Abstract
The development of hemolytic alloantibodies and erythrocyte autoantibodies complicates transfusion therapy in thalassemia patients. The frequency, causes, and prevention of this phenomena among 64 transfused thalassemia patients (75% Asian) were evaluated. The effect of red blood cell (RBC) phenotypic differences between donors (mostly white) and Asian recipients on the frequency of alloimmunization was determined. Additional transfusion and patient immune factors were examined. 14 (22%) of 64 patients (75% Asian) became alloimmunized. A mismatched RBC phenotype between the white population, comprising the majority of the donor pool, and that of the Asian recipients, was found for K, c, S, and Fyb antigens, which accounts for 38% of the alloantibodies among Asian patients. Patients who had a splenectomy had a higher rate of alloimmunization than patients who did not have a splenectomy (36% vs 12.8%; P = .06). Erythrocyte autoantibodies, as determined by a positive Coombs test, developed in 25% or 16 of the 64 patients, thereby causing severe hemolytic anemia in 3 of 16 patients. Of these 16, 11 antibodies were typed immunoglobulin G [IgG], and 5 were typed IgM. Autoimmunization was associated with alloimmunization and with the absence of spleen (44% and 56%, respectively). Transfused RBCs had abnormal deformability profiles, more prominent in the patients without a spleen, which possibly stimulated antibody production. Transfusion of phenotypically matched blood for the Rh and Kell (leukodepleted in 92%) systems compared to blood phenotypically matched for the standard ABO-D system (leukodepleted in 60%) proved to be effective in preventing alloimmunization (2.8% vs 33%; P = .0005). Alloimmunization and autoimmunization are common, serious complications in Asian thalassemia patients, who are affected by donor-recipient RBC antigen mismatch and immunological factors.
Introduction
Life-long red blood cell (RBC) transfusion remains the main treatment for severe thalassemia. The development of anti-RBC antibodies (alloantibodies and/or autoantibodies) can significantly complicate transfusion therapy. Some alloantibodies are hemolytic and may cause, though not invariably, hemolytic transfusion reactions and limit the availability of further safe transfusion. Others are clinically insignificant. Erythrocyte autoantibodies appear less frequently, but they can result in clinical hemolysis and in difficulty in cross-matching blood. Patients with autoantibodies may have a higher transfusion rate and often require immunosuppressive drugs, a splenectomy, or alternative treatments.1,2,3Despite the recognition of autoantibodies as transfusion-associated risks, little is known about the extent and causes of these phenomena among thalassemia patients or the appropriate prevention methods. Approaches for prevention or treatment of alloimmunization are under debate. They range from provision of RBCs matched for all the major antigens associated with clinically significant antibodies to blood matched only for antibodies that have already been made. Reasons for controversy as to the best approach lay in the fact that many alloantibodies are not harmful, and expensive prevention methods may therefore benefit only some patients.4-6 In addition, donor feasibility and the cost of RBC matching affects the approach of individual medical centers. A better knowledge base of the potential harmful alloantibodies among Asian thalassemia patients can assist in considering the appropriate transfusion strategy to use.
World wide, thalassemia is most prevalent in Asians, and due to large population movements, people of Asian descent constitute the majority of patients in many thalassemia centers in Western countries. However, there is very limited data on the RBC phenotypes among Asians, the extent of alloimmunization, and the role of phenotype differences between blood donors and recipients in forming antibodies.
We studied the frequency of alloimmunization and erythrocyte autoimmunization among thalassemia patients who received regular transfusions and in particular looked at the RBC phenotypes of Asians. We investigated factors possibly affecting the antibody formation: RBC phenotypic differences between the local donor pool and Asian recipients; patient- and blood-related immune factors; and preliminary findings of conformational changes of the transfused RBCs, which perhaps trigger antibody response.
Materials and methods
Patients
Clinical and transfusion records of 64 thalassemia patients (mean age, 15 years; range, 2-39 years) who received regular transfusions were analyzed. The transfusion records of all patients were examined for the presence of alloimmunization or autoimmunization, time interval from the start of transfusion, antibody specificity, and isotype. Exposure to nonleukoreduced blood, ethnic background, spleen presence, and age of splenectomy were recorded. The Asian patients participating in this study had origins from China and Southeast Asia. Approval was obtained from the institutional review board for these studies. Informed consent was provided.
Laboratory investigation
Using standard blood bank methods, serum was analyzed prior to each transfusion for detection of new antibodies to RBC antigens. The serum was mixed with saline-suspended red cells with the addition of low ionic-strength saline (LISS) and incubated at 37°C for 15 minutes. A 3-cell antigen panel was used for the antibody screening procedure, and an anti-IgG reagent was used for the antiglobulin phase. If the screen was positive, an extended panel to identify the antibody and a direct antiglobin (DAT) was performed. Absorption methods were employed in patients presenting with a new autoantibody.
In cases of a positive DAT, further investigation using specific reagents to detect IgG, IgM, or a complement were carried out. When an antibody was detected, eluates were prepared and tested against common sample erythrocytes. In some cases polyethylene glycol was used to enhance the reactivity.
Ektacytometry
Blood from 12 thalassemia patients (6 patients with spleens, 6 patients without spleens) who received regular transfusions was drawn in tubes containing acid citrate dextrose (ACD) 3 weeks after the patients received a packed RBC transfusion and just prior to their next transfusion. The blood was immediately characterized by ektacytometry or kept at 4°C for a maximum of 4 days. No difference was found in ektacytometric data as the result of these storage conditions. The ektacytometric osmotic deformability profiles were determined using a Technicon ektacytometer (Technicon, Tarrytown, NY) as previously described.7 Ektacytometry was also obtained on samples from 10 random blood units prior to their transfusion to the same patients.
Donors
Blood donors from the regional blood bank identified their racial background, and a RBC phenotype was performed for the following antigens: A, B, C, c, D, E, Lea, Leb, K, Fya, Fyb, Jka, JKb, M, N, PI, S, and s. The RBC phenotyping of Asian donors was performed during 2 separate time periods. The Asian donors' ethnic origins were China, Southeast Asia, India, Philippines, and Japan.
Statistical analysis
The RBC antigen frequency among Asians was calculated as a weighted average. The Fisher exact test was used for assessment of statistical significant differences between antigen or antibody frequencies when comparing 2 groups.
Results
Patient characteristics
Of the 64 patients in the study, 30 patients had β-thalassemia major, 24 patients had E-β thalassemia, and the remaining 10 patients had hemoglobin H constant spring. Two of the patients died of iron-overload–related complications, and 4 patients underwent successful bone marrow transplantation and discontinued transfusions. There were 37 females and 27 males. Blood exposure prior to antibody formation and the age of antibody formation were available only for a sub-group of patients because many patients had received transfusions and had become alloimmunized prior to transferring their care to the Northern California thalassemia center.
We defined 3 groups based on the type of blood administered. Group I comprised 29 patients who were transfused for a mean of 6.1 years (range, 2-16 years) with standard ABO-D–compatible blood. Of these patients, 26 patients (89%) received leukodepleted blood (bedside or prestorage). In Group II, the 26 patients were transfused first with ABO-D–compatible blood for a mean of 10.5 years (range, 1-20 years). They were then switched to phenotypically matched blood for C, E, and K RBC antigens for a mean of 6.8 years (range, 2-10 years). Of these 26 patients, 3 patients (11%) received leukodepleted blood. The 9 patients of Group III were started up-front on phenotypically matched blood for the above antigens for a mean of 5.4 years (range, 2-9 years), and all patients received leukodepleted blood.
Overall, 26 patients (40%) received nonleukodepleted blood for various time periods (range, 1-21 years). The vast majority of patients (90%) received bedside leukofiltered blood, which is a suboptimal leukodepletion method, during a 6-year period. Subsequently, since 1997 all patients were started on prestorage leukodepleted blood transfusions.
Alloimmunized patients
We identified 19 alloantibodies, 14 of them clinically significant (ie, capable of causing hemolytic transfusion reaction or hemolytic disease of the newborn), in 14 patients (22%) (8 females and 6 males). Three patients developed 2 antibodies, and 3 antibodies were detected in one patient. Only one female gave birth to 3 children, but she formed antibodies prior to her pregnancies. The highest rate of clinically significant alloimmunization occurred among patients from groups I and II, who were transfused with non-phenotypically matched blood, compared to patients from group III, who received phenotypically matched blood (P = .0075) (Table1). Thirteen antibodies were formed by patients who received nonleukodepleted blood (during that time period or after switching to leukoreduced blood). Five antibodies were formed by patients transfused with bedside leukodepleted blood, and one antibody was formed by a patient exposed to all prestorage leukoreduced blood. Of 14 patients, 7 patients (50%) had their spleen removed prior to the time of antibody formation. Overall, of the 25 patients without spleen, 9 patients (36%) became alloimmunized, and of the 39 patients with spleen, 5 patients (12.8%) became alloimmunized (P = .06).
Alloantibodies and RBC autoantibodies detected in thalassemia patients according to the extent of RBC phenotype of the transfused blood
| . | Group I (n = 29) . | Group II (n = 26) . | Group III (n = 35; 9 + 26) . | Total no.in all patients . | Total no.in Asian patients . |
|---|---|---|---|---|---|
| Alloantibodies | |||||
| Anti-K* | 2 | 4 | — | 6 | 4 |
| Anti-c* | 1 | 1 | — | 2 | 1 |
| Anti-E* | 1 | 3 | — | 4 | 1 |
| Anti-JKb* | — | — | 1 | 1 | 1 |
| Anti-Lea | — | 1 | — | 1 | 1 |
| Anti-Kp-a* | — | 1 | — | 1 | 1 |
| Anti-M | — | 1 | — | 1 | 1 |
| Anti-i | 2 | — | — | 2 | 2 |
| HTLA | — | 1 | — | 1 | 1 |
| Total | 6 | 12 | 1 | 19 | 13 |
| Total clinically significant | 4 | 9 | 1 | 14 | 8 |
| Total autoantibodies | 5 | 9 | 2 | 16 | 14 |
| . | Group I (n = 29) . | Group II (n = 26) . | Group III (n = 35; 9 + 26) . | Total no.in all patients . | Total no.in Asian patients . |
|---|---|---|---|---|---|
| Alloantibodies | |||||
| Anti-K* | 2 | 4 | — | 6 | 4 |
| Anti-c* | 1 | 1 | — | 2 | 1 |
| Anti-E* | 1 | 3 | — | 4 | 1 |
| Anti-JKb* | — | — | 1 | 1 | 1 |
| Anti-Lea | — | 1 | — | 1 | 1 |
| Anti-Kp-a* | — | 1 | — | 1 | 1 |
| Anti-M | — | 1 | — | 1 | 1 |
| Anti-i | 2 | — | — | 2 | 2 |
| HTLA | — | 1 | — | 1 | 1 |
| Total | 6 | 12 | 1 | 19 | 13 |
| Total clinically significant | 4 | 9 | 1 | 14 | 8 |
| Total autoantibodies | 5 | 9 | 2 | 16 | 14 |
HTLA indicates high titer, low avidity.
Indicates clinically significant.
Association with erythrocyte autoimmunization
Of the 64 total patients, 6 patients (25%) developed autoantibodies, as determined by a persistent or transient positive DAT that ranged between 1+ and 4+ and was based on a maximum score of 4+. In 7 (44%) of 16 patients, autoantibodies were associated with the presence of alloantibodies, and a similar higher rate was noted among patients from groups I and II compared to patients from group III (non-phenotypically and phenotypically matched blood transfusions, respectively; P = .02) (Table 1). Of 16 patients, 9 patients (56%) underwent a prior splenectomy. Eight antibodies were detected among patients who received nonleukodepleted blood; 6 antibodies were found in patients transfused with bedside leukodepleted blood; and 2 antibodies were formed in patients who had only prestorage leukopoor blood. Eleven antibodies were warm IgG antibodies, with a panagglutinin reaction, and 5 antibodies were cold IgM antibodies, of which 2 had an anti-i specificity. Of the 16 antibodies, 13 were transient and were detected at least twice over one or more periods of several months, as determined by a positive DAT mostly graded as 1+ and not causing significant clinical hemolysis. The remaining 3 patients developed a clinically significant immune hemolytic anemia: IgG-induced in 1, and IgG with complement in the other 2. All 3 patients required prolonged or recurrent treatment with steroids. Two patients had their spleen removed, of whom one patient was subsequently still unable to safely continue transfusions. Laboratory adsorption techniques in these 3 cases proved the antibodies to be true autoantibodies and not alloantibodies.
Effect of racial-related phenotypic differences
Of the total patients, 48 patients were of Asian descent (China, Cambodia, Laos, Thailand, and Vietnam). Most of the remaining patients originated from the Middle East. Of the 14 alloimmunized patients, 10 patients (71%) were Asian. We compared the racial background and RBC phenotype of the local donors to the Asian recipients (Table 2). Of the donors, 83% were white, 6% Asian, 5% black, and 6% Hispanic. We found an antigenic difference in K, c, S, and Fyb antigens between the white donors and Asian patients, which accounted for 38% of the alloantibodies formed in the Asian patients. The same antigenic differences were confirmed when a larger group of people of these racial backgrounds was examined.
Frequency of RBC antigens in the local donor population and RBC phenotype available on 20 Asian thalassemia patients
| RBC antigen . | All local donors (n = 802) . | White donors (n = 352) . | Asian donors (n = 103)† . | Asian donors (n = 435)† . | Asian patients (n = 20) . | Average antigen frequency (Asians) . | P . |
|---|---|---|---|---|---|---|---|
| s* | 93.1 | 91.7 | 90.5 | 99.5 | 100 | 97.86 | — |
| Fya* | 77.1 | 60.2 | 89.5 | 98.2 | 95 | 96.48 | — |
| C* | 51.7 | 50.5 | 84.4 | 95.7 | 95 | 93.59 | — |
| E* | 95.5 | 95.4 | 88.5 | 93.7 | 100 | 92.97 | — |
| M | NA | NA | NA | 83 | 75 | 82.65 | — |
| Jkb* | 57.1 | 70.4 | 67 | 81 | 85 | 78.56 | — |
| N | NA | NA | NA | 72 | 53.8 | 71.20 | — |
| Jka* | 53.7 | 69.3 | 68 | 68 | 75 | 68.25 | — |
| Leb | NA | NA | NA | 64 | 66.6 | 64.11 | — |
| C* | 80.4 | 77.8 | 43.7 | 53.8 | 35 | 51.26 | < .0001 |
| E* | 21.4 | 19 | 32.0 | 43 | 25 | 40.32 | — |
| P1* | NA | NA | NA | 40 | 33.3 | 39.71 | — |
| Lea | NA | NA | NA | 20 | 40 | 20.88 | — |
| Fyb* | 63 | 78.7 | 16.8 | 18 | 20 | 17.85 | < .0001 |
| S* | 40.5 | 51.4 | 15.6 | 12.7 | 20 | 13.50 | .001 |
| K* | —‡ | 9‡ | 0 | 0 | 1.0 | 0.3 | < .0001 |
| RBC antigen . | All local donors (n = 802) . | White donors (n = 352) . | Asian donors (n = 103)† . | Asian donors (n = 435)† . | Asian patients (n = 20) . | Average antigen frequency (Asians) . | P . |
|---|---|---|---|---|---|---|---|
| s* | 93.1 | 91.7 | 90.5 | 99.5 | 100 | 97.86 | — |
| Fya* | 77.1 | 60.2 | 89.5 | 98.2 | 95 | 96.48 | — |
| C* | 51.7 | 50.5 | 84.4 | 95.7 | 95 | 93.59 | — |
| E* | 95.5 | 95.4 | 88.5 | 93.7 | 100 | 92.97 | — |
| M | NA | NA | NA | 83 | 75 | 82.65 | — |
| Jkb* | 57.1 | 70.4 | 67 | 81 | 85 | 78.56 | — |
| N | NA | NA | NA | 72 | 53.8 | 71.20 | — |
| Jka* | 53.7 | 69.3 | 68 | 68 | 75 | 68.25 | — |
| Leb | NA | NA | NA | 64 | 66.6 | 64.11 | — |
| C* | 80.4 | 77.8 | 43.7 | 53.8 | 35 | 51.26 | < .0001 |
| E* | 21.4 | 19 | 32.0 | 43 | 25 | 40.32 | — |
| P1* | NA | NA | NA | 40 | 33.3 | 39.71 | — |
| Lea | NA | NA | NA | 20 | 40 | 20.88 | — |
| Fyb* | 63 | 78.7 | 16.8 | 18 | 20 | 17.85 | < .0001 |
| S* | 40.5 | 51.4 | 15.6 | 12.7 | 20 | 13.50 | .001 |
| K* | —‡ | 9‡ | 0 | 0 | 1.0 | 0.3 | < .0001 |
All local donors include mostly white (85%) and other ethnicities (15%). Asian donor phenotypes were collected during 2 separate time periods. Significant antigen frequency differences between white and Asian RBCs are on the rows with P values given. P was calculated for the antigens appearing in a higher frequency among the white (and total) donor population compared to the average frequency found among Asian patients. NA indicates not available.
Indicates clinically significant antibodies (ie, can cause a hemolytic transfusion reaction or hemolytic disease of the newborn).
Donors' phenotypes were collected in 2 separate time periods.
Frequency of Kell (K) antigen was not always examined. Therefore the known frequency among white people was used.
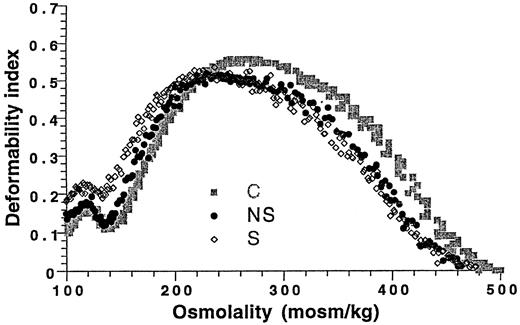
RBC deformability
Conformational changes in the transfused RBCs, as determined by osmotic deformability curves, were analyzed (Figure1). A left shift was shown in both groups of thalassemia patients compared to the control, which indicated a dehydrated, senescent RBC population.8 A more pronounced shift, along with a more heterogenous cell population, was found in the group of patients without spleen. The shift in the deformability curve cannot be attributed to the donor blood unit condition, as donor cells showed ektacytometry patterns within the normal control range.
Erythrocyte osmotic gradient deformability patterns of thalassemia patients with and without spleen 3 weeks after their last transfusion.
The average deformability index (DI) was calculated for 6 patients in each category. A left shift of the deformability curve in the hypotonic region is noted for both groups, which reflects dehydrated RBCs. S indicates patients without a spleen; NS, patients with a spleen; and C, control.
Erythrocyte osmotic gradient deformability patterns of thalassemia patients with and without spleen 3 weeks after their last transfusion.
The average deformability index (DI) was calculated for 6 patients in each category. A left shift of the deformability curve in the hypotonic region is noted for both groups, which reflects dehydrated RBCs. S indicates patients without a spleen; NS, patients with a spleen; and C, control.
Discussion
Only a few studies, mostly in non-Asian transfused thalassemia patients, have investigated the frequency and causes of alloimmunization and autoimmunization. In the present study we examined these elements and defined the common RBC phenotypes among Asians, which have not been previously described.
The factors for alloimmunization are complex and involve at least 3 main contributing elements: The RBC antigenic difference between the blood donor and the recipient; the recipient's immune status; and the immunomodulatory effect of the allogeneic blood transfusions on the recipient's immune system.
A low rate of alloimmunization may be expected when there is homogeneity of RBC antigens between the blood providers and recipients. Previous data on presumed homogenous populations in Greece and Italy showed an overall low rate (5% to 10%) of alloimmunization.9-14 A higher rate of approximately 20% was noted in 2 Greek studies when blood matched only for ABO and Rh-D antigens was used.15,16 We found a high alloimmunization rate of 22% overall and 20.8% among Asian patients. Because the majority of our donor population is white and the majority of blood recipients are Asians, a greater recipient alloimmunization rate could be expected than among homogenous populations, as their erythrocyte antigen distribution differs significantly (Table 2). This antigenic discrepancy accounted for more than one-third (38%) of the alloantibodies detected among the Asian patients and is mostly noted for c, S, K, and Fyb antigens, which are all potentially hemolytic antibodies. However, it did not account for the high frequency (21%) of anti-E antibodies. The alloimmunization rate in the present study is also higher than the 5% rate found in chronically transfused white patients.17 The number of transfusion-dependent Asian thalassemia patients is increasing, while the vast majority of blood donors across the country remain white. As a result, the rate of alloimmunization may be expected to grow further unless prevention methods are applied.
Other factors beyond the antigenic discrepancy, in particular the immune system, are undoubtedly involved. Immune effects of transfused blood, especially immunosuppression of white cells, are well-recognized.18 An overall normal CD4:CD8 ratio was shown in thalassemia patients transfused with post-storage leukocyte-depleted blood.19 However, a marked absolute lymphocytosis, mostly due to an increase in B lymphocytes, predominantly in splenectomized patients was reported with the use of both nonleukoreduced and leukoreduced blood.19,20 The lymphocytosis is accompanied by an increase in serum immunoglobulins, immune complexes, and cells bearing surface immunoglobulins, which are the result of the immunomodulatory effect of blood elements, absence of spleen, and recipient's immune status.18-24 The majority of patients in the present study had a long-term exposure to nonleukopoor and/or suboptimally leukoreduced blood and a higher alloimmunization (and autoimmunization) rate than the patients exposed to all leukodepleted blood. We postulate a similar activated immune system among our patients, thereby increasing the propensity to form antibodies. Patients who had a splenectomy had a higher alloimmunization rate, a finding approaching statistical significance (P = .06). The spleen absence may further enhance the immune response to the infused foreign antigens, which are not effectively filtered.
The association of thalassemia and erythrocyte autoantibodies has not been studied. The true frequency and the clinical spectrum are unknown.1 We found a high frequency (25%) of autoantibody formation, mostly IgG warm antibodies, of which 18% had a significant clinical hemolysis. The antibody development was associated with alloimmunization, exposure to nonleukoreduced blood, and absence of spleen. A high frequency of erythrocyte autoantibodies in association with alloimmunization was recently reported in a sickle cell disease (SCD) study.25 All the patients with SCD in that study were most likely functionally asplenic. We suggest a similar mechanism of conformational changes in the RBCs due to erythrocyte fragmentation, membrane deformation, or alloantibody binding. Our data show altered RBC deformability profiles, more so in patients without spleen than in patients with spleen (Figure 1). These findings are consistent with senescent erythrocytes and may expose new antigens and promote or enhance an immune reaction, as known to occur in aging and impaired RBCs.26,27 It is likely that the absence of an efficient filtering system for removal of damaged erythrocytes enhances the process. In accordance with this hypothesis, a previous study showed increased hemolytic autoantibody response in mice without spleen compared to mice with spleen and a spleen role in regulating this response.28
The immune response may also be affected by the patient's age at the of start of transfusion and the number of blood units a patient receives. Transfusion at an early age (less than 1-3 years old) may offer some immune tolerance and protection against alloimmunization in thalassemia patients.15,16 The relation between the number of blood units transfused and antibody formation is unknown in thalassemia, but it is an important factor for increased alloimmunization in patients, including SCD patients, who receive multiple transfusions.6,17,29 However, some antigen-negative patients may not produce antibodies at all or may form only one antibody despite exposure to antigen-positive cells. Other patients may have serologic findings of a clinically significant alloantibody without evidence of a hemolytic transfusion reaction.4 6 Larger studies in matched thalassemia patient populations are needed.
For prevention of alloimmunization, our results demonstrate that transfusion of blood matched for Rh and K antigens resulted in a significant difference in the alloimmunization rate. Only one new antibody (anti-JKb) was detected in the 35 patients switched to or started on phenotypically matched blood; in 18 (33%) of 55 patients, alloantibodies were formed while the patients received nonphenotypically matched blood (P = .0005). However, the parallel change to all leukodepleted blood could have also contributed to the decrease in alloimmunization. Although RBC matching was shown to be effective in preventing alloimmunization in the present study and in other studies,13,15 16 the expense and the feasibility of antigen-matched blood may not permit such a transfusion approach in some medical centers. Providing matched units for the ABO-D and Kell systems only, particularly in the Asian patients in whom anti-K antibodies were the most frequently detected (30%), is another, possibly more economic, approach. Transfusing matched blood only for patients who first proved to be “antibody producers” may reduce expenses and increase the availability of matched blood; however, patients may still develop clinically significant alloantibodies.
In summary, our data show that alloimmunization to minor erythrocyte antigens and erythrocyte autoimmunization, of variable clinical significance, are frequent findings in transfused thalassemia patients. The causes are not fully understood; however, data suggest that the recipient's immune status, along with the effects of multiple allogeneic blood transfusions, can induce antibody formation. We found an association with the absence of spleen and the presence of deformed ex-vivo RBCs that probably augments these mechanisms. The difference in the RBC antigen profile between the predominantly white donor population and the Asian recipients likely further contributes to the phenomena.
With the growing knowledge base of the immune effects of current blood transfusions23 24 and limited data on the immune status of thalassemia patients, a large study addressing the complex interaction of these factors is needed. Such information may enable understanding and prevention of this serious and common complication. In clinical and transfusion practice, when considering splenectomy, a potential higher risk for future RBC immunization should also be taken into account. We recommend obtaining a RBC antigen phenotype on all thalassemia patients, particularly of Asian ethnicity, who are started on transfusions, and if feasible, providing leukodepleted blood matched for antigens of the ABO-D and Kell systems. Recruitment of Asian blood bank donors, just like recruitment of black donors for SCD patients, can increase the availability of compatible blood for thalassemia patients, who have a life-long transfusion-dependent disease.
Supported in part by the National Institutes of Health E-β-thalassemia grant HL 61186-01.
Submitted March 2, 2000; accepted July 25, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elliott P. Vichinsky, Division of Hematology/Oncology, Children's Hospital Oakland, 747 52nd Street, Oakland, CA 94609; e-mail: evichinsky@mail.cho.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal