The redox properties of iron make this metal a key participant in oxygen-mediated toxicity. Accordingly, L5178Y (LY) mouse lymphoma cell lines, which display a unique inverse cross-sensitivity to ionizing radiation (IR) and hydrogen peroxide (H2O2), are a suitable model for the study of possible differences in the constitutive control of intracellular iron availability. We report here that the level of iron in the cytosolic labile iron pool (LIP), ie, potentially active in the Fenton reaction, is more than 3-fold higher in IR-resistant, H2O2-sensitive (LY-R) cells than in IR-sensitive, H2O2-resistant (LY-S) cells. This difference is associated with markedly greater content of ferritin H-subunits (H-Ft) in LY-S than in LY-R cells. Our results show that different expression of H-Ft in LY cells is a consequence of an up-regulation of H-Ft mRNA in the LY-S mutant cell line. In contrast, posttranscriptional control of iron metabolism mediated by iron-responsive element–iron regulatory proteins (IRPs) interaction is similar in the 2 cell lines, although IRP1 protein levels in iron-rich LY-R cells are twice those in iron-deficient LY-S cells. In showing that LY cell lines exhibit 2 different patterns of intracellular iron regulation, our results highlight both the role of high LIP in the establishment of pro-oxidant status in mammalian cells and the antioxidant role of ferritin.
Iron is found at the active sites of a large number of enzymes, and it participates in crucial biological events, including the processing of dioxygen, an essential substrate of aerobic metabolism for most eukaryotes. The same redox properties that allow iron to be a functional enzymatic cofactor also make this metal a key participant in oxygen-mediated toxicity. The cellular requirement for iron is directly correlated with the cell type, the rate of cell growth, and the stage of cell differentiation. Within most cells, maintenance of intracellular iron homeostasis demands the coordination of iron uptake, utilization, storage and, in some cases, release.1 The master molecular effectors of this coordinated regulation are transferrin receptor (TfR), a protein involved in iron uptake, and ferritin (Ft), an iron-sequestering protein whose synthesis is governed by iron both transcriptionally and posttranscriptionally.1,2 The key elements of this control are iron regulatory proteins (IRP1 and IRP2), which in iron-depleted cells bind to stem-loop structures termed iron-responsive elements (IREs) of messenger RNAs (mRNAs) that encode Ft and TfR. The binding of IRPs to 5′-IREs represses translation of Ft mRNA. Conversely, the binding of IRPs to 3′-IREs stabilizes TfR mRNA.3-5IRP1 is a bifunctional protein: in iron-replete cells, the presence of a [4Fe-4S] cluster in the IRP1 molecule prevents its binding to IRE and determines the aconitase activity of the protein. When iron is scarce, IRP1 loses its cluster, thus enabling its interaction with IRE. In contrast, IRE binding by IRP2 is determined by the rate of its degradation via the proteasome, which is accelerated in the presence of iron.6
All aspects of intracellular iron homeostasis are mirrored in the so-called labile iron pool (LIP), a low-molecular-weight pool of weakly chelated iron that rapidly transits through the cell and constitutes a real crossroads of metabolic pathways of iron-containing compounds. It has been claimed that LIP is a cellular source of iron participating in the Fenton reaction7 as well as a sensor for the IRE-IRP regulatory mechanism.4,8 It seems that under physiologic conditions, a constitutive level of LIP is midway between the cellular need for iron and the hazard of excessive generation of ·OH radical, the most potent oxidant encountered in biological systems. Any fluctuations (depletion or rise) in the LIP may result either in impairment of synthesis of iron-containing proteins or in cell injury by pro-oxidants, as has been clearly demonstrated in erythroid cells overexpressing H-ferritin (H-Ft)9 or exposed to transferrin.7
Mouse lymphoma L5178Y (LY) sublines (LY-R and LY-S) are exceptional among mammalian cell lines because of their unique inverse cross-sensitivity to ionizing radiation (IR) and hydrogen peroxide (H2O2).10-12 A spontaneous conversion from the parental LY-R cell line (IR-resistant) to the mutant LY-S cell line (IR-sensitive) was accompanied by an unexpected increase in H2O2 resistance of LY-S cells.10 This seems to be due to the better antioxidant defense system in LY-S cells13 and the lower content of transition metal ions in the nucleus of these cells.14Higher sensitivity to H2O2 of LY-R cells compared with LY-S cells correlates with the higher initial oxidative damage of DNA bases generated by H2O2treatment.15
It is generally assumed, for the reasons mentioned above, that the availability of iron and its abundance in biological systems are the critical factors of oxygen-mediated toxicity. In this study we characterized and compared the regulation of iron metabolism in the LY sublines to clarify the contribution of iron to their differential sensitivity to H2O2. We show that distinct control of iron homeostasis in LY cells leads to higher H-Ft content of LY-S cells compared with LY-R cells and, also, to a smaller LIP in the former cells, thereby enhancing their resistance to oxidants.
Materials and methods
Materials
Calcein (CA) and calcein acetoxymethyl ester (CA-AM) were from Molecular Probes, Inc (Eugene, OR). [32P]CTP and [32P]dCTP were from NEN Life Science Products—DuPont (Bad Homburg, Germany), and ribonuclease T1 was from Calbiochem (La Jolla, CA). All others chemicals were from Sigma (St. Louis, MO).
Cell cultures
Murine leukemic T lymphoblast LY-R and LY-S were maintained in suspension cultures in Fischer's medium supplemented with 8% bovine serum and antibiotics, as described by Szumiel.16 Asynchronous populations in an exponential phase of growth were used in all experiments.
Determination of LIP
LIP was measured as recently described.17 Cells (2 × 106 cells/mL) were washed in phosphate-buffered saline and loaded with 0.25 μM CA-AM in MEM-HEPES (20 mM HEPES buffer, pH 7.4) medium for 5 minutes at 37°C. Cells were centrifuged, resuspended in MEM-HEPES-BSA (bovine serum albumin) medium (1 mg/mL of BSA), and incubated for 20 minutes at 37°C. After incubation, cells were centrifuged and resuspended in 6 mL of prewarmed HBS medium (20 mM HEPES containing 150 mM NaCl) at a density of 1 × 106 cells/mL. Aliquots (3 mL) of cell suspension were transferred to a stirred thermostated (25°C) cuvette, and fluorescence was monitored at an excitation wavelength of 483 nm and an emission wavelength of 510 nm with a fluorospectrophotometer (Shimadzu, Kyoto, Japan). After obtaining a stable baseline signal, a fluorescence-quenching anticalcein antibody (10 μL/cuvette) (kindly provided by Prof Z.I. Cabantchik, Hebrew University, Jerusalem, Israel)17,18 was added to eliminate extracellular fluorescence. After signal drop and restabilization, 100 μM salicylaldehyde hydrazone (SIH), a highly permeant iron chelator (a generous gift from Prof P. Ponka, McGill University, Montreal, Canada)17,18 was added, causing a rise in fluorescent signal corresponding to the level of LIP. The remaining cells (3 mL) loaded with CA-AM and treated with SIH were used to obtain the CA concentration in the cell suspension and were supplemented sequentially with 1 nM aliquots of CA standards to reach concentrations from 1 to 5 nM. The value of cellular LIP was calculated from the equation given by Epsztejn et al17 using an experimentally determined Kd value of 0.22 μM for the CA-Fe complex.17
Electrophoretic mobility shift assay
IRP-IRE interactions were measured as described previously19,20 by incubating 4 μg of cytosolic extracts with a molar excess of [32P] CTP-labeled IRE transcript from plasmid pSPT-fer (a generous gift from Dr L.C. Kühn, ISREC, Epalinges, Switzerland) in a 20-μL reaction volume of 10 mM HEPES, pH 7.6; 40 mM KCl; 3 mM MgCl2; and 5% glycerol. After 10 minutes of incubation at room temperature, 1 unit of ribonuclease T1 was added and, 10 minutes later, 5 μg of heparin. IRE-protein complexes were run on a nondenaturing 6% polyacrylamide gel. In parallel experiments, samples were routinely treated with 2-mercaptoethanol (2-ME) at a final concentration of 2% prior to addition of the IRE probe to allow full expression of IRE binding activity.21 The IRP-IRE complexes were quantified with a Phosphorimager using Image Quant software (Molecular Dynamics, Sunnyvale, CA)
Determination of aconitase activity
Aconitase activity of cytosolic extracts was measured spectrophotometrically by following the disappearance ofcis-aconitate at 240 nm at 37°C as described previously.22 23 Briefly, the reaction volume (900 μL) contained 80 μg of protein in 100 mM Tris-HCl, pH 7.4, and the reaction was started by the addition of 15 μL of 20 mMcis-aconitate. Units represent nanomoles of substrate consumed per minute (ε = 3.6 mM−1cm−1).
Northern blotting
Total RNA was obtained using TRIzol (Gibco BRL, Gaithersburg, MD) according to the manufacturer's protocol. RNA samples (10 μg) were electrophoresed through denaturing agarose gels (1%) containing formaldehyde, transferred onto a Hybond-N nylon membrane (Amersham Life Sciences, Little Chalfont, England) and UV cross-linked. After prehybridization, the membranes were hybridized overnight at 42°C with random-primed [32P]dCTP-labeled cDNA probes: for mouse TfR, mouse H-Ft (both probes kindly provided by Dr Kühn), mouse L-Ft (kindly provided by Dr C. Beaumont, INSERM 409, Paris, France), and mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH). All blots were exposed to Kodak x-ray film for 3 days.
Western blotting
The cell extracts were obtained by incubating cells in lysis buffer (20 mM HEPES, pH 7.6; 25 mM KCl; 0.5% Nonidet P-40; and 1 mM PMSF) and centrifugation at 15 000g for 30 minutes at 4°C. Proteins (20 μg) were separated on sodium dodecyl sulfate–polyacrylamide gels (8% and 12% for detection of IRP1 and Ft chains, respectively) according to the method of Laemmli24 and were transferred to nitrocellulose membranes (Schleicher & Schuel, Dassel). IRP1 was detected by incubation of the membranes with an anti–IRP1-specific rabbit antibody (kindly provided by Dr Kühn).25 Purified recombinant human IRP1, expressed in Escherichia coli by means of an expression vector constructed in the laboratory of Dr Kühn was used as standard. H-Ft and L-Ft chains were detected with rabbit antisera specific for recombinant mouse H-Ft and L-Ft, respectively. Recombinant mouse H-Ft and L-Ft were used as controls. Anti-Ft antisera as well as recombinant proteins were kindly provided by Dr P. Santambrogio, Milan, Italy.8 All tested proteins were revealed with a horseradish peroxidase–conjugated secondary antibody by visualization with the chemiluminescence ECL kit (Amersham Life Sciences).
Protein determination
The protein content of extracts was determined spectrophotometrically at 595 nm using the Bio-Rad protein assay (Bio-Rad, Munich, Germany) with bovine serum albumin as standard.
Statistical analysis
Statistical evaluation was done with Statistic software (StatSoft, Tulsa, OK). Significance of the difference in mean values was estimated using the Student t test for independent measurements.
Results
LIP levels in LY cells
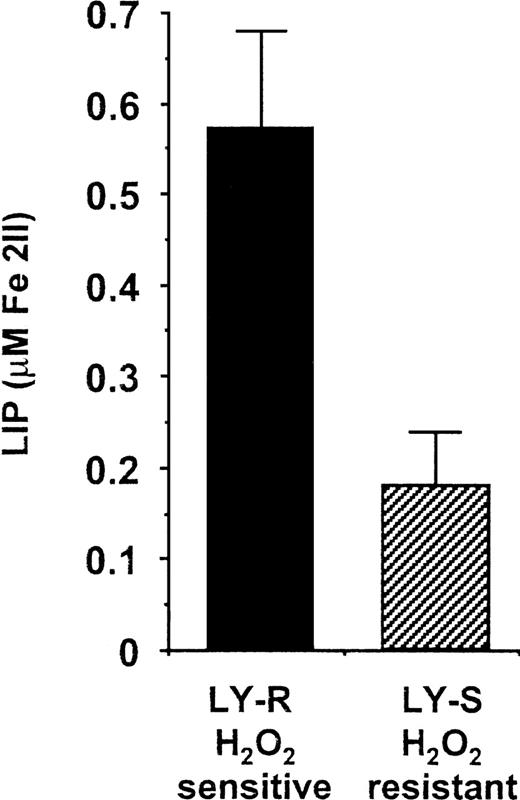
To estimate LIP levels in LY cells, we used a novel noninvasive method based on the fluorescent metallosensitive probe CA, which allows monitoring of the dynamic changes in cytosolic (chelatable) iron.17 Average steady-state LIP levels in LY-R and LY-S cells were 0.57 and 0.18 μM, respectively (Figure1). This clearly shows that the amount of iron potentially active in the Fenton reaction is much higher in H2O2-sensitive LY-R cells. Both LIP values are in the lower range of values estimated for myeloid and erythroid cells.17 This is consistent with the lower rate of iron metabolism in lymphocytes compared with that of other cell types.26 27
LIP levels in LY cells. The cellular chelatable iron content in living LY cells was assessed as described in “Materials and methods” using CA, a metallosensitive fluorescent probe, and SIH, a fast-acting chelator. The estimated values of LIP are expressed as a mean ± SE and were obtained from 5 independent measurements made on separate cultures. The results are significantly different,P < .01.
LIP levels in LY cells. The cellular chelatable iron content in living LY cells was assessed as described in “Materials and methods” using CA, a metallosensitive fluorescent probe, and SIH, a fast-acting chelator. The estimated values of LIP are expressed as a mean ± SE and were obtained from 5 independent measurements made on separate cultures. The results are significantly different,P < .01.
Ft subunit contents in LY cells
To determine whether different LIP levels in LY cells were correlated with Ft subunit content, we estimated H-Ft and L-Ft levels by immunoblotting using antimouse H-Ft and L-Ft antibodies. Interestingly, the low LIP level in H2O2-resistant LY-S cells was associated with a high content of H-Ft, whereas H2O2-sensitive LY-R cells contained a small amount of H-Ft: 11.4 ± 3.5 and 2.3 ± 0.2 arbitrary units, respectively, as measured by densitometry (Figure 2). The L-Ft level was only slightly (1.2 times) higher in LY-S than in LY-R cells.
Western blot analysis of Ft subunit levels in LY cells. Immunoblotting with rabbit antisera specific for recombinant mouse H-Ft and L-Ft was performed as described in “Materials and methods.” About 0.4 μg of each of the 2 recombinant proteins was used as standard. Experiments were repeated 3 times, and representative results are shown.
Western blot analysis of Ft subunit levels in LY cells. Immunoblotting with rabbit antisera specific for recombinant mouse H-Ft and L-Ft was performed as described in “Materials and methods.” About 0.4 μg of each of the 2 recombinant proteins was used as standard. Experiments were repeated 3 times, and representative results are shown.
Activity of IRP1 and IRP2 in LY cells
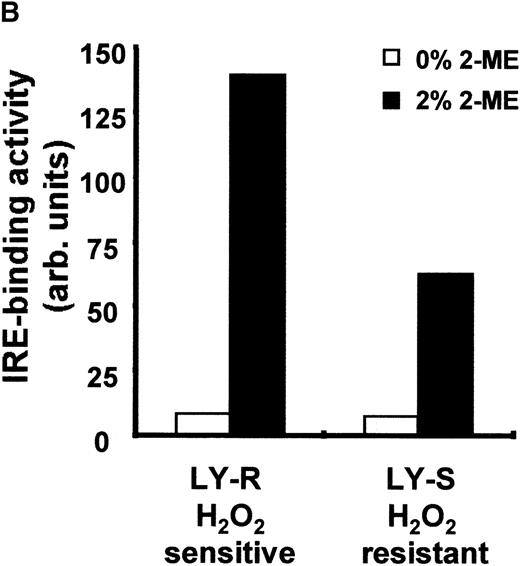
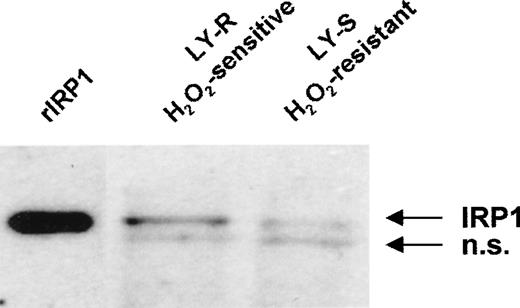
Because IRPs are 2 regulatory proteins that control Ft translation by binding to a specific sequence (IRE) at the 5′ end of its mRNA, we studied their capacity to bind IRE in both LY cell lines. Binding of IRP1 to IRE sequences was low in intact LY cells (Figure3A and 3B) and accounted for 6% and 11% of the total activity obtained after treatment with 2% 2-ME in the LY-R and LY-S lines, respectively. Similarly, there was no difference in basal IRE binding activity of IRP2 (Figure 3C). Keeping in mind thattrans-regulatory activities of IRP1 or IRP2 are directly influenced by the fluctuations in intracellular iron concentration [3-5], it is noteworthy that, despite a marked difference in the LIP levels, the RNA binding activities of IRP1 and IRP2 were similar in the 2 cell lines. To clarify this phenomenon, we attempted to establish whether there is a quantitative relationship in LY cells between LIP and IRP levels. We estimated IRP1 levels using 2 independent assays. First, we determined the total IRE binding capacity of IRP1 after exposure of cytosolic extracts to 2% 2-ME and found that it was 2 times higher in LY-R than in LY-S cells (Figure 3A and 3B). Second, direct determination of IRP1 levels by Western blot using a specific anti-IRP1 antibody again revealed that the IRP1 level in LY-R cells was twice that in LY-S cells (Figure 4). These results tally with the measurements of the cytosolic aconitase activity of IRP1, which was twice as high in LY-R cells as in LY-S cells (Figure5). The correlation between LIP level and potential IRE binding capacity was also detectable in the case of IRP2. Measurement of IRE binding by IRP2 in the presence of 0.5% 2-ME, which allows maximal IRP2 IRE binding activity,28 showed that it was higher in LY-R than in LY-S cells (Figure 3C).
IRE binding activity of IRP1 and IRP2 in LY cells. (A) Cell extracts were prepared as previously described.19 20 Four micrograms of protein were analyzed for IRE binding by IRP1 in the presence or absence of 2% 2-ME. (B) Radioactivity associated with IRE-IRP1 complexes without (open bars) or with (solid bars) 2% 2-ME was quantified with phosphorimaging, and signals are plotted in arbitrary units. (C) Ten micrograms of protein were analyzed for IRE binding by IRP2 in the presence or absence of 0.5% 2-ME. Because signals given by IRP2-IRE complexes are weaker than those of IRP1-IRE complexes, they were amplified and shown separately. Experiments are representative of 5 that gave similar results.
IRE binding activity of IRP1 and IRP2 in LY cells. (A) Cell extracts were prepared as previously described.19 20 Four micrograms of protein were analyzed for IRE binding by IRP1 in the presence or absence of 2% 2-ME. (B) Radioactivity associated with IRE-IRP1 complexes without (open bars) or with (solid bars) 2% 2-ME was quantified with phosphorimaging, and signals are plotted in arbitrary units. (C) Ten micrograms of protein were analyzed for IRE binding by IRP2 in the presence or absence of 0.5% 2-ME. Because signals given by IRP2-IRE complexes are weaker than those of IRP1-IRE complexes, they were amplified and shown separately. Experiments are representative of 5 that gave similar results.
IRP1 protein level in LY cells. Twenty micrograms of cell extracts were separated on an 8% sodium dodecyl sulfate–polyacrylamide gel and transferred to a nitrocellulose membrane. IRP1 was detected with a specific antiserum by the ECL method. Recombinant human IRP1 was used as standard. Two separate experiments produced similar results; n.s. indicates nonspecific band.
IRP1 protein level in LY cells. Twenty micrograms of cell extracts were separated on an 8% sodium dodecyl sulfate–polyacrylamide gel and transferred to a nitrocellulose membrane. IRP1 was detected with a specific antiserum by the ECL method. Recombinant human IRP1 was used as standard. Two separate experiments produced similar results; n.s. indicates nonspecific band.
Aconitase activity of IRP1 in LY cells. Aconitase activity of IRP1 was determined spectrophotometrically by measuring the disappearance of cis-aconitate at 240 nm. Aconitase activity values are expressed as a mean ± SE. Results are obtained from 6 independent measurements made on cytosolic extracts prepared from cells from separate cultures. The results are significantly different,P < .01.
Aconitase activity of IRP1 in LY cells. Aconitase activity of IRP1 was determined spectrophotometrically by measuring the disappearance of cis-aconitate at 240 nm. Aconitase activity values are expressed as a mean ± SE. Results are obtained from 6 independent measurements made on cytosolic extracts prepared from cells from separate cultures. The results are significantly different,P < .01.
Effect of deferoxamine on LIP level and IRP1 activities in LY cells
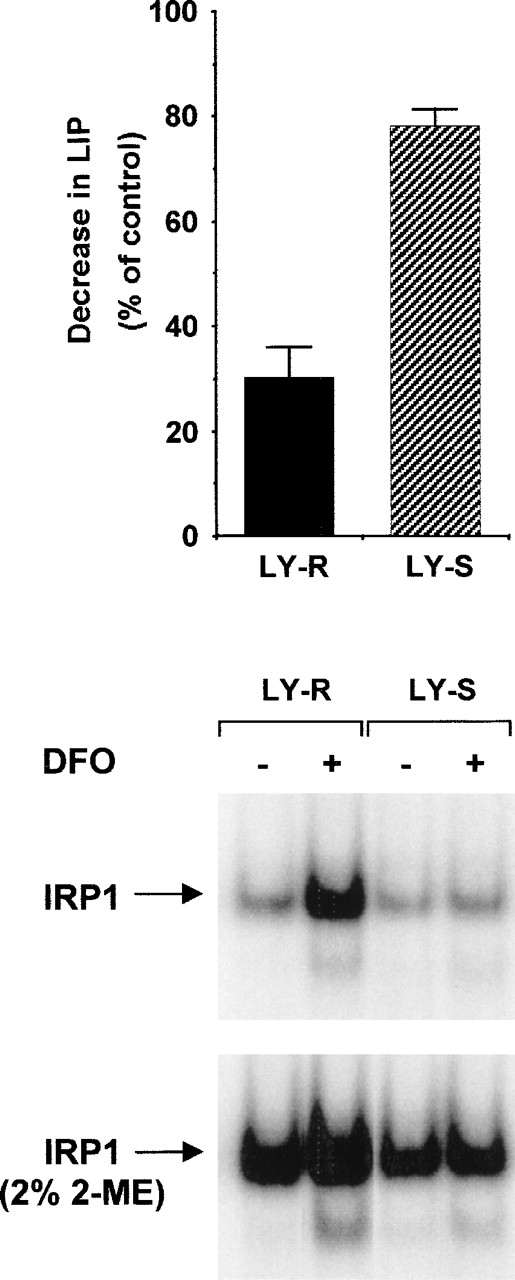
As shown in Figure 6 (upper panel), LIP level was significantly reduced in LY-R cells upon treatment with deferoxamine (DFO). In contrast, a decrease in LIP was barely detectable in LY-S mutant cells. In parallel experiments (Figure 6, lower panel), IRP1 IRE binding activity of DFO-treated LY cells was evaluated. LY-R cells exhibited the classical up-regulation of IRP1 activity upon DFO treatment, but their mutant counterpart displayed no change.
Changes in LIP level and IRP1 activity in LY cells after treatment with DFO. LY cells were exposed to 500μM DFO for 20 hours. Cells were analyzed in parallel for LIP content and IRE binding by IRP1 as described in “Materials and methods.” The upper panel shows LIP levels; the lower panel, IRE binding activity of IRP1. Experiments were performed 3 times, and 1 representative experiment is shown.
Changes in LIP level and IRP1 activity in LY cells after treatment with DFO. LY cells were exposed to 500μM DFO for 20 hours. Cells were analyzed in parallel for LIP content and IRE binding by IRP1 as described in “Materials and methods.” The upper panel shows LIP levels; the lower panel, IRE binding activity of IRP1. Experiments were performed 3 times, and 1 representative experiment is shown.
Ft subunit and TfR mRNA levels in LY cells
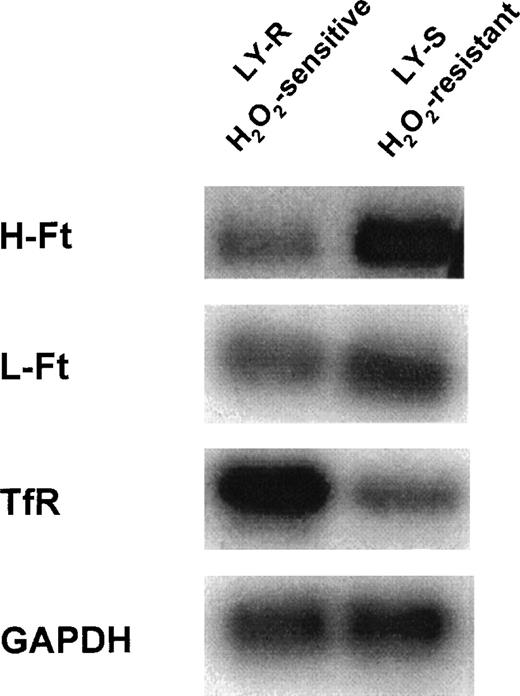
To gain further insight into the regulation of iron metabolism in the LY cell model, we also considered whether different LIP levels could be due to differences in the levels of mRNAs of Ft subunits and TfR in the 2 cell lines. Northern blot analyses provided evidence that mRNAs of Ft and TfR are differentially expressed in LY cells (Figure 7). Interestingly, H-Ft mRNA was far more abundant in LY-S than in LY-R cells (7.4 ± 0.3 vs 2.1 ± 0.1). H-Ft mRNA in LY-R cells was hardly detectable, suggesting that these cells have little potential for H-Ft synthesis. L-Ft mRNA was also slightly but consistently more abundant in LY-S cells (3.1 ± 0.6 vs 2.0 ± 0.3). It is of particular interest that, in addition to low iron-sequestering capacity, LY-R cells may have a high capacity for iron uptake as visualized by a higher level of TfR mRNA as compared with that observed in LY-S cells (2.3 ± 0.1 vs 1.0 ± 0.1).
Northern blot analysis of Ft subunits and TfR mRNA content of LY cells. Ten micrograms of total cellular RNA were electrophoresed on a formaldehyde-agarose gel (1%) and transferred to a nylon membrane. The membrane was hybridized with32P-labeled H-Ft, L-Ft, and TfR cDNA probes as described in “Materials and methods.” The mouse GAPDH probe was used as a control for RNA loading. Data shown are representative of 3 to 5 analyses of RNA samples isolated from cells from separate cultures.
Northern blot analysis of Ft subunits and TfR mRNA content of LY cells. Ten micrograms of total cellular RNA were electrophoresed on a formaldehyde-agarose gel (1%) and transferred to a nylon membrane. The membrane was hybridized with32P-labeled H-Ft, L-Ft, and TfR cDNA probes as described in “Materials and methods.” The mouse GAPDH probe was used as a control for RNA loading. Data shown are representative of 3 to 5 analyses of RNA samples isolated from cells from separate cultures.
Discussion
The phenomenon of iron- or heme-induced and Ft-mediated delayed hyperresistance of cells to pro-oxidant challenges has been documented under a wide range of experimental conditions.29-36However, little is known about how constitutive regulation of intracellular iron metabolism influences the natural sensitivity of cells to oxidants. Here, for the first time, we have characterized iron metabolism in intact, closely related mammalian cell lines differing in sensitivity to H2O2.
The LY cell model has already been explored with regard to the estimation of H2O2-induced cytotoxicity,11,12,14 extent and type of DNA damage,12,14,15 enzymatic antioxidant potential,13 and DNA damage repair.12 As numerous studies emphasize the role of iron in DNA damage under pro-oxidant conditions (reviewed in Meneghini37), it has been suggested that greater availability of iron entering the Fenton reaction makes LY-R cells more sensitive to H2O2.12,14 It is generally assumed that H2O2-dependent DNA damage is due to hydroxyl radicals generated in the presence of iron in the immediate surroundings of DNA.38 Even though LIP was quantified in the cytosol of LY cells, it is not irrelevant to DNA lesions observed in these cells. Indeed, it is likely that there is a general highly positive correlation between iron present in the cytosol and nucleus.37,38 Furthermore, an iron content similar to that found here in the cytosol of LY cells has been previously reported in their nucleus.14
By providing experimental evidence that the pool of cytosolic iron potentially active in the Fenton reaction is more than 3-fold higher in the H2O2-sensitive LY-R cells than in their resistant counterparts (Figure 1), our data point to LIP as one of the critical factors determining the natural sensitivity of mammalian cells to H2O2. This is consistent with other reports showing that an increase in desferrioxamine-chelatable iron in hepatocytes,39,40 as well as high LIP levels in erythroid cells7 exposed to H2O2, are positively correlated with the extent of oxidative cell injury.
The observation of different LIP levels in LY cells raises the question of the overall intracellular Ft concentration as well as of the levels of each of its subunits. The relationship we report here between the amount of H-Ft and the LIP level in LY cells strongly suggests that low LIP in LY-S cells and high LIP in LY-R cells are due to respectively high and low expression of H-Ft, whose ferroxidase activity increases the rate of iron uptake by the Ft molecule.41,42 Our results are in line with recent findings pointing to a key role of H-Ft in the regulation of LIP in genetically manipulated murine erythroleukemia cells overexpressing H-Ft.8 In addition, our data support a recent proposal by Levi et al43 claiming that the availability of H-Ft within the cells is the main determinant of the iron-sequestering capacity of cellular Ft. This is probably so in LY cells because we showed that they display almost the same level of L-Ft but differ markedly in H-Ft content.
There are many controversies surrounding the role of Ft in oxidative stress (reviewed in Lipiński and Drapier44). However, since Balla et al29 clearly demonstrated the in vivo cytoprotective properties of this molecule, the number of arguments for the antioxidant role of Ft has been continuously growing.30-36 Here, we provide experimental data that substantiate the concept of a protective role of Ft under physiologic conditions and show that high expression together with an appropriate ratio of Ft subunits is one of the main strategies in cellular defense against oxidative stress. This does not exclude that under pathologic conditions, eg, in the time course of oxidative stress, Ft may transiently serve as a source of catalytically reactive iron.33 45
The biological importance of LIP may include a control on IRP activities. Thus, keeping in mind the difference in LIP level between the 2 cell lines, it could be expected that constitutivetrans-regulatory activity of IRP1 would be higher in LY-S than in LY-R cells. However, the 2 cell lines display almost the same basal RNA binding by IRP1, suggesting that Ft synthesis is governed similarly with respect to the IRE-IRP system. These results led us to conclude that the physiologically closely related LY cell sublines constitute—in mammalian cells—an exception to the classically accepted converse correlation between the cellular “free” iron content and the IRE binding activity of IRPs.3 5 With regard to IRP1, the similar RNA binding activity observed in the 2 cell lines, despite a marked difference in their LIP content, could be explained by our finding that IRP1 levels in iron-deficient LY-S cells are half those in iron-rich LY-R maternal cells. The proportion of IRP1 molecules active as IRE binding protein is therefore higher in low LIP-containing LY-S mutant cells. In addition, we observed that the major part of IRP1 in both LY cell lines is found as aconitase, ie, in the iron-containing “holo” form. We can thus assume that about twice as much iron provided by LIP is needed for reconstitution of fully assembled [4Fe-4S] clusters in IRP1 molecules in LY-R as in LY-S cells. Taken together, these results suggest that the correlation between the amount of IRP1 (and not only its activity) and the LIP level is relevant for the control of intracellular iron homeostasis. It is interesting that the low LIP in LY-S mutant cells is barely decreased in response to DFO, an iron chelator. It is therefore clear that the major part of the DFO-chelatable iron is absent in these cells. As a consequence, the classical DFO-mediated up-regulation of IRP1 activity, which was conspicuous in LY-R cells, did not occur in their mutant counterpart.
Finally, the results of our Northern blot analysis show that the H2O2-sensitive LY cells exhibit a low level of Ft mRNA, thus stressing the importance of the amount of available mRNA in the expression of H-Ft in these cells. It should be recalled that Ft and TfR are also transcriptionally regulated proteins,1,2even though in recent years the focus has been on the IRP-mediated posttranscriptional regulatory mechanism.3-5 With regard to TfR, transcription of its gene is up-regulated in response to several signals, including growth factors46 and hypoxia.47 48 The different amount of H-Ft found in LY cells, together with similar IRE binding activity of IRP1 and IRP2 in the 2 cell lines, therefore suggests transcriptional control of H-Ft expression. Besides, our results show that H-Ft and TfR mRNA are regulated in opposite fashion in the 2 cell lines. As exemplified in this study by LY-R cells, it is clear that cells equipped to favor uptake of iron via TfR without storing it in Ft develop sensitivity to H2O2 through LIP increase. Conversely, as testified by LY-S mutant behavior, cells that express high H-Ft and low TfR mRNA are more able to resist iron-catalyzed Fenton-type reactions. In conclusion, our findings point out a direct relationship between a high expression of intracellular Ft, ie, an increased capacity of cells to scavenge iron active in the Fenton reaction, and their resistance to oxidative damage. Further, they support the idea that minimizing LIP in mammalian cells is one of the most efficient mechanisms for reducing cell susceptibility to oxidative stress.
Acknowledgment
We thank Prof Irena Szumiel for critical reading of the manuscript.
Supported by the Institute of Nuclear Chemistry and Technology and the Institute of Genetics and Animal Breeding, and by the Direction des Relations Internationales et le Programme “Physique et Chimie du Vivant” du Centre National de la Recherche Scientifique (The International Relations Department and the program “Physics and Chemistry of Living Forms,” from CNRS), Gif-sur-Yvette, France.
Portions of this work were presented at the World Congress on Iron Metabolism BioIron, May 23-28, 1999, Sorrento, Italy.
Reprints:Pawel Lipiński, Institute of Genetics and Animal Breeding, Polish Academy of Sciences, Jastrzebiec, 05-551 Mroków, Poland; e-mail: lipinskip@rocketmail.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal