We have studied the effect of transforming growth factor beta (TGFβ) on erythropoiesis in cultures from adult peripheral blood, using flow cytometric enumeration of fetal hemoglobin (HbF)-containing cells. TGFβ caused a dramatic increase in the proportions of cells that accumulated HbF together with adult hemoglobin (HbA) (F+A+ cells). This highly significant (P < .0001) increase in F+ cell proportion was achieved by TGFβ treatment during the first 4 days of culture and was sustained during further culture expansion in the absence of TGFβ. The increase in F+ cell proportions did not depend on the cytokine combination (EPO+SCF+IL3, EPO+SCF, EPO+IL3, SCF+IL3) used during the phase of TGFβ treatment. Increased F+ cell proportions were paralleled by an increased molecular ratio of HbF/ HbF+ HbA, measured by cation exchange high-performance liquid chromatography (HPLC). In addition to the effect on F+ cell proportions, TGFβ caused a dramatic increase in overall cell division potential. By the time cultures reached terminal growth arrest (12-14 days in controls and 18-26 days after TGFβ), the overall numbers of F+ cells produced per initially seeded clonogenic cell was approximately 10 times higher in the TGFβ-treated cultures than in the controls. We propose to investigate whether the TGFβ-induced increase in relative and absolute numbers of nucleated F+ cells, as demonstrated in vitro, can be translated into increased F+ erythrocytes in vivo, allowing therapeutic application for some beta-hemoglobinopathies.
Peripheral blood contains clonogenic cells that produce erythroid colonies and bursts in semisolid culture, given the appropriate combination of growth factors. Individual cells in such colonies can accumulate fetal hemoglobin (HbF), adult hemoglobin (HbA), or a combination of both. The pattern of hemoglobin expression and accumulation is different in cultures from fetal and adult blood. Fetal erythroid cells express only HbF for at least 1 week of culture, which is followed by low levels of HbA expression only after cells have reached maximum HbF levels.1,2 In cultures from adult blood, nucleated red cells accumulate either only HbA (F−A+) or a combination of HbF and HbA (F+A+).2-5 Individual colonies contain both F+ and F− cells, which means that both types are progeny from the same circulating stem cells.5,6 The proportion of F+ cells developing in cultures from normal adult peripheral blood is not preprogrammed in vivo but depends on culture conditions. A shift into the combined HbF and HbA expression pathway can, for example, be achieved in vitro by high serum concentrations,2 5-7 based on an unidentified activity that can be absorbed on activated charcoal. Thus, a mechanism must exist whereby cells during the early stages of development in a culture execute an option whether or not to express HbF.
The possibility to manipulate adult erythroid stem and progenitor cell development toward fetal erythropoiesis is of direct clinical interest because hemoglobin disorders such as sickle cell anemia and the beta-thalassemias are ameliorated by increased HbF production (reviewed by Jane, Cunningham, Olivieri, and Bunn8-10). Agents that stimulate HbF in vivo include 5-aza-cytidine, 11,12 hydroxyurea,13 and butyrates.14,15 These agents act via different mechanisms that are not yet completely understood. Their effectiveness has been demonstrated in several clinical trials, but is limited by unwanted side effects and variability in patient responses.8,9Although even minimal increases in HbF levels are helpful in sickle cell disease, beta-thalassemias require much higher increases that are not reliably achieved by any of the currently used agents.9Therefore, there is a need for additional and novel HbF-increasing agents.
We found in erythroid cell cultures from adult peripheral blood that TGFβ treatment caused a dramatic increase in the proportions of cells accumulating HbF, exceeding the effect of the highest usable serum concentrations. TGFβ has multiple, mostly inhibitory effects on hemopoiesis. It causes growth arrest and premature maturation in erythroid cultures,16-18 induces hemoglobin accumulation in erythroid cell lines,18-20 and also reversibly suppresses hemopoiesis in vivo.21-23 A role of TGFβ in the regulation of in vivo erythropoiesis might be implicated from a recent study showing a positive correlation between elevated plasma levels of TGFβ and HbF in patients with sickle cell disease.24 We show here that a relatively brief exposure to TGFβ during the first few days of cell culture causes a lasting increase in the proportions of HbF-expressing cells during continued clonal expansion, as well as an increase of overall division potential until terminal growth arrest. This effect did not depend on the combination of cytokines used to support erythropoiesis during TGFβ treatment. We suggest exploring whether short-term TGFβ treatment of stem cell preparations ex vivo, or a pulsed treatment in vivo, could be used for a therapeutic increase in fetal hemoglobin production.
Materials and methods
Blood samples
Peripheral venous blood samples were collected in vacuum glass tubes containing ethylenediamine tetraacetic acid (EDTA) as an anticoagulant (Becton-Dickinson, Franklin Lakes, NJ) from adult donors under a protocol approved by the Institutional Review Board at New England Medical Center.
Sera
Fetal calf serum (FCS) (dialyzed with 10 000 MW cutoff) was purchased from Sigma (St Louis, MO). Human umbilical cord blood was collected without anticoagulant, the clots removed, the remaining blood centrifuged at 3000 rpm for 20 minutes and the supernatant serum (CHS) collected. CHS was extracted twice with 3 volumes of chloroform, then charcoal treated twice by suspending 1 g of acid-washed charcoal (Norit SX4; Norit, Amsterdam, The Netherlands) in 50 mL serum for 30 minutes at 4°C. The charcoal was sedimented by centrifugation. Chloroform extraction removed some growth-inhibitory activities, and charcoal treatment removed all HbF-stimulating activity.
Cell culture
Blood samples were kept at room temperature and processed as soon as possible (between 2 and 24 hours after collection). The blood was diluted 1:4 with phosphate-buffered saline (PBS), the mononuclear cells isolated by density gradient (density 1.077), washed in PBS with 1% bovine serum albumin (BSA), and cultured without further processing in standard 6-well plates, 3 mL per well, at a maximum density of 0.3 million per milliliter. The standard medium (referred to as “control” medium) was a mixture of two-thirds Iscoves MDM and one-third RPMI1640, containing methylcellulose (0.9%); charcoal-treated (C)-CHS (1%); erythropoietin (EPO) (1 U/mL); stem cell factor (SCF) (20 ng/mL); interleukin 3 (IL3) (10 ng/mL); insulin (3 μg/mL); iron-saturated transferrin (70 μg/mL); and mercaptoethanol (0.7 mmol/L). Cytokines were from R&D Systems or Genzyme (Cambridge, MA). FCS was used at 30%. Recombinant human TGFβ1, 2, and 3 were from R&D Systems (Minneapolis, MN). TGFβ (rhTGFβ1) was added at 10 ng/mL, unless indicated otherwise. TGFβ or FCS was removed from cultures by washing the cultures twice in PBS/BSA, then reseeding in fresh control medium. At selected times, whole cultures containing a large number of colonies were harvested (ie, the colonies mixed into a single-cell suspension) and the cells processed for flow cytometric analysis.
Cell labeling
Cells were fixed with 5% formaldehyde in PBS at 37°C for 1 hour, exposed to 100% methanol for 5 minutes at room temperature, then permeabilized in Solution B of the Caltag Fix & Perm kit (Caltag; Burlingame, CA) during incubation with phycoerythrin (PE)-conjugated antibodies to the gamma chain of hemoglobin (HbF) (Cortex, San Leandro, CA) and fluorescein isothiocyanate (FITC)-conjugated antibodies to the beta chain of hemoglobin (HbA) (Wallac, Akron, OH) or FITC-conjugated antibodies specific for sickle cell hemoglobin (HbS) (Wallac). After labeling, cells were washed twice and suspended in PBS with 1% formaldehyde and 0.2 μg/mL Hoechst 33342.
Flow cytometry
Cells were processed in a Beckton-Dickinson Vantage flow cytometer/cell sorter with dual, displaced-beam laser excitation. Hoechst 33324 fluorescence (430 nm) was excited by ultraviolet, and PE and FITC were excited at 488 nm and measured at 530 and 575 nm, respectively. Correlated fluorescence values were recorded with color compensation for PE and FITC. The accuracy of color compensation over 4 logs of fluorescence values was limited.
Intact nucleated cells were selected for all display and numerical analysis by gating on DNA-specific Hoechst fluorescence. Most nonproliferative cells that had died (by apoptosis or necrosis) during the time between seeding and cell harvest were not included in the DNA-gated profiles because they disintegrated in culture and/or during the multiple preparation steps from harvest to analysis. This was confirmed by tests comparing viable cell counts before fixation with Hoechst-gated cell counts at the time of hemoglobin analysis.
The proportion (given in percentage) of F+ cells is defined as the number of all cells that label with HbF antibody (F+A− plus F+A+, as indicated in the first profile of Figure1), divided by the number of all cells labeling with any of the 2 hemoglobin antibodies (ie, the sum of F+A−, F+A+, and F−A+ cells). Cells negative for both antibodies (eg, nonerythroid cells) were not included in the calculation.
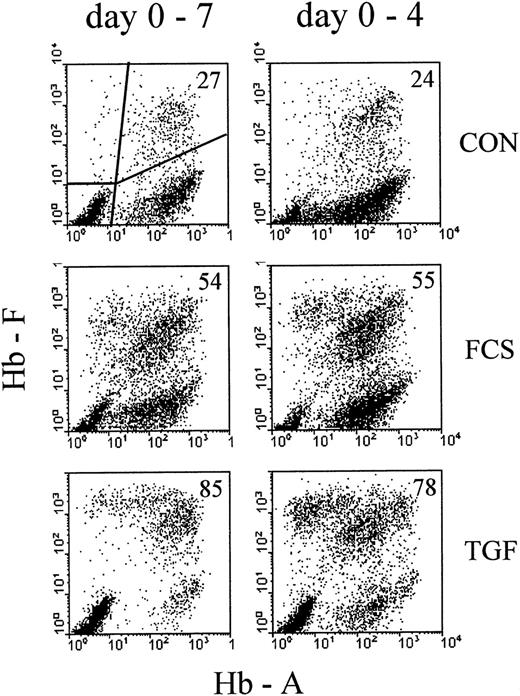
Profiles of correlated cellular hemoglobin contents after treatment with 30% FCS or 10 ng/ mL rhTGFβ1.
Comparison between continuous exposure (days 0-7, left column) and exposure for the first 4 days of culture (days 0-4, right column). Analysis on day 7 of culture. Cultures were mixed into single-cell suspensions and the correlated contents of HbF and HbA for each individual cell measured by 2-color flow cytometry. Each analyzed cell sample results from a mixture of about 100 colonies. Each profile results from 10 000 intact nucleated cells with normal DNA content, gated by Hoechst fluorescence (see “Materials and methods”). The proportions of F+ cells (percentage of all Hb+ cells, see “Materials and methods”) are indicated in the upper right corner of each profile.
Profiles of correlated cellular hemoglobin contents after treatment with 30% FCS or 10 ng/ mL rhTGFβ1.
Comparison between continuous exposure (days 0-7, left column) and exposure for the first 4 days of culture (days 0-4, right column). Analysis on day 7 of culture. Cultures were mixed into single-cell suspensions and the correlated contents of HbF and HbA for each individual cell measured by 2-color flow cytometry. Each analyzed cell sample results from a mixture of about 100 colonies. Each profile results from 10 000 intact nucleated cells with normal DNA content, gated by Hoechst fluorescence (see “Materials and methods”). The proportions of F+ cells (percentage of all Hb+ cells, see “Materials and methods”) are indicated in the upper right corner of each profile.
To determine absolute cell numbers in any area of the hemoglobin profiles, known amounts of fluorescent plastic beads (Immunobrite Level IV; Coulter, Miami, FL) were added to each cell suspension. The absolute cell numbers per sample could then be calculated from the ratio of cells to beads, multiplied by the number of beads added to the sample.25 For the display of Hb profiles, the beads were gated out. The flow cytometric study of erythropoiesis as used here is further described and discussed in Bohmer.26
Statistics
Each analyzed cell sample resulted from a mixture of large numbers of colonies. Experiments in which cultures turned out to contain far less than 100 colonies (per condition analyzed) were not used for this presentation, because the variation of profiles between individual colonies is significant.27 From each prepared cell sample, at least 20 000 particles (intact nucleated cells plus added beads) were analyzed in the flow cytometer, and each hemoglobin profile is presented with 10 000 cells. The standard error of measurement between replicate identical cultures within the same experiment, or between replicate flow cytometry runs from the same harvested culture, was found to be reliably less than ±5% of the measured value. Between cultures derived from different donors, the variability of numerical results was more substantial, and time course data from separate experiments could not be pooled with meaningful normalization. Therefore, some kinetic results are demonstrated in form of an individual representative experiment, and the statistics between independent experiments are shown separately.
High-performance liquid chromatographic analysis of hemoglobins
Hemoglobins present in the cell lysates were separated by high-performance liquid chromatography (HPLC) using a PolyCAT A cation exchange column (PolyLC, Columbia, MD), 200 × 4.6 mm using the buffer system described in Ou and Rognerud.28 The buffer gradient was delivered via a Rainin HPX HPLC system (Rainin Instrument Corp, Woburn, MA) and hemoglobins detected at 415 nm using a Perkin-Elmer 785A UV/Vis detector (Perkin-Elmer, Norwalk, CT). Hemoglobins in the lysates were identified by comparing retention times with known hemoglobin standards used for isoelectric focusing (Wallac).
Results
Relative increase in fetal hemoglobin–containing cells by serum or transforming growth factor beta
To compare the well-known effect of high serum concentrations with the effect of TGFβ, we cultured mononuclear cells from adult blood with 30% FCS or 10 ng/mL rhTGFβ-1 (TGF). The control (CON) contained 1% charcoal-treated human cord serum, a supplementation that allows fast colony growth while minimizing the proportions of HbF-containing cells.27 On day 4, part of the cultures were washed and reseeded in control medium without FCS and TGF, the other part remaining unmodified until the time of harvest. On day 7, cultures containing about 100 colonies were turned into single-cell suspensions and the correlated contents of HbA and HbF measured by flow cytometry (Figure 1). In the control cultures, the large majority of nucleated red cells were clustered with a range of HbA levels but with little or no HbF (F−A+). A small proportion contained HbF, together with HbA (F+A+). Some cells were F+A−, and some were spread over other areas of the profile. The subdivision chosen for numerical evaluations is indicated in the first profile, and the percentage of F+ cells (see “Material and methods” for definition) is indicated in each profile. More data on the time course of hemoglobin accumulation in erythroid cultures are shown elsewhere.2,26 27 TGFβ increased the proportions of F+ cells more strongly than 30% FCS. Four days of exposure to the HbF-inducing agents (right column of Figure 1) increased the proportions of F+ cells nearly as effectively as continuous exposure up to the day of analysis.
In contrast to the 4-day treatment, continuous exposure to TGFβ (day 0-7) resulted in small colonies that appeared to consist mostly of apoptotic/necrotic cells. Therefore, continuous TGFβ treatment was not considered of further interest for the purpose of this study.
Near-maximum F+ proportions by brief transforming growth factor beta treatment in the early culture phase
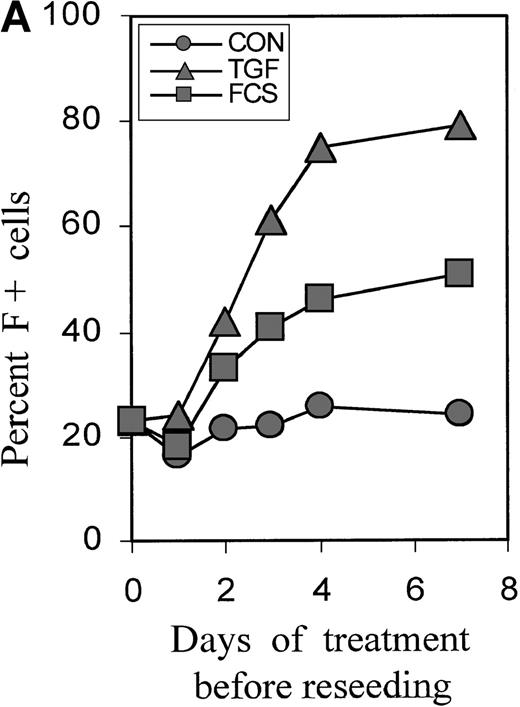
Cultures were treated with TGFβ or FCS for 0, 1, 2, 3, and 4 days, then reseeded without TGFβ, and the proportions of F+ cells were determined on day 7. Figure 2A shows the time dependence using a representative experiment (Figure 2B). A small increase in F+ cells could be seen after a 2-day treatment, and the effect was nearly maximal with a 4-day treatment. The time course was similar for FCS and TGFβ. The proportions of F+ cells reached a plateau at approximately 50% with 30% FCS and at approximately 80% with TGFβ. Baseline and induced plateau levels varied between experiments from different blood donors. Further experiments focused on TGFβ as the more powerful inducer of F+ cells.
Short-term incubation with FCS or TGFβ1.
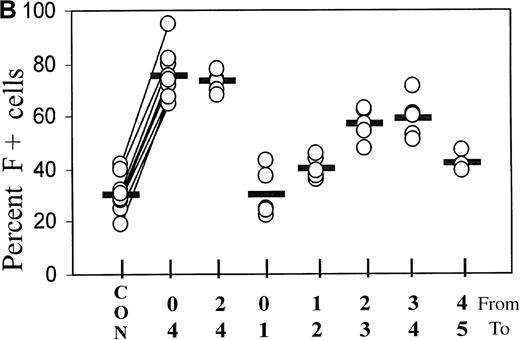
(A) Cultures initiated in the presence of TGFβ1 or FCS were washed and reseeded in control medium after 0, 1, 3, and 4 days. The percentage of F+ cells was determined on day 7 of culture. The data are from 1 representative experiment. (B) Statistics of the TGFβ1 effect in cultures from different donors, comparing various treatment timings between days 0 and 5. Measurements on day 7. The values from all individual cases (open circles) are shown to indicate range and distribution, and the median values are shown as horizontal bars. On the x-axis label, the upper row of numbers (from) indicates the starting day of treatment, and the lower row (to) the ending day of treatment. In the case of 4-day treatment, data pairs are connected. There is a strong positive correlation (r = +0.84) between the variations of TGF-induced F+ cell proportions and the variations of the corresponding baseline F+ cell proportions. The effect of 4-day treatment is statistically highly significant withP < .0001.
Short-term incubation with FCS or TGFβ1.
(A) Cultures initiated in the presence of TGFβ1 or FCS were washed and reseeded in control medium after 0, 1, 3, and 4 days. The percentage of F+ cells was determined on day 7 of culture. The data are from 1 representative experiment. (B) Statistics of the TGFβ1 effect in cultures from different donors, comparing various treatment timings between days 0 and 5. Measurements on day 7. The values from all individual cases (open circles) are shown to indicate range and distribution, and the median values are shown as horizontal bars. On the x-axis label, the upper row of numbers (from) indicates the starting day of treatment, and the lower row (to) the ending day of treatment. In the case of 4-day treatment, data pairs are connected. There is a strong positive correlation (r = +0.84) between the variations of TGF-induced F+ cell proportions and the variations of the corresponding baseline F+ cell proportions. The effect of 4-day treatment is statistically highly significant withP < .0001.
Figure 2B further explores the timing of TGFβ treatment, showing the statistics of experiments with the blood from many different donors. Treatment from day 0 to 4 is compared with treatments using narrower intervals (days 2 to 4 and 1-day intervals within the first 5 days of culture). The difference between baseline and F+ proportions induced by 4-day TGFβ treatment was statistically highly significant, withP < .0001. The value pairs are connected and demonstrate a positive correlation between the variations of baseline values and inducible F+ proportions (r = +0.84). Treatment from day 2 to day 4 was as effective as treatment from day 0 to day 4. Although the treatment between day 0 and day 1 had no significant effect, a treatment for as little as 1 day between day 2 and day 4 caused a substantial increase in the proportions of F+ cells. After day 4, the potential of TGFβ to induce F+ cells decreased (see also Figure 4). The effects of TGFβ treatments for less than one cell cycle duration (ie, less than 18 hours) will be the subject of a separate study.
Relative effectiveness of different forms of transforming growth factor beta
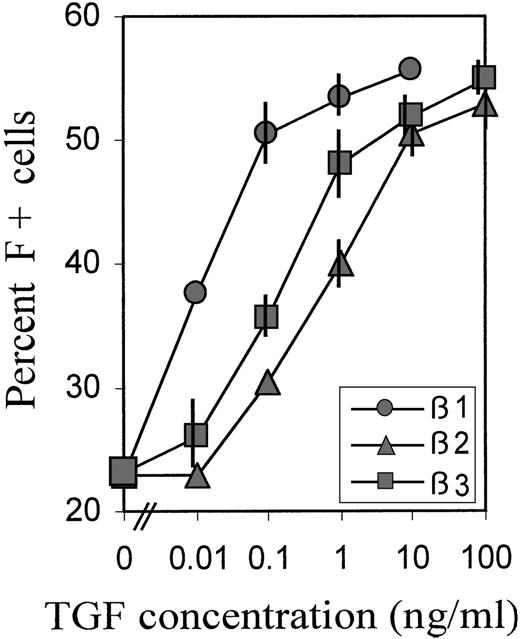
To investigate the relative potencies of different forms of TGFβ to increase the proportions of F+ cells, we titrated TGFβ 1, 2, and 3 over a wide range of concentrations. The averaged data of 2 experiments with blood cells from different donors are shown in Figure3. For the titrations, we chose a relatively brief (38 hours) TGFβ exposure between days 2 and 4, during the TGF-sensitive culture phase (Figure 2B), because we observed that, in this case, the titration curves shifted to lower concentrations, compared with treatments from day 0 to day 4. This may be due to some TGF degradation from the beginning of its presence in culture. TGFβ-1 was approximately 100 times more potent than TGFβ-2, and approximately 50 times more potent than TGFβ-3, with potency defined by the concentration required to achieve half-maximal effect. However, the maximum achievable (plateau) levels of F+ cell proportions were the same with all 3 forms of TGFβ, indicating that this level was determined by the treatment schedule.
Relative potency of different forms of TGFβ.
Cultures were treated with a wide range of concentrations of TGFβ 1, 2, and 3 between day 2 and day 4 (hour 52 → hour 90), then reseeded and grown in TGFβ-free medium. The percentage of F+ cells was determined on day 8. Data are average values from 2 independent experiments with blood from different donors. The range of values is indicated by vertical bars. Some bars are not visible because they fall within the extension of the symbols.
Relative potency of different forms of TGFβ.
Cultures were treated with a wide range of concentrations of TGFβ 1, 2, and 3 between day 2 and day 4 (hour 52 → hour 90), then reseeded and grown in TGFβ-free medium. The percentage of F+ cells was determined on day 8. Data are average values from 2 independent experiments with blood from different donors. The range of values is indicated by vertical bars. Some bars are not visible because they fall within the extension of the symbols.
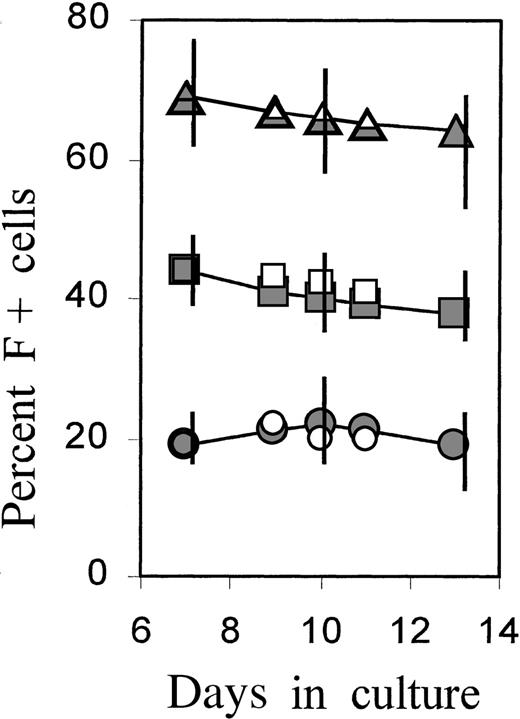
F+ cell proportions persist during further culture expansion
The proportions of F+ cells during further culture development were monitored in separate experiments (Figure4). After a 4-day treatment with TGFβ or serum, cultures were grown in control medium and further subcultivated on days 7 and 10 to minimize the exhaustion of medium components or the potential accumulation of inhibitory factors by the rapidly expanding cell mass. The time course is shown as a representative experiment in which all conditions were investigated simultaneously on cells from the same donor. The data from 3 further independent experiments are given as vertical bars, indicating the range of measured values. The increased proportions of F+ cells, as introduced by brief TGFβ or serum treatment, were maintained during further culture growth without TGFβ or serum, indicating that F+ and F−A+ cells were expanding at approximately the same rate. A gradual small decline in F+ proportions was observed in the TGFβ-treated cultures, but the proportions did not approach the levels of untreated cultures (see also Figure 7A).
Persistence of F+ cell proportions during the later culture phase.
Cultures were incubated with TGFβ or FCS for 4 days, then washed and reseeded in fresh control medium. On days 7 and 10, the cultures were further diluted (1:10 and 1:5, respectively) in fresh control medium (full symbols) or medium supplemented with TGFβ1 (open symbols). Percentage of F+ cells was determined at selected times between day 7 and day 13. The time course is shown from 1 individual experiment in which all conditions were investigated together on the cells from the same donor. The vertical bars on days 7, 10, and 13 show the ranges of results obtained in 4 independent experiments with blood from 4 different donors.
Persistence of F+ cell proportions during the later culture phase.
Cultures were incubated with TGFβ or FCS for 4 days, then washed and reseeded in fresh control medium. On days 7 and 10, the cultures were further diluted (1:10 and 1:5, respectively) in fresh control medium (full symbols) or medium supplemented with TGFβ1 (open symbols). Percentage of F+ cells was determined at selected times between day 7 and day 13. The time course is shown from 1 individual experiment in which all conditions were investigated together on the cells from the same donor. The vertical bars on days 7, 10, and 13 show the ranges of results obtained in 4 independent experiments with blood from 4 different donors.
To assess the effect of TGFβ at this later culture phase, parts of the cultures in the same experiment were newly supplied with TGFβ beginning on day 7 (data included with open symbols in Figure 4). Added at this later stage of culture, TGFβ had no effect on F+ proportions but caused near-complete growth arrest after 3 to 4 days (not shown). Thus, growth inhibition by TGFβ occurred with the same kinetics in F+ and F−A+ cells. In parallel, TGFβ caused an accelerated accumulation of HbA in both F+ and F− cells. These data are not within the scope of this presentation and will be shown in a separate study. The findings are in agreement with a previous report demonstrating that TGFβ caused growth arrest, combined with accelerated maturation and hemoglobin accumulation.17
Stimulation of F+A− cells and transition to F+A+
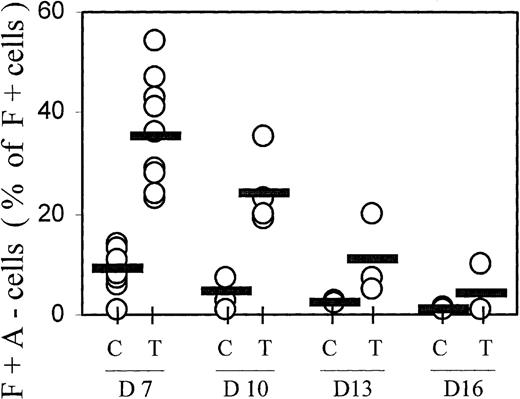
The hemoglobin profiles of TGFβ-treated cultures showed not only increased proportions of F+ cells, but also a change in the distribution within the F+ cell population, with increased proportions of cells containing high levels of HbF but little HbA (F+A−) (see profiles in Figures 1 and 8). This population of F+A− cells is reminiscent of nucleated red cells in cultures from fetal blood, in which all cells initially accumulate only HbF for at least 1 week, and do not begin any HbA accumulation before they have reached maximum HbF.2 27 The data from many independent experiments are compiled in Figure 5. On day 7, the proportions of F+A− cells (given as a percentage of F+ cells, not of all hemoglobin-containing cells) averaged approximately 10% in controls and approximately 40% in TGFβ-treated cultures. The difference in F+A− proportions between TGFβ-treated and control cultures is statistically significant with P < .0001. With increasing culture time, the proportions of F+A− cells within the F+ population declined gradually. This decline suggests that the TGFβ-induced F+A− population moved into the F+A+ compartment during further culture growth.
Progression from F+A− to F+A+ with time in culture.
F+A− cells (defined in Figure 1) were quantitated at different culture times (days 7, 10, 13, and 16) and expressed as a percentage of all F+ cells (note: not of all Hb+ cells). Results from all individual cases are shown (open circles) to indicate range and distribution of values. Averages are shown by horizontal bars. C = control; T = TGFβ treatment. The difference in F+A− proportions between TGFβ-treated and control cultures on day 7 is significant with P < .0001.
Progression from F+A− to F+A+ with time in culture.
F+A− cells (defined in Figure 1) were quantitated at different culture times (days 7, 10, 13, and 16) and expressed as a percentage of all F+ cells (note: not of all Hb+ cells). Results from all individual cases are shown (open circles) to indicate range and distribution of values. Averages are shown by horizontal bars. C = control; T = TGFβ treatment. The difference in F+A− proportions between TGFβ-treated and control cultures on day 7 is significant with P < .0001.
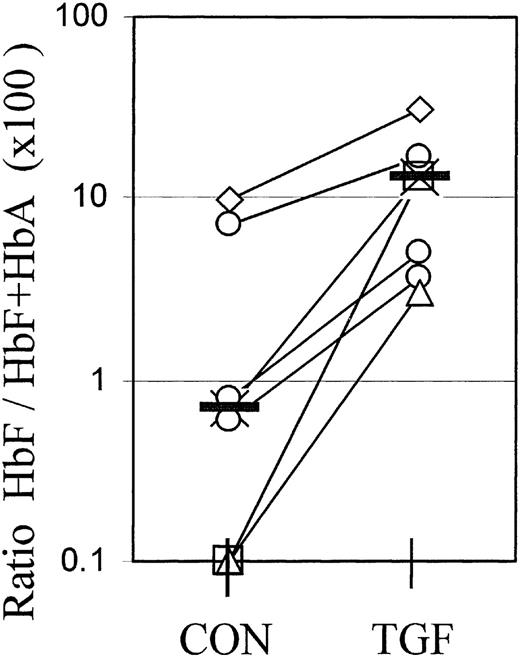
Molecular ratios of HbF/HbA+HbF in whole-culture lysates
For reasons elaborated in “Discussion,” flow cytometry cannot provide a calibrated measurement of absolute hemoglobin contents. However, the molecular proportions of HbF (HbF/HbA+HbF) or the relative synthesis rates of gamma and beta chains are the gold standard to assess the efficacy of agents to modify the balance between fetal and adult hemoglobin expression from adult progenitors. Therefore, we used cation exchange HPLC to measure the molecular ratios of HbF/HbA+HbF in whole-culture lysates at different times after a 4-day TGFβ treatment. Figure 6 shows the data from 7 independent experiments, with cultures from individual blood donors harvested at different times between day 8 and day 19. The value pairs from each experiment are connected. TGFβ treatment caused a significant (P < .01) increase in the molecular ratios of HbF/HbF+HbA in whole-culture lysates, in accordance with the increased proportions of F+ cells in those cultures.
Molecular ratios of HbF/HbF+HbA in whole-culture lysates.
Cultures were lysed and the relative amounts of fetal hemoglobin measured by cation exchange HPLC. The ratios HbF/HbF+HbA are multiplied by 100 to give the percentage of HbF. The 2 values plotted at the 0.1% level are from samples in which the HbF peak was too small for quantitation. Each value pair (connected) represents a separate, independent experiment in which all cells were harvested at 1 time point between day 8 and day 19. Different culture times are distinguished by symbols: Diamonds: day 8; crosses: day 11; circles: day 13 (3 different experiments); triangles: day 16; squares: day 19. The effect of TGFβ is significant with P < .01.
Molecular ratios of HbF/HbF+HbA in whole-culture lysates.
Cultures were lysed and the relative amounts of fetal hemoglobin measured by cation exchange HPLC. The ratios HbF/HbF+HbA are multiplied by 100 to give the percentage of HbF. The 2 values plotted at the 0.1% level are from samples in which the HbF peak was too small for quantitation. Each value pair (connected) represents a separate, independent experiment in which all cells were harvested at 1 time point between day 8 and day 19. Different culture times are distinguished by symbols: Diamonds: day 8; crosses: day 11; circles: day 13 (3 different experiments); triangles: day 16; squares: day 19. The effect of TGFβ is significant with P < .01.
Effect of TGFβ on absolute cell numbers and long-term proliferation
A change in relative F+ cell numbers could be based on different mechanisms that might be distinguishable by comparing absolute cell counts per culture. Cultures that were treated with TGFβ from days 0 to 4 were subcultivated again on days 7, 10, and 13, and the total production of F+ and F−A+ cells per culture were determined on days 7, 10, 13, and 16. Figure 7A shows the time course from a representative experiment: Between days 7 and 10, the total numbers of F+ cells (full symbols) in TGFβ-treated and control cultures were approximately equal, whereas F−A+ cells (open symbols) were dramatically reduced in the TGFβ-treated cultures, suggesting a selective inhibition or deletion of F−A+ cells during or shortly after the treatment phase. Between days 7 and 10, both cell types in both cultures proliferated rapidly at approximately the same rate. After day 10, in the control culture, the proliferation decreased strongly and equally for both F+ and F−A+ cells, maintaining the ratio. No secondary colonies were seen after the subcultivation of control cultures on day 13, in any experiment. In contrast, the proliferation in the TGFβ-treated culture continued for much longer for both F+ and F−A+ cells, leading to a much higher overall production of F+ cells during the entire culture life span. This conclusion from cell counts is supported by photographs of cultures on day 18, after reseeding on day 13, showing the growth of large cell clusters in TGF-treated cultures but not in the controls (Figure 7A insert). The F−A+ cell numbers were gradually reducing the gap with F+ cells, but F+ cell proportions remained above 50%. The number of days for which the proliferation of TGFβ-treated cultures could be maintained was highly variable between donors (between 18 and 26 days), whereas the proliferative life spans of untreated control cultures were less variable (12-14 days). We define the proliferative life span as the time after which further subcultivation does not lead to any detectable growth of cell clusters of any size.
Absolute numbers of F+ and F−A+ cells.
Cultures were treated with TGFβ from day 0 to day 4, then propagated without TGFβ, with further subcultivation and dilution on days 7 (1:10), 10 (1:5), and 13 (1:5). Absolute numbers of F+ and F−A+ cells per culture were determined on days 7, 10, 13, and 16. (A) Time course of proliferation. Representative example from one experiment. Overall cell production is calculated by multiplying total cell numbers per culture with all preceding culture dilution factors. Open symbols: F−A+ cells; full symbols: F+ cells. Triangles: TGFβ-treated cultures; circles: controls. Inset: photographs of day 18 cultures, after reseeding on day 13. Left: control; right: TGF-treated. (B) Ratios of cell numbers in TGFβ-treated and control cultures (N[TGF] / N[CON]), as a function of culture time. Pairs of F+ and F−A+ values from individual experiments are identified by connecting lines. Median values are indicated by horizontal bars, and individual values are shown (open circles) to indicate range and distribution. The increase in the ratios (N[TGF]/N[CON]) between day 7 and day 16 is statistically significant, with P < .03 for F+ cells andP < .02 for F−A+ cells. The difference in the ratios N(TGF)/N(CON) between F+ and F−A+ cells is significant withP < .001 at all time points.
Absolute numbers of F+ and F−A+ cells.
Cultures were treated with TGFβ from day 0 to day 4, then propagated without TGFβ, with further subcultivation and dilution on days 7 (1:10), 10 (1:5), and 13 (1:5). Absolute numbers of F+ and F−A+ cells per culture were determined on days 7, 10, 13, and 16. (A) Time course of proliferation. Representative example from one experiment. Overall cell production is calculated by multiplying total cell numbers per culture with all preceding culture dilution factors. Open symbols: F−A+ cells; full symbols: F+ cells. Triangles: TGFβ-treated cultures; circles: controls. Inset: photographs of day 18 cultures, after reseeding on day 13. Left: control; right: TGF-treated. (B) Ratios of cell numbers in TGFβ-treated and control cultures (N[TGF] / N[CON]), as a function of culture time. Pairs of F+ and F−A+ values from individual experiments are identified by connecting lines. Median values are indicated by horizontal bars, and individual values are shown (open circles) to indicate range and distribution. The increase in the ratios (N[TGF]/N[CON]) between day 7 and day 16 is statistically significant, with P < .03 for F+ cells andP < .02 for F−A+ cells. The difference in the ratios N(TGF)/N(CON) between F+ and F−A+ cells is significant withP < .001 at all time points.
Despite substantial variations in the time course and life span (which made it impossible to pool the growth curves from different experiments), both the initial selective reduction of F−A+ cells and the increase in overall cell production was strictly reproduced in 4 experiments from different donors. The data are summarized in Figure7B. The ratios of absolute cell numbers in TGFβ-treated to control cultures (N[TGF] / N[CON]) are shown on different days, with pairs of F+ and F−A+ values for individual samples identified by connecting lines, and medians indicated as horizontal bars. Measured on day 7, absolute F+ cell numbers were little affected by TGFβ (ratio = 1), whereas the F−A+ cell numbers were reduced approximately 10-fold (ratio = 0.1). This difference in the effect of TGFβ on F+ and F−A+ cells was highly significant withP < .0001. The day 7 values are shown from 12 experiments, and 4 experiments were extended until terminal growth arrest in the control cultures. With increasing culture time, the ratios increased for both F+ and F−A+ cells, indicating that both types of cells in TGFβ-treated cultures were able to outproliferate the controls, as exemplified in Figure 7A. On average, the F+ population of TGFβ-treated cultures grew to nearly 10-fold higher levels than the controls. The increase from day 7 to day 16 was significant withP < .03. In 2 experiments, the TGFβ-treated cultures kept proliferating and producing secondary colonies beyond 3 weeks, with the ratios N(TGF)/N(CON) for F+ cells exceeding 100. Data beyond day 16 are not shown because cell counts in control cultures decreased because of cellular disintegration or enucleation, making the ratio appear meaningless during this phase. Under our general culture conditions, a proliferative life span beyond 3 weeks is observed only in cultures from fetal blood at an early gestational age.
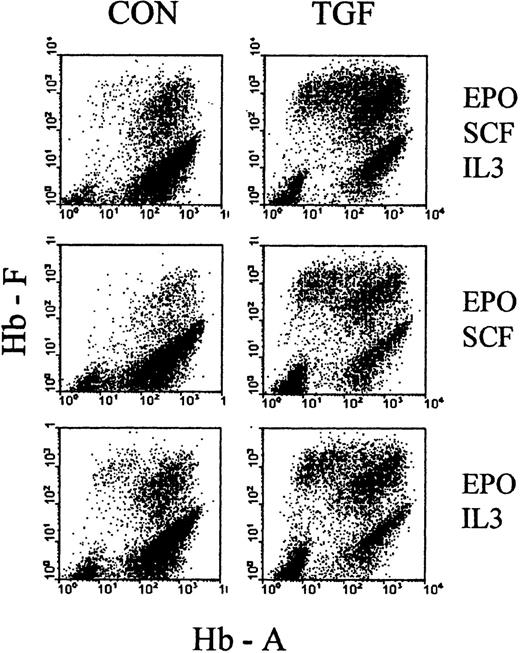
Transforming growth factor β effect does not depend on the combination of cytokines
As a first attempt to explore the basis of TGFβ action during the time of treatment, we examined how it would be affected by the choice of cytokine cocktail, supporting erythropoiesis during the phase of TGFβ treatment. We initiated cultures in EPO+SCF+IL3, EPO+SCF, EPO+IL3, and SCF+IL3, and treated them with TGFβ for the first 4 days. The cultures were then reseeded, without TGFβ, in the full cytokine cocktail (EPO+SCF+IL3) and analyzed between days 7 and 9 of culture. Figure 8 shows examples of the resulting profiles, and Table 1 shows the numerical evaluations, with proportions of F+ cells, F+A− cells, absolute cell numbers, as well as the numbers of secondary colonies. In all cytokine combinations, TGFβ increased the proportions of F+ cells, based mostly on a strong reduction of F−A+ cells (ratio approximately 0.1) and accompanied by a moderate (approximately 2×) decrease in secondary colonies.
Effect of TGFβ under different cytokine combinations.
Cultures were initiated in media with different combinations of cytokines (SCF + IL3 + EPO, SCF + EPO, IL3 + EPO) in the presence or absence of TGFβ (as indicated in the figure). After 4 days, all cultures were reseeded without TGFβ in fresh medium with the full complement of SCF, IL3, and EPO. Examples of hemoglobin profiles from day 8 cultures (4 days after reseeding). See Table 1 for numerical evaluations.
Effect of TGFβ under different cytokine combinations.
Cultures were initiated in media with different combinations of cytokines (SCF + IL3 + EPO, SCF + EPO, IL3 + EPO) in the presence or absence of TGFβ (as indicated in the figure). After 4 days, all cultures were reseeded without TGFβ in fresh medium with the full complement of SCF, IL3, and EPO. Examples of hemoglobin profiles from day 8 cultures (4 days after reseeding). See Table 1 for numerical evaluations.
Effect of transforming growth factor β in different cytokine cocktails
| . | . | ESI . | ES . | EI . | SI . |
|---|---|---|---|---|---|
| Proportion F+ cells | CON | 30 | 20 | 34 | 18 |
| (percentage of Hb+) | TGF | 76 | 75 | 72 | 65 |
| Proportion F+A− cells | CON | 9.4 | 5.6 | 14 | 7.8 |
| (percentage of F+) | TGF | 39 | 47 | 41 | 29 |
| Relative TGF effect | F+ | 1.15 | 1.15 | 0.65 | 1.05 |
| (N[TGF]/N[CON]) | F−A+ | 0.14 | 0.08 | 0.13 | 0.12 |
| Secondary colonies | CON | 100 | 26 | 38 | 74 |
| (normalized) | TGF | 61 | 18 | 19 | 39 |
| . | . | ESI . | ES . | EI . | SI . |
|---|---|---|---|---|---|
| Proportion F+ cells | CON | 30 | 20 | 34 | 18 |
| (percentage of Hb+) | TGF | 76 | 75 | 72 | 65 |
| Proportion F+A− cells | CON | 9.4 | 5.6 | 14 | 7.8 |
| (percentage of F+) | TGF | 39 | 47 | 41 | 29 |
| Relative TGF effect | F+ | 1.15 | 1.15 | 0.65 | 1.05 |
| (N[TGF]/N[CON]) | F−A+ | 0.14 | 0.08 | 0.13 | 0.12 |
| Secondary colonies | CON | 100 | 26 | 38 | 74 |
| (normalized) | TGF | 61 | 18 | 19 | 39 |
Cultures were initiated in media with different combinations of cytokines in the presence or absence of TGFβ. After 4 days, all cultures were reseeded without TGFβ in fresh medium with the full complement of SCF, IL3, and EPO. Flow cytometric analysis on day 8 of culture. Secondary colonies were counted on day 14 and normalized to 100 for the control cultures in EPO, SCF, and IL3. Data represent the averaged values from 2 independent experiments with blood from different donors. Numerical results from both experiments were all within 10% of each other, so that the display of ranges is omitted for simplicity.
ESI = EPO + SCF + IL3; ES = EPO + SCF; EI = EPO + IL3; SI = SCF + IL3.
It must be pointed out that suboptimal cytokine combinations lead to a reduced cell cycle rate during the first few days of culture, which is reflected by the reduced secondary colony numbers (in control cultures without TGFβ) (Table 1). Therefore, a TGFβ treatment timing that is optimal for one cytokine cocktail may not be optimal for another, so that a quantitative comparison of the magnitudes of TGFβ effect between cytokine conditions has limited significance in this experiment. (Further kinetic exploration will be presented in a separate study.) Nevertheless, the data of Figure 8 and Table 1indicate that the 4-day TGFβ treatment had a similar relative effect in all cytokine combinations, indicating that it may not be based on an interference with the support of survival, proliferation, or maturation by those cytokines.
Discussion
We have shown that TGFβ treatment leads to dramatically increased proportions of HbF-containing cells in erythroid cultures from adult peripheral blood. This effect was achieved by TGFβ treatment within the first 4 days of culture. Both the baseline and TGFβ-induced proportions of F+ cells remained nearly constant during further culture expansion, with F+ and F−A+ cells proliferating at approximately the same rate.
An increase in the proportions of F+ cells, initiated early in culture and measured after 1 week or later, could be due to different mechanisms: a reversal of the hemoglobin switch, a selective proliferative boost to F+ cells, or a selective inhibition of F−A+ cells. A pure reversal of the hemoglobin switch would lead to increased numbers of F+ cells at the numerically equal expense of F−A+ cells. A selective increase in the rate of F+ cell proliferation would leave F−A+ cell numbers unchanged. Finally, a selective suppression of F−A+ cells would leave F+ cell numbers unchanged. None of the above is seen in our experiments. The data indicate a complex situation that may be explained by the superposition of many effects. A selective deletion of progenitors that are programmed to develop into cells expressing only HbA would explain the strongly and selectively decreased F−A+ numbers measured between day 7 and day 10. However, F−A+ cells of treated cultures eventually catch up with controls, and F+ cell numbers of treated cultures far exceed those of controls during the late stages of culture. The increased overall cell production excludes mechanisms in which TGFβ merely deletes any progenitor subpopulation, or transiently delays their proliferation. The initial shift to F+A− cells within the compartment of F+ cells appears to fit better with a reversal of the hemoglobin switch than with a selective deletion of F−A+ cells, because the latter would leave the distribution of cells within the F+ compartment unchanged. One could speculate that a reversal of the hemoglobin switch might be coupled with an increased expansion potential of newly initiated F+ cells. However, that would leave the high division potential of initially suppressed F−A+ cells unexplained. Alternatively, TGFβ might suppress negative growth control by autocrine/paracrine signaling, similar to the transforming effects of TGFβ in some other cell systems.
We emphasize that our flow cytometric data and conclusions deal with the distinction and enumeration of F+ and F−A+ cells, without quantitating absolute hemoglobin contents in cells. In the lower ranges of cellular hemoglobin content, which prevail up to approximately day 8, the fluorescent antibody signal is likely to be approximately proportional to hemoglobin content. However, at later stages of erythroid maturation, the large amounts of hemoglobin per cell lead to saturation effects that prevent the resolution of quantitative differences by fluorescent antibody label.26 Therefore, although the distinction between F+ and F− cells remains reliable throughout the erythroid development, the use of a complementary method of hemoglobin measurement was required. Unsurprisingly, increased proportion of F+ cells in a culture were reflected by an increased ratio of HbF/HbF+HbA in that culture. The numerical relationship between F+ cell proportions and the molecular HbF proportions in whole cultures is complex because F+ cells also accumulate HbA, so that numerical comparisons between the data from the different assays are not possible. However, the HPLC results in Figure 6 support our conclusion that brief TGFβ treatment increased the relative HbF levels during further culture development.
Apart from the relative and absolute numbers of F+A+ erythrocytes in a patient, an important question is whether the hemoglobin ratios within individual F+A+ erythrocytes are affected by TGFβ treatment of their early progenitors. Considering that the final stages of erythroid maturation in vitro may not reflect the situation in vivo, the terminal maturation of TGFβ-treated erythroid progenitors will have to be studied in animal models.
The effects of TGFβ were similar in different cytokine combinations, suggesting that this effect is not based on interference with the survival- and division-supporting activity of cytokines. This result appears surprising, considering the reported effects of TGFβ on the signaling of SCF and IL3.29,30 A regulatory role of TGFβ on HbF levels via the SCF-signaling mechanism has been suggested to explain a negative correlation between SCF and TGFβ plasma levels in patients with sickle cell.24 However, our data may suggest a more direct causal link between high levels of TGFβ and high HbF levels in these patients.
Serum was less effective than TGFβ in increasing the proportions of F+ cells, but was also less inhibitory to cell proliferation (data not shown). The similar kinetics of the serum effect suggest that the mechanism of action may be the same. However, different experiments will be needed for quantitative comparisons, because of the superimposed effects of serum-contained additional growth factors. Various tests (TGFβ quantitation in sera by enzyme-linking immunosorbent assay [ELISA], neutralizing antibodies, and latency-associated peptide [LAP]) indicated that the serum activity cannot be explained by its content of TGFβ (unpublished data).
Different forms of TGFβ (beta 1, 2, and 3) had different potencies to increase F+ cell proportions, with potency defined as the concentration required to achieve half-maximal effect. The maximum achievable effect (ie, the plateau level of F+ proportions at saturating TGFβ concentration) was the same for TGFβ 1, 2, and 3. It will be interesting to explore if and how the potencies of different TGFβ forms to increase F+ cell proportions correlate with their potencies to cause growth inhibition and acceleration of erythroid maturation.17
Flow cytometric measurement of the correlated cellular contents of different hemoglobin types provides a novel tool for the study of erythropoiesis in culture, permitting the detection of novel features, as well as the quick screening of the effects of cytokines and drugs. After substituting HbS for HbA antibodies, the hemoglobin profiles of nucleated red cells are also suitable to study sickle cell erythropoiesis, and to monitor the condition and responses of patients with sickle cell disease.
We were able to demonstrate the same effect of TGFβ in a culture from a patient with sickle cell disease (data not shown). We now propose to study whether our results have any potential application for the treatment of hemoglobin disorders that are ameliorated by an increase in the relative amounts of cellular HbF or the relative numbers of F+ cells. Potential clinical avenues may include the repeated transplantation from a bank of TGFβ-treated autologous progenitor cells (eg, from preserved cord blood), or in vivo treatment by brief TGFβ pulses at intervals that avoid overall suppression of hemopoiesis.
Supported by a research grant from Genzyme Genetics.
Reprints:Ralph M. Bohmer, New England Medical Center, Box 394, 750 Washington St, Boston, MA 02111; e-mail: ralph.bohmer@es.nemc.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 7. Absolute numbers of F+ and F−A+ cells. / Cultures were treated with TGFβ from day 0 to day 4, then propagated without TGFβ, with further subcultivation and dilution on days 7 (1:10), 10 (1:5), and 13 (1:5). Absolute numbers of F+ and F−A+ cells per culture were determined on days 7, 10, 13, and 16. (A) Time course of proliferation. Representative example from one experiment. Overall cell production is calculated by multiplying total cell numbers per culture with all preceding culture dilution factors. Open symbols: F−A+ cells; full symbols: F+ cells. Triangles: TGFβ-treated cultures; circles: controls. Inset: photographs of day 18 cultures, after reseeding on day 13. Left: control; right: TGF-treated. (B) Ratios of cell numbers in TGFβ-treated and control cultures (N[TGF] / N[CON]), as a function of culture time. Pairs of F+ and F−A+ values from individual experiments are identified by connecting lines. Median values are indicated by horizontal bars, and individual values are shown (open circles) to indicate range and distribution. The increase in the ratios (N[TGF]/N[CON]) between day 7 and day 16 is statistically significant, with P < .03 for F+ cells andP < .02 for F−A+ cells. The difference in the ratios N(TGF)/N(CON) between F+ and F−A+ cells is significant withP < .001 at all time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/9/10.1182_blood.v95.9.2967.009k21_2967_2974/5/m_bloo00921007aw.jpeg?Expires=1770406331&Signature=RulTz-kuU3osDWPqbnrdifqGK5PsW8QxWXMcyrT4YntZtdbgEvW-s-omF3F96gprzFdTrX~C~PufvUCnTaahngIgO~br2X1MGdm3a4n4YHMWqxVn6J6oJkb2UJgxBMthrzDBrgwQMFMkykeij97KumS1yyd5WmPaFDimCBUY4b50CSXmnMHngaqEmmAo5jR~HeA3Wxg8X~S6rq8apepBHPjcvkCcwMjauGF6GDLgm3asI2DJropag3OEOhWYxGT2TTeYl~x5Uww1je5jpFWFlb-EihonqHbNV6psCdH0Y~LFUbRJHnefgIYHuLcnbysY~MCkZSjy28LqUwYSkqMIAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Absolute numbers of F+ and F−A+ cells. / Cultures were treated with TGFβ from day 0 to day 4, then propagated without TGFβ, with further subcultivation and dilution on days 7 (1:10), 10 (1:5), and 13 (1:5). Absolute numbers of F+ and F−A+ cells per culture were determined on days 7, 10, 13, and 16. (A) Time course of proliferation. Representative example from one experiment. Overall cell production is calculated by multiplying total cell numbers per culture with all preceding culture dilution factors. Open symbols: F−A+ cells; full symbols: F+ cells. Triangles: TGFβ-treated cultures; circles: controls. Inset: photographs of day 18 cultures, after reseeding on day 13. Left: control; right: TGF-treated. (B) Ratios of cell numbers in TGFβ-treated and control cultures (N[TGF] / N[CON]), as a function of culture time. Pairs of F+ and F−A+ values from individual experiments are identified by connecting lines. Median values are indicated by horizontal bars, and individual values are shown (open circles) to indicate range and distribution. The increase in the ratios (N[TGF]/N[CON]) between day 7 and day 16 is statistically significant, with P < .03 for F+ cells andP < .02 for F−A+ cells. The difference in the ratios N(TGF)/N(CON) between F+ and F−A+ cells is significant withP < .001 at all time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/9/10.1182_blood.v95.9.2967.009k21_2967_2974/5/m_bloo00921007bx.jpeg?Expires=1770406331&Signature=lNNGpfKeV15RMh93taTEiI5p~JOZKW6HhcO8rm25zmiHi~--MGyaDNrfVNukciosmLq~6LO9UXNHJ5-Gy8Sq-mieuIbBUldhYcbJUiTHh7CPg6H6dZBuqaXRm9-HK3TLxHmdXV0zOcfCXWfrF-rqZNRlTFORkDqEUPJFbWvgZH33LzwpD-NyG~U0QTNf10RwWOvaaFIuABlOyodSnTz5wFQYv1lPXmvlGrQOCUJw~YXDQZxF0KGbWCOS4PKC8uO1GkXJzQ4N~Uv7p6wKVb7ILEPH-~jKR48dos1prWQFcIv2UythrZ2HKlpE~QgaESGjKDm7W~GAjhBO3KGBjQBMNQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal