Interleukin (IL)-16 is a chemoattractant cytokine for CD4+ leukocytes. Because delayed-type hypersensitivity (DTH) reaction is mediated by T helper 1 (Th1) cells and CD4+ T cells can be chemoattracted by IL-16, we have investigated the involvement of IL-16 in the DTH reaction. Immunohistochemical analysis revealed the IL-16 expression in infiltrating cells and epithelial cells in the DTH footpads. The IL-16 expression was also detected intracellularly in the infiltrating cells. In addition, markedly increased production of IL-16 was detected in the DTH footpad extracts, but not in the control footpad extracts, by an enzyme-linked immunosorbent assay and also by Western blot analysis. The DTH footpad extracts exhibited a strong chemoattractant activity toward splenic T cells, which was significantly inhibited by the inclusion of neutralizing monoclonal antibody (mAb) against IL-16 in the migration assay. Furthermore, treatment of sensitized mice in vivo with the anti-IL-16 neutralizing mAb significantly suppressed the footpad swelling induced by an antigen challenge, together with decreased infiltration of leukocytes including not only CD4+ T cells but also CD8+ T cells and macrophages into the DTH footpads. Decreased production of macrophage inflammatory protein 1 was also observed in the DTH footpad extracts by the mAb treatment. These results suggest that IL-16 plays an important role in the recruitment of leukocytes—presumably including antigen-specific Th1 cells, which secrete cytokines and chemokines mediating the following hypersensitivity reaction after activation by the interaction with Langerhans cells carrying the antigen—for the elicitation of DTH response.
Interleukin (IL)-16 is a chemoattractant cytokine for CD4+ leukocytes1,2 and has been shown to up-regulate IL-2 receptor α chain (CD25) and major histocompatibility complex class II on T cells1 and also induce the transient loss of responsiveness via the T-cell receptor (TCR) stimulation.3,4 IL-16 is first synthesized as an 80-kd precursor, pro-IL-16, which is constitutively and most exclusively expressed in lymphoid organs, such as spleen, lymph node, and thymus, including both CD4+ and CD8+ T cells,5-8 and also expressed in activated epithelium.9 The amino terminus of the pro-IL-16 is proteolytically processed by caspase-3 upon cell activation to become a 14-kd mature IL-16, which then autoaggregates to form a bioactive homotetramer.5,7,8,10 IL-16 has been reported to play a critical role in diseases characterized by CD4+T-cell involvement, such as allergic asthma,9,11-13rheumatoid arthritis,14 multiple sclerosis,15,16 and acquired immunodeficiency syndrome.17-20 Thus, IL-16 is postulated to be a proinflammatory and immunoregulatory molecule playing an important role in recruitment and activation of CD4+ T cells at the site of inflammation.
One of the important mechanisms for the clearance of pathogen from infected host is the cell-mediated response that potentiates the infiltration of leukocytes, especially lymphocytes and macrophages, into infected tissues. Delayed-type hypersensitivity (DTH) is the typical in vivo manifestation of the cell-mediated immunity, and the response can be measured easily and semiquantitatively. A typical DTH reaction is characterized by activation and recruitment predominantly of T cells and macrophages and by resultant swelling at 24 to 48 hours at the site of intradermal antigen (Ag) injection in previously sensitized hosts. DTH is well known to be mediated by T helper 1 (Th1) cells secreting predominantly interferon-γ and IL-2 and provides a useful model to study the role of putative inflammatory mediators in leukocyte recruitment in vivo. However, how leukocytes are attracted to the Ag site in a sensitized host has not been fully elucidated yet, although a cascade of multiple cytokines and chemokines has been considered to be involved in the DTH reaction. Interferon-γ was previously demonstrated to be a major mediator of lymphocyte recruitment into the DTH reaction site.21 Chemokines such as IL-8,22monocyte chemoattractant protein 1 (MCP-1)23 and macrophage inflammatory protein 1α (MIP-1α),24 and macrophage migration inhibitory factor (MIF)25 were demonstrated to be involved in the recruitment of leukocytes to the DTH reaction site. Moreover, the expression of interferon-inducible protein 10 (IP-10)26 and RANTES27 (regulated on activation normal T expressed and secreted) was reported to be detected in a contact sensitivity reaction and in DTH granulomas, respectively.
Considering that the DTH reaction is thus mediated by Th1 cells and CD4+ T cells can be chemoattracted by IL-16, we have reasonably investigated the involvement of IL-16 in the DTH reaction. In the present study, we have found that bioactive IL-16 is produced by an Ag challenge in the DTH footpads and that treatment of sensitized mice with neutralizing monoclonal antibody (mAb) against IL-16 immediately before the Ag challenge suppresses significantly the DTH response, suggesting that IL-16 plays an important role for the development of the DTH reaction in the elicitation phase. This is the first report on the detection of IL-16 in the DTH footpads and the involvement of IL-16 in the DTH reaction.
Materials and methods
Mice
Female C57BL/6 mice (6-8 weeks of age) were purchased from Japan SLC (Hamamatsu, Japan).
DTH response
DTH response to methylated bovine serum albumin (mBSA, Sigma, St. Louis, MO) was estimated by footpad swelling as described.28 Briefly, mice were sensitized to mBSA by an intradermal injection of 50 μL of 2.5-mg/mL mBSA emulsified with complete Freund's adjuvant (Difco, Detroit, MI) at 2 sites on the abdomen. Seven days after the immunization, the mice were challenged by an injection of 30 μL of 5-mg/mL mBSA in phosphate-buffered saline (PBS) into 1 rear footpad, while the other rear footpad received a comparable volume of PBS as a control. Footpad swelling was measured using a dial caliper (Mitutoyo Corp, Tokyo, Japan) at a given time after the challenge. The magnitude of the DTH response was determined as the difference in footpad thickness between mBSA- and PBS-injected footpads.
Immunohistochemical examination
Histologic examination of the DTH footpad was carried out as described.28 Soft tissue samples from each footpad were collected 24 hours after the challenge with mBSA, immersed in OCT embedding medium, and snap-frozen in liquid nitrogen. Endogenous peroxidase activity in tissue section was blocked by treatment with 0.6% H2O2 and 0.2% sodium azide for 10 minutes. Tissue section was then incubated for 20 minutes at 37°C in PBS containing 2% normal mouse or rabbit serum and 1% BSA to block nonspecific immunoglobulin (Ig) G binding. For detection of IL-16, tissue section was incubated with biotinylated antihuman/mouse IL-16 (clone 17.1, mouse IgG1)7 or isotype-matched control mAb (MOPC-21, mouse IgG1, Pharmingen, San Diego, CA) (5 μg/mL) in PBS containing 1% BSA overnight at 4°C. For detection of CD4+ T cells, CD8+ T cells, and macrophages, tissue section was incubated with antimouse primary rat mAb to the respective markers (5 μg/mL) in PBS containing 1% BSA for 1 hour at room temperature, and the bound primary rat mAb was then labeled with rabbit antirat IgG conjugated with biotin for 1 hour at room temperature. The following primary mAbs were used for the immunostaining: antimouse CD4 (GK1.5, rat IgG2b, American Type Cell Culture [ATCC], Rockville, MD), antimouse CD8 (53.6.7, rat IgG2a, ATCC), and antimouse macrophage (F4/80, rat IgG2b, Pharmingen). The isotype-matched control mAbs of rat IgG2a and IgG2b used were clone R35-95 and R35-38 (Pharmingen), respectively. Detection was performed with a streptavidin-horseradish peroxidase conjugate and diaminobenzidine substrate. The section was counterstained with hematoxylin to detect cell nuclei, dehydrated in graded ethanol solutions, equilibrated with xylene, and coverslipped. Positively stained cells were counted in 10 randomly selected fields (each [100 μm]2) as described.28
Intracellular staining
Infiltrating cells into the DTH footpad were isolated as described.29 Pooled infiltrating cells prepared from 10 mice 24 hours after the Ag challenge were stained intracellularly with phycoerythrin (PE)-conjugated anti-IL-16 as described.7Briefly, infiltrating cells in staining buffer (PBS containing 2% fetal calf serum and 0.1% sodium azide) were blocked with anti-FcγII/III receptor mAb (2.4G2, Pharmingen), incubated with fluorescein isothiocyanate–conjugated mAb to the desired surface Ag, and fixed in PBS containing 1% paraformaldehyde for 20 minutes. After washing, the cells were incubated in 50 μL of permeabilization buffer (0.1% saponin in the staining buffer) containing 0.1 μg of PE-conjugated antihuman/mouse IL-16 (clone 14.1, mouse IgG2a, Pharmingen) or isotype-matched control mAb (G155-178, mouse IgG2a, Pharmingen) for 30 minutes. For blocking, PE-conjugated anti-IL-16 (0.1 μg) preincubated with recombinant human IL-16 (1 μg)6in 50 μL of permeabilization buffer for 30 minutes at 4°C was incubated with the cells. Then, the cells were washed with permeabilization buffer and analyzed by flow cytometry using a FACScan (Becton Dickinson, Mountain View, CA).
Footpad cytokine extraction
Footpad cytokine extraction was prepared as described.30Briefly, at a given time after the Ag challenge into the footpad of sensitized mice, their footpads were cut off, minced in cold PBS (1 mL), and incubated on ice for approximately 1 hour to release soluble materials from the tissue. The supernatant was collected after centrifugation and frozen at −80°C until used.
Detection of IL-16 and MIP-1 by enzyme-linked immunosorbent assay
The sandwich enzyme-linked immunosorbent assay (ELISA) for mouse IL-16 was carried out using anti-IL-16 (14.1)9 as capture mAb, biotinylated anti-IL-16 (17.1)7 as detection mAb, and recombinant IL-166 as a standard. The ELISA for mouse MIP-1α was performed by using a kit (Genzyme-Techne, Minneapolis, MN) according to the manufacturer's instructions.
Immunoprecipitation and Western blot analyses
Protein G Sepharose beads preincubated with anti-IL-16 (14.1) or an isotype-matched control mAb (MOPC-173, mouse IgG2a, Pharmingen) were added to an aliquot (approximately 0.4 mL) of each footpad extract and incubated overnight at 4°C. The beads were washed, resuspended in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and boiled, and released materials were electrophoresed on an SDS-polyacrylamide (10-20% gradient) gel. After electrophoresis, proteins were transferred to a polyvinylidene difluoride microporous membrane (PVDF, Immobilon, Millipore, Bedford, MA), and the membrane was probed with biotinylated anti-IL-16 (17.1) or isotype-matched control mAb (MOPC-21) followed by incubation with a streptavidin-horseradish peroxidase conjugate. Visualization of the signal was by electrochemiluminescence (Amersham, Oakville, Canada).
Migration assay
Migration assay was performed using a modified Boyden chemotaxis chamber as described.9 Briefly, T cells were prepared by passing spleen cells through a nylon wool column. A total of 107 T cells in 50 μL of Medium 199 enriched with 0.4% BSA were loaded into the upper well of the chamber, and 30 μL of the sample to be tested were placed in the lower well. The upper and lower well were separated by a nitrocellulose filter with a pore size of 8 μm. The chamber was incubated for 3 hours, and afterward the filter was fixed and stained with hematoxylin. Migration was quantified by counting the number of cells that migrated beyond a depth of 50 μm using an Optomax automated image analyzer (Burlington, MA). All migration data are expressed as the number of cells per high-power field (hpf). All samples were performed in triplicate. On average, 14 to 16 cells/hpf were counted under control conditions. Counts were compared with control (medium alone) migration, which was normalized to 100%. For blocking experiments, 5 μg/mL of anti-IL-16 neutralizing mAb (14.1)9 or the control mAb (MOPC-173) were added to the lower well.
Neutralization of IL-16 in vivo with monoclonal antibodies
To neutralize endogenous IL-16, sensitized mice were injected intraperitoneally with 0.5 mg/injection of anti-IL-16 (14.1) twice at 15 to 18 hours and immediately before the Ag challenge.9 As an isotype-matched control mAb, anti-SRBC (S-S.1, mouse IgG2a, ATCC) was similarly injected. These mAbs were purified from ascites on a protein G column.
Statistical analysis
Statistical analysis was performed by Student t test.P values <.05 were considered statistically significant.
Results
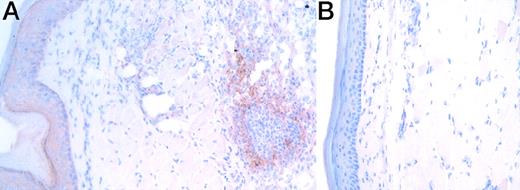
To explore whether IL-16 expression is induced by challenge with Ag in footpads of sensitized mice for the elicitation of a DTH response, we first analyzed immunohistochemically the IL-16 expression in the footpads 24 hours after the Ag challenge. IL-16 immunoreactivity was detected in the mass of infiltrating cells and also in the epithelium in footpads challenged with mBSA (Figure1A), but it was hardly or much less detected in footpads challenged with PBS (Figure 1B) or in footpads of nontreated mice (data not shown). No staining with control mAb was detected in footpads challenged with mBSA (data not shown). Then, the infiltrating cells into DTH footpads were isolated 24 hours after the Ag challenge and stained intracellularly for IL-16. Most infiltrating cells, including mainly Mac-1+ cells and also CD4+ and CD8+ T cells (data not shown), were positively stained with PE-conjugated anti-IL-16 but not with PE-conjugated control mAb (Figure 2). The intracellular detection of IL-16 was completely blocked by preincubating PE-conjugated anti-IL-16 with recombinant IL-16 (Figure 2). These results suggest the induction of IL-16 expression in the footpads of sensitized mice by Ag challenge in the elicitation phase of the DTH response.
Immunohistochemical detection of IL-16 in DTH footpads.
The section of mBSA- and PBS-challenged footpads (A and B, respectively) was immunohistochemically analyzed for the expression of IL-16 using anti-IL-16 (17.1) 24 hours after the Ag challenge. No staining was observed when an isotype-matched control mAb (MOPS-21) was used (data not shown). Similar results were obtained in 3 independent experiments.
Immunohistochemical detection of IL-16 in DTH footpads.
The section of mBSA- and PBS-challenged footpads (A and B, respectively) was immunohistochemically analyzed for the expression of IL-16 using anti-IL-16 (17.1) 24 hours after the Ag challenge. No staining was observed when an isotype-matched control mAb (MOPS-21) was used (data not shown). Similar results were obtained in 3 independent experiments.
Intracellular detection of IL-16 in infiltrating cells into DTH footpads.
Infiltrating cells into DTH footpads were isolated 24 hours after the Ag challenge and stained intracellularly with PE-conjugated anti-IL-16 (14.1) or isotype-matched control mAb (G155-178). For blocking, PE-conjugated anti-IL-16 was preincubated with recombinant IL-16 and used for the staining. Similar results were obtained in 3 independent experiments.
Intracellular detection of IL-16 in infiltrating cells into DTH footpads.
Infiltrating cells into DTH footpads were isolated 24 hours after the Ag challenge and stained intracellularly with PE-conjugated anti-IL-16 (14.1) or isotype-matched control mAb (G155-178). For blocking, PE-conjugated anti-IL-16 was preincubated with recombinant IL-16 and used for the staining. Similar results were obtained in 3 independent experiments.
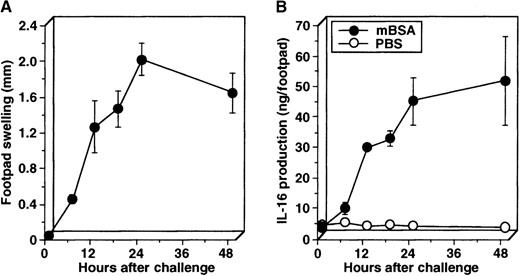
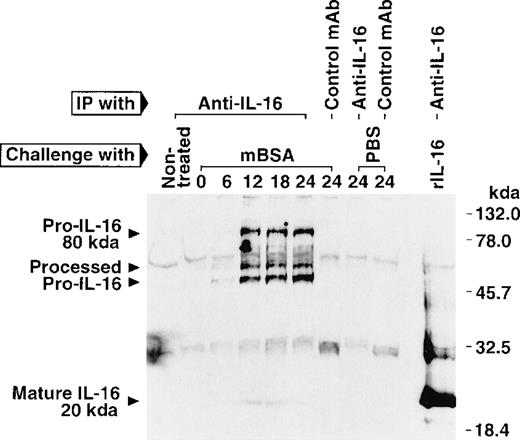
To further explore for the IL-16 production kinetically in the DTH footpads, the swelling was measured at a given time after the challenge, and then swollen footpads were cut off and minced in cold PBS, and resultant supernatant was assayed for IL-16 by ELISA. The amount of IL-16 produced in the DTH footpad extracts became detectable about 6 hours after the challenge and increased rapidly (Figure3B) as the footpad swelling enlarged (Figure 3A). In contrast, in the control footpad extracts almost no IL-16 protein was detected (Figure 3B). Because IL-16 is first synthesized as a precursor (pro-IL-16) and then cleavaged upon cell activation to form and secrete mature bioactive IL-165,7,8and our ELISA cannot distinguish pro-IL-16 from mature IL-16, we then examined the molecular size of IL-16 proteins immunoprecipitated from the DTH footpad extracts by Western blotting using anti-IL-16. The extracts of footpad challenged with mBSA contained not only 80-kd pro-IL-16 but, also, lower molecular mass proteins (approximately 45 and 50 kd), which are presumably processed forms of pro-IL-16 seen after activation (Figure 4).7In addition, the 20-kd mature IL-16 was quite slightly, but certainly, detected (Figure 4). The mature IL-16 was not detected in most experiments but was detected in a few experiments, including this one, probably due to the low production of mature IL-16 protein to be detected in this assay. In contrast, no IL-16 proteins were detected in the extracts of footpads challenged with PBS or in footpads of nontreated mice (Figure 4). To further examine for bioactive IL-16 production, we then measured the chemoattractant activity of footpad extracts toward splenic T cells. The extracts of footpads challenged with mBSA exhibited a strong chemoattractant activity, and this activity was significantly inhibited by the inclusion of neutralizing mAb against IL-16 in the migration assay (Figure 5). The inclusion of control mAb did not affect the migration assay (data not shown). On the other hand, neither extracts of footpads challenged with PBS nor footpad extracts of nontreated mice showed the activity (Figure 5). These results further suggest the production of bioactive IL-16 in the footpads of sensitized mice by Ag challenge for the elicitation of the DTH response.
Kinetic analysis on the IL-16 production in DTH footpads.
Sensitized mice were challenged with mBSA into footpads, and the footpad swelling was measured at a given time (A). Then, swollen footpads were cut off, minced in cold PBS, and briefly incubated on ice. Resultant supernatant was analyzed for IL-16 by ELISA (B). Similar results were obtained in 2 independent experiments.
Kinetic analysis on the IL-16 production in DTH footpads.
Sensitized mice were challenged with mBSA into footpads, and the footpad swelling was measured at a given time (A). Then, swollen footpads were cut off, minced in cold PBS, and briefly incubated on ice. Resultant supernatant was analyzed for IL-16 by ELISA (B). Similar results were obtained in 2 independent experiments.
Western blot analysis of IL-16 produced in DTH footpads.
Sensitized mice were challenged with mBSA into footpads, and swollen footpads were cut off, minced in cold PBS, and briefly incubated on ice. Resultant supernatant was immunoprecipitated by anti-IL-16 (14.1) or an isotype-matched control mAb (MOPS-173), electrophoresed on an SDS-polyacrylamide (10%-20% gradient) gel, and subjected to Western blot analysis using biotinylated anti-IL-16 (17.1) or isotype-matched control mAb (MOPC-21). Similar results were obtained in 2 independent experiments.
Western blot analysis of IL-16 produced in DTH footpads.
Sensitized mice were challenged with mBSA into footpads, and swollen footpads were cut off, minced in cold PBS, and briefly incubated on ice. Resultant supernatant was immunoprecipitated by anti-IL-16 (14.1) or an isotype-matched control mAb (MOPS-173), electrophoresed on an SDS-polyacrylamide (10%-20% gradient) gel, and subjected to Western blot analysis using biotinylated anti-IL-16 (17.1) or isotype-matched control mAb (MOPC-21). Similar results were obtained in 2 independent experiments.
Production of bioactive IL-16 in DTH footpads.
Sensitized mice were challenged with mBSA into footpads, and swollen footpads were cut off 24 hours after the Ag challenge, minced in cold PBS, and briefly incubated on ice. Resultant supernatant was then analyzed for IL-16 chemoattractant activity in the presence and absence of neutralizing mAb against IL-16 (14.1) using a migration assay. The inclusion of an isotype-matched control mAb (MOPS-173) did not affect the migration assay (data not shown). Data are shown as mean ± SD of 4 mice. *indicates P < .001 compared with PBS-challenged footpads of sensitized mice and footpads of nontreated mice. **indicates P < .001 compared with the absence of anti-IL-16. Similar results were obtained in 3 independent experiments.
Production of bioactive IL-16 in DTH footpads.
Sensitized mice were challenged with mBSA into footpads, and swollen footpads were cut off 24 hours after the Ag challenge, minced in cold PBS, and briefly incubated on ice. Resultant supernatant was then analyzed for IL-16 chemoattractant activity in the presence and absence of neutralizing mAb against IL-16 (14.1) using a migration assay. The inclusion of an isotype-matched control mAb (MOPS-173) did not affect the migration assay (data not shown). Data are shown as mean ± SD of 4 mice. *indicates P < .001 compared with PBS-challenged footpads of sensitized mice and footpads of nontreated mice. **indicates P < .001 compared with the absence of anti-IL-16. Similar results were obtained in 3 independent experiments.
Next, to examine whether the IL-16 activity is involved in the DTH response, we treated sensitized mice with neutralizing mAb against IL-16 and challenged with mBSA. The mAb treatment significantly reduced the footpad swelling by approximately 50% in comparison to the swelling of mice treated with control mAb (Figure6). These results suggest that the bioactive IL-16 is involved in the induction of the DTH response in terms of footpad swelling. To further examine the role of bioactive IL-16 in the DTH response, we performed the immunohistochemical analysis of DTH footpads of mice treated with anti-IL-16 or control mAb using mAbs against CD4+ T cells, CD8+ T cells, and macrophages. The treatment with anti-IL-16 attenuated the dermal hypertrophy (Figure 7A and B) and reduced significantly the number of infiltrating CD4+ T cells (Figure 7A-C) and also CD8+ T cells and macrophages (Figure7C). Furthermore, we measured MIP-1α production in DTH footpads, because MIP-1α, which is chemotactic for T lymphocytes, macrophages, and neutrophils,24,31,32 was reported to be produced and involved in the DTH reaction.24 In addition to IL-16, we observed increased production of MIP-1α in the DTH footpad extracts but almost not in the control footpad extracts, which was determined by ELISA (Figure 8A). Moreover, the anti-IL-16 treatment slightly but significantly reduced the MIP-1α production in the DTH footpads, which was measured 12 hours after the Ag challenge (Figure 8B). Taken together, these results suggest that IL-16 plays an important role in the recruitment of leukocytes—presumably including Ag-specific Th1 cells, which secrete cytokines and chemokines mediating the following hypersensitivity reaction after the interaction with Langerhans cells carrying the Ag—into the footpads in the elicitation phase of the DTH response.
Inhibition of the DTH response by anti-IL-16 treatment.
Sensitized mice were treated with neutralizing mAb against IL-16 (14.1) or an isotype-matched control mAb (S-S.1) and challenged with mBSA into footpads. Footpad swelling was measured 0, 24, 48, and 72 hours after the Ag challenge. Data are shown as mean ± SD of 5 mice. *indicates P < .01 compared with control mAb. Similar results were obtained in 3 independent experiments.
Inhibition of the DTH response by anti-IL-16 treatment.
Sensitized mice were treated with neutralizing mAb against IL-16 (14.1) or an isotype-matched control mAb (S-S.1) and challenged with mBSA into footpads. Footpad swelling was measured 0, 24, 48, and 72 hours after the Ag challenge. Data are shown as mean ± SD of 5 mice. *indicates P < .01 compared with control mAb. Similar results were obtained in 3 independent experiments.
Inhibition of cellular infiltration into DTH footpads by anti-IL-16 treatment.
Sensitized mice were treated with neutralizing mAb against IL-16 (14.1, B) or an isotype-matched control mAb (S-S.1, A) and challenged with mBSA into footpads. Soft tissue samples from each footpad were collected 24 hours after the Ag challenge, and the tissue section was immunohistochemically analyzed using anti-CD4 (GK1.5, A and B). The section was also stained with anti-CD8 and antimacrophage mAbs (53.6.7 and F4/80, respectively). No staining was observed when isotype-matched control mAbs (R35-95 and R35-38) were used (data not shown). Positively stained cells were counted in 10 randomly selected fields (each [100 μm]2) (C). Data are shown as mean ± SD of 5 anti-IL-16–treated mice and 8 control mAb-treated mice. *indicates P < .01 compared with control mAb. Similar results were obtained in 2 independent experiments.
Inhibition of cellular infiltration into DTH footpads by anti-IL-16 treatment.
Sensitized mice were treated with neutralizing mAb against IL-16 (14.1, B) or an isotype-matched control mAb (S-S.1, A) and challenged with mBSA into footpads. Soft tissue samples from each footpad were collected 24 hours after the Ag challenge, and the tissue section was immunohistochemically analyzed using anti-CD4 (GK1.5, A and B). The section was also stained with anti-CD8 and antimacrophage mAbs (53.6.7 and F4/80, respectively). No staining was observed when isotype-matched control mAbs (R35-95 and R35-38) were used (data not shown). Positively stained cells were counted in 10 randomly selected fields (each [100 μm]2) (C). Data are shown as mean ± SD of 5 anti-IL-16–treated mice and 8 control mAb-treated mice. *indicates P < .01 compared with control mAb. Similar results were obtained in 2 independent experiments.
Decreased production of MIP-1 in DTH footpads by anti-IL-16 treatment.
Sensitized mice were challenged with mBSA into footpads, and swollen footpads were cut off, minced in cold PBS, and briefly incubated on ice. Resultant supernatant was then analyzed for MIP-1α by ELISA (A). Sensitized mice were treated with neutralizing mAb against IL-16 (14.1) or an isotype-matched control mAb (S-S.1) and challenged with mBSA into footpads. Swollen footpads were then cut off 12 hours after the Ag challenge, minced in cold PBS, and briefly incubated on ice, and resultant supernatant was analyzed for MIP-1α by ELISA (B).
Decreased production of MIP-1 in DTH footpads by anti-IL-16 treatment.
Sensitized mice were challenged with mBSA into footpads, and swollen footpads were cut off, minced in cold PBS, and briefly incubated on ice. Resultant supernatant was then analyzed for MIP-1α by ELISA (A). Sensitized mice were treated with neutralizing mAb against IL-16 (14.1) or an isotype-matched control mAb (S-S.1) and challenged with mBSA into footpads. Swollen footpads were then cut off 12 hours after the Ag challenge, minced in cold PBS, and briefly incubated on ice, and resultant supernatant was analyzed for MIP-1α by ELISA (B).
Discussion
In the present study, we have investigated the involvement of IL-16 in the DTH response. First of all, immunohistochemical analysis revealed the expression of IL-16 in infiltrating cells and epithelial cells in footpads challenged with mBSA (Figure 1). Similar findings are reported in the airways after repeated ovalbumin inhalation in sensitized mice.9 In addition, the intracellular staining for IL-16 elucidated that most infiltrating cells such as, mainly, Mac-1+ and, also, CD4+ and CD8+ T cells into DTH footpads were positive for the IL-16 expression (Figure2). IL-16 was first reported to be produced by CD8+ T cells33,34 and subsequently by eosinophils,35mast cells,36 epithelial cells,9CD4+ T cells,8 and recently by dendritic cells.37 Therefore, the results of intracellular analysis imply that macrophages may be another candidate for producer cells of IL-16. Moreover, these results could suggest the possible secretion of IL-16 protein from infiltrating cells and epithelial cells, potentially including Langerhans cells, which are premature dendritic cells resting in the skin,37 in the DTH footpads. Indeed, we observed increased production not only of pro-IL-16 but also its processed forms and mature IL-16 in the DTH footpad extracts by ELISA (Figure 3B) and Western blot analysis (Figure 4). Although we could detect the mature IL-16 protein in the DTH footpad extracts in a few experiments, including this one (Figure 4), we could not detect it in most experiments. This is because that mature IL-16 protein seems to be difficult to detect by physicochemical techniques, probably due to the low production even when its bioactivity is detected by a migration assay.7 A strong chemoattractant activity toward splenic T cells was constantly detected in the DTH footpad extracts in vitro, and approximately 50% of the activity was inhibited by the inclusion of anti-IL-16 neutralizing mAb (Figure 5B). These results suggest that bioactive IL-16 was produced in the DTH footpads and that it could be involved in the recruitment of CD4+ T cells to the DTH reaction site. Because MIP-1α,24 MCP-1,23IL-8,22 and MIF25 were reported to be involved in the recruitment of lymphocytes into the DTH reaction site, the rest of the chemoattractant activity would be ascribed to some of these factors. Indeed, we detected increased production of MIP-1α in addition to IL-16 in the DTH footpad extracts by ELISA (Figure 8).
Consistent with the in vitro results, footpad swelling was significantly suppressed by in vivo treatment of sensitized mice with anti-IL-16 immediately before the Ag challenge (Figure 6). Infiltration not only of CD4+ T cells but also CD8+ T cells and macrophages was significantly reduced by the anti-IL-16 treatment (Figure 7). Considering that CD4 is the only receptor for IL-16,2 these results imply that IL-16 may act in both direct and indirect ways in the recruitment of these leukocytes. That is, IL-16 potentially produced from Langerhans cells carrying Ag, as suggested recently,37 may chemoattract the Ag-specific Th1 cells, which are the key molecules to initiate the DTH reaction,38 into the DTH reaction site. Then, these Th1 cells are activated by the interaction with the Langerhans cells, resulting in the secretion of cytokines and chemokines, which mediate the following hypersensitivity reaction. In addition, degranulation of mast cells occur, resulting in the release of cytokines and vasoactive amines such as serotonin and histamine. These amines were previously shown to induce the rapid release of bioactive IL-16 from CD8+ T cells, which contain preformed mature IL-16.33,34 CD4+ T cells also produce mature IL-16 after activation by CD3/TCR ligation.8 Thus, most cell sources for IL-16, such as dendritic cells (Langerhans cells),37 CD4+ T cells,8CD8+ T cells,33 mast cells,36eosinophils,35 and epithelial cells9 are implicated in the local inflammatory response accompanying DTH reactivity, which may support a direct mechanism of action by IL-16 in the DTH response. Moreover, mutual interaction through IL-16 among these cells may further amplify the DTH response, which makes the role of IL-16 in the DTH reaction more complicated. Consistent with the hypothesis, the production of one of these chemokines, MIP-1α, was also reduced by the anti-IL-16 treatment (Figure 8). In addition, it has been recently demonstrated that IL-16 enhances the production of MIP-1α, MIP-1β, IL-6, and tumor necrosis factor-α from CD8+ T cell–depleted phytohemagglutinin-activated peripheral blood mononuclear cells from HIV-infected patients.20 Moreover, the incubation of CD4+ T cells with IL-16 in the presence of IL-2 was reported to increase the interferon-γ production.39 In contrast, it has been also reported that preincubation of CD4+ T cells with IL-16 reduces the proliferative response to CD3/TCR ligation3 and also the IL-2 production in response to the stimulation with concanavalin A.40 Although the latter in vitro reports may implicate inhibitory activity of IL-16 on the DTH response, the transient unresponsiveness might impart temporary protection from Fas-mediated activation-induced cell death in the recruited lymphocytes and thereby increase the efficiency of the immune response in vivo, as discussed in the paper.3 Moreover, consistent with the present results using mBSA as an Ag, preliminary data revealed that increased production of IL-16 was also detected by ELISA in the DTH footpad extracts of mice sensitized with bacille Calmette-Guérin and then challenged with purified protein derivative of tuberculin and that the anti-IL-16-treatment slightly but significantly reduced the footpad swelling. Taken together, these results suggest that IL-16 plays an important role in the recruitment of leukocytes, presumably including Ag-specific Th1 cells, into the DTH reaction site for the initiation of the elicitation phase of the DTH response.
IL-16 was recently demonstrated to inhibit the replication of macrophage- and T-cell–tropic human immunodeficiency virus (HIV)-1 strains in acutely infected, CD8+ T-cell–depleted lymphocytes.17 The mechanism of HIV suppression was reported to be mediated through repression of HIV-1 promoter activity following CD4 cross-linking by IL-16.18 Moreover, it has been recently demonstrated that serum IL-16 levels are significantly increased in HIV-1-infected patients during the asymptomatic phase and drop on progression to disease.20 Taken together, these results further suggest that IL-16 would be playing an important role in the development of cell-mediated immunity such as the DTH reaction against the infection with HIV in addition to its direct anti- HIV effect.
Supported by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture, Japan, and from the Japanese Ministry of Public Health and Welfare.
W.W.C. is a recipient of a Clinical Investigator Award from the American Lung Association.
T.Y.'s present address is Intractable Disease Research Center, Tokyo Medical University, 6-1-1 Shinjuku, Shinjuku-ku, Tokyo 160-8402, Japan; e-mail: yoshimot@tokyo-med.ac.jp.
Reprints:Takayuki Yoshimoto, Department of Allergology, Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; e-mail:yoshimot@ims.u-tokyo.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




![Fig. 7. Inhibition of cellular infiltration into DTH footpads by anti-IL-16 treatment. / Sensitized mice were treated with neutralizing mAb against IL-16 (14.1, B) or an isotype-matched control mAb (S-S.1, A) and challenged with mBSA into footpads. Soft tissue samples from each footpad were collected 24 hours after the Ag challenge, and the tissue section was immunohistochemically analyzed using anti-CD4 (GK1.5, A and B). The section was also stained with anti-CD8 and antimacrophage mAbs (53.6.7 and F4/80, respectively). No staining was observed when isotype-matched control mAbs (R35-95 and R35-38) were used (data not shown). Positively stained cells were counted in 10 randomly selected fields (each [100 μm]2) (C). Data are shown as mean ± SD of 5 anti-IL-16–treated mice and 8 control mAb-treated mice. *indicates P < .01 compared with control mAb. Similar results were obtained in 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/9/10.1182_blood.v95.9.2869.009k18_2869_2874/5/m_bloo00918007ay.jpeg?Expires=1767735666&Signature=jr0uByQ9A9yj4a2pasjw8SHnLT6~4GE8PccAx4MjIgmQAcPP9kvu9r4FMRDrHygu8E4WfP26v4C0EMnWpaxZexYc2IMo1Mc7PMkAPIm10q3eisD8GpLA43wfy5E7CFNdJAuyzPwCQw1GriIqtU-dcOp~gHwPdvFqFvJobJ3HipDnq0TkTlyaYVph80nKqb~ImBrtf-Nxax5ljPIUXFx3MSrj7r4ybFPecwCYC06J0KmD0-6KZynRf3xISLNVClB9Iw4M6UbU9~jKIDOwjMnykE6MhbnB6ijtk~CHGnn85oZ6hVGTPs-1Ciap8qHXl8KUcxlvtYYaSh2tLenzRP39LQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Inhibition of cellular infiltration into DTH footpads by anti-IL-16 treatment. / Sensitized mice were treated with neutralizing mAb against IL-16 (14.1, B) or an isotype-matched control mAb (S-S.1, A) and challenged with mBSA into footpads. Soft tissue samples from each footpad were collected 24 hours after the Ag challenge, and the tissue section was immunohistochemically analyzed using anti-CD4 (GK1.5, A and B). The section was also stained with anti-CD8 and antimacrophage mAbs (53.6.7 and F4/80, respectively). No staining was observed when isotype-matched control mAbs (R35-95 and R35-38) were used (data not shown). Positively stained cells were counted in 10 randomly selected fields (each [100 μm]2) (C). Data are shown as mean ± SD of 5 anti-IL-16–treated mice and 8 control mAb-treated mice. *indicates P < .01 compared with control mAb. Similar results were obtained in 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/9/10.1182_blood.v95.9.2869.009k18_2869_2874/5/m_bloo00918007cx.jpeg?Expires=1767735666&Signature=d3mRfsVGVavlKfxkoU20r2hsVongw2YfCzd3Mc4hRFWnWB34VcOIfmHgeMJngH2l7ng5-h0U6ArFB0AoweobKC07Z4fkl893yXgP03whsYYRxt2dPb9z7KpZwghCalN6ONftY2tvLbqnOJ4YnzB-2orGKkgNIAgWfGS9y4XYvhfrpuojJykNlgRfhCtaR~zkc9Ucee8U-01obPeCqc2G5WFJBCtxEH3ti6M7k0LMaGMcT7sx08Hymd7REoVp5K7WL6PXAbSNjTXFW3srSifWT3J85hBci4n7vEy1ezLp8keQr5FDX3Q7~P-IwrvnyjnL2SaX6OXMdUXQxx6laFeOhg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal