Abstract
Carriers of a mutation in the prothrombin (clotting factor II) or factor V gene have a 2- to 4-fold greater risk for venous thromboembolism than subjects without the mutations. Whether both mutations also predispose to recurrent venous thromboembolism is unclear. Outpatients who had a first episode of proven symptomatic deep-vein thrombosis and a long-term follow-up were studied. The outcome measure was the cumulative incidence of confirmed venous thromboembolic complications. Two hundred fifty-one patients were enrolled in the study. Mean duration of follow-up was 8.3 years. The prothrombin gene mutation was demonstrated in 27 patients (prevalence, 10.8%; 95% CI, 6.9 to 14.6), and the factor V gene mutation was demonstrated in 41 patients (prevalence, 16.3%; 95% CI, 11.8 to 20.9). The cumulative incidence of venous thromboembolic complications after 10 years was 61.3% (95% CI, 35.7 to 87.9), and the hazard ratio was 2.4 (95% CI, 1.3 to 4.7; P = .004) in patients with the prothrombin gene mutation); the cumulative incidence of venous thromboembolic complications after 10 years was 55.2% (95% CI, 36.4 to 74.0), and the hazard ratio was 2.4 (95% CI, 1.4 to 4.1;P = .001) in patients with the factor V gene mutation. In comparison, the cumulative incidence of venous thromboembolic complications after 10 years was 23.1% (95% CI, 16.2 to 30.1) in patients without the mutations. Prothrombin and factor V gene mutations occur frequently in patients with venous thrombosis and are associated with an increased risk for recurrent venous thromboembolic complications
Introduction
In the last decades several hereditary coagulation abnormalities, including antithrombin and protein C and protein S defects, have been identified that predispose patients to venous thromboembolism.1-3 Carriers of these defects who have experienced a venous thrombotic episode remain at risk for recurrent disease.4,5 Recently, a mutation in the factor V gene6-10 and a mutation in the prothrombin gene11-18 were identified and related to an increased risk for venous thromboembolism. The mutation in the factor V gene results in a substitution of glutamine for arginine at position 506 in the coagulation factor V molecule causing resistance to activated protein C.8 The mutation in the prothrombin (clotting factor II) gene is characterized by a G-to-A transition at position 20210 in the 3′ untranslated region and results in elevated prothrombin levels.11 Combined, these 2 mutations can be identified in approximately 25% of patients with venous thromboembolic disorders.19
Reports on the relation between the factor V gene mutation and recurrent venous thromboembolism have been conflicting,20-28 whereas 3 reports fail to show an association between an increased risk for recurrences and the carrier state for the prothrombin gene mutation.25,28 29 It is important to identify the true relation between these mutations and the risk for recurrent venous thromboembolism. If the mutations are indeed associated with an increased risk for recurrent venous thromboembolism, their identification could influence the treatment of known carriers.
Recently, we reported20 on the association between recurrent venous thromboembolism and the presence of the factor V gene mutation in a large cohort of patients with a first episode of venography-proven deep-vein thrombosis. In the current analysis of the same cohort, we evaluated the influence of both the factor V and the prothrombin gene mutations on recurrent venous thromboembolism.
Materials and methods
Identification of inception cohort
All outpatients who were referred to the thrombosis unit of the University of Padua between January 1986 and June 1994 because of a first episode of venography-proven deep-vein thrombosis and who had long-term follow-up were potentially eligible for the study.4,20,27 Patients were excluded if they had malignant disease or an abnormality in the coagulation or fibrinolytic system, including antithrombin, protein C and protein S defects, plasminogen, fibrinogen, and antiphospholipid antibody syndrome. Patients who needed vitamin K antagonist therapy for reasons other than venous thromboembolism were also excluded. Surviving patients who met the eligibility criteria and who provided informed consent had factor V and prothrombin gene mutation assessment according to standard methods.20 Laboratory tests were performed by technicians unaware of the clinical details of the patients. The study protocol was approved by the institutional review board.
Treatment and follow-up
All patients were treated with therapeutic dosages of low-molecular–weight or unfractionated heparin followed by 3 to 6 months of oral anticoagulants (target international normalized ratio, 2.5; range, 2.0 to 3.0). Follow-up visits were conducted every 6 months until 8 years. Patients were instructed to return if symptoms or signs suggestive of recurrent venous thromboembolism developed. Patients with suspected recurrent venous thrombosis or pulmonary embolism had confirmatory testing as described earlier.4 30 Briefly, recurrent deep-vein thrombosis was demonstrated by venography or compression ultrasonography, according to standard methods. If the venogram was not diagnostic, recurrent venous thrombosis was diagnosed on the basis of abnormal results of fibrinogen I 125 leg imaging or results of noninvasive tests that had changed from normal to abnormal. Patients with suspected pulmonary embolism underwent venography if they had concurrent leg symptoms or lung imaging. Patients with low or intermediate lung scans underwent pulmonary angiography. Lung imaging and pulmonary angiography were performed and interpreted according to standard procedures. A diagnosis of fatal pulmonary embolism was based on the findings of autopsy or the opinion of an independent physician. Recurrent venous thromboembolic events were adjudicated by a committee that was unaware of further clinical details (including factor V and prothrombin gene mutation status) of the patients.
Analysis
The cumulative incidence of venous thromboembolic complications in patients with and without the prothrombin mutation, the factor V gene mutation, or both was calculated according to the method of Kaplan and Meier. A multivariate Cox proportional hazards model was used to assess the significance of the difference between groups and to determine the hazard ratios and their 95% confidence intervals. Furthermore, the hazard ratio for the presence of the mutations was adjusted for the effects of age and idiopathic versus secondary thrombosis. Secondary vein thrombosis was defined as deep-vein thrombosis occurring in association with surgery, trauma, fracture, pregnancy/childbirth (all within 3 months), or use of estrogens (oral contraceptives or hormone replacement therapy).P < .05 was considered statistically significant.
Results
Patients
Of the 517 consecutive patients with a first episode of proven deep-vein thrombosis, 170 were excluded from the study. Reasons for exclusion were malignant disease (n = 101), other abnormalities in the coagulation system (n = 51), and necessity for long-term oral anticoagulation (n = 18). Of the remaining 347 patients, 85 (24.5%) were not available for genetic testing because they died (n = 74) or were lost to follow-up (n = 11). The demographic and clinical characteristics of these 85 patients are presented in Table1 and are comparable to those enrolled in the study. Of the remaining 262 patients, 251 gave their informed consent and were included in the study. The demographic and clinical characteristics of the 251 study patients are presented in Table2. Patients with the prothrombin gene mutation were on average younger than those with the factor V gene mutation or those without mutations. Five years of follow-up was completed in 232 patients (92%), and 10 years of follow-up was completed in 100 patients (40%), for a median duration of follow-up of 8.3 years. The prothrombin gene mutation was demonstrated in 27 patients (prevalence, 10.8%; 95% CI, 6.9 to 14.6), and the factor V gene mutation was demonstrated in 41 patients (prevalence, 16.3%; 95% CI, 11.8 to 20.9). Three patients carried both mutations. All carriers of the prothrombin or factor V gene mutations were heterozygous for these abnormalities. The duration of anticoagulant therapy was similar for patients with and without the gene mutations.
Demographic and clinical characteristics of patients not tested for the prothrombin gene mutation compared with those of investigated patients
| . | Patients not tested (N = 85) . | Patients tested (N = 251) . |
|---|---|---|
| Age (y) (median, range) | 65 (20-86) | 62 (23-84) |
| Sex (% male) | 58 | 52 |
| PDD (d) (median, range) | 7 (1-30) | 7 (1-30) |
| Risk factors for venous thrombosis (%) | ||
| recent surgery | 16 (19) | 53 (21) |
| recent trauma/fracture | 20 (24) | 51 (20) |
| estrogen therapy | 4 (5) | 18 (7) |
| pregnancy/childbirth | 1 (1) | 11 (4) |
| APC resistance/factor V mutation | 13 (15) | 41 (16) |
| Extent of deep-vein thrombosis (%) | ||
| isolated distal | 6 (7) | 15 (6) |
| proximal | 79 (93) | 236 (94) |
| Initial treatment (%) | ||
| unfractionated heparin | 59 (69) | 172 (69) |
| LMWH | 23 (27) | 65 (26) |
| other treatments | 3 (4) | 14 (6) |
| Recurrent VTE in 10-year follow-up (%) | 21 (25) | 68 (27) |
| . | Patients not tested (N = 85) . | Patients tested (N = 251) . |
|---|---|---|
| Age (y) (median, range) | 65 (20-86) | 62 (23-84) |
| Sex (% male) | 58 | 52 |
| PDD (d) (median, range) | 7 (1-30) | 7 (1-30) |
| Risk factors for venous thrombosis (%) | ||
| recent surgery | 16 (19) | 53 (21) |
| recent trauma/fracture | 20 (24) | 51 (20) |
| estrogen therapy | 4 (5) | 18 (7) |
| pregnancy/childbirth | 1 (1) | 11 (4) |
| APC resistance/factor V mutation | 13 (15) | 41 (16) |
| Extent of deep-vein thrombosis (%) | ||
| isolated distal | 6 (7) | 15 (6) |
| proximal | 79 (93) | 236 (94) |
| Initial treatment (%) | ||
| unfractionated heparin | 59 (69) | 172 (69) |
| LMWH | 23 (27) | 65 (26) |
| other treatments | 3 (4) | 14 (6) |
| Recurrent VTE in 10-year follow-up (%) | 21 (25) | 68 (27) |
PDD, patient-doctor delay, defined as the period elapsed between occurrence of first symptoms and referral to thrombosis center; APC,; LMWH, low-molecular–weight heparin; VTE, venous thromboembolism.
Demographic, clinical, and treatment-related characteristics of patients with and without the prothrombin or factor V gene mutation
| . | No mutation (N = 186) . | FIIM (N = 24) . | FVM (N = 38) . | Combined mutation (N = 3) . |
|---|---|---|---|---|
| Age (y) (median, range) | 62 (23-86) | 52 (25-83) | 64 (23-87) | 45 (19-64) |
| Male sex (%) | 94 (51) | 12 (50) | 23 (61) | 2 (67) |
| PDD (d) (median, range) | 10 (1-52) | 7 (2-30) | 7 (1-30) | 12 (8-20) |
| Presentation of venous thrombosis | ||||
| idiopathic etiology (%) | 96 (77) | 8 (7) | 18 (15) | 2 (1.6) |
| secondary to risk factors (%) | 90 (71) | 16 (13) | 20 (16) | 1 (0.8) |
| Extent of DVT | ||||
| isolated distal (%) | 11 (6) | 1 (4) | 3 (8) | 0 (0) |
| proximal (%) | 175 (94) | 23 (96) | 35 (92) | 3 (100) |
| Duration of anticoagulant treatment | ||||
| 3 mo (%) | 179 (96) | 22 (92) | 34 (89) | 3 (100) |
| 6 mo (%) | 7 (4) | 2 (8) | 4 (11) | 0 (0) |
| . | No mutation (N = 186) . | FIIM (N = 24) . | FVM (N = 38) . | Combined mutation (N = 3) . |
|---|---|---|---|---|
| Age (y) (median, range) | 62 (23-86) | 52 (25-83) | 64 (23-87) | 45 (19-64) |
| Male sex (%) | 94 (51) | 12 (50) | 23 (61) | 2 (67) |
| PDD (d) (median, range) | 10 (1-52) | 7 (2-30) | 7 (1-30) | 12 (8-20) |
| Presentation of venous thrombosis | ||||
| idiopathic etiology (%) | 96 (77) | 8 (7) | 18 (15) | 2 (1.6) |
| secondary to risk factors (%) | 90 (71) | 16 (13) | 20 (16) | 1 (0.8) |
| Extent of DVT | ||||
| isolated distal (%) | 11 (6) | 1 (4) | 3 (8) | 0 (0) |
| proximal (%) | 175 (94) | 23 (96) | 35 (92) | 3 (100) |
| Duration of anticoagulant treatment | ||||
| 3 mo (%) | 179 (96) | 22 (92) | 34 (89) | 3 (100) |
| 6 mo (%) | 7 (4) | 2 (8) | 4 (11) | 0 (0) |
PDD, patient-doctor delay, defined as the period elapsed between occurrence of first symptoms and referral to thrombosis center.
Venous thromboembolic complications on follow-up
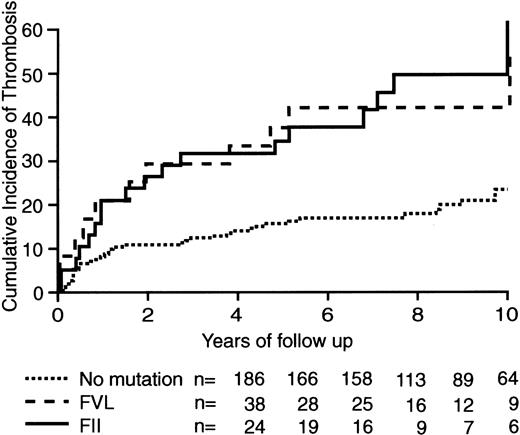
One hundred sixty-seven episodes of suspected deep-vein thrombosis or pulmonary embolism occurred in 113 patients and could be objectively confirmed in 68 patients (deep-vein thrombosis, n = 57; pulmonary embolism, n = 11). Recurrent venous thromboembolic complications were ruled out by the diagnostic work-up in the remaining 45 patients. Venous thromboembolic complications developed in 13 patients with the prothrombin gene mutation and in 19 patients with the factor V gene mutation. One of these recurrences occurred in 1 of 3 patients with a combined abnormality. Another 37 recurrent events were observed in patients without the defects. The distribution of recurrences between patients with secondary and idiopathic deep-vein thrombosis in relation to carrier status is given in Table 3. The cumulative incidence of venous thromboembolic complications after 10 years was 61.3 (95% CI, 35.7 to 87.9%) in patients with the prothrombin gene mutation and 55.2% (95% CI, 36.4 to 74.0%) among carriers of the factor V gene mutation. This compares with 23.1% (95% CI, 16.2 to 30.1) in the 186 patients without these mutations (Figure1). The hazard ratios for venous thromboembolic complications among patients with the prothrombin or factor V gene mutation, compared to patients without these mutations, were 2.4 (95% CI, 1.3 to 4.7; P = .004) and 2.4 (95% CI, 1.4 to 4.1; P = .001), respectively. Adjustment for age and idiopathic thrombosis resulted in similar estimates for the hazard ratios of both mutations (2.8 and 2.4, respectively). The influence of age was statistically nonsignificant, whereas the risk in patients with initially idiopathic rather than secondary thrombosis was increased by a factor 1.5 (P = .06). None of the introduced interaction terms were statistically significant.
Distribution of the 68 recurrent venous thromboembolic episodes
| . | None (%) . | FIIM (%) . | FVM (%) . | Both (%) . |
|---|---|---|---|---|
| Idiopathic | 25/96 (26) | 6/8 (75) | 8/18 (44) | 1/2 (50) |
| Secondary | 12/90 (13) | 6/16 (38) | 10/20 (50) | 0/1 (0) |
| . | None (%) . | FIIM (%) . | FVM (%) . | Both (%) . |
|---|---|---|---|---|
| Idiopathic | 25/96 (26) | 6/8 (75) | 8/18 (44) | 1/2 (50) |
| Secondary | 12/90 (13) | 6/16 (38) | 10/20 (50) | 0/1 (0) |
Based on findings in the 124 patients with idiopathic and the 127 patients with secondary venous thrombosis in relation to presence or absence of the prothrombin or the factor V gene mutation.
Cumulative incidence of recurrent thromboembolism after a first episode of symptomatic deep-vein thrombosis.
Incidences occurred in patients with the factor V mutation, in those with the prothrombin gene mutation, and in those without genetic mutations.
Cumulative incidence of recurrent thromboembolism after a first episode of symptomatic deep-vein thrombosis.
Incidences occurred in patients with the factor V mutation, in those with the prothrombin gene mutation, and in those without genetic mutations.
Discussion
The results of our study demonstrate that patients with venous thrombosis who are carriers of the prothrombin or factor V gene mutation remain at higher risk for further venous thromboembolic complications than patients without a known thrombophilic condition (antithrombin, protein C, protein S, antiphospholipid syndrome, prothrombin, or factor V gene mutation). Among carriers of either gene mutation, a relative risk of 2.4 was found. This risk translated to a cumulative incidence of recurrent venous thromboembolism of more than 55% during 10 years of follow-up.
The prothrombin gene mutation occurred less frequently than the factor V mutation (10.8% vs 16.3%), and combined mutations were found at an expected prevalence rate. According to the results of our study, both mutations are independent risk factors and expose patients to similar risks for recurrent venous thromboembolic complications. The hazard ratios observed in the current analysis are (substantially) higher than reported in earlier studies. In our first report patients with a deficiency of antithrombin, protein C, or protein S had a hazard ratio for recurrent venous thromboembolism of 1.4.4 However, in that analysis there was a large proportion of subjects with a (then unknown) prothrombin or factor V gene mutation in the reference group. Similarly, in our report20 on the increased risk for recurrent venous thromboembolism in patients with factor V gene mutation, the controls harbored a (then unknown) 11% prevalence rate for the prothrombin gene mutation. Consequently, in the current study in which the control group is devoid of these highly prevalent thrombophilic conditions, the estimated hazard ratios are substantially higher.
We believe that our results are representative of the general population because of the following. First, only patients with a first episode of symptomatic deep-vein thrombosis without conditions confounding the risk for recurrences were prospectively enrolled and treated with anticoagulants for a period of 3 to 6 months. Second, interpretation bias was avoided by having the adjudication of recurrent events and genetic testing performed by operators unaware of the other patient details. Third, patients with malignancy or thrombophilic defects were excluded from our analysis because prolonged anticoagulant treatment was given to these specific patients groups only. Given that anticoagulant treatment is known to be highly effective in preventing venous thromboembolism,19 these patients were excluded from our cohort. Finally, though approximately 25% of potentially eligible patients could not be tested for the mutations, the demographic and clinical characteristics of these patients were fully comparable with those of enrolled patients, as were the rate of recurrent thromboembolism (Table 1) and the prevalence of resistance to activated protein C.20 Therefore, it is unlikely that an important bias influenced our results.
Our findings are in keeping with the results of 2 prospective studies21,22 in patients with a first episode of venous thromboembolism. In contrast, in other recent reports25-29,31,32 on cohorts of patients with venous thromboembolism, no relation was found between the presence of the factor V or prothrombin gene mutation and recurrent venous thromboembolism. What could be the explanations for these contradictory findings? Several important design features can influence the ability of a clinical study to detect differences in prognosis in subgroups of patients with venous thrombosis. These include prospective or retrospective conduct of clinical follow-up, formation of a proper inception cohort, comparability of (duration of) treatment between groups, adequate documentation of outcome events, and independent assessment of laboratory tests and clinical outcomes. Retrospective studies have the potential to be unreliable because numerous biases could apply, including inadequate identification of (recurrent) thrombotic disease, differences in treatment exposure, and patient selection. Many subgroups of patients with venous disease may have not only a different risk for recurrent venous thromboembolism but also a varying association between this risk and the presence of factor V and prothrombin gene mutations. Thus, patients with a first episode of idiopathic proximal deep vein thrombosis are clearly at a higher risk than patients with (secondary) isolated calf vein thrombosis. Deep-vein thrombosis of the arm, which often occurs in association with clear risk factors (such as indwelling catheters with or without chemotherapy), is less likely to produce new thrombotic episodes if the triggering event is avoided or controlled. Moreover, pulmonary embolism, though closely related to venous thrombosis of the leg, has a weaker association with the factor V gene mutation.24Because anticoagulant therapy is highly effective in preventing recurrent thrombotic events, differences in exposure between patients with and without the gene mutations could lead to false estimations of the risk for recurrence. Adequate documentation of venous thrombosis, not only at baseline but also at follow-up in case of a suspected recurrent episode, is crucial because clinical diagnosis is highly unspecific. In fact, state-of-the-art evaluation of suspected (recurrent) venous thrombosis could refute a diagnosis of recurrence in approximately two thirds of such patients.19
Of the 5 prospectively conducted cohort studies,22,25,27-29 only one study demonstrated a statistically significant increased risk for recurrent venous thromboembolism in carriers of the factor V gene mutation. Of the remaining 4 studies, one lacks the potential for detecting clinically relevant differences between patients with and without genetic mutations because of a low number of patients and a short duration of follow-up.25 The 3 other reports deal with a study population substantially different from that enrolled in our study.27-29 As many as 15% to 30% of patients were recruited because of pulmonary embolism, and patients with isolated calf-vein thrombosis formed a substantial proportion (35%-40%) of the initial cohort, which is clinically unusual among consecutive symptomatic patients with venography proved deep-vein thrombosis.33,34 Moreover, patients with arm-vein thrombosis were also included in 2 studies.27,29 As a result of this pattern, patients with proximal-vein thrombosis represented only 30% to 40% of enrolled patients compared to more than 90% of patients in our cohort. Because the prevalence of genetic abnormalities may vary according to the modality of clinical presentation24 and because the incidence of recurrent venous thromboembolism is highly dependent on the extension of the initial thrombosis, the profound heterogeneity in the characteristics of patients recruited in the various reports makes it problematic to compare their results. In recent case-control studies that concluded that the gene mutations were not associated with recurrent venous thrombosis, the potential for bias was considerable not only because of the retrospective design of the study but also because of the absence of an inception cohort.31 32
Our observation of an increased risk for recurrent venous thromboembolism in symptomatic carriers of either the factor V or factor II gene mutation is in biologic consistency with the observation of an increased risk for a first thromboembolic event in carriers of these mutations9-18 and with the increased risk for recurrences in carriers of other inherited or acquired coagulation abnormalities.4,5 35-38
In summary, both the prothrombin and factor V gene mutations occur relatively frequently in patients with venous thrombosis, and both are associated with an increased risk for venous thromboembolic complications. This high incidence of complications challenges the widely adopted short-term anticoagulant regimen in these patients. On the other hand, prolonged anticoagulation is associated with a risk for bleeding25,39 40; therefore, prospective studies are needed to evaluate the benefit-to-risk ratio of different durations of anticoagulation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anthonie W. A. Lensing, Centre for Vascular Medicine, Academic Medical Centre F4-237, Meibergdreef 9, 1105 AZ Amsterdam, Netherlands; e-mail: ton@lensing.demon.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal