Notch signaling has been shown to play a key role in cell fate decisions in numerous developmental systems. Using a reverse transcriptase-polymerase chain reaction (RT-PCR) assay, we reported the expression of human Notch-1 in CD34+ progenitors. In this study, we evaluated the expression of human Notch-1 and Notch-2 protein by hematopoietic cells. In immunofluoresence study, we detected low amounts of Notch-1 and Notch-2 protein in both CD34+ and CD34+Lin− cells, high amounts in CD14+ monocytes as well as B and T cells, but no expression in CD15+ granulocytes. We further found that an immobilized truncated form of the Notch ligand, Delta-1, induced apoptosis in monocytes in the presence of macrophage colony-stimulating factor (M-CSF), but not granulocyte-macrophage colony-stimulating factor (GM-CSF). The widespread expressions of Notch proteins suggest multiple functions for this receptor during hematopoiesis. These studies further indicate a novel role for Notch in regulating monocyte survival.
In the hierarchical development of the hematopoietic system, the progeny of stem cells progressively lose self-renewal and proliferative capacity while committing to differentiate along distinct pathways.1 The complexity of lineage determination during hematopoiesis has led to the conflicting concepts of instructive and stochastic models. In instructive models, cytokines have generally been thought to determine lineage. However, current evidence indicates that the lineage specificity of cytokine activity results from restricted expression of their receptors rather than determination properties of the actual cytokine receptor.2 In purely stochastic models, lineage fate determination has been thought to result from a probabilistic event(s), followed by cytokine-mediated expansion of cell numbers within a particular lineage.3,4 Although this represents a workable hypothesis, in other developmental systems the potential of the progeny of multipotent precursor cells to adopt a particular fate is often modified as a result of cell-cell interactions, eg, those mediated via the Notch receptor.5 6
The evolutionary conserved Notch transmembrane receptor has been found to be a fundamental cell-fate controlling mechanism that involves signals between neighboring precursor cells.5-7 In general, a precursor cell progresses to a more differentiated state in response to specific signals. Notch is not associated with the transmission of these signals, but appears to control fates by modulating a precursor cell's ability to respond to signals. Although not confined to a particular precursor type, Notch signaling is generally capable of influencing the ability of a cell to progress from a less to a more differentiated state. For example, during neurogenesis, a single cell within a cluster of neuroepithelial precursor cells upon commitment to neural development, initiates Notch-mediated signaling in adjacent cells, inhibiting their neural potential, while allowing their development along the epithelial lineage.8
Four paralogs of the Notch gene, Notch-1,-2,-3, and -4,9-13 and 5 Notch ligands, including Jagged-1, and -2, and Delta-1,-2, and -3,14-21 have been identified in vertebrates. Notch interactions have been shown to affect cell-fate decisions in multiple systems in both invertebrates and vertebrates. Because successive cell-fate choices mediated by cell-cell interactions may underlie hematopoietic processes, Notch signaling could potentially play an important role.22 In support of this, we have previously reported expression of Notch-1 messenger RNA (mRNA) in bone marrow cells, including the CD34+ subset,23 and have recently identified expression of Notch-1 and Notch-2 protein by purified murine hematopoietic precursors.24 We and others further demonstrated the expression of the Notch-1 ligand, Jagged-1, by hematopoietic stroma cells.24-26 It has also been shown that the abnormal activation of Notch signaling in either the murine or human hematopoietic system, by expressing mutant, constitutively activated forms of the receptor, profoundly affects hematopoietic lineages.27-30 Most importantly, we have shown that incubation of hematopoietic precursor cells with purified or cell-bound Jagged-1 generated an increase in the formation of a primitive precursor cell population, HPP-mix.24 Taken together, these results provide ample evidence for a role of Notch signaling in influencing cell-fate decisions during hematopoietic development.
To gain further insight into the specific cell-fate decision points where Notch signaling may influence hematopoietic decisions, we determined the protein expression of 2 members of this receptor family,Notch-1 and Notch-2, in cells from marrow and peripheral blood. We detected relatively high amounts of these receptors in monocytes as well as in T and B lymphocytes, lower levels in developing precursor cells, and no expression of Notch-1 or Notch-2 in granulocytes. Because high levels of Notch receptors were found in monocytes, we further investigated the potential role of Notch signaling in the development of these cells. To activate Notch receptors in monocytes, we used a truncated form of the Notch ligand, Delta-1, consisting of the extracellular domain without the transmembrane and cytoplasmic domains. We have previously found this ligand, when immobilized on a plastic surface, inhibits the differentiation of C2 myoblast cells, indicative of Notch activation (Varnum-Finney et al, submitted). We found that incubation of monocytes with the plastic-immobilized Notch ligand in the presence of macrophage colony-stimulating factor (M-CSF) led to apoptotic cell death, but did not in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF). These studies therefore demonstrate widespread expression of Notch receptors during hematopoiesis, suggesting multiple roles for Notch signaling, and further demonstrated a novel role for Notch in the cytokine specific regulation of monocyte survival.
Materials and methods
Separation of marrow cells and peripheral blood
Human marrow samples were obtained after informed consent. Cells were separated by Ficoll-Hypaque density gradient centrifugation (density 1.077), washed, and resuspended in phosphate-buffered saline (PBS) with 2% human-type AB serum. Peripheral blood cells were obtained from healthy volunteers and separated over a 2-layer density gradient (density 1.090 and 1.108) by mixing Ficoll-Hypaque (density 1.077) and Hypaque (density 1.3) to separate monocytes and granulocytes. The monocyte-rich layer was harvested and resuspended in PBS with 2% human-type AB serum. The cells were further purified by depleting T, B, and NK cells using antibodies 9.6 (anti-CD2), HD37 (anti-CD19), B1 (anti-CD20), CD56 (Immunotech, Marseilles, France), and R10 (antiglycophorin A), followed by goat antimouse IgG (Dynabeads M-450, Oslo, Norway), and then immunomagnetic bead depletion according to the manufacture's instructions. Monocytes were further purified using fluorescence-activated cell sorting by selecting cells with forward and side-scatter properties characteristic of monocytes and by excluding propidium iodide (Sigma, St Louis, MO) positive dead cells. The proportion of monocytes in the isolated cell populations were verified by staining with anti-CD14 antibody, with more than 95% of sorted cells showing positive staining in all experiments.
Antibodies and immunofluoresence studies
Cells were incubated in 96-well v-bottom plates with antibodies against hematopoietic differentiation antigens, including phycoerythrin (PE)-labeled IgG antibodies against CD2, CD4, CD8, CD20, and CD33 (all from Becton Dickinson, Sunnyvale, CA), and PE-labeled antiglycophorin A (Pharmingen, San Diego, CA). Three color studies were performed using the PE-Cy5 tandem dye directly conjugated to either CD14 or CD34 (both from Immunotech, Westbrook, ME). For studies of granulocytes, the unlabeled mouse IgM anti-CD15 antibody, 1G10, was used. PE-labeled affinity purified antimouse IgM (Amac, Westbrook, ME) was used to detect CD15 positive cells. Briefly, for 2-color or 3-color analysis, cells were incubated at room temperature with PE-Cy5 antibodies and/or with 1G10. After this, cells were washed twice and the 1G10-labeled cells were incubated at room temperature with the PE antimouse IgM antibody. The antihuman myc antibody F(ab′)2 was prepared from the 9E10 hybridoma in our laboratory.31 The F(ab′)2 fragment of the 9E10 antibody was prepared by incubation of purified 9E10 (2 mg/mL in 0.1 mol/L sodium citrate pH 3.5) with Pepsin (Sigma Chemical Co, St Louis, MO; ratio of enzyme to antibody was 1:200). The mixture was incubated at 37°C for 4 hours, neutralized with 3 mol/L Tris pH 8.0 and chromatographed on HiTrap Protein A Column (Amersham Pharmacia Biotech AB, Uppsala, Sweden).
For Notch-1 or -2 expression, cells were then washed twice and made permeable by incubation with PermeaFix (Ortho, Raritan, NJ), washed twice, and then incubated at room temperature with the rat antihuman Notch-1 antibody 18G or antihuman Notch-2 antibody Bhn6 (kindly provided by S. Artavanis and P. Zagouras, Massachusetts General Hospital, Boston, MA). The preparation and characterization of these antibodies that detect the intracellular domain of Notch-1 and -2 has been described previously.32 Cells were again washed twice, incubated at 4°C with mouse-adsorbed rabbit antirat antiserum, conjugated to biotin (Vector Laboratories, Burlingame, CA), washed twice, and then incubated at 4°C with streptavidin-fluorescein (Biosouce/Tago, Camarillo, CA.) Control staining was measured using isotype-matched directly labeled or unlabeled antibody of irrelevant specificity, except for the IgG2a CD14-PE-Cy5 antibody in which an IgG1-PE-Cy5 control was used instead. Cells were analyzed and sorted using a Vantage flow cytometer or FACScan (Becton Dickinson Co, Mountain View, CA).
Immunohistochemical staining for Notch
Immunohistochemistry was performed on formalin-fixed, formic acid-ethylenediamine tetraacetic acid (EDTA) decalcified, paraffin-embedded tissues using the TechMate automated histostainer (Ventana Medical Systems, Tucson, AZ). Sections 4 μm thick were cut onto slides and allowed to dry for at least 1 hour in a 57°C oven. After deparaffinization and rehydration to water, tissue sections were heated in 0.01 mol/L citric acid buffer, pH 6.0, for 20 minutes in a vegetable steamer and cooled for 5 minutes at room temperature. Tissues were pretreated with 5% normal goat serum, 2% bovine serum albumen (BSA), 0.1% Tween-20 for 10 minutes. Primary rat anti–Notch-1 or control antibody was diluted in PBS, 1% BSA, and 0.03% Tween-20 and was applied for 30 minutes at room temperature. After washing, biotinylated rabbit antirat antibody (Dako, Carpinteria, CA), was applied for 30 minutes, followed by quenching of endogenous peroxidase in 3% H2O2 for 7.5 minutes. The biotinylated secondary antibody was detected with avidin-biotin complex (ABC) (Vector, Burlingame, CA), followed by the chromogen diaminobenzidine 0.5mg/mL, 0.1% NiCl2, 0.01% H2O2. Sections were counterstained with 0.1% Acridine Orange/ 0.05% Safranin O, dehydrated, and mounted in a toluene-based mounting media (Polymount, Poly Scientific, Bay Shore, NY).
Double staining for Notch and glycophorin was performed using simultaneous biotin-streptavidin peroxidase and mouse APAAP techniques. Briefly, anti–Notch-1 (18G) and mouse antiglycophorin antibodies (Dako, Carpinteria, CA) were applied together, followed by simultaneous application of biotinylated rabbit antirat and rabbit antimouse antibody (Dako Carpinteria, CA). They were detected with pooled streptavidin peroxidase (Zymed, South San Francisco, CA) and mouse APAAP (Dako, Carpinteria, CA). Vector Red chromogen (Vector) was applied after the diaminobenzidine step. Double staining for myeloperoxidase and Notch was performed by applying rabbit antimyeloperoxidase antibody (Dako, Carpinteria, CA), which was detected with alkaline phosphatase-conjugated goat antirabbit antibody (Dako, Carpinteria, CA), followed by Vector Red chromogen. After steam pretreatment in citrate buffer, Notch staining was performed as above.
Extracellular domain of Delta-1 generation
These constructs were engineered to encode the extracellular domain of human Delta-1(amino acids 1-536, GenBank accession number AF003722, kindly provided by G. Gray, R. Mann, and S. Artavanis). cDNA sequences were subcloned into the pcDNA3, adding a Cla-1 site to the 3′ end of the truncated Delta-1. A HindIII and Cla-1 fragment containing the Delta-1 sequence was subcloned into PCS2 vector that contained sequences encoding 6 consecutive myc epitopes (kindly provided by David Turner and Hal Weintraub), so that 6 myc epitopes were expressed from the carboxy terminus. An Eco-R1 fragment containing the truncated Delta-1 and the myc tags was then subcloned into the expression vector pcDNA3.1/amp (Invitogen, San Diego, CA) that added sequences to encode 6 histidines and a stop codon at the carboxy terminus.24NSO myeloma cells were electroporated with constructs to generate stable cell lines for protein production. G418-resistant clones were screened for secretion of the fusion proteins using a quantitative enzyme-linked immunosorbent assay (ELISA). In brief, wells were coated with the 9E10 anti-myc antibody, washed, and then blocked with gelatin. Supernatant material generated by transfected cells was then added. After a period of incubation, Deltaext-myc protein was detected, using a biotinylated anti-myc tag antibody. Clones that generated the highest amounts of Deltaext-myc were expanded in roller bottles and the medium collected. The medium was then concentrated and the truncated ligand purified using methods previously reported for the purification of a truncated Jagged-1 ligand.24 This procedure involved the binding of concentrated medium to a nickel column (Ni-NTA Agarose, Qiagen, Chatsworth, CA) using the His-Bind buffer Kit (Novagen, Madison, WI), according to the manufacturer's instructions. After elution with imidizol (5 mmol/L-1 mol/L), fractions that contained Deltaext-myc were identified with Western blots using the 9E10 antibody. Positive fractions were then pooled, concentrated, and dialyzed with PBS. The protein concentration was determined by OD280. The control conditioned medium was collected from nontransfected NSO and was similarly prepared using all the steps used for purifying the ligand.
Monocyte cultures
Isolated monocytes were incubated in tissue culture wells containing Deltaext-myc or control medium. In some cases, a mouse anti-Myc antibody, 9E10, in the form of an F(ab′)2fragment, or a control murine anti-CD20 antibody F(ab′)2 fragment (kindly provided by D. Maloney, Fred Hutchinson Cancer Research Center), was adhered to a 96-well plate (Corning Incorporated, Corning, NY) at the concentration of 10 μg/mL for 30 minutes at 37°C. After washing, coated wells were blocked with Iscove's Modified Dulbecco's Medium (IMDM) containing 20% fetal bovine serum (FBS) (Hyclone, Logan, UT) for 30 minutes at 37°C. After washing, Deltaext-myc or control medium was applied to the coated well for 30 minutes at 37°C in a humidified atmosphere of 5% CO2 in air. Isolated monocytes were then cultured in these wells in the presence of 10% FCS IMDM, containing 10 ng/mL M-CSF (Peprotech, Rocky Hill, NJ) or 100 ng/mL GM-CSF (Amgen, Thousand Oaks, CA). Cultured cells were harvested by incubation with PBS, containing 200 μmol/L EDTA (Gibco, Grand Island, NY) for 15 minutes on ice to enhance the detachment of adherent cells.
Apoptosis assays
Annexin V assays were performed according to the manufacture's specifications. Briefly, cultured cells were collected, washed with binding buffer, and incubated in 200 μL of binding buffer containing 5 μL of annexin V-FITC (Pharmingen, San Diego, CA) and 1.25 μg of propidium iodide at room temperature. Apoptotic cells were analyzed using a FACScan (Becton Dickinson Co, Mountain View, CA).
Statistical analysis
A Student t test was used to determine statistical significance.
Results
Immunocytochemistry of normal bone marrow
We determined the expression of Notch-1 in formalin-fixed biopsy specimens of normal human bone marrow. As seen in Figure1A, significant staining was observed, with stained cells appearing immature, including cells with features of monocytic and/or primitive erythroid cells. Background staining with an isotype-matched control antibody was minimal (Figure 1B). To assess Notch-1 expression in erythroid cells, we double-stained sections with both anti–Notch-1 and antiglycophorin antibodies. As shown in Figure1C, a portion of glycophorin-expressing cells also expressed Notch-1. The remaining portion of Notch negative, glycophorin positive cells had the appearance of more mature erythroid cells, such as acidophilic normoblasts. To assess Notch-1 expression in granulocyte precursors, we double-stained sections with both anti–Notch-1 and antimyeloperoxidase antibodies. No overlap of staining was seen, indicating the absence of Notch-1 expression in granulocytic cells (Figure 1D). These results therefore demonstrate that substantial numbers of maturing cells expressed the Notch-1 receptor, and mainly include monocytic and erythrocytic elements.
Immunocytochemistry of normal bone marrow.
(A) shows normal bone marrow stained with anti–Notch-1 antibody. Positive staining is brown-black and the counterstain is a light-blue hematoxylin. Positive staining is seen in the cytoplasm and appears to be on the cell membrane in both erythroid precursors (seen in a patch on the left as a narrow cytoplasmic rim in round cells with round nuclei) and in myeloid precursors (seen in a patch on the right as cytoplasmic staining of polygonal cells). Background staining with an isotype-matched antibody of irrelevant specificity was minimal (B). (C) shows a double stain for Notch-1 and glycophorin, with the latter seen as red. A portion of the glycophorin-staining cells also contained Notch-1. This portion contained mainly the larger more immature glycophorin-staining cells, which the more mature, smaller erythroid precursors did not stain for Notch 1. A few cells in this field contain both. It appears that Notch-1 is lost as the cells mature and gain glycophorin. (D) shows double-staining for Notch-1 and myeloperoxidase, with the myeloperoxidase staining red. The majority of cells in this field are of the granulocytic lineage, staining with myeloperoxidase and sometimes showing polylobulated nuclei characteristic of mature neutrophils. The Notch-1 stained cells appear not to be myeloperoxidase positive, even when lightly stained. Some of the Notch-1 stained cells are clearly small, round erythroid precursors, others have a more polygonal cytoplasmic shape and sometimes a clefted nucleus consistent with a monocytic cell. All photomicrographs were taken at magnification × 60.
Immunocytochemistry of normal bone marrow.
(A) shows normal bone marrow stained with anti–Notch-1 antibody. Positive staining is brown-black and the counterstain is a light-blue hematoxylin. Positive staining is seen in the cytoplasm and appears to be on the cell membrane in both erythroid precursors (seen in a patch on the left as a narrow cytoplasmic rim in round cells with round nuclei) and in myeloid precursors (seen in a patch on the right as cytoplasmic staining of polygonal cells). Background staining with an isotype-matched antibody of irrelevant specificity was minimal (B). (C) shows a double stain for Notch-1 and glycophorin, with the latter seen as red. A portion of the glycophorin-staining cells also contained Notch-1. This portion contained mainly the larger more immature glycophorin-staining cells, which the more mature, smaller erythroid precursors did not stain for Notch 1. A few cells in this field contain both. It appears that Notch-1 is lost as the cells mature and gain glycophorin. (D) shows double-staining for Notch-1 and myeloperoxidase, with the myeloperoxidase staining red. The majority of cells in this field are of the granulocytic lineage, staining with myeloperoxidase and sometimes showing polylobulated nuclei characteristic of mature neutrophils. The Notch-1 stained cells appear not to be myeloperoxidase positive, even when lightly stained. Some of the Notch-1 stained cells are clearly small, round erythroid precursors, others have a more polygonal cytoplasmic shape and sometimes a clefted nucleus consistent with a monocytic cell. All photomicrographs were taken at magnification × 60.
Immunofluoresence analysis of normal bone marrow and peripheral blood
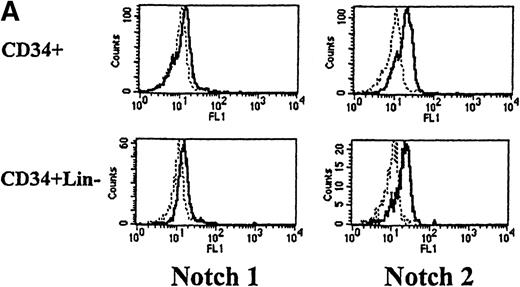
We next used flow cytometry to study Notch-1 and Notch-2 expression by marrow cells. In the CD34+ population, there was a small homogenous shift in the fluorescence intensity curve after staining with either Notch-1 or Notch-2 antibodies, indicating relatively low expression of both Notch-1 and Notch-2 by essentially all cells (see representative examples in Figure 2A). This result was reproducibly seen in 4 individuals. Similarly, low levels of Notch-1 and Notch-2 were also found on the less differentiated CD34+ CD33−Lin− subpopulation (excludes cells expressing antigens associated with T [CD2] or B [CD19] cells). To assess Notch expression in more mature myeloid populations, marrow cells were double-stained with either Notch-1 or Notch-2 antibodies and either an anti-CD14 antibody to identify monocytes or an anti-CD15 antibody to identify granulocytes. Notch-1 and Notch-2 were seen in substantial levels on a major portion of cells that expressed CD14 (Figure 2B). The fluorescence intensity of Notch-1 staining was greater on the CD14+ cells than on CD34+ cells. In contrast, no Notch-1 or Notch-2 was detected on CD15+ cells. To assess Notch expression in developing erythroid cells, marrow cells were double-stained with either Notch-1 or Notch-2 antibodies and with antiglycophorin A antibodies. Little or no Notch-1 and Notch-2 was detected in cells that expressed glycophorin A (Figure 2B). However, further analysis revealed low levels of Notch-1 in cells expressing low levels of glycophorin A, suggesting that, as in the immunocytochemistry studies, immature erythroid precursors express Notch-1 (data not shown). Notch-2 was not detected in cells expressing either high or low levels of glycophorin A (data not shown).
Expression of Notch-1 and Notch-2 by human bone marrow cells and peripheral blood.
(A) Fluorescence histograms of human bone marrow cells stained with anti–Notch-1 and -2 antibodies and CD34, or CD34 and the absence of other antigens associated with lineage commitment. The x-axis represents log fluorescence intensity and the y-axis represents cell number. The solid line represents staining with anti–Notch-1 and -2 antibodies, and the dashed line represents staining with an isotype-matched control antibody of irrelevant specificity. (B) Notch-1 and -2 expression by human bone marrow cells committed to monocytic (CD14), granulocytic (CD15), and erythroid (glycophorin A) lineages. The x-axis represents log fluorescence intensity and the y-axis represents cell number. The solid line represents staining with anti–Notch 1-and 2-antibodies, and the dashed line represents staining with an isotype-matched control antibody. (C) Notch-1 and -2 expression by human peripheral blood cells. Fluorescence histograms for 2 color studies gated on cells committed to monocytic (CD14), granulocytic (CD15), T-cell (CD4, 8), or B-cell (CD20) lineages are shown. The x-axis represents log fluorescence intensity and the y-axis represents cell number. The solid line represents staining with anti–Notch-1 and -2 antibodies, and the dashed line represents staining with an isotype-matched control antibody.
Expression of Notch-1 and Notch-2 by human bone marrow cells and peripheral blood.
(A) Fluorescence histograms of human bone marrow cells stained with anti–Notch-1 and -2 antibodies and CD34, or CD34 and the absence of other antigens associated with lineage commitment. The x-axis represents log fluorescence intensity and the y-axis represents cell number. The solid line represents staining with anti–Notch-1 and -2 antibodies, and the dashed line represents staining with an isotype-matched control antibody of irrelevant specificity. (B) Notch-1 and -2 expression by human bone marrow cells committed to monocytic (CD14), granulocytic (CD15), and erythroid (glycophorin A) lineages. The x-axis represents log fluorescence intensity and the y-axis represents cell number. The solid line represents staining with anti–Notch 1-and 2-antibodies, and the dashed line represents staining with an isotype-matched control antibody. (C) Notch-1 and -2 expression by human peripheral blood cells. Fluorescence histograms for 2 color studies gated on cells committed to monocytic (CD14), granulocytic (CD15), T-cell (CD4, 8), or B-cell (CD20) lineages are shown. The x-axis represents log fluorescence intensity and the y-axis represents cell number. The solid line represents staining with anti–Notch-1 and -2 antibodies, and the dashed line represents staining with an isotype-matched control antibody.
To assess Notch expression in peripheral blood, cells were double-stained with antibodies to either Notch-1 or Notch-2 and antibodies to CD14, CD15, CD4, and CD8 (antigens expressed by subsets of T lymphocytes) and CD20 (antigen expressed by B cells). As observed in marrow, both Notch-1 and Notch-2 were detected in CD14+ monocytes, but not CD15+ granulocytes (Figure 2C). Both Notch-1 and Notch-2 were also found on CD4+ and CD8+ cells. Moreover, as shown in Figure 2C, a slightly greater staining intensity was seen with Notch-1 in the CD8+ cells, compared with the CD4+ T cells. This difference was seen in 7 of 10 individuals studied (data not shown). Notch-2 staining, however, was essentially equivalent in both CD4+ and CD8+ T cells. In addition, both Notch-1 and Notch-2 were detected in CD20+ B cells.
Effect of Notch ligand on CD14+ monocytes in the presence of M-CSF
Because monocytes express the highest amounts of Notch-1 and Notch-2 within the myeloid lineages, we further investigated the effect of Notch activation on isolated monocytes in vitro. Monocytes were isolated from Ficoll-Hypaque–separated peripheral blood mononuclear cells, based on forward and right angle light-scatter properties. Isolated cells were shown to be more than 95% positive for CD14 staining (data not shown). These cells were incubated with a truncated form of the extracellular domain of the Notch ligand, Delta-1, which contained the highly conserved DSL domain, thought to be critical for activation of Notch and all the epidermal growth factor (EGF) repeats. The truncated Delta-1 was further engineered to contain 6 myc tag epitopes at the carboxy-terminus, Deltaext-myc (Figure3A). Previous studies have demonstrated that the differentiation of C2 myoblasts is inhibited by overexpressing the constitutively active intracellular domain of Notch-1 or exposure to cell-membrane–expressed Notch ligand.14 33 More recent studies in our laboratory revealed that Deltaext-mycinhibited differentiation of C2 cells, but only if it was first immobilized on the plastic surface of the culture wells (Varnum-Finney, submitted). We therefore tested the effect of Deltaext-mycin a solution as well as in an immobilized form on monocyte growth and differentiation. Deltaext-myc was immobilized by attaching it to plastic via its myc tags, by first binding 9E10 anti-myc F(ab′)2 antibody fragments to the plastic surface. We used F(ab′)2 fragments because the whole antibody appeared to affect monocytes in culture, presumably as a result of the effects of the Fc domain.
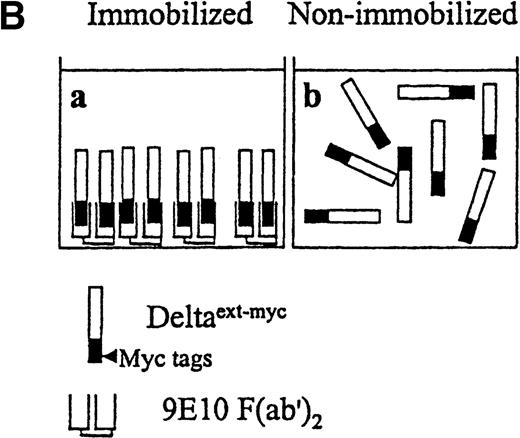
Presentation of Deltaext-myc.
(A) A schematic diagram of the truncated form of the extracellular domain of Delta-1, Deltaext-myc. The construct contains the extracellular domain of Delta, including the signal peptide (SP), conserved DSL domain, EGF repeats, and 6 myc tag epitopes. (B) A schematic diagram of the presentation of Deltaext-myc. Each panel represents a mode of presentation of Deltaext-myc. In Panel A, Deltaext-myc is presented in immobilized form, bound to 9E10 F(ab′)2 antibody fragments that is bound to the plastic. In Panel B, Deltaext-myc is presented in the absence of antibody fragments (see “Materials and methods”).
Presentation of Deltaext-myc.
(A) A schematic diagram of the truncated form of the extracellular domain of Delta-1, Deltaext-myc. The construct contains the extracellular domain of Delta, including the signal peptide (SP), conserved DSL domain, EGF repeats, and 6 myc tag epitopes. (B) A schematic diagram of the presentation of Deltaext-myc. Each panel represents a mode of presentation of Deltaext-myc. In Panel A, Deltaext-myc is presented in immobilized form, bound to 9E10 F(ab′)2 antibody fragments that is bound to the plastic. In Panel B, Deltaext-myc is presented in the absence of antibody fragments (see “Materials and methods”).
Increased amounts of Deltaext-myc were added to tissue culture wells with or without anti-myc F(ab′)2antibody fragments bound to the plastic (Figure 3B). After 30 minutes, monocytes were incubated in these wells in the presence of M-CSF, a cytokine known to promote monocyte survival and differentiation into macrophages.34 35 After 6 days, there was a substantially lower number of cells in cultures containing Deltaext-myc that was immobilized by attachment to plastic-bound antibody fragments in comparison with cultures with only nonimmobilized Deltaext-myc (Figure4A). The reduction in cell numbers in cultures containing immobilized Deltaext-myc was maximal at 1 μg/mL of ligand, consistent with ELISAs demonstrating maximal saturation of the plastic-bound antibody by this dose of ligand (data not shown). As additional controls, we evaluated the effect of medium that was collected from nontransfected NSO cells and purified identically to Deltaext-myc (control medium), and also evaluated the effect of Deltaext-myc in wells coated with a control F(ab′)2 fragment. We found no effect of the control material in the presence of the plastic-bound 9E10 antibody fragments, compared with Deltaext-myc (Figure 4B). Nor did we find an effect of Deltaext-myc in the presence of the control, isotype-matched anti-CD20 F(ab′)2 antibody fragments, compared with the anti-myc antibody fragments, whereas significant decreases in cell numbers were again seen in wells containing both Deltaext-myc and the plastic-bound anti-myc antibody fragments (Figure 4B).
Effect of Deltaext-myc on monocyte survival.
Ten thousand monocytes were cultured for 6 days with 10% FCS IMDM with 10 ng/mL of M-CSF. In panel A, the effect of immobilized and nonimmobilized ligand was compared by adding the soluble Deltaext-myc (0, 0.3, 1, 3, and 10 μg/mL) to plastic wells that were previously coated (●) or not coated (○) with 10 μg/mL of 9E10 F(ab′)2 anti-myc antibody fragments. In panel B, controls consisting of purified control medium from noninfected NSO cells and control antibody fragments are shown. Monocytes (5 × 103) were cultured with M-CSF in the presence of 1 μg/mL of Deltaext-myc or control medium in wells coated with 9E10 F(ab′)2 or anti-CD20 F(ab′)2 antibody fragments. (*) represents statistical differences between cultures containing Deltaext-myc in the presence of 9E10 F(ab′)2 antibody fragments and either cultures containing control medium in the presence of 9E10 F(ab′)2 antibody fragments or cultures containing Deltaext-myc in the presence of control, anti-CD20 F(ab′)2 antibody fragments.
Effect of Deltaext-myc on monocyte survival.
Ten thousand monocytes were cultured for 6 days with 10% FCS IMDM with 10 ng/mL of M-CSF. In panel A, the effect of immobilized and nonimmobilized ligand was compared by adding the soluble Deltaext-myc (0, 0.3, 1, 3, and 10 μg/mL) to plastic wells that were previously coated (●) or not coated (○) with 10 μg/mL of 9E10 F(ab′)2 anti-myc antibody fragments. In panel B, controls consisting of purified control medium from noninfected NSO cells and control antibody fragments are shown. Monocytes (5 × 103) were cultured with M-CSF in the presence of 1 μg/mL of Deltaext-myc or control medium in wells coated with 9E10 F(ab′)2 or anti-CD20 F(ab′)2 antibody fragments. (*) represents statistical differences between cultures containing Deltaext-myc in the presence of 9E10 F(ab′)2 antibody fragments and either cultures containing control medium in the presence of 9E10 F(ab′)2 antibody fragments or cultures containing Deltaext-myc in the presence of control, anti-CD20 F(ab′)2 antibody fragments.
Time course of reduction of monocyte numbers
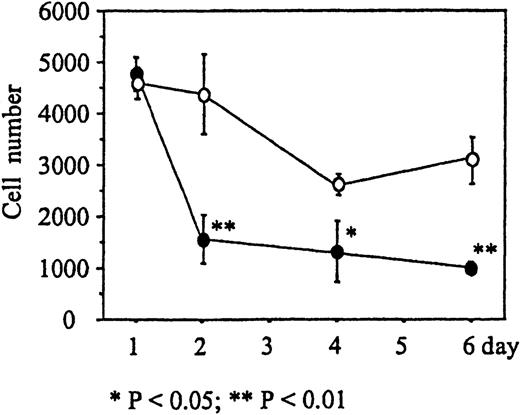
We next determined the time course of the reduction of cell numbers due to immobilized Deltaext-myc. Monocytes were incubated in wells with 9E10 antibody F(ab′)2 fragments attached to the surface containing either Deltaext-myc or control medium. M-CSF was then added to the wells and, after 1, 2, 4, and 6 days, the cells were collected and counted. The number of monocytes in the presence of Deltaext-myc decreased dramatically until day 3, where 36% of the cell numbers in the control well was seen, and afterward declined at a more gradual rate similar to that seen in control cultures (Figure 5). This rapid reduction of the number of cells during the initial 2 days of culture was observed using monocytes obtained from each of 3 different individuals (Table 1).
Effect of Deltaext-myc on monocyte survival.
The number of monocytes present in cultures containing 10% FCS IMDM and 10 ng/mL M-CSF in the presence of 1 μg/mL of Deltaext-myc (•) or control (○). Tissue culture wells were coated with anti-myc antibody, 9E10 F(ab′)2 to attach the myc containing Deltaext-myc to the plastic surface. Ten thousand monocytes were added to each well and after 1, 2, 4, and 6 days, cells were collected and counted. (*) represents statistical difference compared with the respective control.
Effect of Deltaext-myc on monocyte survival.
The number of monocytes present in cultures containing 10% FCS IMDM and 10 ng/mL M-CSF in the presence of 1 μg/mL of Deltaext-myc (•) or control (○). Tissue culture wells were coated with anti-myc antibody, 9E10 F(ab′)2 to attach the myc containing Deltaext-myc to the plastic surface. Ten thousand monocytes were added to each well and after 1, 2, 4, and 6 days, cells were collected and counted. (*) represents statistical difference compared with the respective control.
Effects of Deltaext-myc on monocyte survival
| . | Cell Number at Day 0 . | Cell Number at Day 2 . | PValue . |
|---|---|---|---|
| Exp 1 | |||
| Delta | 10 000 | 1562.7 ± 472.1 | <.01 |
| Cont | 4375.0 ± 780.7 | ||
| Exp 2 | |||
| Delta | 5,000 | 798.7 ± 597.2 | <.05 |
| Cont | 3096.0 ± 841.1 | ||
| Exp 3 | |||
| Delta | 5,000 | 261.4 ± 104.2 | <.01 |
| Cont | 1176.0 ± 279.5 |
| . | Cell Number at Day 0 . | Cell Number at Day 2 . | PValue . |
|---|---|---|---|
| Exp 1 | |||
| Delta | 10 000 | 1562.7 ± 472.1 | <.01 |
| Cont | 4375.0 ± 780.7 | ||
| Exp 2 | |||
| Delta | 5,000 | 798.7 ± 597.2 | <.05 |
| Cont | 3096.0 ± 841.1 | ||
| Exp 3 | |||
| Delta | 5,000 | 261.4 ± 104.2 | <.01 |
| Cont | 1176.0 ± 279.5 |
The number of monocytes present after 2 days in cultures containing 10% FCS IMDM and 10 ng/mL M-CSF in the presence of 1 μg/mL Deltaext-myc or control. Tissue culture wells were coated with anti-myc antibody, 9E10 F(ab′)2 to attach the myc-containing Deltaext-myc construct to the plastic surface. The 5-10 × 103 monocytes from different individuals were added to each well and, after 2 days, cells were collected and counted.
Apoptosis of monocytes in the presence of Deltaext-myc and macrophage colony-stimulating factor
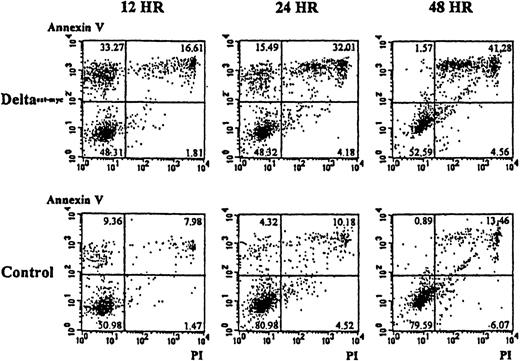
To determine whether the lower numbers of cells present in cultures of monocytes with immobilized Deltaext-myc and M-CSF resulted from an increase in programmed cell death, apoptosis was evaluated using annexin V and, as an indication of cell viability, propidium iodide. As shown in Figure 6A, cultures containing the immobilized Deltaext-myc showed an approximately 3-fold increase in the proportion of cells undergoing apoptosis that stained positively for annexin V but did not stain with propidium iodide, compared with cells from control cultures at 12 hours. By 48 hours, there was an increase in the portion of cells staining positively with propidium iodide as well as annexin V, suggesting the cells proceeded to a necrotic state (Figure 6). This result was seen with cells from 2 additional individuals (data not shown).
Induction of apoptosis in monocytes by Deltaext-myc in the presence of M-CSF.
Ten thousand monocytes were cultured with 10% FCS IMDM containing M-CSF (10 ng/mL) in the presence of Deltaext-myc (1 μg/mL) or as a control, control material from nontransfected NSO cells. All wells were coated with anti-myc 9E10 F(ab′)2 fragments. Cells were harvested at 12, 24, and 48 hours, washed with binding buffer, and incubated in binding buffer containing annexin V-FITC and PI. The x-axis represents log fluorescence intensity with PI staining; the y-axis represents log fluorescence intensity with annexin V staining.
Induction of apoptosis in monocytes by Deltaext-myc in the presence of M-CSF.
Ten thousand monocytes were cultured with 10% FCS IMDM containing M-CSF (10 ng/mL) in the presence of Deltaext-myc (1 μg/mL) or as a control, control material from nontransfected NSO cells. All wells were coated with anti-myc 9E10 F(ab′)2 fragments. Cells were harvested at 12, 24, and 48 hours, washed with binding buffer, and incubated in binding buffer containing annexin V-FITC and PI. The x-axis represents log fluorescence intensity with PI staining; the y-axis represents log fluorescence intensity with annexin V staining.
We also investigated the morphology and nonspecific esterase staining of cells surviving in the presence or absence of immobilized Delta. After 6 days of culture with M-CSF and immobilized Deltaext-myc, cytocentrifuge slides of the cells were prepared and stained with hematoxylin and eosin, and with nonspecific esterase. Cells obtained from cultures with or without ligand had the appearance of macrophages and stained positively with nonspecific esterase, indicating that in each case these cells had differentiated into macrophages (data not shown).
Apoptosis occurs in the presence of macrophage colony-stimulating factor but not granulocyte-macrophage colony-stimulating factor
To determine whether the induction of apoptosis by Deltaext-myc was specific for M-CSF, we evaluated the effect of another cytokine known to support the proliferation and differentiation of monocytes, ie, GM-CSF.36-38 Although substantial proportion of cells in cultures with M-CSF and immobilized Deltaext-myc underwent apoptosis (annexin V+ propidium iodide−) after 12 hours, we saw no significant difference between cultures containing GM-CSF, or GM-CSF plus M-CSF (Figure7). This result was seen with cells from 2 additional individuals (data not shown). These data indicate that apoptosis due to Deltaext-myc is induced in a cytokine-specific manner. Furthermore, because apoptosis was not seen in the presence of GM-CSF and M-CSF, GM-CSF appears to abrogate the expected apoptosis induced by Deltaext-myc in the presence of M-CSF.
Induction of apoptosis in monocytes by Deltaext-myc in the presence of M-CSF compared to GM-CSF.
Monocytes were cultured with 10% FCS IMDM containing M-CSF (10 ng/mL), GM-CSF (100 ng/mL), or M-CSF plus GM-CSF in the presence of Deltaext-myc (1 μg/mL) or purified control medium. All wells were coated with anti-myc 9E10 F(ab′)2fragments. Cells were harvested at 12 hours, washed with binding buffer, and incubated in binding buffer containing annexin V-FITC and PI. The mean and standard error of annexin V positive cells in the PI negative window for the 3 wells are shown. (*) represents statistical difference compared with respective control.
Induction of apoptosis in monocytes by Deltaext-myc in the presence of M-CSF compared to GM-CSF.
Monocytes were cultured with 10% FCS IMDM containing M-CSF (10 ng/mL), GM-CSF (100 ng/mL), or M-CSF plus GM-CSF in the presence of Deltaext-myc (1 μg/mL) or purified control medium. All wells were coated with anti-myc 9E10 F(ab′)2fragments. Cells were harvested at 12 hours, washed with binding buffer, and incubated in binding buffer containing annexin V-FITC and PI. The mean and standard error of annexin V positive cells in the PI negative window for the 3 wells are shown. (*) represents statistical difference compared with respective control.
Discussion
To determine cell lineages in which Notch-mediated interactions may be relevant in cell-fate decisions, we evaluated the maturation-dependent expression of 2 members of the Notch receptor family, Notch-1 and Notch-2, using immunocytochemistry and quantitative immunofluoresence. We detected low levels of Notch-1 and Notch-2 protein on both CD34+ and CD34+ Lin− cells and found that these levels in CD34+ cells increased as they matured into monocytes, but decreased as they differentiated into granulocytes. Surprisingly, the greatest amounts of Notch receptor expression were in the more mature cells, with monocytes expressing the highest amounts of Notch-1 within the myeloid lineages. It was thought possible that the Notch activity in monocytes may affect their survival and/or subsequent decisions regarding further differentiation into macrophages. Because the survival of monocytes and their differentiation into macrophages are promoted by the cytokine, M-CSF, we first determined the effect of a truncated Notch ligand, Delta-1, on monocytes in the presence of this cytokine.34 35 Previous studies in our laboratory have indicated that the presentation of this ligand, Deltaext-myc in immobilized form, but not in solution, induced inhibition of muscle differentiation by C2 myoblast cells, indicative of Notch activation (Varnum-Finney et al, submitted). In those studies, a second construct, Deltaext-IgG, consisting of the extracellular domain of Delta-1 and the Fc portion of human IgG1, and a control construct, ControlIgG, consisting of the Jagged-1 signal peptide fused to the Fc portion of human IgG1, were also studied. Immobilized Deltaext-IgG, as well as Deltaext-myc, but not immobilized ControlIgG, inhibited C2 myoblast differentiation, further demonstrating the specificity of the effect (Varnum-Finney et al, submitted). It was not possible, however, to use these latter constructs for studies of monocytes, because the Fc domain of the constructs affected monocyte survival. Deltaext-myc was therefore presented in an immobilized form tethered to the surface of the culture well by plastic-bound anti-myc F(ab′)2 antibody fragments, which recognized myc-tags included in the engineered ligand.
In this study, we observed significant apoptosis in monocytes after incubation with immobilized Deltaext-myc in the presence of M-CSF. Although immobilization of the truncated ligand was required for the induction of cell death in monocytes, other studies have found that nonimmobilized soluble forms of a Notch ligand can inhibit oligodendrocyte differentiation or neurite retraction in vitro as indicators of Notch activation.39,40 Moreover, conflicting data has also been observed in vivo, where soluble, truncated forms have caused inhibitory as well as activating effects on Notch signaling.41 42 Further studies will be required to determine the responsible mechanisms and reasons for differences between different cell systems and possibly between the different ligand forms used in these experiments.
Monocytes are known to undergo spontaneous apoptosis in the absence of specific survival signals, but on differentiation into macrophages, become more resistant to cell death.43-45 Because M-CSF is known to promote the survival of monocytes and their differentiation into macrophages,34 35 it is tempting to speculate that Notch activation inhibited M-CSF–induced signaling, thereby enhancing apoptosis. A portion of cells did, however, survive in cultures containing Deltaext-myc. These cells, which were nonspecific esterase positive macrophages, may have been derived from more mature cells that are more resistant to apoptosis, or from cells less sensitive to effects of Deltaext-myc.
We further found that apoptosis was induced in the presence of M-CSF but not GM-CSF. Thus, the effect of Notch signaling is a cytokine specific one. Although the mechanism for this specificity is not known, studies in 32D cells have shown that the constitutively active forms of the intracellular domains of Notch-1 and Notch-2 inhibited the differentiation of 32D cells in response to specific cytokines.28 These studies found that the activated, intracellular domain of Notch-1 was inhibitory to G-CSF–induced differentiation, and the Notch-2 intracellular domain was inhibitory to GM-CSF–induced differentiation. These studies further suggested that a specific Notch domain, termed the Notch cytokine response region, contributed to this cytokine specificity.28 Alternatively, Notch signaling inhibits the transcriptional expression of genes that are normally induced by M-CSF and are part of the differentiation and survival program required for M-CSF–induced terminal macrophage differentiation. In this case, the expression of those genes induced by GM-CSF–induced signaling may not be inhibited by the Notch pathway. Consistent with this notion is the observation that M-CSF and GM-CSF generate macrophages that are phenotypically and functionally distinct.37,38 We are currently exploring whether, in the presence of GM-CSF, Notch signaling allows the survival of cells with the appearance of macrophages, but which are not terminally committed and retain the capacity to either further differentiate along the macrophage lineage, or along an alternative cellular pathway, ie, dendritic cells.46-48
A role for Notch signaling in preventing apoptosis has already been determined in T-cell development. Notch-1–mediated signaling has been found obligatory for T-cell development,49 and further evidence indicates an antiapoptotic effects of Notch-1 signaling in immature CD4+ CD8+ T cells and T-cell lines. In one study, overexpression of constitutively active intracellular domain of Notch-1 inhibited glucocorticoid-induced apoptosis of a T-cell leukemic cell line.50 Further studies have shown that Notch-mediated effects on cell survival in T cells are mediated by Nur-77.51 The finding in this study of an inductive rather than inhibitory effect on apoptosis in monocytes, taken together with results of studies of T cells, clearly indicates a more general role for Notch signaling in regulating hematopoietic cell survival. The widespread expression of at least 2 Notch receptors in developing hematopoietic cells and their lineage-restricted expression by the progeny of multipotent precursors is consistent with a role for Notch signaling in a variety of cell-fate decision during hematopoietic development, including decisions by more mature blood elements. Relatively little is known, however, about potential differences in signaling induced by the different Notch receptors. We found that Notch-2 staining levels were slightly higher relative to Notch-1 in CD34+ and CD34+ Lin− cells, whereas staining levels with Notch-1 were relatively greater than with Notch-2 on monocytes and T cells, and only Notch-1 was detected in erythroid precursors. These differences in the relative expression of Notch-1 and Notch-2 by cell subsets suggest the possibility of distinct roles for the different Notch receptors. Recent evidence that Notch-1 and Notch-2 may affect distinct cytokine-activated signaling pathways further supports the notion of distinct activities for the different Notch paralogs.28 It is also not known whether the ligand used in this study preferentially activates 1 or more of the different Notch receptors.
Although this study has focused on Notch-1 and Notch-2, at least 4 members of the Notch family9-13 and 5 Notch ligands14-21 have been identified in vertebrates. Thus, a more complex story of Notch and Notch ligand interactions is likely to unfold. Nonetheless, it is clear that future evaluations of the ontogeny of expression of these molecules in hematopoietic development may provide important insights into their role in development as well as insights in guiding experiments to determine their physiologic role. These studies also indicated a role for Notch signaling in regulating more mature myeloid elements, suggesting further investigations of the role of Notch–mediated cellular interactions on monocyte differentiation.
Acknowledgments
We thank Monica Yu, Carolyn Brashem-Stein, Steven Staats, Doug Wilson, and Rita E. Ryncarz for their excellent technical assistance; Dr Robert G. Andrews and Dr Louise E. Purton for critically reviewing the manuscript; and Lynn Planet for preparation of the manuscript.
Supported by NIH grants HL54881, CA-18029, and CA-15704. K.O. is a Fellow of the Leukemia Society of America. I.D.B is supported as a Clinical Research Professor by the American Cancer Society.
Reprints:Irvin D. Bernstein, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, C1-169, Seattle WA 98109.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal