) | 3 (6) | 5 (11) | |||
| > 100 | 64 (71) | 3 (3) | 23 (26) | |||
| Factor . | Category . | No. of patients (%) . | P value* . | P value† . | ||
|---|---|---|---|---|---|---|
| Nontraumatic . | TLP− . | TLP+ . | ||||
| Age (y) | < 1 | 15 (79) | 0 (0) | 4 (21) | .30 | .96 |
| ≥ 1 to < 10 | 306 (80) | 36 (9) | 43 (11) | |||
| ≥ 10 | 111 (78) | 18 (13) | 13 (9) | |||
| WBC (× 109/L) | ≤ 50 | 329 (80) | 48 (12) | 32 (8) | < .001 | .12 |
| > 50 to ≤ 100 | 39 (83) | 3 (6) | 5 (11) | |||
| > 100 | 64 (71) | 3 (3) | 23 (26) | |||
TLP indicates traumatic lumbar puncture; WBC, white blood cell count.
From comparison of TLP+ versus others.
From comparison of TLP+ or TLP− versus nontraumatic.
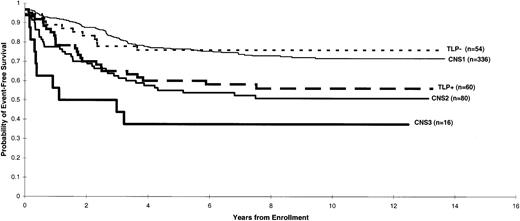
The 5-year EFS estimates (± 1 SE) for patients in each group were as follows: CNS 1 (77 ± 2%), CNS 2 (55 ± 6%), CNS 3 (38 ± 11%), TLP− (76 ± 6%), and TLP+(60 ± 6%) (Figure 1). Event-free survival estimates were almost identical between CNS 1 and TLP− groups (P = .85); thus, for subsequent analyses patients who had a TLP without blast cells were combined with patients classified as having CNS 1 status. The EFS of patients with a TLP positive for leukemic blast cells (TLP+ status) was worse than that of those with CNS 1 status even after stratifying for treatment protocol and NCI/Rome risk criteria (P = .026) and was comparable to that of patients with CNS 2 status (P = .59).
Distribution of EFS estimates according to the various CNS status categories after the initial diagnostic lumbar puncture.
Distribution of EFS estimates according to the various CNS status categories after the initial diagnostic lumbar puncture.
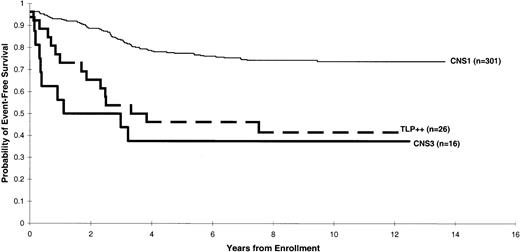
We then studied the effect of the second lumbar puncture results on the estimates of EFS. Twenty-six patients had 2 consecutive TLPs with blast cells (TLP++). Such patients fared significantly worse than those with CNS 1 status, even after stratifying for treatment protocol and NCI/Rome risk criteria (P = .001; Figure2). In fact, treatment outcome of the patients with TLP++ was comparable to that of patients with overt CNS disease (CNS 3 status) (P = .84; Figure 2). In a Cox multiple regression analysis stratified by treatment protocol, the hazard of adverse events was found to be 2.39 times more likely for patients with TLP++ than for those with CNS 1 status (95% confidence interval, 1.36-4.20), after adjustment for NCI/Rome risk criteria, DNA index, and immunophenotype (Table2).
Distribution of EFS estimates according to the following risk categories: CNS 1, CNS 3, and TLP++.
Distribution of EFS estimates according to the following risk categories: CNS 1, CNS 3, and TLP++.
Results of the Cox proportional hazards model evaluating the prognostic importance of having two consecutive positive traumatic lumbar punctures (TLP++) in patients whose final CNS classification was CNS 1 or TLP++*
| Adverse feature . | Hazard ratio . | 95% CI . | P value . |
|---|---|---|---|
| TLP++ | 2.39 | (1.36, 4.20) | .003 |
| NCI high-risk group | 2.04 | (1.29, 3.24) | .002 |
| DNA index < 1.16 or > 1.6 | 1.53 | (0.83, 2.80) | .17 |
| T-cell ALL | 1.51 | (0.85, 2.67) | .16 |
| Adverse feature . | Hazard ratio . | 95% CI . | P value . |
|---|---|---|---|
| TLP++ | 2.39 | (1.36, 4.20) | .003 |
| NCI high-risk group | 2.04 | (1.29, 3.24) | .002 |
| DNA index < 1.16 or > 1.6 | 1.53 | (0.83, 2.80) | .17 |
| T-cell ALL | 1.51 | (0.85, 2.67) | .16 |
CNS indicates central nervous system; CI, confidence interval; NCI, National Cancer Institute; ALL, acute lymphoblastic leukemia.
Stratified for treatment protocol and simultaneously adjusting for NCI/Rome criteria, DNA index, and immunophenotype.
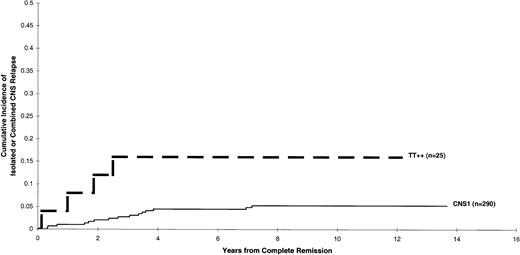
The effect of having 2 consecutive TLPs with blast cells on the cumulative incidence of developing either isolated or combined CNS relapse is depicted in Figure 3. Results indicated that the cumulative incidence for patients with TLP++ was higher than that of those with CNS 1 status (5-year estimates: 16 ± 8% and 4 ± 1%, respectively) after stratification for study number and NCI/Rome risk criteria (P = .084). Likewise, the cumulative incidence of isolated hematologic relapse was higher in patients with TLP++compared with that of those with CNS 1 status (5-year estimates: 32 ± 10% and 11 ± 2%, respectively) after stratification for study number and NCI/Rome risk criteria (P = .017).
Cumulative incidence of an isolated or combined CNS relapse for patients with CNS 1 status and TLP++status.
Cumulative incidence of an isolated or combined CNS relapse for patients with CNS 1 status and TLP++status.
Discussion
Our results show that TLPs with blast cells at the time of diagnosis negatively affect the treatment outcome of patients with newly diagnosed ALL. The adverse prognosis was largely accounted for by the subgroup of patients who had 2 consecutive TLPs with blast cells. The risk of treatment failure was 2.39-fold higher for these patients than for patients who did not have blast cells in the CSF in both procedures.
The presence of leukemic cells in the CSF at the time of diagnosis generally indicates a poor outcome. Leukemic cells in the CSF arise from the cranial arachnoid tissue.20-23 The circulating leukemic cells reach the walls of the superficial veins where they extend through the superficial arachnoid into the arachnoid, surrounding the arteries, veins, arterioles, and venules as they course into and through the brain. With increasing mass, the leukemic cells reduce the caliber of the vessels, producing cerebral hypoperfusion. Eventually, the leukemic cells can move out of the arachnoid trabeculae into the CSF, resulting in leukemic meningitis.24 An increasing number of leukemic cells in the CSF reflects either more aggressive leukemia or more advanced disease. The presence of 1 leukemic blast cell per microliter of CSF corresponds to approximately 105 leukemic cells in the entire CSF compartment. TLP at the time of diagnosis, when most patients have circulating blast cells, may be another way of introducing leukemic blasts from the systemic circulation into the CSF.25-27 As expected, in this study TLP with blast cells was associated with a higher presenting leukocyte count (hence, blast cell count).
Children's Cancer Group investigators have demonstrated that in patients with intermediate-risk ALL patients with low number of blasts in the CSF at diagnosis, treatment outcome is comparable to those children who have no blasts in their diagnostic CSF. In contrast, we7 and Lauer et al8 have previously shown that patients with CNS 3 status fared worse than those with CNS 2 status, who in turn have a poorer outcome than patients with no leukemic cells in the CSF (CNS 1 status). In this study we have demonstrated that iatrogenic introduction of leukemic cells into the CSF may also adversely affect treatment outcome.
Why did patients with 2 consecutive TLPs with blast cells have a particularly dismal outcome? First, it is conceivable that more leukemic cells were introduced into the CSF. Second, it is possible that these patients did not receive adequate early intrathecal treatment. A TLP just before instillation of intrathecal therapy may indicate that the tip of the spinal needle is not in the proper position. Not recognizing the prognostic effect of this finding, we did not give additional intrathecal therapy to patients with a TLP with blast cells; in fact, these patients received the same treatment as those with CNS 1 status and were not given the second intrathecal treatment until 3 weeks later. This relatively long delay in treatment may have allowed leukemic cells to seed and grow in the meninges. Third, some of these patients may, in fact, have had CNS 2 status or CNS 3 status that was obscured by the traumatic finding. Hence, without proper therapeutic intervention, these patients would have had an increased risk of CNS relapse, not to mention a poorer overall EFS.
How could the prognosis of patients with TLPs with blast cells be improved? Because early intensive systemic treatment can more effectively eradicate leukemic blasts and forestall the development of a drug-resistant leukemic clone, frequent intrathecal therapy early in the treatment course should improve the outcome of patients who have TLPs with blast cells. In our subsequent Total Therapy Study XIII, patients with CNS 2 status, CNS 3 status, or TLPs with blast cells were given intrathecal therapy weekly for 4 doses during both remission induction and consolidation treatment, and then every 4 weeks during the first year of continuation treatment.28 This intensified intrathecal therapy has virtually eliminated CNS relapses in Total Therapy Study XIII and boosted the overall 5-year EFS estimate to 80%.28 29
Notwithstanding this improved treatment outcome in recent studies, every attempt should be made to prevent TLP, because this occurrence adversely affects the patient's quality of life by making additional intrathecal therapy necessary. Hence, we have implemented several steps to decrease the frequency and consequence of TLP. The procedure is now routinely performed by one of our more experienced clinicians and with the patient under short-acting general anesthesia.30-32Moreover, intrathecal therapy is now given with the first diagnostic lumbar puncture performed after the diagnosis of leukemia has been established. In the event of a TLP, this approach may reduce the likelihood that contaminated leukemic cells will seed the meninges.
Acknowledgments
The authors would like to thank Flo Witte, Director of Scientific Editing, for editorial consultation, and Patsy Burnside for word processing assistance.
Supported by grants CA21765, CA20180, R37CA36401, and CA51001 from the National Cancer Institute, by a Center of Excellence Grant from the State of Tennessee, and by the American Lebanese Syrian Associated Charities (ALSAC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Amar Gajjar, Department of Hematology-Oncology, St Jude Children's Research Hospital, 332 North Lauderdale, Memphis, TN 38105; e-mail: amar.gajjar@stjude.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal