Abstract
With the use of intravital microscopy, a new type of platelet–endothelial interaction in mouse mesenteric venules at low shear (80-100 seconds−1) is described. Stimulation of these vessels with calcium ionophore A23187, a known secretagogue of Weibel-Palade bodies, induced immediate platelet adhesion (within 15 seconds) and translocation without the formation of aggregates. This stop-and-go process reached a maximum in approximately 1 minute, when approximately 25 000 platelets adhered/mm2·s, and then adhesion progressively decreased. This adhesion process was dependent on von Willebrand factor (vWF) and independent of P-selectin. Immunohistologic analysis showed that the venules were not denuded withA23187 treatment, suggesting that platelets adhered to vWF secreted on the luminal face of the endothelial cells. Histamine treatment induced a similar adhesion phenomenon. Platelet adhesion was not abolished in β3-deficient mice or when the platelets were treated with inhibitory antibodies to PECAM-1 or PSGL-1, indicating that these molecules are not required for platelet–endothelium interaction at low shear. The adhesion was mediated by platelet glycoprotein Ibα (GPIbα) because the adhesion of murine platelets expressing exclusively the human GPIbα could be prevented by a pretreatment with mocarhagin, a snake venom protease that cleaves human GPIbα. The results indicate that vWF released from Weibel-Palade bodies can dramatically increase the concentration of platelets along the vessel wall through an interaction with GPIbα. It is proposed that this process may rapidly recruit platelets to sites of injury or inflammation in veins.
Introduction
In normal physiologic conditions, circulating platelets do not interact with nonactivated endothelium.1However, any endothelial denudation will lead to immediate platelet adhesion and aggregation at the site of injury.2 In addition to this well-defined process, another type of platelet–vessel wall interaction has been increasingly studied in the past decade. Indeed, platelets are able to interact with activated endothelium and even roll on it as was previously described for leukocytes.3 The molecular mechanisms involved in platelet–endothelium interactions are not yet totally identified, though there is some evidence that platelet glycoprotein (GP) IIb/IIIa (integrin αIIbβ3),4,5 platelet–endothelial cell adhesion molecule-1 (PECAM-1),6 fibrinogen,7and the β1 integrin8 are implicated in this process, depending on how the endothelium was activated. In addition, different receptors seem to be involved according to the activation state of the platelets. One phenomenon that is independent of platelet activation is the platelet rolling observed by intravital microscopy. Our laboratory has shown that both nonactivated and activated platelets can roll in vivo on P-selectin expressed at the surface of activated and inflamed endothelial cells in small mesenteric venules characterized by a high shear rate of approximately 500 seconds-1.9,10This rolling process on P-selectin has also been described in a model of ischemia–reperfusion injury in mice.11 In addition to the smooth P-selectin–dependent platelet rolling, we observed that many platelets were captured transiently onto the vessel wall, even when P-selectin–deficient platelets were injected in doubly P- and E-selectin–deficient mice, indicating a selectin-independent mechanism.10 The appearance of P-selectin at the cell surface is the consequence of Weibel-Palade body secretion, an early hallmark of endothelial cell activation. These storage granules contain not only P-selectin, but also von Willebrand factor (vWF),12 a very important ligand of platelet receptors GPIbα and αIIbβ3 involved in platelet adhesion and aggregation during vascular injury.13 Resting platelets have been shown to bind and translocate in a stop-and-go motion on vWF-coated glass coverslips under flow conditions.14 vWF is a multimeric protein, and the high molecular weight multimers that are the most active biologically are stored and secreted from Weibel-Palade bodies.15 In vitro, some of the released vWF appears to remain transiently associated with the endothelial cell surface.15 This led us to investigate whether released vWF could recruit platelets in veins in vivo and could account for the selectin-independent platelet adhesion to endothelium that we have observed.10 Using an intravital microscopy model, we compared platelet interactions with the vessel wall in activated mesenteric venules of wild-type and vWF-deficient mice.16
Materials and methods
Animals
All mice used in this study were on a mixed background C57BL/6J/129Sv. As platelet donors for intravital microscopy, we used both male and female mice of any age and of the following genotypes: wild-type (WT), vWF−/−,16 P-selectin−/− (P−/−),17 β3−/−,18 or mice expressing exclusively the human GPIbα (mGPIb−/−;TghGPIb mice).19As recipients for intravital microscopy, we used young male mice weighing 14 to 19 g. The genotypes of recipients were the same as those of donors or as indicated in Table1. Experimental procedures were approved by the Animal Care and Use Committee of the Center for Blood Research.
Hemodynamic characteristics of A23187-activated venules
| Genotype donor platelets . | Treatment . | Genotype recipient . | Venular diameter (μm) . | Centerline velocity (mm/s) . | Shear rate (s−1) . |
|---|---|---|---|---|---|
| WT | — | WT | 191 ± 14 | 3.3 ± 0.46 | 93 ± 17 |
| P−/− | — | P−/− | 188 ± 11 | 3.31 ± 0.42 | 92 ± 17 |
| vWF−/− | — | vWF−/− | 174 ± 12 | 3.22 ± 0.49 | 98 ± 18 |
| β3−/− | — | β3−/− | 157 ± 20 | 2.66 ± 0.61 | 84 ± 14 |
| WT | Anti–PSGL-1 | WT | 174 ± 13 | 2.88 ± 0.19 | 84 ± 5 |
| WT | Anti–PECAM-1 | WT | 165 ± 18 | 2.9 ± 0.28 | 91 ± 16 |
| P−/− | — | GPIb−/− | 177 ± 14 | 2.97 ± 0.25 | 87 ± 13 |
| mGPIb−/−;TghGPIb | — | P−/− | 185 ± 16 | 3 ± 0.37 | 81 ± 4 |
| mGPIb−/−;TghGPIb | Mocarhagin | P−/− | 192 ± 2 | 2.86 ± 0.23 | 75 ± 6 |
| WT | Mocarhagin | P−/− | 171 ± 12 | 2.35 ± 0.31 | 74 ± 17 |
| Genotype donor platelets . | Treatment . | Genotype recipient . | Venular diameter (μm) . | Centerline velocity (mm/s) . | Shear rate (s−1) . |
|---|---|---|---|---|---|
| WT | — | WT | 191 ± 14 | 3.3 ± 0.46 | 93 ± 17 |
| P−/− | — | P−/− | 188 ± 11 | 3.31 ± 0.42 | 92 ± 17 |
| vWF−/− | — | vWF−/− | 174 ± 12 | 3.22 ± 0.49 | 98 ± 18 |
| β3−/− | — | β3−/− | 157 ± 20 | 2.66 ± 0.61 | 84 ± 14 |
| WT | Anti–PSGL-1 | WT | 174 ± 13 | 2.88 ± 0.19 | 84 ± 5 |
| WT | Anti–PECAM-1 | WT | 165 ± 18 | 2.9 ± 0.28 | 91 ± 16 |
| P−/− | — | GPIb−/− | 177 ± 14 | 2.97 ± 0.25 | 87 ± 13 |
| mGPIb−/−;TghGPIb | — | P−/− | 185 ± 16 | 3 ± 0.37 | 81 ± 4 |
| mGPIb−/−;TghGPIb | Mocarhagin | P−/− | 192 ± 2 | 2.86 ± 0.23 | 75 ± 6 |
| WT | Mocarhagin | P−/− | 171 ± 12 | 2.35 ± 0.31 | 74 ± 17 |
Blood sampling and platelet preparation
Blood was harvested from the retro-orbital venous plexus by puncture and collected in 1-mL polypropylene tubes (Eppendorf; Marsh Biochemical Products, Rochester, NY) containing 10% final volume of acid–citrate–dextrose, 38 mmol/L citric acid, 75 mmol/L trisodium citrate, and 100 mmol/L dextrose). Platelet-rich plasma was obtained by centrifugation at 220g for 7 minutes. The plasma and the buffy coat were gently transferred to a fresh polypropylene tube containing 0.7 mL of a washing buffer (129 mmol/L NaCl; 13.6 mmol/L trisodium citrate; 11.1 mmol/L dextrose, 1.6 mmol/L KH2PO4, pH 6.8) in the presence of 1 μmol/L prostaglandin E1 (Sigma, St. Louis, MO), and centrifuged at 160g for 4 minutes. The supernatant was transferred to 2 polypropylene tubes containing 3 mL of the washing buffer. After centrifugation at 2000g for 10 minutes, the pellet was resuspended in resuspension buffer (137 mmol/L NaCl; 4 mmol/L KCl; 0.5 mmol/L MgCl2; 0.5 mmol/L sodium phosphate; 11.1 mmol/L dextrose; 0.1% bovine serum albumin; 10 mmol/L HEPES, pH 7.4). Platelets were fluorescently labeled with calcein AM 0.25 μg/mL (Molecular Probes, Eugene, OR) for 15 minutes at room temperature. Fluorescent platelets (5 × 109 platelets/kg) were infused through the tail vein. In some experiments, washed platelets were incubated in the presence of 2 mg/kg inhibitory monoclonal antibody (mAb) against PECAM-1 (clone 390, Pharmingen, San Diego, CA)6 or 1 mg/kg inhibitory mAb against PSGL-1 (a kind gift of Dr D. Vestweber, Zentrum fur Molekularbiologie der Entzundung, Institut fur Zellbiologie, Munster, Germany)20 and then infused into the animals without removing the excess antibody. In other experiments, platelets from normal or mGPIb−/−;TghGPIb mice were resuspended in 0.01 mol/L Tris, 0.15 mol/L NaCl, pH 7.4 and incubated with 10 μg/mL mocarhagin (a kind gift of Dr M. C. Berndt, Baker Medical Research Institute, Melbourne, Australia) for 15 minutes at room temperature in the presence of 1 mmol/L CaCl2. The activity of mocarhagin was blocked by adding EDTA (0.01 mol/L final concentration). The treated platelets were then centrifuged at 2000g for 10 minutes and resuspended in the resuspension buffer described above, labeled with calcein AM, and injected intravenously.
Intravital microscopy
Immediately after infusion of fluorescent platelets, mice were anesthetized with 2.5% tribromoethanol (0.15 mL/10 g). An incision was made through the abdominal wall to expose the mesentery, and mesenteric venules of 100- to 200-μm diameter were studied. The shear rate was calculated using an optical Doppler velocimeter as described.10 Venules were visualized using a Zeiss (Oberkochen, Germany) Axiovert 135 inverted microscope (objective 32 ×, 0.4 NA) equipped with a 100-W HBO fluorescent lamp source (Opti Quip, Highland Mills, NY) with a narrow-band fluorescein isothiocyanate filter set (Chroma Technology, Brattleboro, VT) and a silicon-intensified tube camera C2400 (Hamamatsu, Tokyo, Japan) connected to an SVHS video recorder (AG-6730; Panasonic, Tokyo, Japan). One venule per animal was filmed for 4 minutes before the A23187superfusion (30 μL of a 10- μmol/L solution) and for 6 minutes thereafter. For the platelet adhesion study using histamine, recipient mice were treated intraperitoneally with 200 μL of a 1-mmol/L solution 15 minutes before the surgical procedure. Then 4 to 6 venules were sequentially observed for 4 minutes during the hour after the surgical procedure.
Video analysis
Platelets classified as adherent were platelets transiently captured by the endothelium that then translocated over a maximum distance of 15 μm (in a stop-and-go fashion) before being washed away in less than 3 seconds. The number of adhering fluorescent platelets was counted before and after A23187 superfusion. The number was determined over a 250-μm long and 150-μm wide venular segment visible on a given frame lasting 0.033 seconds. It was then translated to the number of fluorescent platelets adhering/mm2/frame.
Immunohistochemistry
Mouse mesentery was collected 1 minute after A23187 superfusion and fixed in phosphate-buffered saline containing 2% paraformaldehyde for 40 minutes. The biopsies were embedded in OCT, frozen in a methanol–dry ice mixture and stored at −80°C. Six-micrometer–thick cryostat sections were cut and transferred onto poly-l-lysine–coated slides (Sigma). Endogenous peroxidase activity was quenched by treating tissue sections with 3% hydrogen peroxide in phosphate-buffered saline for 10 minutes. PECAM-1 was detected on mouse mesenteric venules using a rat antimouse PECAM-1 monoclonal antibody (1:20 dilution; Pharmingen). A biotinylated rabbit antirat antibody (1:50 dilution; Vector Laboratories, Burlingame, CA) was used as the second antibody. Sections were then washed and treated sequentially with a streptavidin–peroxidase conjugate and a substrate–chromogen mixture according to an immunohistologic kit (Zymed, San Francisco, CA). Sections were counterstained with hematoxylin for 1 minute. Negative controls were obtained by omission of the primary antibody.
Platelet aggregation study
Normal washed platelets or mGPIb−/−;TghGPIb platelets were prepared as described above and resuspended in buffer (137 mmol/L NaCl; 4 mmol/L KCl; 0.5 mmol/L MgCl2; 0.5 mmol/L sodium phosphate; 11.1 mmol/L dextrose; 0.1% bovine serum albumin; 10 mmol/L HEPES, pH 7.4). The platelets (3 × 108 platelets/mL), were incubated with 1.5 mg/mL ristocetin (Sigma) in the presence of 1 mmol/L CaCl2 and 5 μg/mL human vWF (gift from Genetics Institute, Cambridge, MA). In some experiments, washed platelets from mGPIb−/−;TghGPIb mice were pretreated with 10 μg/mL mocarhagin for 15 minutes at room temperature before activation with ristocetin.
Platelet aggregation was initiated by stirring the platelet suspensions at 1000 rpm for 5 minutes at 37°C using a lumi-aggregometer (Sienco, Morrison, CO). The extent of aggregation was expressed in light transmission units.
Statistics
Results are reported as the mean ± SEM. The statistical significance of the difference between means was assessed by the Student t test.
Results
Large numbers of platelets adhere to and translocate on venules activated by calcium ionophore
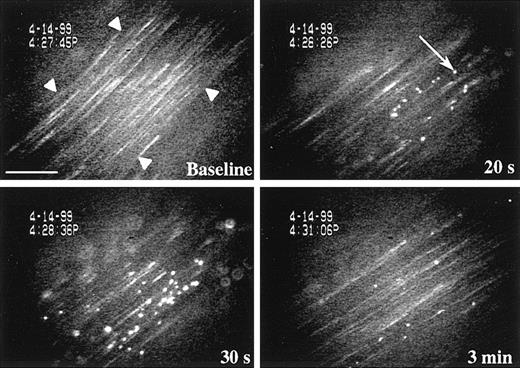
Platelet rolling on P-selectin expressed on endothelium activated with the secretagogue calcium ionophore A23187 or tumor necrosis factor-α was previously observed in small mesenteric venules (diameter, 25-35 μm; shear rate, approximately 500-600 seconds−1).9 10 In addition, we also detected in these venules a selectin-independent platelet adhesion that appeared to be particularly striking in larger venules (diameter, 100-200 μm) with lower shear. This prompted us to investigate platelet adhesion toA23187-stimulated large venules. The shear rate and size of the venules were similar in all the different genotypes studied (Table 1). Occasionally, a slight reduction in vessel diameter (10%-15%) was observed in the first 3 minutes after A23187 superfusion, but this did not significantly affect the shear rate (data not shown). Before treatment in wild-type animals, only 3 to 5 fluorescent platelets adhered transiently to the endothelium per minute. Fifteen seconds after treatment with A23187, many platelets began to adhere to and translocate on the endothelium (Figure1). This process reached a maximum (peak of adhesion) between 30 seconds and 1 minute (Figure2). An estimation of the number of adherent platelets per second was established at the peak of adhesion. Because platelets adhere for approximately 1 to 2 seconds, each adherent platelet was counted only once, at its first appearance, regardless of whether it was visible on subsequent frames. We found that approximately 2500 fluorescent platelets/mm2·s adhered at the peak of adhesion. Because approximately 10% of total platelets are fluorescent, a total of approximately 25 000 platelets/mm2·s adhered at the peak of adhesion, creating a carpet of adherent platelets on the vessel wall. We estimate that several platelets adhere per endothelial cell. This phenomenon progressively decreased with time, showing approximately 250 fluorescent platelets per second adhering 6 minutes after stimulation.
Resting platelets adhere on A23187-activated mesenteric venules.
Calcein AM-labeled platelets were visualized in mesenteric venules using a fluorescent microscope. Fluorescent resting WT platelets were transfused into WT recipient mice and began to adhere to the endothelium 15 seconds after topical superfusion of the calcium ionophore A23187. The adhesion process reached a maximum between 30 seconds and 1 minute and progressively decreased with time. Platelet aggregation was not observed. Arrowheads indicate the edge of the mesenteric venule. Arrow points to a fluorescent platelet adhering to the vessel wall. Bar, 75 μm.
Resting platelets adhere on A23187-activated mesenteric venules.
Calcein AM-labeled platelets were visualized in mesenteric venules using a fluorescent microscope. Fluorescent resting WT platelets were transfused into WT recipient mice and began to adhere to the endothelium 15 seconds after topical superfusion of the calcium ionophore A23187. The adhesion process reached a maximum between 30 seconds and 1 minute and progressively decreased with time. Platelet aggregation was not observed. Arrowheads indicate the edge of the mesenteric venule. Arrow points to a fluorescent platelet adhering to the vessel wall. Bar, 75 μm.
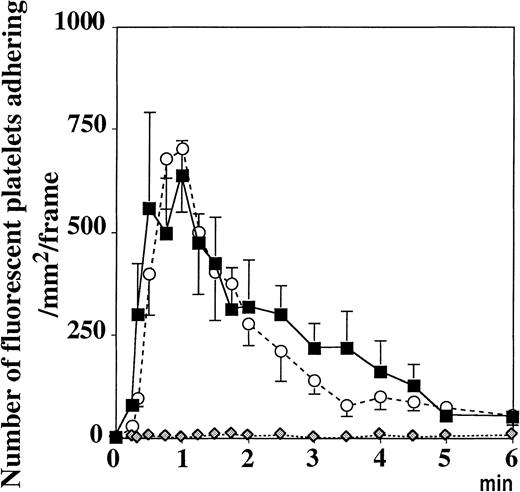
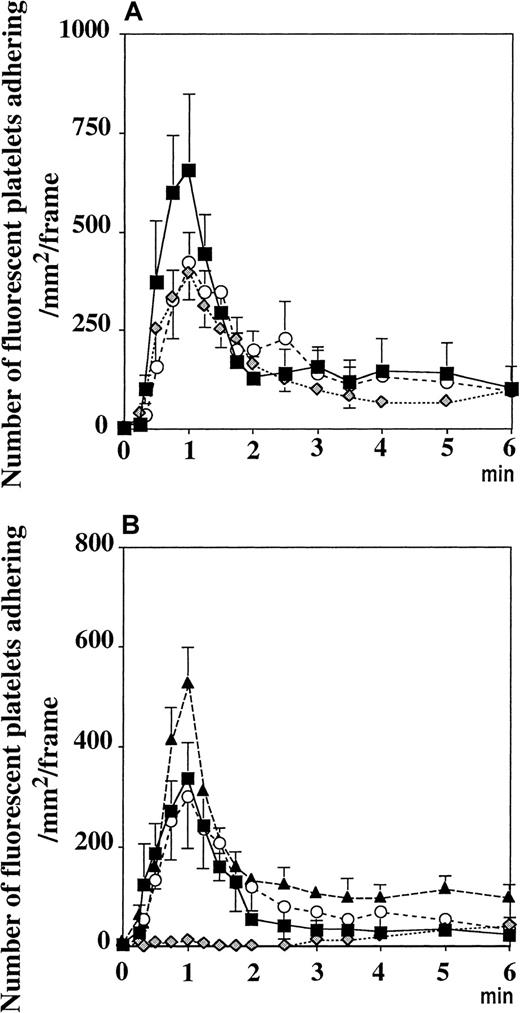
Resting platelets adhere to vWF in activated mesenteric venules.
Topical superfusion of A23187 on mesenteric venules induced the same platelet adhesion in WT (▪) and P−/− mice (○), n = 5 and n = 7, respectively. This process was absent in vWF−/− mice ( , n = 10, P < .001 vs WT mice for 6 minutes after A23187treatment). Values were determined on a 250-μm long, 150-μm wide vessel segment and were translated as the number of adherent fluorescent platelets/mm2·frame (see “Materials and methods”).
, n = 10, P < .001 vs WT mice for 6 minutes after A23187treatment). Values were determined on a 250-μm long, 150-μm wide vessel segment and were translated as the number of adherent fluorescent platelets/mm2·frame (see “Materials and methods”).
Resting platelets adhere to vWF in activated mesenteric venules.
Topical superfusion of A23187 on mesenteric venules induced the same platelet adhesion in WT (▪) and P−/− mice (○), n = 5 and n = 7, respectively. This process was absent in vWF−/− mice ( , n = 10, P < .001 vs WT mice for 6 minutes after A23187treatment). Values were determined on a 250-μm long, 150-μm wide vessel segment and were translated as the number of adherent fluorescent platelets/mm2·frame (see “Materials and methods”).
, n = 10, P < .001 vs WT mice for 6 minutes after A23187treatment). Values were determined on a 250-μm long, 150-μm wide vessel segment and were translated as the number of adherent fluorescent platelets/mm2·frame (see “Materials and methods”).
Platelets adhere to and translocate on vWF in activated venules
Because vWF is secreted from the Weibel-Palade bodies with A23187treatment, we performed the same experiment in vWF−/− mice perfused with platelets of the same genotype. The platelet adhesion process was totally absent (Figure 2). Similar results were obtained when wild-type platelets were infused in vWF−/− mice, suggesting that the vWF present in the endothelium or in the plasma, not in the platelet vWF, is responsible for this adhesion (not shown). In contrast, the injection of fluorescent P−/− platelets in P−/− mice resulted in platelet adhesion and translocation in A23187-treated venules similar to that observed in wild-type mice, indicating that this process was P-selectin independent (Figure 2).
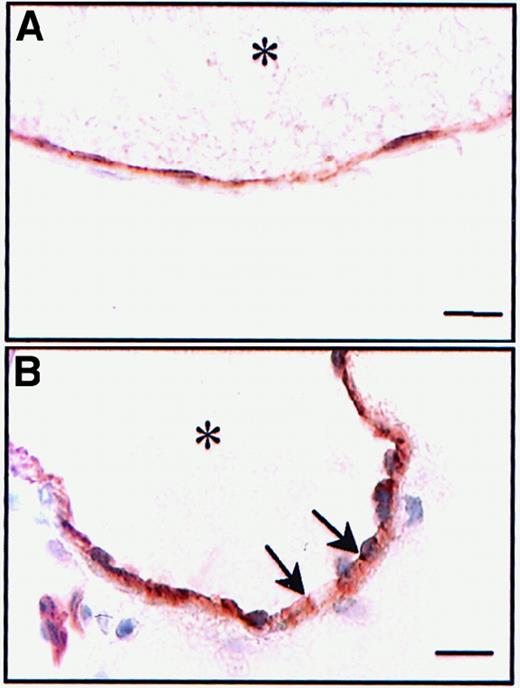
Calcium ionophore did not destroy the endothelial monolayer
In vitro, A23187 does not disrupt the endothelial monolayer,21 and though there is no indication that it does so in vivo,9 we decided to investigate this matter further. Indeed, because vWF is present not only in Weibel-Palade bodies of endothelial cells but also in the subendothelium, it was important to determine which compartment mediated the platelet adhesion. Immunohistochemistry was performed with an anti–PECAM-1 (CD31) on untreated vessels and on treated vessels, fixed 1 minute after A23187 superfusion. PECAM-1 staining at the junctions between endothelial cells was preserved after A23187 treatment (Figure3), indicating that the endothelial cell monolayer was not disrupted by the treatment. Thus, it appears that the released vWF presented by the luminal surface of the endothelial cell mediates platelet adhesion and translocation.
Persistence of the physical integrity of the endothelial layer after A23187 treatment.
Cryostat sections of untreated (A) or A23187-treated mesenteric venules (B) were incubated with a mAb directed against PECAM-1. The activated venules were collected 1 minute after A23187 treatment (peak of platelet adhesion). Immunoreactivity for PECAM-1 was not affected byA23187. Asterisk indicates vessel lumen. Bar, 10 μm.
Persistence of the physical integrity of the endothelial layer after A23187 treatment.
Cryostat sections of untreated (A) or A23187-treated mesenteric venules (B) were incubated with a mAb directed against PECAM-1. The activated venules were collected 1 minute after A23187 treatment (peak of platelet adhesion). Immunoreactivity for PECAM-1 was not affected byA23187. Asterisk indicates vessel lumen. Bar, 10 μm.
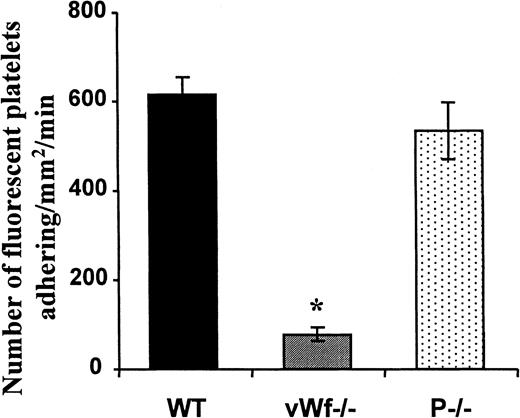
Platelets adhere to and translocate on vWF in histamine-treated venules
To examine whether vWF released from Weibel-Palade bodies could recruit platelets in inflamed venules, we injected 1 mmol/L histamine intraperitoneally in WT, P−/−, and vWF−/− mice and observed the platelet–vessel wall interactions 15 minutes later through intravital microscopy. In these conditions 2 different phenomena were observed. The first was P-selectin–dependent platelet rolling seen only in WT and vWF−/− mice (not shown). The second consisted of adhesion of platelets and stop-and-go translocation, similar to that observed inA23187-treated venules. This last adhesion process was identical in WT and P−/− mice but was close to absent in vWF−/− animals, indicating that vWF was also involved in the recruitment of platelets to the endothelium of vessels exposed to histamine (Figure4).
Resting platelets adhere to vWF in histamine-treated mesenteric venules.
Histamine (1 mmol/L) was injected intraperitoneally into recipient mice. Ten minutes later, washed and fluorescently labeled platelets from WT, P−/−, and vWF−/− mice were transfused into recipient mice of the same genotype. Analysis of the platelet–vessel wall interactions started immediately after platelet transfusion. The number of adherent fluorescent platelets/mm2·min, determined in a 4-minute period, was averaged from 4 to 6 venules in each animal. n = 4 to n = 6 animals; *P < .001 vWF−/− vs WT or P−/− mice.
Resting platelets adhere to vWF in histamine-treated mesenteric venules.
Histamine (1 mmol/L) was injected intraperitoneally into recipient mice. Ten minutes later, washed and fluorescently labeled platelets from WT, P−/−, and vWF−/− mice were transfused into recipient mice of the same genotype. Analysis of the platelet–vessel wall interactions started immediately after platelet transfusion. The number of adherent fluorescent platelets/mm2·min, determined in a 4-minute period, was averaged from 4 to 6 venules in each animal. n = 4 to n = 6 animals; *P < .001 vWF−/− vs WT or P−/− mice.
Identification of the receptor(s) for vWF
Pretreatment of the platelets with a mAb directed against PECAM-1 did not interfere with the adhesion process (Figure5A), indicating that PECAM-1–PECAM-1 interaction was not required. Similarly, pretreatment of the fluorescent platelets with an antibody directed against mouse PSGL-1 did not prevent the platelet adhesion process induced by A23187 (Figure5A), though this antibody did abolish leukocyte rolling (not shown). This showed that PSGL-1, which can mediate platelet rolling on P-selectin,20 was not the platelet receptor for vWF.
Platelets adhere to vWF released from activated endothelial cells in venules through the GPIbα receptor.
(A) Incubation of washed platelets with antibodies directed against PECAM-1 (○) or PSGL-1 ( ) did not affect their adhesion on mesenteric venules superfused withA23187. Similarly, β3−/− platelets transfused into β3−/− recipient mice adhered normally (▪). (B) Fluorescent P−/− platelets adhered to the activated vessel wall of GPIbα-deficient mice (▴), as did platelets with human GPIbα onto P−/− venules (○). Mocarhagin treatment of mGPIb−/−;TghGPIb platelets (
) did not affect their adhesion on mesenteric venules superfused withA23187. Similarly, β3−/− platelets transfused into β3−/− recipient mice adhered normally (▪). (B) Fluorescent P−/− platelets adhered to the activated vessel wall of GPIbα-deficient mice (▴), as did platelets with human GPIbα onto P−/− venules (○). Mocarhagin treatment of mGPIb−/−;TghGPIb platelets ( ), but not WT platelets (■), prevented the adhesion process. Values are expressed as number of adherent fluorescent platelets/mm2·frame (see “Materials and methods”).
), but not WT platelets (■), prevented the adhesion process. Values are expressed as number of adherent fluorescent platelets/mm2·frame (see “Materials and methods”).
Platelets adhere to vWF released from activated endothelial cells in venules through the GPIbα receptor.
(A) Incubation of washed platelets with antibodies directed against PECAM-1 (○) or PSGL-1 ( ) did not affect their adhesion on mesenteric venules superfused withA23187. Similarly, β3−/− platelets transfused into β3−/− recipient mice adhered normally (▪). (B) Fluorescent P−/− platelets adhered to the activated vessel wall of GPIbα-deficient mice (▴), as did platelets with human GPIbα onto P−/− venules (○). Mocarhagin treatment of mGPIb−/−;TghGPIb platelets (
) did not affect their adhesion on mesenteric venules superfused withA23187. Similarly, β3−/− platelets transfused into β3−/− recipient mice adhered normally (▪). (B) Fluorescent P−/− platelets adhered to the activated vessel wall of GPIbα-deficient mice (▴), as did platelets with human GPIbα onto P−/− venules (○). Mocarhagin treatment of mGPIb−/−;TghGPIb platelets ( ), but not WT platelets (■), prevented the adhesion process. Values are expressed as number of adherent fluorescent platelets/mm2·frame (see “Materials and methods”).
), but not WT platelets (■), prevented the adhesion process. Values are expressed as number of adherent fluorescent platelets/mm2·frame (see “Materials and methods”).
To determine whether the integrin αIIbβ3 was the receptor for vWF, we used β3−/− mice18 as platelet donors and recipients. A23187 induced the same response, indicating that αIIbβ3 did not mediate the binding to vWF and that integrin αvβ3 could not be the receptor either (Figure 5A).
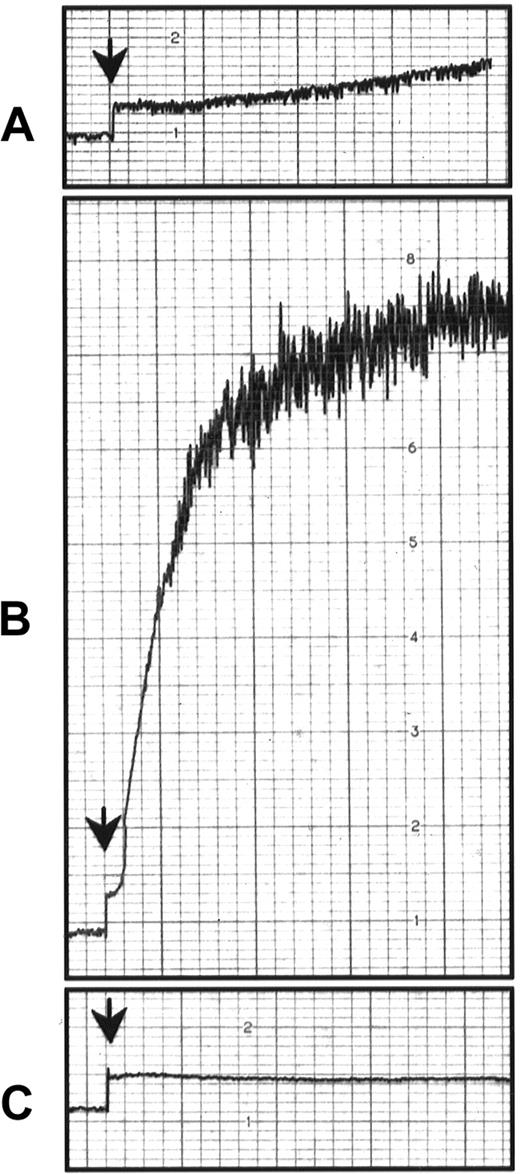
To determine a potential role of endothelial GPIbα in mediating platelet adhesion, we injected GPIbα−/− mice19 with P−/− fluorescent platelets. A23187 superfusion induced platelet adhesion, showing that endothelial GPIbα did not mediate the observed platelet adhesion (Figure 5B). To determine whether platelet GPIbα mediated the adhesion to vWF presented by endothelial cells in vivo, we used P−/− mice as recipient mice. This prevented the rolling of leukocytes after A23187 treatment and thus allowed us to concentrate on pure platelet–endothelial cell interactions. The investigation of the role of platelet GPIbα in mice is difficult because antibodies against GPIbα induce severe thrombocytopenia22,23 and GPIbα−/− mice have low numbers of platelets of aberrant size.19 Furthermore, mocarhagin, the cobra venom metalloproteinase generating the amino-terminal fragment (aa 1 to 282) of human GPIbα,24 does not cleave the mouse GPIbα (our personal observations; Figure 5B). To overcome this problem, we used platelets from the mGPIb−/−;TghGPIb mice, which express exclusively human GPIbα at the surface.19 It is known that human GPIbα can recognize mouse vWF and that mouse GPIbα does not recognize human vWF.25 We found that A23187 induced adhesion to and translocation of mGPIb−/−;TghGPIb platelets in P−/− mice. This process was abolished when the mGPIb−/−;TghGPIb platelets were preincubated with mocarhagin, whereas mocarhagin treatment did not affect the adhesion of WT platelets (Figure 5B). The GPIbα cleavage by mocarhagin was confirmed by its inhibition of the ristocetin-induced agglutination of washed mGPIb−/−;TghGPIb platelets in the presence of 5 μg/mL human vWF and 1 mmol/L CaCl2 (Figure6). This indicates that, similar to platelet adhesion to subendothelium, platelet adhesion to vWF bound on activated endothelial cells is mediated by platelet GPIbα.
Effect of mocarhagin on ristocetin-induced platelet aggregation in mGPIb−/−;TghGPIb mice.
Ristocetin is an antibiotic that causes platelet aggregation of human but not mouse platelets in the presence of human vWF.25Washed platelets (3 × 108) from WT mice (A) or mGPIb−/−;TghGPIb mice (B) were treated under stirring conditions (1000 rpm) at 37°C in a lumi-aggregometer, with 1.5 mg/mL ristocetin and 5 μg/mL multimeric human vWF. (C) Washed mGPIb−/−;TghGPIb platelets were incubated with 10 μg/mL mocarhagin before ristocetin treatment. Aggregation was followed for 2 minutes.
Effect of mocarhagin on ristocetin-induced platelet aggregation in mGPIb−/−;TghGPIb mice.
Ristocetin is an antibiotic that causes platelet aggregation of human but not mouse platelets in the presence of human vWF.25Washed platelets (3 × 108) from WT mice (A) or mGPIb−/−;TghGPIb mice (B) were treated under stirring conditions (1000 rpm) at 37°C in a lumi-aggregometer, with 1.5 mg/mL ristocetin and 5 μg/mL multimeric human vWF. (C) Washed mGPIb−/−;TghGPIb platelets were incubated with 10 μg/mL mocarhagin before ristocetin treatment. Aggregation was followed for 2 minutes.
Discussion
Platelet interactions with the vessel wall have been studied extensively in arteries, because platelet-rich thrombi form at sites of severe vascular injury and atherosclerotic lesions are critical events leading to arterial thrombosis and cardiovascular disease.26-28 Although venous thrombosis is generally thought to be initiated by factors of the plasma coagulation cascade, leading to the formation of red thrombi rich in erythrocytes and fibrin, platelets are also involved.29 However, the significance of platelet–vessel wall interactions in veins is not clearly defined. In this report we describe a prominent interaction between resting platelets and mesenteric venules in vivo that is both inducible and reversible. We have found that platelets can adhere transiently to stimulated endothelium and that this phenomenon is mediated by vWF as shown using gene-targeted mice (Figure 2). Our first concern on making this observation was whether the endothelium could be disrupted, leading to the exposition of subendothelial vWF. Several observations ruled out this possibility: (1) platelets adhered transiently on the activated surface without forming aggregates, suggesting that they were not adhering to subendothelium components that would allow activation and spreading30; (2) the platelet adhesion process in WT animals was paralleled by a rapid decrease in rolling leukocyte velocity (not shown), which demonstrated increased surface expression of P-selectin,17 likely from Weibel-Palade body exocytosis; (3) A23187 superfusion of veins in mice expressing the green fluorescent protein in endothelial cells30 did not affect the fluorescence intensity of these cells (not shown); (4) immunostaining for PECAM-1 revealed the typical junctional staining pattern for this protein and demonstrated that the endothelial cell layer was not denuded by the ionophore treatment (Figure 3). Thus, although the existence of small gaps between endothelial cells cannot be excluded, these observations strongly suggest that the circulating platelets translocated on the intact endothelium (ie, on vWF released from Weibel-Palade bodies). This adhesion phenomenon was different from the smooth rolling of platelets over P-selectin that has been described in small mesenteric venules superfused with calcium ionophore.9 In our experiment, we also observed P-selectin–dependent platelet rolling after calcium ionophore treatment (approximately 8 fluorescent platelets/mm2·s adhered to the vessel wall), but this was relatively minor when compared with the vWF-dependent adhesion–translocation of platelets (2500 fluorescent platelets/mm2·s at the peak of adhesion).
The extent of the adhesion phenomenon on vWF is even more striking if one considers that the calcium ionophore has been shown in vitro to induce a regulated secretion of vWF, of which 90% occurs in a basolateral direction.21 Thus, the 10% of vWF secreted on the luminal face of the endothelial cells is sufficient to induce this major platelet recruitment in veins. Interestingly, after this first peak of adhesion, we observed a rapid decrease in the number of adherent platelets, suggesting that the endothelial surface becomes less adhesive with time, possibly because the vWF is progressively washed away. This could explain why this adhesion process was more important in large venules than in small venules with a higher shear.
We next addressed the question of the platelet receptor for vWF under these low shear conditions. By modulating the vWF-GPIbα axis, we identified platelet GPIbα as the vWF receptor on platelets. The transient interaction and stop-and-go motion of the platelets suggested that the vWF–GPIbα interaction was insufficient to engage αIIbβ3 in an irreversible adhesion process to vWF. In that sense, it resembled the rolling/translocation of platelets or transfected cells expressing GPIbα on a vWF matrix observed in vitro under low shear conditions.14 31 In our model, the vWF/GPIbα axis seemed to be the only necessary pathway modulating this interaction because the blockade of P-selectin, PECAM-1, PSGL-1, αIIbβ3, and αvβ3 had no effect on the platelet adhesion process.
Our attempts to define the endothelial receptor for vWF have not been successful. However, we could exclude several important candidates: αvβ3 and GPIbα (Figure 5A). The presence of GPIbα on endothelial cells is still controversial.23,32-34 Although our study does not address this controversy, we did not find evidence that the released vWF is retained on the endothelial surface by GPIbα (Figure 5B). Our working hypothesis is that the adhesion of vWF to the endothelial cell surface is mediated by electrostatic charges presented by glycosaminoglycans because vWF has at least 2 well-defined heparin-binding sites.35 36
The physiologic relevance of the vWF-mediated platelet adhesion in veins is underlined by the fact that, in inflamed venules after histamine treatment, the same adhesion process occurred. Although we might have missed the peak of adhesion in that experiment, histamine seems to have a longer-acting effect (observed for more than 1 hour) than A23187, which may have a more synchronized release of Weibel-Palade bodies. A vWF-mediated platelet adhesion has also been described in vitro with human umbilical vein endothelial cells infected with the herpes virus.37 The virus induces Weibel-Palade body secretion leading to a vWF-GPIbα–mediated platelet adhesion to the infected cells. Actually, any situation that causes Weibel-Palade body exocytosis and exposure of the highly reactive forms of vWF and P-selectin is likely to lead to a rapid and important local accumulation of platelets and leukocytes. There is a great variety of such stimuli: hypoxia,38 viral or bacterial infection,37,39 thrombin,40,41 inflammatory mediators,42,43 complement component C5b-9,44oxygen radicals,45 or fibrin deposition.46Secretagogues for Weibel-Palade bodies are also likely to be produced in various pathologic situations, provoking an immediate adhesive response by “patrolling” platelets. The outcome of the platelet adhesion process would then depend on the severity of the endothelium disturbance. If platelet activation also occurs, platelets have the capacity to orchestrate additional responses. They release a number of potent proinflammatory cytokines and consequently modulate leukocyte function. Alternatively, their capacity to release growth factors, such as the platelet-derived growth factor or vascular endothelium growth factor, could allow platelets to play a role in angiogenesis.47 Platelet-endothelial cell adhesion may therefore represent a defense mechanism by which platelets would accumulate in the vicinity of an injury, making them available for immediate response. Unfortunately, in pathological settings, these platelets recruited to endothelium could initiate venous thrombosis.
In conclusion, we have described a new type of platelet–endothelium interaction in veins mediated by the vWF-GPIbα axis and resulting from the exocytosis of the Weibel-Palade bodies. Learning how to control the release of these storage granules would be of significant clinical importance to modulate both platelet and leukocyte recruitment.
Acknowledgments
We thank Dr M. Berndt for the mocarhagin and Dr D. Vestweber for monoclonal antibody to PSGL-1. We also thank Maria Economopoulos and Sangeetha Subbarao for mouse husbandry, and Lesley Cowan for help with the preparation of the manuscript.
Supported by National Institutes of Health grants P01HL56949 and R37HL41002 to D.D.W. and a Singer-Polignac grant to P.A.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Denisa D. Wagner, The Center for Blood Research, 800 Huntington Ave, Boston, MA 02115.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal