Abstract
We recently reported increased sensitivity of B-cell chronic lymphocytic leukemia (B-CLL) lymphocytes to apoptotic death activation by the proteasome-specific inhibitor lactacystin. Here, we show that only specific—not nonspecific—proteasomal inhibitors can discriminate between malignant and normal lymphocytes in inducing the apoptotic death response. Indeed, lactacystin and its active metaboliteclasto-lactacystin β-lactone induced apoptotic death in CLL but not in normal lymphocytes. This difference was completely abolished when tripeptide aldehydes such as MG132 or LLnL (which can also inhibit calpains) were used as less specific proteasomal inhibitors. Moreover, B-CLL cells exhibited a constitutive altered ubiquitin-proteasome system, including a threefold higher chymotrypsin-like proteasomal activity and high levels of nuclear ubiquitin-conjugated proteins compared with normal lymphocytes. Interestingly, B-CLL cells also displayed altered proteolytic regulation of wild-type p53, an apoptotic factor reported to be a substrate for the ubiquitin-proteasome system. Nuclear wild-type p53 accumulated after lactacystin treatment used at the discriminating concentration in malignant, but not in normal, lymphocytes. In contrast, p53 was stabilized by MG132 or LLnL in malignant and normal cells undergoing apoptosis, indicating that in normal lymphocytes p53 is regulated mainly by calpains and not by the ubiquitin-proteasome system. This work raises the possibility that two different proteolytic pathways controlling p53 stability may be pathologically imbalanced. This could result in modification of apoptosis control, since in CLL-lymphocytes a highly upregulated ubiquitin-proteasome system, which controls p53 stability among other apoptotic factors, was correlated with an increased propensity of these cells to apoptosis triggered by lactacystin.
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by an accumulation of quiescent monoclonal CD5+ B cells, which arise owing to undefined defects in apoptotic cell death.1 Investigations of the molecular mechanisms involved in resistance and sensitivity to drug- and radiation-induced apoptosis in these cells should offer new therapeutic strategies for the treatment of CLL.
We and other groups are interested in the possible role of the ubiquitin-proteasome system in this malignancy and its altered apoptotic death control. The ubiquitin-proteasome system consists of the covalent attachment of ubiquitin to proteins, targeting their degradation and/or activation through proteolytic processing by a multicatalytic complex, the proteasome.2,3 This system is an essential component of many cellular processes, such as cell cycle progression, transcriptional regulation, and antigen presentation.4,5 Several lines of evidence indicate that alterations in the ubiquitin system are involved in a broad range of processes related to tumor progression, including escape from immune control, drug resistance, alteration of cell cycle controls,6 and angiogenesis.7 Activation of the ubiquitin system is specifically associated with the initiation of radiation-induced apoptosis in normal human lymphocytes,8and proteasome activation occurs upstream from mitochondrial changes and caspase activation during apoptosis induced by dexamethasone or etoposide in thymocytes.9 These data and other reported studies demonstrate that the ubiquitin-proteasome system itself can play a central role in this pathway.10
An attractive feature is that inhibition of the proteasome has been shown to induce apoptosis in neoplastic cells, such as U937 myeloid leukemia cells,11 human leukemic (HL)-60 cells,12 and B-CLL cells.13,14The mechanisms underlying this pathway remain unclear and might involve the balance between proapoptotic and antiapoptotic factors regulated by the ubiquitin-proteasome system. Inactivation of the antiapoptotic nuclear factor (NF)–κB by proteasomal inhibition has been reported to promote apoptosis induction or apoptosis sensitization to tumor necrosis factor (TNF)–α,13 death-inducing ligands, or other drugs,15 but this is not a general rule,16 suggesting that other factors are involved.
One of the ubiquitin targets is the tumor suppressor p53 protein, which has a very short half-life within the cell and whose accumulation in response to stress, including DNA damage, is reported to play a central proapoptotic role.17,18 In human papillomavirus (HPV)–transformed cells, the well-defined E6/E6AP complex targets degradation of p53 by the ubiquitin-proteasome system while promoting oncogenicity.19 The regulation of p53 proteolysis is still obscure in non–HPV-transformed cells although evidence based primarily on the use of specific and nonspecific proteasomal inhibitors implicates the ubiquitin-proteasome system,20-24calpains, or both proteolytic systems.25-27Lactacystin, a Streptomyces-derived metabolite,28is a highly specific inhibitor of the proteasome. It requires transformation to an active analog, clasto-lactacystin β-lactone, in order to cross the cell membrane and further covalently modify the amino-terminal threonine of the mammalian proteasome subunit X/MB1.29,30 In contrast, tripeptide aldehydes, such as MG132, are less specific inhibitors of the proteasome since they can also inhibit calpains and cathepsins in vitro.31
We have recently reported that lactacystin induces apoptosis in lymphocytes derived from CLL. Interestingly, the same concentration of lactacystin failed to induce apoptosis in normal human lymphocytes,13 16 suggesting that the ubiquitin system might be altered and/or that apoptotic factors might be differentially expressed or regulated by this system.
Here we report that the sensitivity to apoptosis induction by MG132 or lactacystin in normal human lymphocytes is quite different and should therefore be linked to their respective specificity for proteolytic pathway inhibition. In an attempt to define the observed discrepancy in the sensitivity to lactacystin-induced apoptosis between normal and B-CLL cells, we studied the chymotrypsin-like activity of the proteasome, whole-protein ubiquitination, and regulation of the wild-type p53 protein, as a potential substrate of the ubiquitin system involved in apoptosis and tumor cell growth. Our results demonstrate that proteolytic pathways, especially the ubiquitin-proteasome system, may be modified in malignant cells, and that this may form the basis for altered sensitivity to apoptosis induction and cellular transformation.
Materials and methods
Isolation of human lymphocytes
Peripheral blood lymphocytes were recovered in heparin-coated tubes from normal and leukemic donors with their informed consent. This study was approved by the Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale (Paris-Cochin Ethics Committee). Lymphocytes from 50 leukemic donors were obtained at the time of diagnosis (stage A, before any treatment). After gradient separation (at 400g and 18°C for 40 minutes) in lymphocyte separation medium (BioWhittaker, Boehringer Ingelheim, Heidelberg, Germany), the mononuclear cell layer was washed twice with phosphate-buffered saline (PBS) solution. Monocyte cell populations were separated by adhesion on cell culture flasks (TPP, ATGC Biotechnology, Noisy-le-Grand, France) in RPMI 1640 (BioWhittaker) culture medium supplemented with 10% (vol/vol) heat-inactivated (30 minutes at 56°C) fetal calf serum (D. Dutscher, Brumath, France), 2 mmol/L L-glutamine, and nonessential amino acids (BioWhittaker) at 37°C in a humidified 5% CO2 atmosphere. After 30 minutes to 1 hour of cell culture, nonadherent cells (more than 90% of B lymphocytes) were recovered and cultured in the same medium under the same culture conditions.
Apoptotic cell detection
Chromatin-DNA was labeled with bisbenzimide Hoechst H33342(Molecular Probe Europe BV, Leiden, Netherlands) at 0.1 μg/mL for 30 minutes at 37°C and examined directly in Petriperm dishes (Bachoffer, Reutlingen, Germany) under an inverted fluorescence microscope (Leica, Wetzlar, Germany). Apoptotic cells with characteristic chromatin-associated fluorescence morphology (such as chromatin condensation and nuclear fragmentation) were visually counted immediately after lymphocyte isolation and also after 24 hours of cell culture and different cell treatments. At least 1000 cells were counted for each sample, and apoptosis was evaluated as mean percentage values (and standard deviations were calculated).
Cell treatments
Lactacystin 2.5 mmol/L,28clasto-lactacystin-β-lactone 2.5 mmol/L (Biomol, Plymouth Meeting, PA), LLnL 25 mmol/L (N-acetyl-leu-leu-norleucinal, calpain inhibitor I, Bachem, France), and MG132 1 mmol/L (N-carbobenzoxyl-leu-leu-leucinal, Biomol), were prepared in dry dimethyl sulfoxide and stored at −20°C prior to use. These inhibitors were added directly to the cell culture at the indicated final concentrations.
Determination of the chymotrypsin-like activity of the proteasome
Untreated lymphocytes (5.106 in 200 μL of PBS, pH 7.4) were incubated for 30 minutes at 37°C with the fluorogenic substrate N-succinyl-L-leucyl- L-leucyl-L-valyl-L-tyrosine-7-amido-4-methylcoumarin (Bachem) (100 μmol/L), and 7-amido-4-methylcoumarin fluorescence was measured in a spectrofluorometer (excitation wavelength, 380 nm; emission wavelength, 460 nm). Background values obtained after 30 minutes of treatment with 2.5 μmol/Lclasto-lactacystin β-lactone (or lactacystin) in peripheral blood lymphocytes (PBL) cells or B-CLL cells were subtracted from the experimental values. The activity was calculated as mean percentage values (with standard deviations).
Western blot analyses
Lymphocyte total protein extracts or cytoplasmic and nuclear protein extracts were prepared as previously reported.8 32 After sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), the proteins were transferred to pilyvinlidene fluoride (PVDF) membranes (Immobilion, Millipore, Bedford, MA) by means of a liquid transfer system (BioRad Laboratories, Hercules, CA). After overnight incubation in 4% nonfat milk in tris buffered saline (TBS) solution (10 mmol/L Tris and 150 mmol/L NaCl, pH 7.4) at 4°C, the membranes were incubated with p53 monoclonal antibodies DO-7 (a kind gift from T. Soussi) at a dilution of 1:1000 in TBS/Tween 20 (0.05%, vol/vol) for 2 hours at room temperature. We used β-catenin antibody (Transduction Laboratory, Lexington, KY) at 1:1000, actin antibody (Calbiochem-Novabiochem, La Jolla, CA) at 1:6000, and ubiquitin antibody (Calbiochem-Novabiochem) at 1:500 final concentration. A peroxidase-conjugated second antibody (Sigma Immunochemicals, St Louis, MO) and enhanced chemiluminescence system (Super Signal, Pierce, Rockford, IL) were used for detection at 1:20 000 dilution.
Results
Different sensitivity of CLL and normal lymphocytes to apoptosis induction by specific but not by nonspecific inhibitors of the proteasome
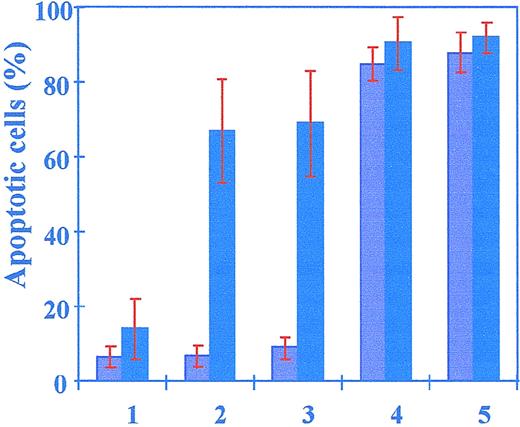
We have previously reported that a very low concentration of lactacystin (2.5 μmol/L) enhanced apoptosis in B-CLL but not in normal human lymphocytes, in which a tenfold higher concentration only weakly induced apoptosis.33 To confirm that this difference was indeed due to specific inhibition of the proteasome and not to an impaired mechanism of uptake and/or metabolism of the drug, B-CLL and normal cells were treated with clasto-lactacystin β-lactone (clasto-LA), reported to be the active lactacystin metabolite that can enter the cell. Clasto-LA (2.5 μmol/L) induced significant apoptosis in CLL-derived lymphocytes (69% ± 14%) but not in PBL (or normal B-lymphocytes derived from PBL after separation, not shown), where spontaneous apoptosis was weakly modified (6.5% ± 2.7%, 24 hours in culture without any treatment compared with 9% ± 1.7%, 24 hours after clasto-LA) (Figure 1). The difference in apoptosis induction between malignant and normal lymphocytes was confirmed with the use of the same concentration of lactacystin (2.5 μmol/L) (65% ± 13% for B-CLL cells versus 6.7% ± 3% for normal cells), suggesting that the entry of the inhibitor into the cell was not a limiting step in the sensitivity to lactacystin-induced apoptosis. Moreover, a tenfold higher concentration ofclasto-LA was necessary to weakly induce apoptosis (less than 30%, not shown) in normal human lymphocytes, and the difference between lactacystin- and clasto-LA–induced apoptosis was not significant in either malignant or normal cells, showing that both inhibitors were equally discriminating between the 2 cell types.
Apoptosis induction by specific and nonspecific inhibitors of the proteasome.
B-CLL cells (dark columns) and PBL cells (light columns) were untreated (lane 1) or treated with 2.5 μmol/L lactacystin (lane 2), 2.5 μmol/L clasto-lactacystin β-lactone (lane 3), 1 μmol/L MG-132 (lane 4), or 25 μmol/L LLnL (lane 5). Apoptotic values were determined 24 hours after cell treatments. The results represent analyses of cells from 50 patients and 20 normal donors. Error bars indicate the standard deviation.
Apoptosis induction by specific and nonspecific inhibitors of the proteasome.
B-CLL cells (dark columns) and PBL cells (light columns) were untreated (lane 1) or treated with 2.5 μmol/L lactacystin (lane 2), 2.5 μmol/L clasto-lactacystin β-lactone (lane 3), 1 μmol/L MG-132 (lane 4), or 25 μmol/L LLnL (lane 5). Apoptotic values were determined 24 hours after cell treatments. The results represent analyses of cells from 50 patients and 20 normal donors. Error bars indicate the standard deviation.
Surprisingly, tripeptide aldehydes, such as MG132 or LLnL, induced apoptosis in the same manner in both CLL and normal lymphocytes. Apoptosis was significantly enhanced in B-CLL and in normal cells treated with 1 μmol/L MG132 (90% ± 6.8% and 85% ± 4.3%, respectively) or 25 μmol/L LLnL (92% ± 4% and 88% ± 5.3%, respectively), and the difference between normal and B-CLL cells was not significant. Thus, tripeptide aldehydes reported to be nonspecific proteasomal inhibitors may alter intracellular factors involved in apoptosis control that are distinct from those targeted by lactacystin or clasto-LA, since they efficiently induced apoptosis in both malignant and normal lymphocytes. These results suggest that specific, but not nonspecific, inhibitors of the proteasome discriminate between B-CLL and normal cells.
Constitutive upregulated ubiquitin-proteasome system in B-CLL
In order to better define the molecular basis of the hypersensitivity of B-CLL to lactacystin-induced apoptosis, we studied the chymotrypsin-like activity of the proteasome, 1 of the 2 proteasomal proteolytic activities reported to be irreversibly inhibited by lactacystin. Untreated B-CLL cells showed threefold higher chymotrypsin-like activity (317 ± 34 arbitrary units) than untreated PBL (100 ± 20 arbitrary units) (Figure2). We next determined if the increased proteasomal activity in B-CLL cells could be associated with an altered level of ubiquitinated cellular proteins. The overall pattern of ubiquitin-conjugated proteins observed by Western blot was increased in nuclei of untreated B-CLL compared with unstimulated normal human lymphocytes (Figure 3). This was specific to nuclei-derived protein extracts since the same pattern was observed in cytoplasmic extracts between B-CLL cells and PBL. This is, to our knowledge, the first demonstration of a constitutive alteration of the ubiquitin system in these malignant B-CLL cells.
Constitutive proteasomal chymotrypsin-like activity.
Untreated B-CLL cells (dark columns) and PBL (light columns) were incubated with the fluorogenic substrate N-suc-L-leu-L-leu-L-val-L-tyr-AMC (100 μmol/L), and AMC fluorescence was measured in a spectrofluorometer (excitation wavelength, 380 nm; emission wavelength, 460 nm). The figure represents assays on cells from 20 patients and 20 normal donors. Error bars indicate the standard deviation.
Constitutive proteasomal chymotrypsin-like activity.
Untreated B-CLL cells (dark columns) and PBL (light columns) were incubated with the fluorogenic substrate N-suc-L-leu-L-leu-L-val-L-tyr-AMC (100 μmol/L), and AMC fluorescence was measured in a spectrofluorometer (excitation wavelength, 380 nm; emission wavelength, 460 nm). The figure represents assays on cells from 20 patients and 20 normal donors. Error bars indicate the standard deviation.
High levels of ubiquitinated nuclear proteins in B-CLL.
Cytoplasmic (A) or nuclear (B) ubiquitin-conjugated proteins of untreated PBL (lane 1) or untreated B-CLL cells (lane 2) were determined by Western blot in 10% SDS-PAGE. An equal amount of protein derived from 1.5 × 106 cells was deposited in each lane. The results represent analyses of cells from 10 different patients and 10 different normal donors.
High levels of ubiquitinated nuclear proteins in B-CLL.
Cytoplasmic (A) or nuclear (B) ubiquitin-conjugated proteins of untreated PBL (lane 1) or untreated B-CLL cells (lane 2) were determined by Western blot in 10% SDS-PAGE. An equal amount of protein derived from 1.5 × 106 cells was deposited in each lane. The results represent analyses of cells from 10 different patients and 10 different normal donors.
Modification of the proteolytic balance of wild-type p53 in B-CLL cells
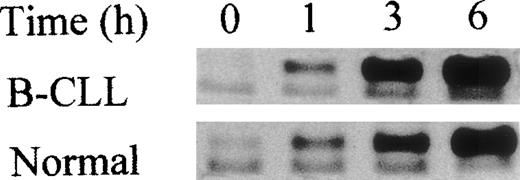
In an attempt to determine whether the abnormal sensitivity of B-CLL to specific proteasomal inhibitor-induced apoptosis could be linked to the activation of the wild-type p53 protein, we studied its accumulation in both normal and malignant nuclear extracts after proteasome inhibitor treatments. A very low concentration of lactacystin (2.5 μmol/L) caused a marked and rapid accumulation (as early as 3 hours) of wild-type p53 in B-CLL but not in normal cells (Figure 4). We observed that constitutive levels of p53 proteins and the levels of total proteins, evaluated with the actin antibody, were the same in both normal and malignant cells. Interestingly, accumulation of β-catenin at the same lactacystin concentration occurred in the same manner in malignant and normal cells (as early as 3 hours, Figure 4), suggesting that p53 regulation is not controlled principally by the proteasome in normal lymphocytes. This hypothesis was strengthened by the observation that a tenfold higher concentration of lactacystin, which initiated about 30% apoptotic death in normal cells, very weakly induced p53 accumulation in normal cells (data not shown). Since tripeptide aldehydes are nonspecific inhibitors of the proteasome and were found to induce apoptosis in the same manner (same concentration) in both malignant and normal lymphocytes, we studied p53 stabilization after tripeptide aldehyde treatment used at concentrations that efficiently induced apoptosis in both cell types. In both normal and malignant cells, MG132 (1 μmol/L) or LLnL (25 μmol/L, not shown) led to the accumulation of p53, which could be detected as early as 3 hours and increased for up to 6 hours after treatment (Figure 5). This suggests that proteolysis of p53 is controlled mainly by the calpain activities in normal lymphocytes. The alteration of the ubiquitin-proteasome system in B-CLL was associated with a modification of the physiological proteolytic balance of p53. This observation correlated with lactacystin-induced apoptosis in B-CLL but not in normal lymphocytes.
p53 accumulation after lactacystin treatment in B-CLL but not in normal lymphocytes.
Western blot analyses of β-catenin and nuclear wild-type p53 at different times after lactacystin (2.5 μmol/L) treatment of B-CLL or PBL compared with untreated cells (time 0). Note that an equal amount of protein (derived from 1.5 × 106 cells) was deposited in each lane and visualized by actin protein levels. The results represent analyses of cells from 10 different patients and 10 different normal donors.
p53 accumulation after lactacystin treatment in B-CLL but not in normal lymphocytes.
Western blot analyses of β-catenin and nuclear wild-type p53 at different times after lactacystin (2.5 μmol/L) treatment of B-CLL or PBL compared with untreated cells (time 0). Note that an equal amount of protein (derived from 1.5 × 106 cells) was deposited in each lane and visualized by actin protein levels. The results represent analyses of cells from 10 different patients and 10 different normal donors.
p53 accumulation in both B-CLL and normal lymphocytes after MG132.
Western blot analyses of the nuclear wild-type p53 at different times after MG132 (1 μmol/L) treatment of PBL or B-CLL cells versus untreated cells (time 0). An equal amount of protein (derived from 1.5 × 106 cells) was deposited in each lane. The same accumulation was obtained with LLnL (25 μmol/L) in both cell types. The results represent analyses of cells from 10 different patients and 10 different normal donors.
p53 accumulation in both B-CLL and normal lymphocytes after MG132.
Western blot analyses of the nuclear wild-type p53 at different times after MG132 (1 μmol/L) treatment of PBL or B-CLL cells versus untreated cells (time 0). An equal amount of protein (derived from 1.5 × 106 cells) was deposited in each lane. The same accumulation was obtained with LLnL (25 μmol/L) in both cell types. The results represent analyses of cells from 10 different patients and 10 different normal donors.
Lactacystin-induced apoptosis is independent of p53 transcriptional function
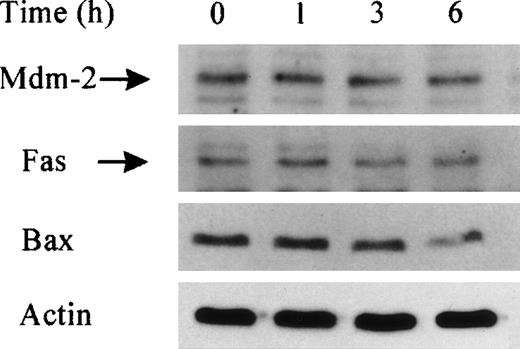
B-CLL cells with a mutated P53 gene (8% of all B-CLL so far studied) displayed the same sensitivity to lactacystin-induced apoptosis as B-CLL with wild-type p53, suggesting that factors independent of p53 and/or independent of its transcriptional function may be involved in this pathway. In order to verify this hypothesis, we studied the expression of 3 p53 reporter genes that may be involved in apoptosis control. Our data showed that the levels of mouse double minute (Mdm)-2 and of Fas and Bax proteins observed by Western blot (Figure 6) (or the messenger RNA [mRNA] of p21 and Bax, not shown) remained unaltered in B-CLL at different times after lactacystin treatment at concentrations that induced significant apoptosis. Our results indicate that lactacystin-induced apoptosis in malignant cells can occur without a transcriptionally active p53 and without the induction of some reporter genes. Further analysis is necessary to determine how lactacystin specifically induces apoptosis in B-CLL cells and the relationship between the reported increase in the ubiquitin system in malignant cells and apoptosis/oncogenesis controls.
Unaltered levels Mdm-2, Fas, and Bax in B-CLL after lactacystin treatment.
Western blot analyses of Mdm-2, Fas, and Bax in B-CLL total cell extracts at different times after lactacystin (2.5 μmol/L) treatment or without any treatment (time 0). Note that an equal amount of protein (derived from 1.5 × 106 cells) was deposited in each lane, visualized by actin protein levels.
Unaltered levels Mdm-2, Fas, and Bax in B-CLL after lactacystin treatment.
Western blot analyses of Mdm-2, Fas, and Bax in B-CLL total cell extracts at different times after lactacystin (2.5 μmol/L) treatment or without any treatment (time 0). Note that an equal amount of protein (derived from 1.5 × 106 cells) was deposited in each lane, visualized by actin protein levels.
Discussion
The functional complexity of the ubiquitin system in the living cell and the limitations of experimental approaches (ie, the use of inhibitors for which nonspecific actions in vivo cannot be ruled out a priori) may explain some conflicting published results. Here we demonstrate that only specific inhibitors of the proteasome used at very low concentrations can discriminate between B-CLL and normal human cells. Thus, B-CLL cells showed an increased sensitivity to lactacystin-induced apoptosis compared with normal cells. This discrepancy was also observed between B-CLL and clinical remission–derived cells.33 Compared with lactacystin, the same results were obtained withclasto-lactacystin β-lactone, showing that this difference was not caused by an impairment in the metabolism or intracellular uptake of the inhibitor.
In contrast, the discrepancy between normal versus CLL lymphocytes was not observed when tripeptide aldehydes were used, suggesting that these inhibitors have distinct targets. Indeed, tripeptide aldehydes have been directly or indirectly shown to inhibit proteases other than the chymotrypsin-like activity of the proteasome, including calpains in vitro and also in vivo.31,34,35 A hypothetical role for the ubiquitin-proteasome system in apoptotic death control should thus be interpreted with caution when only peptide aldehydes are used as proteasome inhibitors, especially because calpains themselves are reported to be involved in apoptosis regulation.36 The use of lactacystin, which has no apparent calpain inhibitor activity,34 should allow discrimination between specific and nonspecific proteasome-dependent apoptotic control.
In addition to the role of the ubiquitin system in apoptosis control, our data lend support to the involvement of this system in malignant transformation. Interestingly, untreated B-CLL exhibited a constitutive altered ubiquitin-proteasome system, including threefold higher chymotrypsin-like activity of the proteasome and higher nuclear ubiquitin-conjugated protein expression than unstimulated normal lymphocytes. These results are in agreement with other studies showing an increased level of ubiquitin-conjugated proteins and expression of the ubiquitin genes specific to various tumor cells37-39and abnormally high constitutive expression of the protein and/or mRNA of some of the proteasome subunits in human leukemic cells,40 human primary cancer liver and kidney cells,38 and breast cancer tissues.41 However, the role of the constitutive alteration of the ubiquitin system in transformation of B-CLL cells remains to be elucidated. We and others reported specific abnormal sensitivity to lactacystin-induced apoptosis in neoplastic cells such as B-CLL cells,13,14,16 Ewing's sarcoma cells,42 and c-myc-transformed Epstein-Barr virus–immortalized human lymphoblasts,43 as well as an inhibitory effect on angiogenesis,7 suggesting an anti-neoplastic role for lactacystin. Nevertheless, the precise mechanisms by which lactacystin acts remain to be established, since the complexity of the ubiquitin system (at least the part of the system already known) implies that it is involved in apoptotic control at several levels. One might suppose that the result of specific inhibition of the proteasome would depend on the balance between antiapoptotic versus proapoptotic substrates.
In a previous work, we investigated the proposed antiapoptotic role of NF-κB in CLL. However, we found that only in rare cases of B-CLL cells could the proteasome-dependent regulation of NF-κB and especially IκBα protein levels be directly associated with apoptosis induction or sensitization to TNF-α–induced apoptosis by lactacystin.13 In addition, we recently showed that this is not a general rule in B-CLL cells,16suggesting an involvement of other factors controlling apoptotic death processes.
Thus, we asked whether the stabilization of wild-type p53, a potential substrate of the ubiquitin system, occurred within cells after proteasomal inhibition as an event that might be involved in apoptosis control. Our results strongly suggest that in normal human lymphocytes, wild-type p53 stability is not principally regulated by the proteasome but rather by calpains. In contrast, in B-CLL cells, both proteasome and calpain activities might be involved in p53 regulation. Wild-type p53 protein accumulated in the nucleus of B-CLL cells treated with lactacystin at concentrations that induced apoptosis in malignant but not in normal lymphocytes. A tenfold higher lactacystin concentration giving about 30% apoptosis induction in normal cells correlated with only a very weak accumulation of p53 (not shown). In contrast, tripeptide aldehydes induced p53 stabilization and apoptosis in both normal and B-CLL lymphocytes. This is in agreement with other recent data reporting that p53 may also be regulated by calpains in certain cell/stimuli conditions, including MCF-7 breast carcinoma cells,26 Jurkat and ts20 cells expressing a thermosensitive activating–ubiquitin enzyme E1,27 as well as in normal human fibroblasts.44 Our conclusion is further strengthened by the observed accumulation of β-catenin, described as a specific substrate of the ubiquitin-proteasome system,45 after lactacystin treatment in malignant and normal cells.
Although B-CLL cells with a mutated P53 gene were also hypersensitive to lactacystin-induced apoptosis, a transcription-independent role of p53 in lactacystin-induced apoptosis cannot yet be ruled out, according to our work and other data reporting a proapoptotic role for p53 independent of its transcriptional activity.46-49 Indeed, Bax, Fas, and Mdm-2, whose gene expression can be controlled by p53, were not induced in parallel to lactacystin-induced apoptosis and wild-type p53 stabilization in B-CLL nuclei. This hypothesis is supported by Mimnaugh et al, who demonstrated that lactacystin inhibits the general transcription machinery in human breast tumor cells.50 Finally, our study provides strong evidence that the ubiquitin-proteasome system is pathologically upregulated in B-CLL cells, possibly accounting for the imbalance in proteolytic regulation of wild-type p53, among other factors and, consequently, contributing to altered apoptosis control and malignant transformation.
Acknowledgments
We are grateful to healthy and leukemic blood donors, to Mounira Amor-Guéret (UMR 1598, IGR, Villejuif) for helpful discussions and suggestions, Thierry Soussi (Institut Curie, Paris) for generously providing the p53 antibodies and helpful suggestions, and the Service d'Iconographie for help with the figures.
Supported by La Ligue Nationale Contre le Cancer, Commissariat àl'Energie Atomique (CEA/DSV), Institut Curie, and Electricité de France.
Reprints:Jozo Delic, Laboratoire de Recherche Correspondant n°2 du CEA (DSV-DRR)/Laboratoire de Radiopathologie, Institut Curie, 26, rue d'Ulm, 75005 Paris, France; e-mail:jozo.delic@curie.net.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal